Microscopic Image Segmentation and Morphological Characterization of Novel Chitosan/Silica Nanoparticle/Nisin Films Using Antimicrobial Technique for Blueberry Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Films Preparation and Production
2.1.2. Sample Treatments
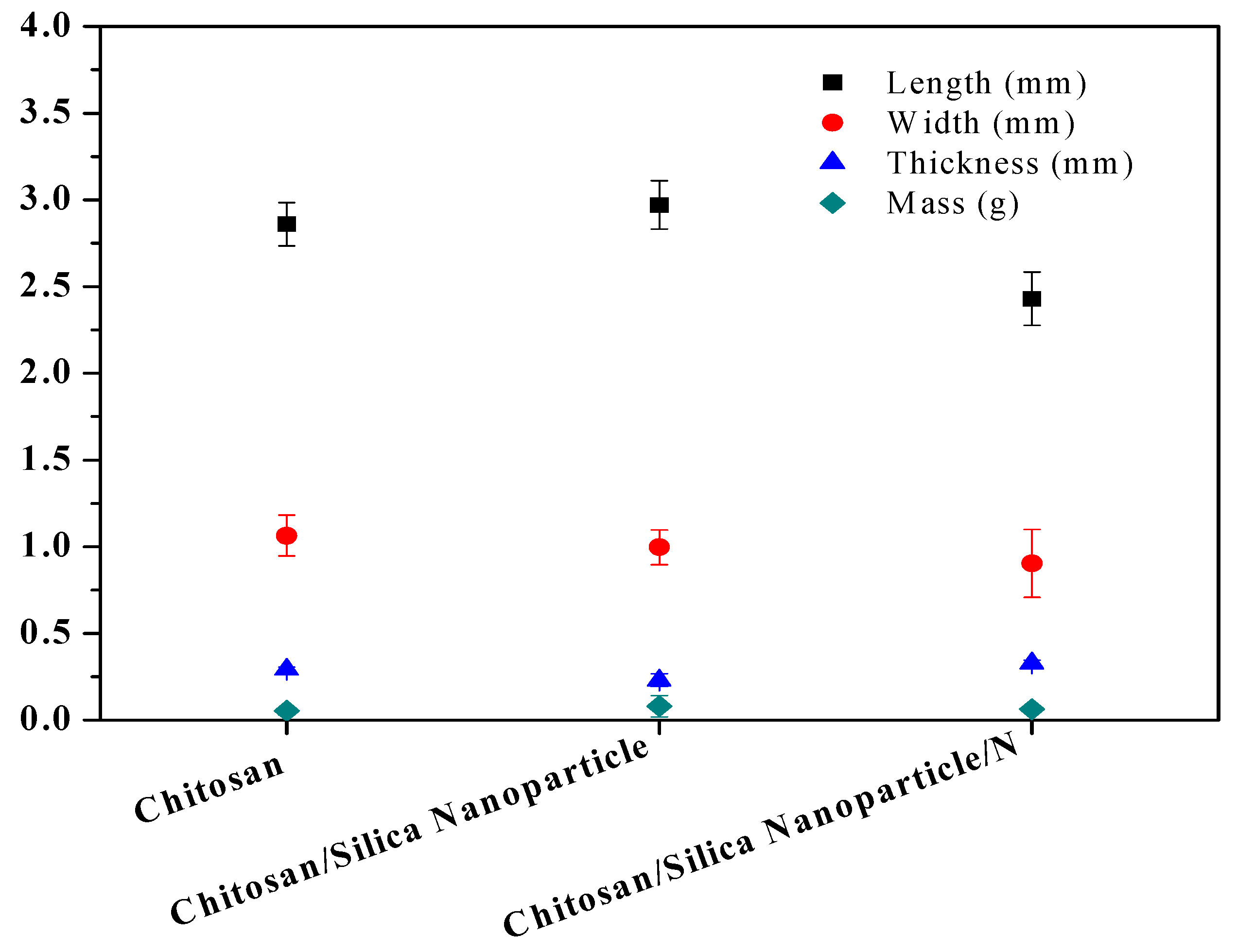
2.1.3. Determination of Morphological Properties
2.1.4. Determination of Film Color
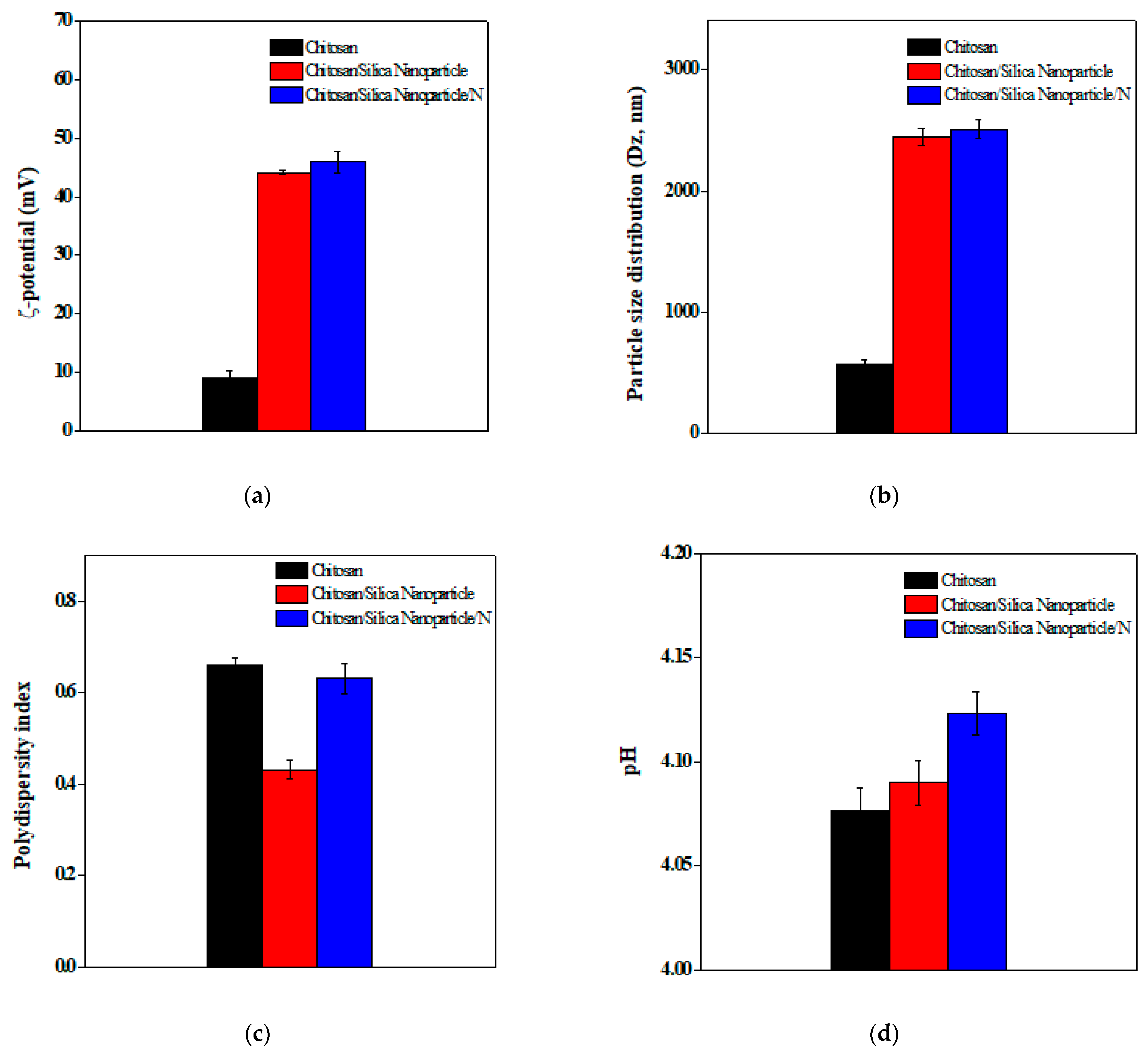
2.1.5. Determination of ζ-Potential, Particle Size Distribution, and Polydispersity Index
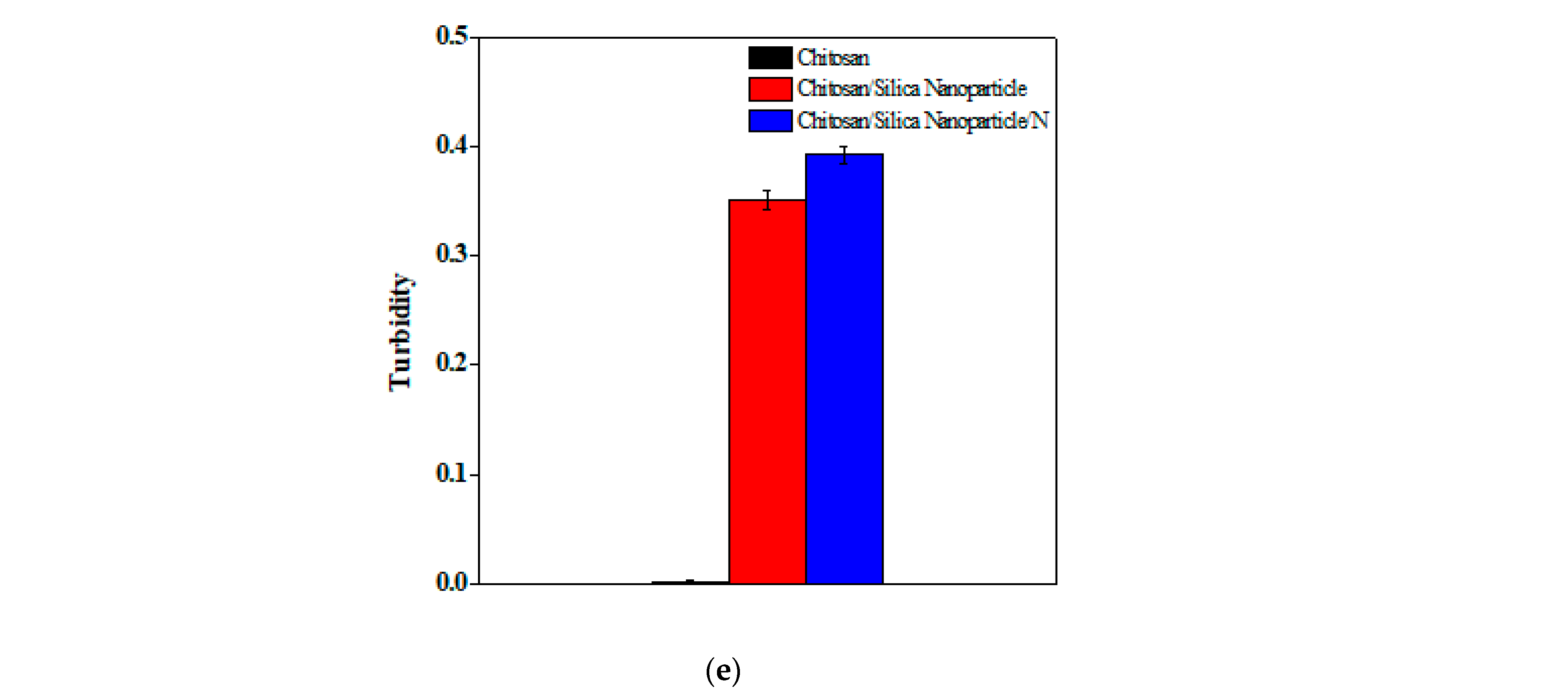
2.1.6. Determination of Acidity and Turbidity
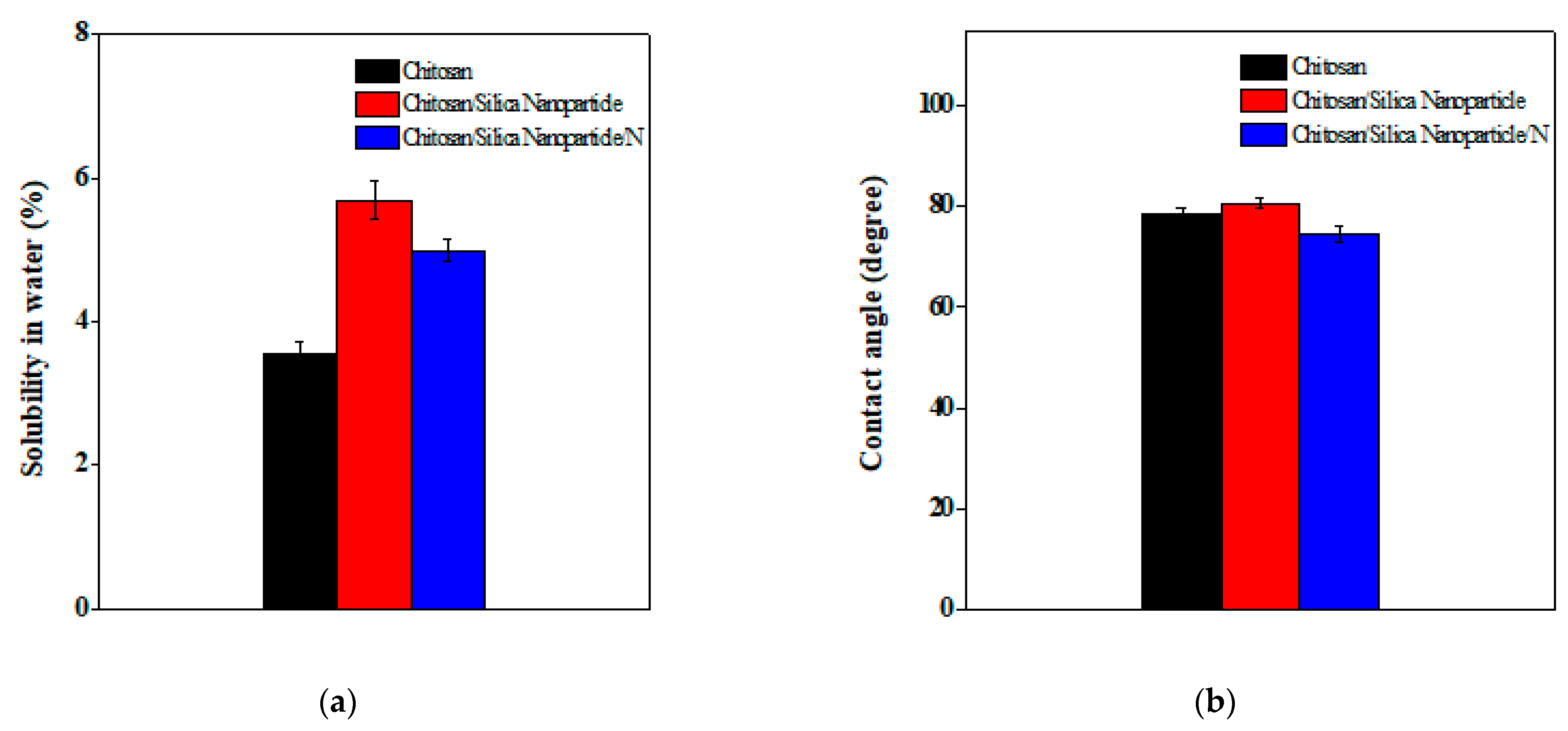
2.1.7. Determination of Solubility in Water and Contact Angle
2.1.8. Determination of Mechanical Tensile Strength Tests
2.1.9. Determination of Microbial Contamination Analysis
2.1.10. Microscopic Images Dataset
2.2. Microscopic Image Segmentation Methodology
2.2.1. Image Processing Steps
2.2.2. Image Enhancement (Pre-Processing)
2.2.3. Segmentation (Processing)
2.2.3.1. Conversion Step RGB to L*a*b
2.2.3.2. K-Means Clustering
2.2.3.3. Median Filter and Binarization
2.2.4. Regions Characterization (Post-Processing)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Physical–Chemical Characteristics
3.2. Solubility in Water and Contact Angle
3.3. Mechanical Properties
3.4. Microbial Contamination Analysis
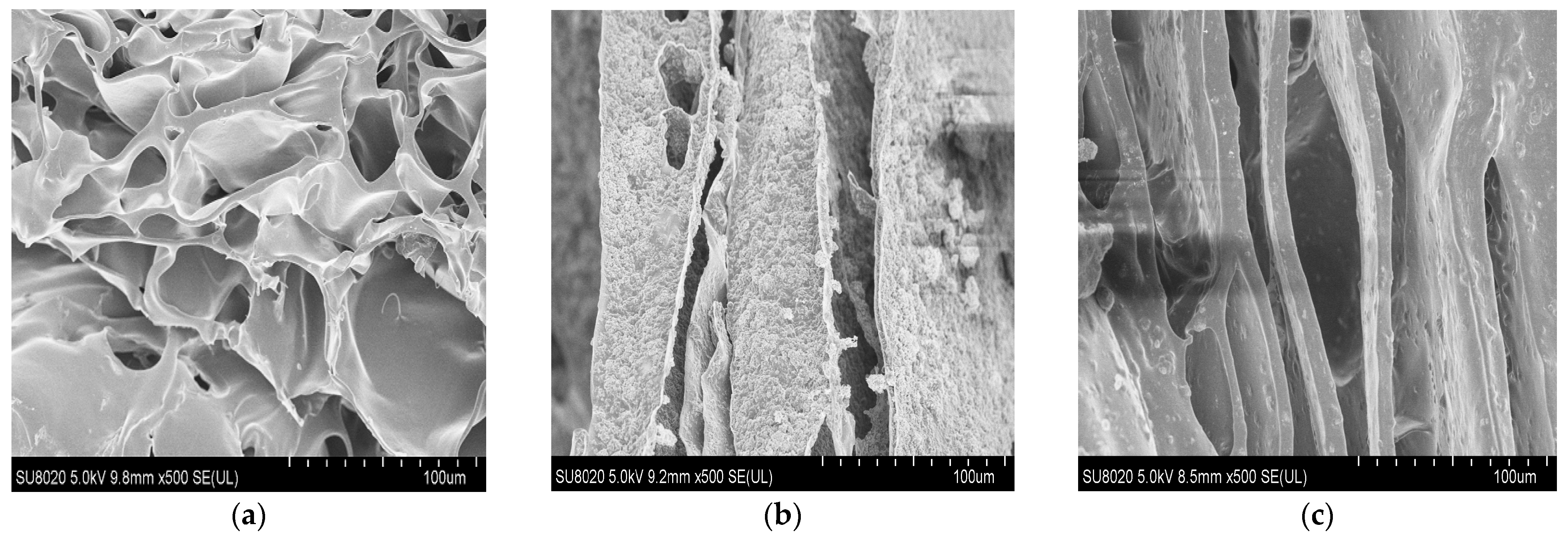
3.5. Morphological Properties
3.6. Color Index
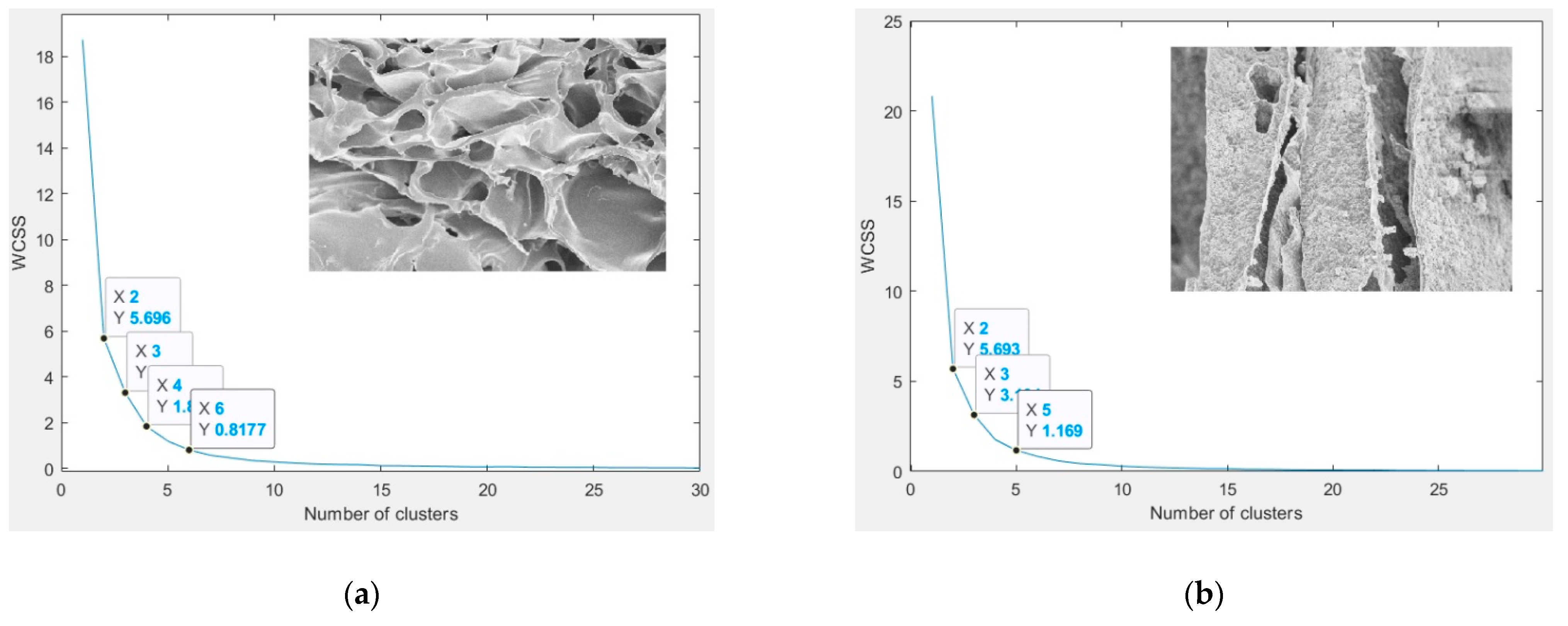
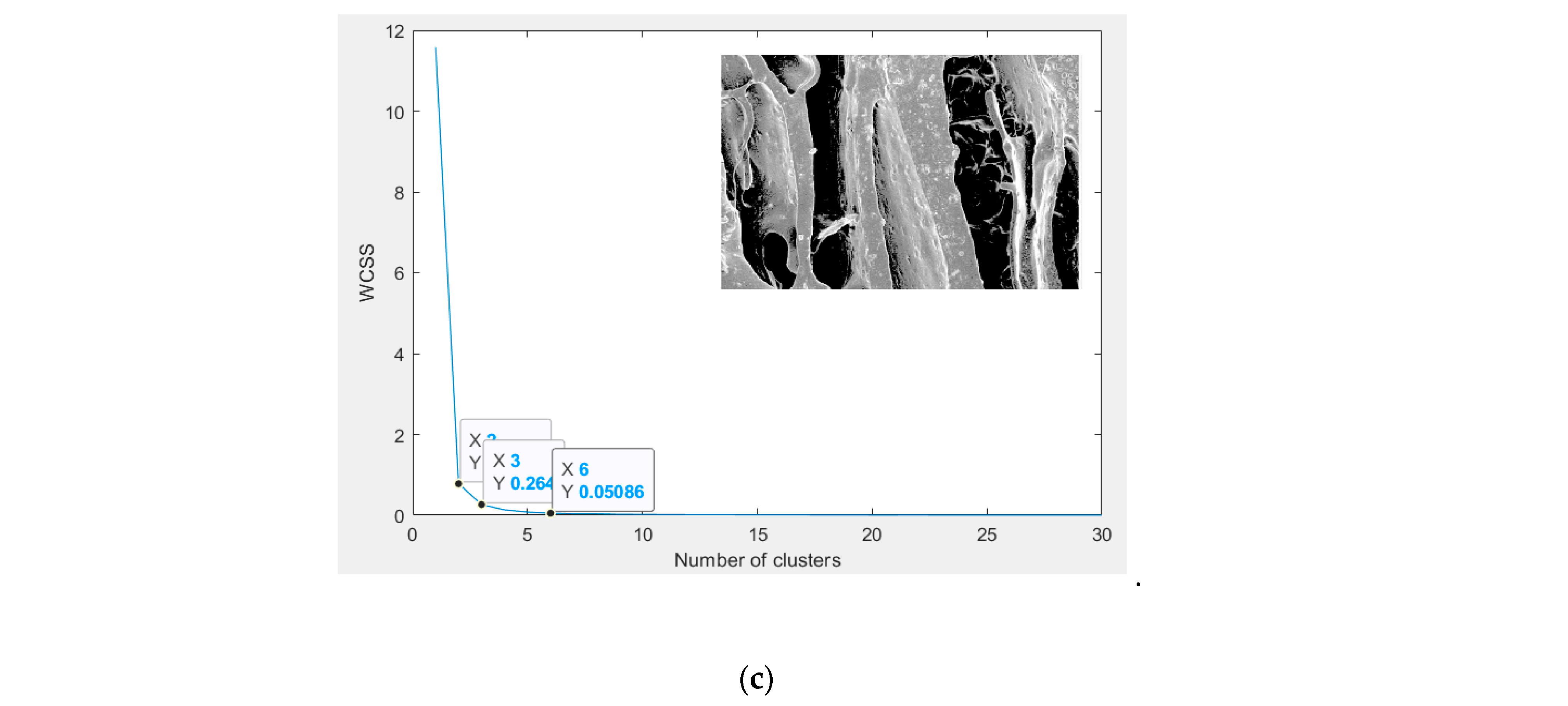
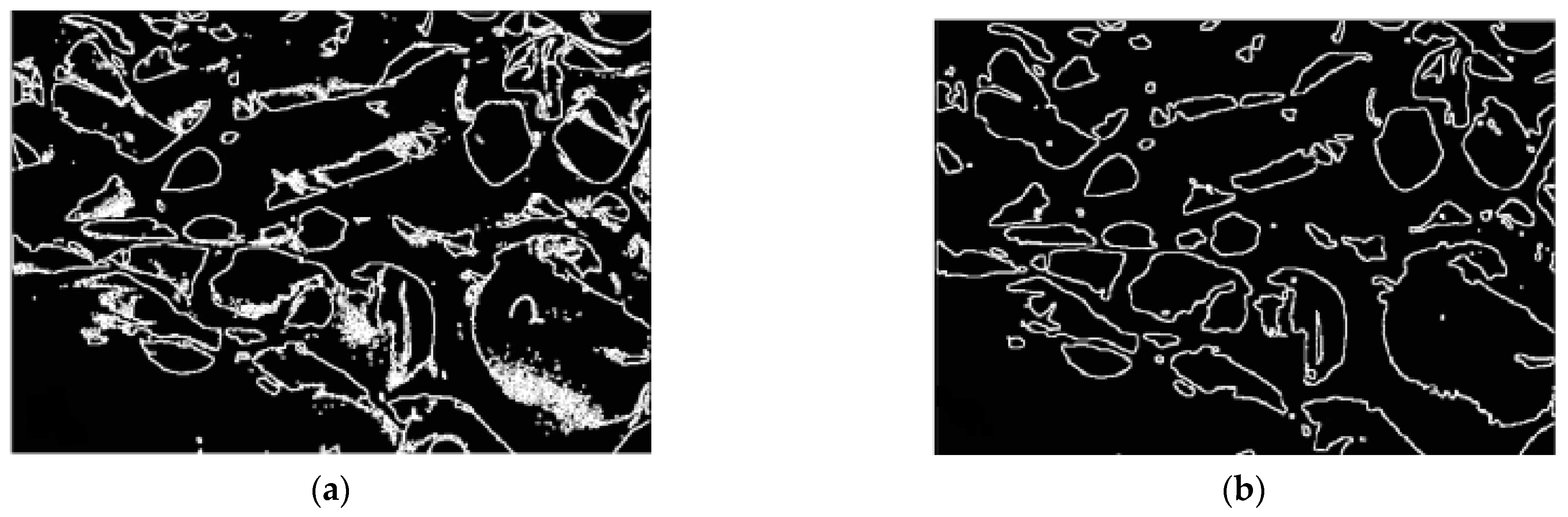
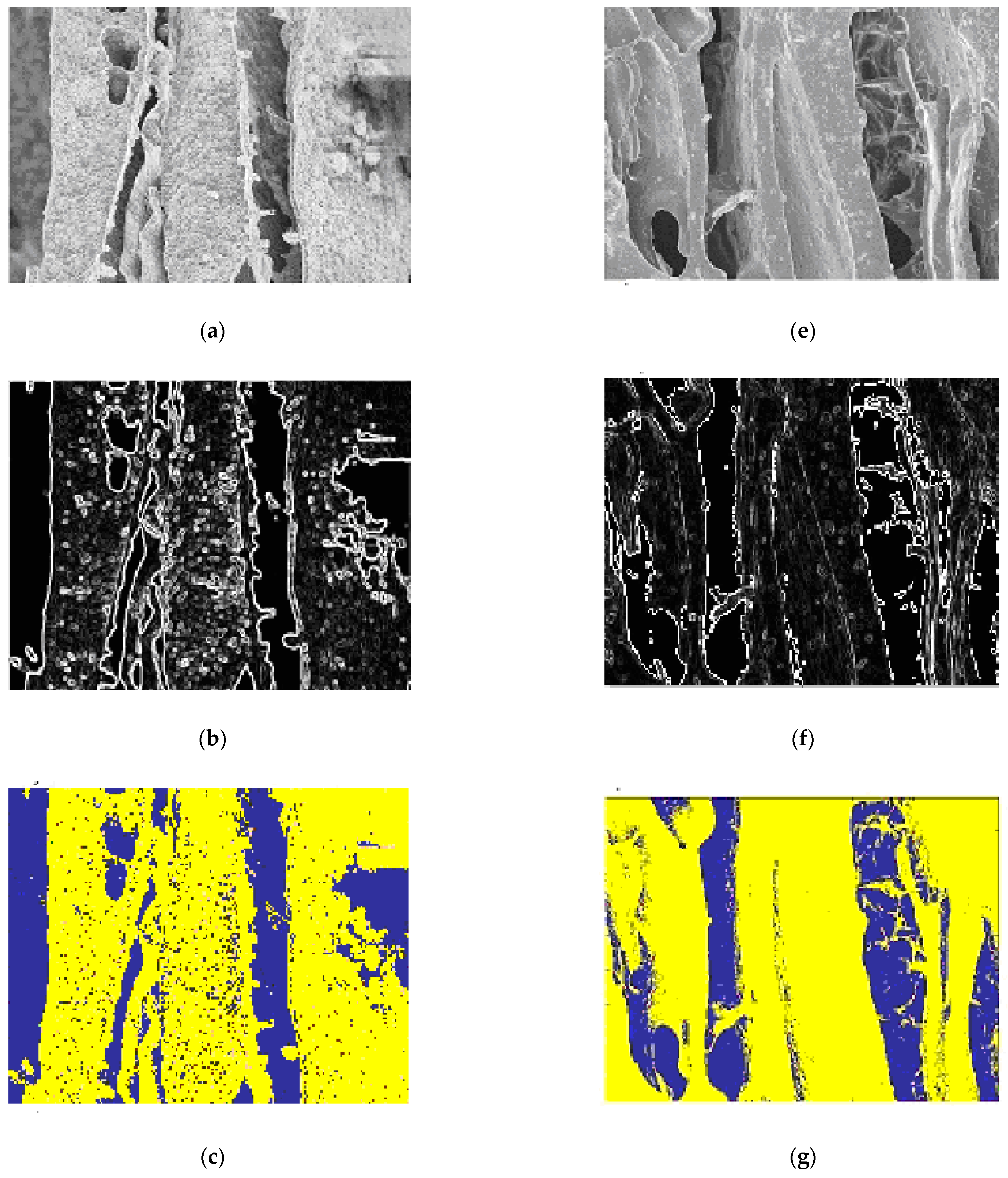
3.7. Image Segmentation
3.7.1. Chitosan (CH) Film
3.7.2. Chitosan/Silica Nanoparticle (CH-SN) Film
3.7.3. Chitosan/Silica Nanoparticle/Nisin (CH-SN-N) Film
3.7.4. Characterization and Comparison of the Different Classes of Films
3.7.5. Results on Blueberry by Using the Three Classes of Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arl, D.; Rogé, V.; Adjeroud, N.; Pistillo, B.R.; Sarr, M.; Bahlawane, N.; Lenoble, D. SiO2 thin film growth through a pure atomic layer deposition technique at room temperature. RSC Adv. 2020, 10, 18073–18081. [Google Scholar] [CrossRef]
