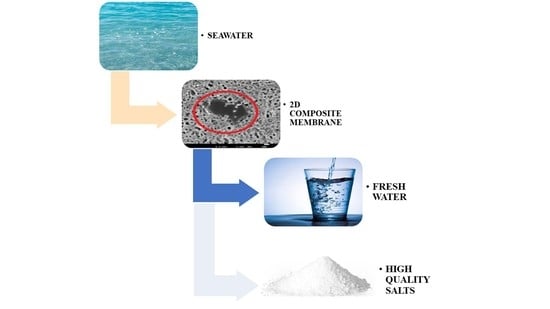
Performance of PVDF Based Membranes with 2D Materials for Membrane Assisted-Crystallization Process
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Membrane Crystallization Experiments
3. Results and Discussion
3.1. Membrane Preparation and Characterizations
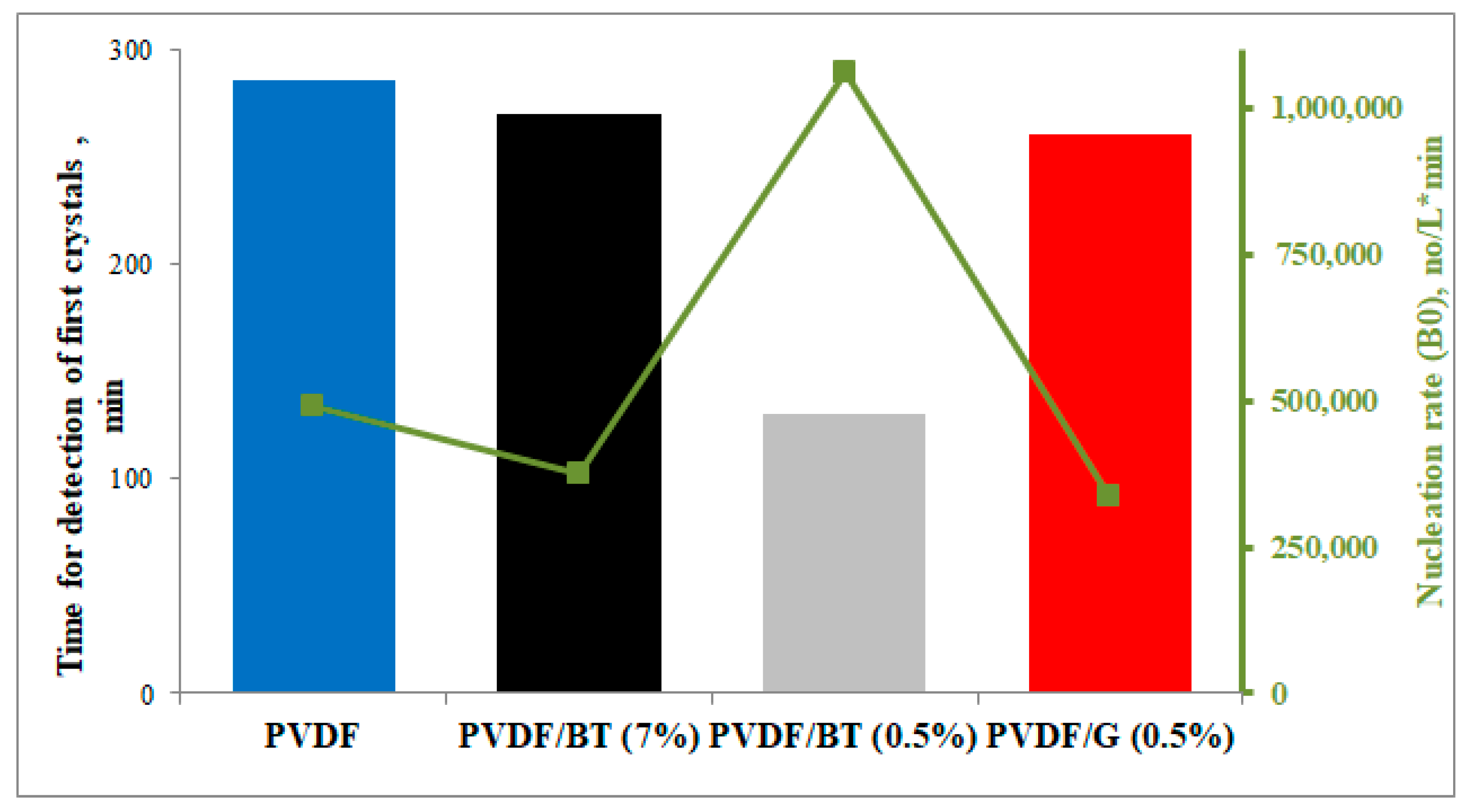
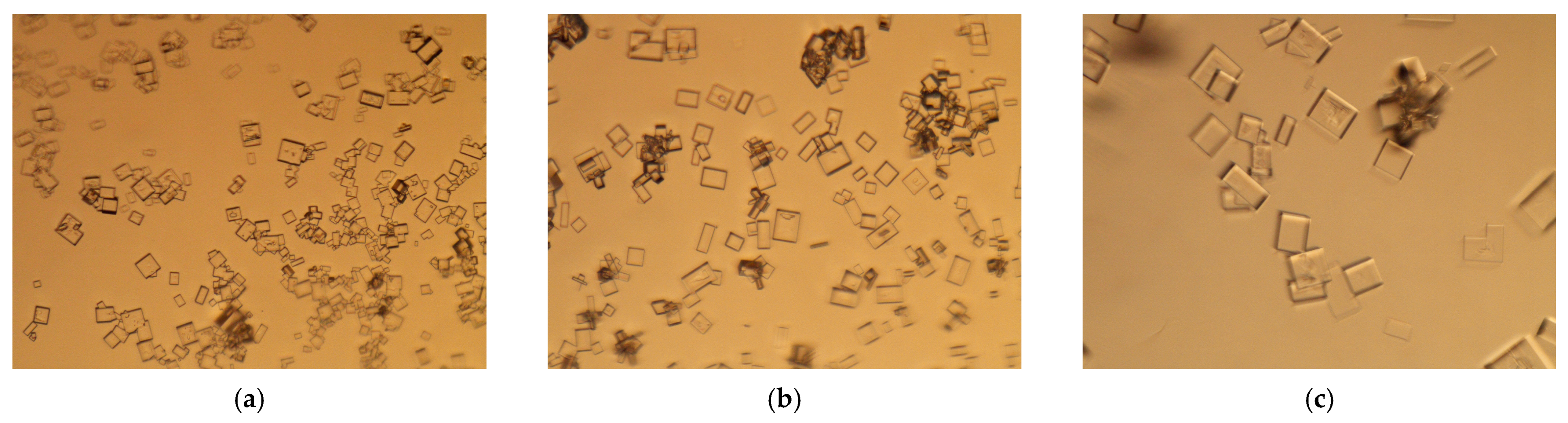
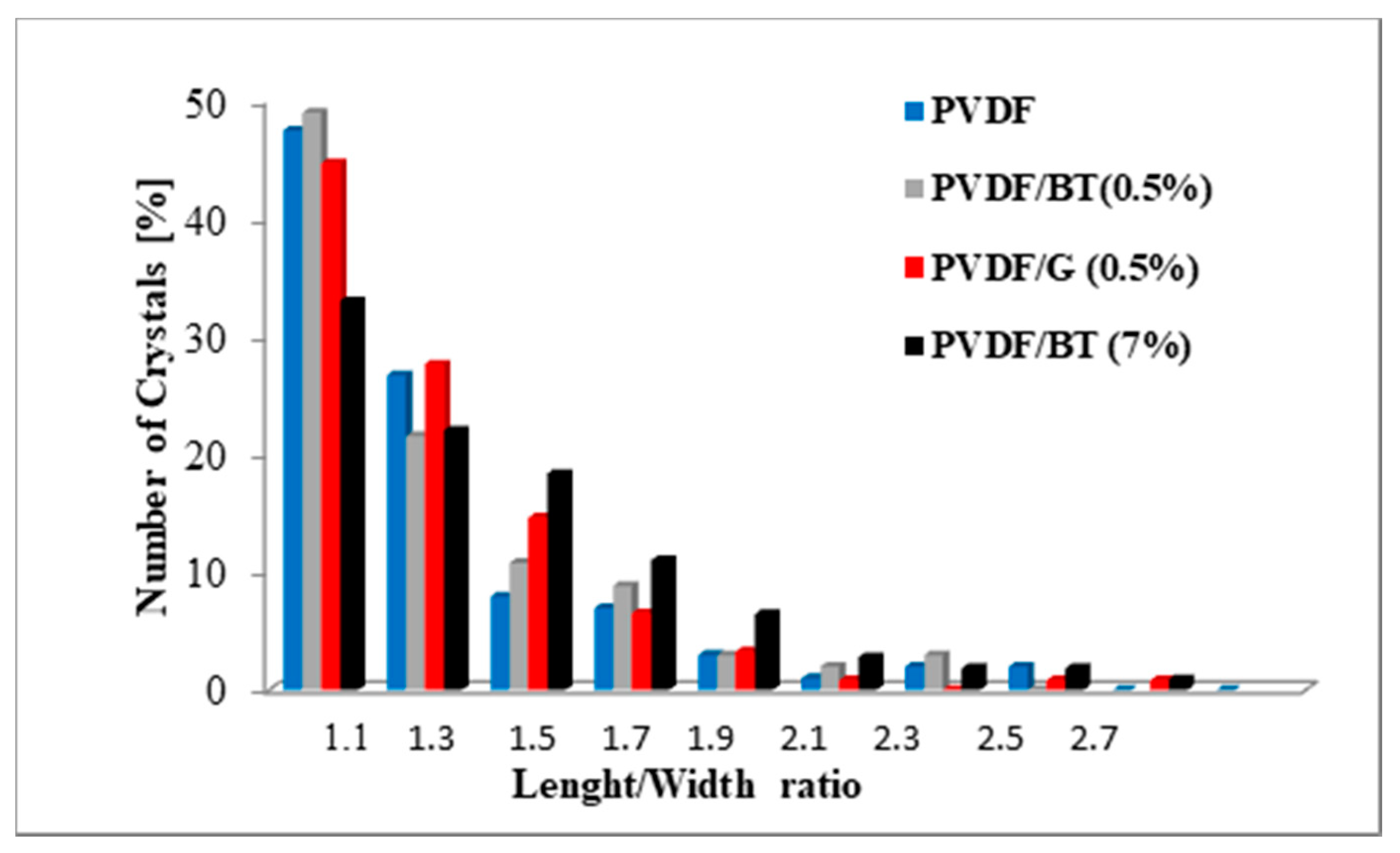
3.2. Membrane Crystallization Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification—An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Ko, C.-C.; Ali, A.; Drioli, E.; Tung, K.-L.; Chen, C.-H.; Chen, Y.-R.; Macedonio, F. Performance of ceramic membrane in vacuum membrane distillation and in vacuum membrane crystallization. Desalination 2018, 440, 48–58. [Google Scholar] [CrossRef]
- Salmón, I.R.; Luis, P. Membrane crystallization via membrane distillation. Chem. Eng. Process. Process. Intensif. 2018, 123, 258–271. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E. Hydrophobic membranes for salts recovery from desalination plants. Desalin. Water Treat. 2010, 18, 224–234. [Google Scholar] [CrossRef]