- Eldib, R. Application of Nano-coating and Chitosan Combination Films on Cantaloupe Preservation. Pak. J. Biol. Sci. 2020, 23, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Glackin, C.A.; Horwitz, M.A.; Zink, J.I. Nanomachines and Other Caps on Mesoporous Silica Nanoparticles for Drug Delivery. Acc. Chem. Res. 2019, 52, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cheng, C.-A.; Lee, B.-Y.; Clemens, D.L.; Huang, W.-Y.; Horwitz, M.A.; Zink, J.I. Facile Strategy Enabling Both High Loading and High Release Amounts of the Water-Insoluble Drug Clofazimine Using Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 31870–31881. [Google Scholar] [CrossRef]
- Monadi Sefidan, A. Novel silicon dioxide -based nanocomposites as an antimicrobial in poly (lactic acid) nanocomposites films. Nanomed. Res. J. 2018, 3, 65–70. [Google Scholar] [CrossRef]
- Wu, G.; Liu, S.; Jia, H.; Dai, J. Preparation and properties of heat resistant polylactic acid (PLA)/Nano-SiO2 composite filament. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 164–171. [Google Scholar] [CrossRef]
- Sobahan, K.M.A.; Park, Y.J.; Kim, J.J.; Hwangbo, C.K. Nanostructured porous SiO2 films for antireflection coatings. Opt. Commun. 2011, 284, 873–876. [Google Scholar] [CrossRef]
- Rokayya, S.; Khojah, E.; Elhakem, A.; Benajiba, N.; Chavali, M.; Vivek, K.; Iqbal, A.; Helal, M. Investigating the Nano-Films Effect on Physical, Mechanical Properties, Chemical Changes, and Microbial Load Contamination of White Button Mushrooms During Storage. Coatings 2021, 11, 44. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; AlMatrafi, M.; Abdelazez, A.; Helal, M. Evaluation of Antioxidant Activities, Oxidation Enzymes, and Quality of Nano-Coated Button Mushrooms (Agaricus Bisporus) during Storage. Coatings 2021, 11, 149. [Google Scholar] [CrossRef]
- Il’Ves, V.G.; Zuev, M.G.; Sokovnin, S.Y. Properties of Silicon Dioxide Amorphous Nanopowder Produced by Pulsed Electron Beam Evaporation. J. Nanotechnol. 2015, 2015, 417817. [Google Scholar] [CrossRef]
- Talebian, N.; Zare, E. Structure and antibacterial property of nano-SiO2 supported oxide ceramic. Ceram. Int. 2014, 40, 281–287. [Google Scholar] [CrossRef]
- Murthy, R.V.V.R.; Chavali, M.; Mohammad, F. Synergistic effect of nano-silica slurries for cementing oil and gas wells. Pet. Res. 2020, 5, 83–91. [Google Scholar] [CrossRef]
- Llanos, J.H.R.; Vercik, L.C.D.O.; Vercik, A. Physical Properties of Chitosan Films Obtained after Neutralization of Polycation by Slow Drip Method. J. Biomater. Nanobiotechnology 2015, 6, 276–291. [Google Scholar] [CrossRef]
- Qiao, G.; Xiao, Z.; Ding, W.; Rok, A. Effect of Chitosan/Nano-Titanium Dioxide/Thymol and Tween Films on Ready-to-Eat Cantaloupe Fruit Quality. Coatings 2019, 9, 828. [Google Scholar] [CrossRef]
- Rokayya, S.; Jia, F.; Li, Y.; Nie, X.; Xu, J.; Han, R.; Yu, H.; Amanullah, S.; Almatrafi, M.M.; Helal, M. Application of nano-titanum dioxide coating on fresh Highbush blueberries shelf life stored under ambient temperature. LWT 2021, 137, 110422. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; AlMatrafi, M.; Jing, J.; Helal, M. Effect of Titanium Dioxide Nanocomposite Material and Antimicrobial Agents on Mushrooms Shelf-Life Preservation. Processes 2020, 8, 1632. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Almushhin, A.; Alharbi, M.; Almatrafi, M.; Benajiba, N.; Fikry, M.; Helal, M. Enhancement in physicochemical parameters and microbial populations of mushrooms as influenced by nano-coating treatments. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Ahmad, V.; Khan, M.S.; Jamal, Q.M.S.; Alzohairy, M.A.; Al Karaawi, M.A.; Siddiqui, M.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation. Int. J. Antimicrob. Agents 2017, 49, 1–11. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Woods, R.E. Digital Image Processing, 4th ed.; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 2021. [Google Scholar]
- Soltane, S. Adaptive approach to detect Optimal Edge Orientation using the Variogram. In Proceedings of the WiDSTaif 2021: International Conference of Women in Data Science at Taif University, Online, 30–31 March 2021. [Google Scholar]
- George, G.; Oommen, R.M.; Shelly, S.; Philipose, S.S.; Varghese, A.M. A Survey on Various Median Filtering Techniques for Removal of Impulse Noise from Digital Image. In Proceedings of the 2018 Conference on Emerging Devices and Smart Systems (ICEDSS), Tiruchengode, India, 2–3 March 2018; pp. 235–238. [Google Scholar]
- Paulin Mini, P.; Mary Reeja, Y. Robust Edge Extraction for Detecting Discontinuities. In Proceedings of the 2019 International Conference on Recent Advances in Energy-efficient Computing and Communication (ICRAECC), Nagercoil, India, 7–8 March 2019; pp. 1–6. [Google Scholar]