- Tun, C.M.; Fane, A.G.; Matheickal, J.T.; Sheikholeslami, R. Membrane distillation crystallization of concentrated salts—Flux and crystal formation. J. Membr. Sci. 2005, 257, 144–155. [Google Scholar] [CrossRef]
- Meng, S.; Ye, Y.; Mansouri, J.; Chen, V. Fouling and crystallisation behaviour of superhydrophobic nano-composite PVDF membranes in direct contact membrane distillation. J. Membr. Sci. 2014, 463, 102–112. [Google Scholar] [CrossRef]
- Perrotta, M.L.; Macedonio, F.; Tocci, E.; Giorno, L.; Drioli, E.; Gugliuzza, A. Graphene stimulates the nucleation and growth rate of NaCl crystals from hypersaline solution via membrane crystallization. Environ. Sci. Water Res. Technol. 2020, 6, 1723–1736. [Google Scholar] [CrossRef]
- Cui, Z.; Li, X.; Zhang, Y.; Wang, Z.; Gugliuzza, A.; Militano, F.; Drioli, E.; Macedonio, F. Testing of three different PVDF membranes in membrane assisted-crystallization process: Influence of membrane structural-properties on process performance. Desalination 2018, 440, 68–77. [Google Scholar] [CrossRef]
- Ray, S.S.; Bakshi, H.S.; Dangayach, R.; Singh, R.; Deb, C.K.; Ganesapillai, M.; Chen, S.-S.; Purkait, M.K. Recent Developments in Nanomaterials-Modified Membranes for Improved Membrane Distillation Performance. Membranes 2020, 10, 140. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Enhanced performance of PVDF nanocomposite membrane by nanofiber coating: A membrane for sustainable desalination through MD. Water Res. 2016, 89, 39–49. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Fane, A.G. Engineering superhydrophobic surface on poly(vinylidene fluoride) nanofiber membranes for direct contact membrane distillation. J. Membr. Sci. 2013, 440, 77–87. [Google Scholar] [CrossRef]
- Athanasekou, C.; Sapalidis, A.; Katris, I.; Savopoulou, E.; Beltsios, K.; Tsoufis, T.; Kaltzoglou, A.; Falaras, P.; Bounos, G.; Antoniou, M.; et al. Mixed Matrix PVDF/Graphene and Composite-Skin PVDF/Graphene Oxide Membranes Applied in Membrane Distillation. Polym. Eng. Sci. 2019, 59, 262–278. [Google Scholar] [CrossRef]
- Moradi, R.; Karimi-Sabet, J.; Shariaty-Niassar, M.; Koochaki, M.A. Preparation and Characterization of Polyvinylidene Fluoride/Graphene Superhydrophobic Fibrous Films. Polymers 2015, 7, 1444–1463. [Google Scholar] [CrossRef]
- Woo, Y.C.; Kim, Y.; Shim, W.-G.; Tijing, L.D.; Yao, M.; Nghiem, L.D.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Graphene/PVDF flat-sheet membrane for the treatment of RO brine from coal seam gas produced water by air gap membrane distillation. J. Membr. Sci. 2016, 513, 74–84. [Google Scholar] [CrossRef]