- Soltane, S. Adaptive Approach to Edge Detection on Blood Image Microscopic by Using Variograms. In Proceedings of the BIOMEIC’14 Biomedical Engineering IEEE International Conference, Tlemcen, Algeria, 15–16 October 2014; pp. 110–116. [Google Scholar]
- Cheng, G.; Liu, L. Survey of Image Segmentation Methods Based on Clustering. In Proceedings of the 2020 IEEE International Conference on Information Technology, Big Data and Artificial Intelligence (ICIBA), Chongqing, China, 6–8 November 2020; pp. 1111–1115. [Google Scholar]
- Soltane, S.; Kerkeni, N.; Angué, J.C. The use of 2D DCT for an adaptive approach to image segmentation. In Proceedings of the IS&T/SPIE’s, Image and Video Processing, San Jose, CA, USA, 1–2 February 1996 pp. 242–251. [Google Scholar]
- Zheng, X.; Lei, Q.; Yao, R.; Gong, Y.; Yin, Q. Image segmentation based on adaptive K-means algorithm. EURASIP J. Image Video Process. 2018, 2018, 68. [Google Scholar] [CrossRef]
- Adollah, R.; Mashor, M.Y.; Nasir, N.F.M.; Rosline, H.; Mahsin, H.; Adilah, H. Blood Cell Image Segmentation: A Review. IFMBE Proc. 2008, 21, 141–144. [Google Scholar]
- Lin, L.; Wang, W.; Chen, B. Leukocyte recognition with convolutional neural network. J. Algorithms Comput. Technol. 2018, 13, 13. [Google Scholar] [CrossRef]
- Ajala Funmilola, A.; Oke, O.A.; Adedeji, T.O.; Alade, O.M.; Adewusi, E.A. Fuzzy k-c-means Clustering Algorithm for Medical Image Segmentation. J. Inf. Eng. Appl. 2012, 2, 21–32. [Google Scholar]
- Khashman, A.; Al-Zgoul, E. Image Segmentation of Blood Cells in Leukemia Patients. In Proceedings of the 4th WSEAS International Conference on Computer Engineering and Applications, Cambridge, MA, USA, 25–27 January 2012; pp. 104–109. [Google Scholar]
- Sami, R.; Khojah, E.; Elgarni, E.; Aljumayi, H. Morphological-Mechanical Proprieties of Five Different Tomato Varieties in Kingdom of Saudi Arabia for High Techniques in Harvesting, Handling and Manufacturing. Tikrit J. Agric. Sci. 2016, 16, 1–11. [Google Scholar]
- Sami, R.; Khojah, E. Evaluation of physical properties of okra (Abelmoschus esculentus L.) pods with different structural characteristics. Res. Crop. 2019, 20, 73–78. [Google Scholar] [CrossRef]
- Rokayya, S.; Khojah, E. Physical-mechanical Estimation of Pepper (Capsicum annuum L.) Fruit Varieties. J. Northeast. Agric. Univ. Engl. Ed. 2016, 23, 61–69. [Google Scholar] [CrossRef]
- Rokayya, S. Some aspects of physical-mechanical properties of apple (Malus domestica) cultivars for high techniques in manufacturing. J. Northeast. Agric. Univ. Engl. Ed. 2017, 24, 31–39. [Google Scholar]
- Elhakem, A.H.; Benajiba, N.; Koko, M.Y.; Khojah, E.; Rok, A. DPPH, FRAP and TAEC Assays with Postharvest Cabbage (Brassica oleracea) Parameters During the Packaging Process. Pak. J. Biol. Sci. 2021, 24, 182–187. [Google Scholar] [CrossRef]
- Ma, W.; Rokayya, S.; Xu, L.; Sui, X.; Jiang, L.; Li, Y. Physical-Chemical Properties of Edible Film Made from Soybean Residue and Citric Acid. J. Chem. 2018, 2018, 4026831. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Li, Y.; Tong, X.; Regenstein, J.M.; Huang, Y.; Ma, W.; Sami, R.; Qi, B.; Jiang, L. Effect of the condition of spray-drying on the properties of the polypeptide-rich powders from enzyme-assisted aqueous extraction processing. Dry. Technol. 2019, 37, 2105–2115. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Qi, B.; Rokayya, S.; Ma, W.; Liang, J.; Sui, X.; Zhang, Y.; Jiang, L. Heating Quality and Stability of Aqueous Enzymatic Extraction of Fatty Acid-Balanced Oil in Comparison with Other Blended Oils. J. Chem. 2014, 2014, 530787. [Google Scholar] [CrossRef]
- Zhou, Y.; Teng, F.; Tian, T.; Sami, R.; Wu, C.; Zhu, Y.; Zheng, L.; Jiang, L.; Wang, Z.; Li, Y. The impact of soy protein isolate-dextran conjugation on capsicum oleoresin (Capsicum annuum L.) nanoemulsions. Food Hydrocoll. 2020, 108, 105818. [Google Scholar] [CrossRef]
- Abdelazez, A.; Muhammad, Z.; Zhang, Q.-X.; Zhu, Z.-T.; Abdelmotaal, H.; Sami, R.; Meng, X.-C. Production of a Functional Frozen Yogurt Fortified with Bifidobacterium spp. BioMed Res. Int. 2017, 2017, 6438528. [Google Scholar] [CrossRef] [PubMed]
- Khouri, J.; Penlidis, A.; Moresoli, C. Heterogeneous method of chitosan film preparation: Effect of multifunctional acid on film properties. J. Appl. Polym. Sci. 2020, 137, 48648. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Chitosan reinforced with modified CaCO3 nanoparticles to enhance thermal, hydrophobicity properties and removal of cu(II) and cd(II) ions. J. Polym. Res. 2017, 24, 86. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Cheng, M. Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Bambace, M.F.; Gerard, L.M.; Moreira, M.D.R. An approach to improve the safety and quality of ready-to-eat blueberries. J. Food Saf. 2019, 39, e12602. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hsiao, J.-Y.; Hsieh, C.-P. An Adaptive Median Filter for Image Denoising. In Proceedings of the 2008 Second International Symposium on Intelligent Information Technology Application, Shanghai, China, 20–22 December 2008; pp. 346–350. [Google Scholar] [CrossRef]
- Parasar, D.; Rathod, V.R. Particle swarm optimisation K-means clustering segmentation of foetus ultrasound image. Int. J. Signal Imaging Syst. Eng. 2017, 10, 95. [Google Scholar] [CrossRef]
- Inbarani, H.H.; Azar, A.T.; G, J. Leukemia Image Segmentation Using a Hybrid Histogram-Based Soft Covering Rough K-Means Clustering Algorithm. Electronics 2020, 9, 188. [Google Scholar] [CrossRef]
- Neelima, N.; Rao, P.K.; Praneeth, N.S.; Mamatha, G.N. An Integrated Framework to Image Retrieval Using L*a*b Color Space ans Local Binary Pattern, RTIP2R. In Proceedings of the International Conference on Recent Trends in Image Processing and Pattern Recognition, RTIP2R, Bidar, India, 16–17 December 2016. [Google Scholar]
- Shi, P.; Zhong, J.; Huang, R.; Lin, J. Automated Quantitative Image Analysis of Hematoxylin-Eosin Staining Slides in Lymphoma Based on Hierarchical Kmeans Clustering. In Proceedings of the 2016 8th International Conference on Information Technology in Medicine and Education (ITME), Fuzhou, China, 23–25 December 2016; pp. 99–104. [Google Scholar]
- Marutho, D.; Handaka, S.H.; Wijaya, E.; Muljono. The Determination of Cluster Number at k-Mean Using Elbow Method and Purity Evaluation on Headline News. In Proceedings of the 2018 International Seminar on Application for Technology of Information and Communication, Semarang, Indonesia, 21–22 September 2018; pp. 533–538. [Google Scholar]
- Sidauruk, H.; Munandar, A.; Haryati, S. The Production of Nanoparticles Chitosan from Crustaceans Shell Using the Top-down Method. E3S Web Conf. 2020, 147, 03025. [Google Scholar] [CrossRef]
- Vijayalakshmi, V.; Kousar, P.A.H.; Das, S. Optimization and characterization of chitosan based nanocarrier for the application of cancer drug delivery. J. Crit. Rev. 2020, 7, 762–769. [Google Scholar] [CrossRef]
- O’Callaghan, K.A.M.; Kerry, J.P. Preparation of low- and medium-molecular weight chitosan nanoparticles and their antimicrobial evaluation against a panel of microorganisms, including cheese-derived cultures. Food Control. 2016, 69, 256–261. [Google Scholar] [CrossRef]
- Xiong, J.; Sheng, C.; Wang, Q.; Guo, W. Toughened and water-resistant starch/TiO2 bio-nanocomposites as an environment-friendly food packaging material. Mater. Res. Express 2019, 6, 055045. [Google Scholar] [CrossRef]
- Ngadiman, N.H.A.; Yusof, N.M.; Idris, A.; Misran, E.; Kurniawan, D. Development of highly porous biodegradable γ-Fe2O3/polyvinyl alcohol nanofiber mats using electrospinning process for biomedical application. Mater. Sci. Eng. C 2017, 70, 520–534. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Eifert, J.D.; Williams, R.C.; Marcy, J.E.; Welbaum, G.E. Shelf Life Determination of Fresh Blueberries (Vaccinium corymbosum) Stored under Controlled Atmosphere and Ozone. Int. J. Food Sci. 2015, 2015, 164143. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Graciano-Verdugo, A.Z.; Rodriguez-Félix, F.; Castillo-Ortega, M.M.; Yépiz-Gómez, M.S.; Plascencia-Jatomea, M. Chitosan composite films: Thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Biduski, B.; da Silva, F.T.; da Silva, W.M.; Halal, S.L.D.M.E.; Pinto, V.Z.; Dias, A.R.G.; Zavareze, E.D.R. Impact of acid and oxidative modifications, single or dual, of sorghum starch on biodegradable films. Food Chem. 2017, 214, 53–60. [Google Scholar] [CrossRef]






| Films | Elongation-at -Break | Tensile Strength, σ | Elastic Modulus, E | Breaking Force, BF | Fracture Stress, σF | Extensibility |
|---|---|---|---|---|---|---|
| % | MPa | MPa | gf | MPa | mm | |
| Chitosan | 38.64 ± 2.95 a | 16.53 ± 0.66 a | 2034.52 ± 215.83 a | 600.85 ± 17.71 a | 18.92 ± 0.84 a | 7.86 ± 0.49 a |
| Chitosan/Silica Nanoparticle | 12.49 ± 1.25 b | 7.65 ± 0.41 b | 2233.03 ± 257.58 a | 194.73 ± 12.69 b | 11.85 ± 0.90 a | 3.75 ± 0.57 b |
| Chitosan/Silica Nanoparticle/N | 5.25 ± 1.80 b | 0.90 ± 0.30 b | 569.19 ± 107.07 b | 27.78 ± 1.30 c | 0.94 ± 0.31 b | 1.57 ± 0.54 b |
| Days | Untreated | Chitosan | Chitosan/Silica Nanoparticle | Chitosan/Silica Nanoparticle/N |
|---|---|---|---|---|
| Aerobic bacteria counts (log CFU/g) | ||||
| 0 | 1.767 ± 0.049 a | 1.700 ± 0.013 a | 1.500 ± 0.035 b | 1.033 ± 0.015 c |
| 3 | 2.200 ± 0.023 a | 2.033 ± 0.072 b | 1.900 ± 0.059 c | 1.367 ± 0.024 d |
| 6 | 3.000 ± 0.045 a | 2.633 ± 0.027 c | 2.867 ± 0.032 b | 1.933 ± 0.025 d |
| 9 | 4.227 ± 0.038 a | 3.901 ± 0.053 b | 3.733 ± 0.019 c | 2.823 ± 0.079 d |
| Molds and yeasts counts (log CFU/g) | ||||
| 0 | 2.033 ± 0.049 a | 1.900 ± 0.011 b | 1.867 ± 0.031 b | 1.800 ± 0.010 c |
| 3 | 2.433 ± 0.012 a | 2.267 ± 0.093 b | 2.400 ± 0.051 a | 2.067 ± 0.039 c |
| 6 | 3.200 ± 0.050 a | 3.067 ± 0.029 b | 3.267 ± 0.034 a | 2.467 ± 0.026 c |
| 9 | 4.622 ± 0.034 a | 4.090 ± 0.055 a | 4.000 ± 0.010 a | 3.580 ± 0.025 b |
| Films | Color Index | ||
|---|---|---|---|
| L* | a* | b* | |
| Chitosan | 09.59 ± 0.05 c | −1.90 ± 1.29 ab | 2.54 ± 0.49 b |
| Chitosan/Silica Nanoparticle | 49.39 ± 0.26 b | −2.50 ± 0.18 b | −1.78 ± 0.06 c |
| Chitosan/Silica Nanoparticle/N | 58.24 ± 0.41 a | −0.67 ± 0.12 a | 10.34 ± 0.32 a |
| Films | Region Number | Total Area | % Area | Average Area Size |
|---|---|---|---|---|
| CH | 94 | 42,211 | 9.61 | 449.10 |
| CH-SN | 169 | 22,060 | 8 | 130.53 |
| CH-SN-N | 79 | 23,016 | 5.19 | 291.34 |
| Case | Area/Pixels | Perimeter | Edge/Number of Point | Segmentation/Color Edge |
|---|---|---|---|---|
| Control (H2O) | 1785 | 715.2 | 514 | Yellow |
| CH | 1663 | 663 | 474 | Blue |
| CH-SN | 1803 | 707.8 | 500 | Red |
| CH-SN-N | 1983 | 760 | 518 | Green |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sami, R.; Soltane, S.; Helal, M. Microscopic Image Segmentation and Morphological Characterization of Novel Chitosan/Silica Nanoparticle/Nisin Films Using Antimicrobial Technique for Blueberry Preservation. Membranes 2021, 11, 303. https://doi.org/10.3390/membranes11050303
Sami R, Soltane S, Helal M. Microscopic Image Segmentation and Morphological Characterization of Novel Chitosan/Silica Nanoparticle/Nisin Films Using Antimicrobial Technique for Blueberry Preservation. Membranes. 2021; 11(5):303. https://doi.org/10.3390/membranes11050303
Chicago/Turabian StyleSami, Rokayya, Schahrazad Soltane, and Mahmoud Helal. 2021. "Microscopic Image Segmentation and Morphological Characterization of Novel Chitosan/Silica Nanoparticle/Nisin Films Using Antimicrobial Technique for Blueberry Preservation" Membranes 11, no. 5: 303. https://doi.org/10.3390/membranes11050303
APA StyleSami, R., Soltane, S., & Helal, M. (2021). Microscopic Image Segmentation and Morphological Characterization of Novel Chitosan/Silica Nanoparticle/Nisin Films Using Antimicrobial Technique for Blueberry Preservation. Membranes, 11(5), 303. https://doi.org/10.3390/membranes11050303