- Frappa, M.; Castillo, A.E.D.R.; Macedonio, F.; Politano, A.; Drioli, E.; Bonaccorso, F.; Pellegrini, V.; Gugliuzza, A. A few-layer graphene for advanced composite PVDF membranes dedicated to water desalination: A comparative study. Nanoscale Adv. 2020. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Macedonio, F.; Politano, A.; Drioli, E. Prospects of 2D materials-based membranes in water desalination. Chem. Eng. Trans. 2019, 73. [Google Scholar] [CrossRef]
- Perrotta, M.L.; Macedonio, F.; Giorno, L.; Jin, W.; Drioli, E.; Gugliuzza, A.; Tocci, E. Molecular insights on NaCl crystal formation approaching PVDF membranes functionalized with graphene. Phys. Chem. Chem. Phys. 2020, 22, 7817–7827. [Google Scholar] [CrossRef]
- Politano, A.; Bonaccorso, F.; Del Rio Castillo, A.; Drioli, E.; Gugliuzza, A.; Macedonio, F.; Pellegrini, V. Procedimento per la Fabbricazione di una Membrana Composita con Cristalli Bidimensionali per Esfoliazione di Materiali Stratificati Mediante Tecniche di Wet-Jet Milling. Italian Patent IT102018000020641, 21 December 2018. [Google Scholar]
- Gontarek, E.; Macedonio, F.; Militano, F.; Giorno, L.; Lieder, M.; Politano, A.; Drioli, E.; Gugliuzza, A. Adsorption-assisted transport of water vapour in super-hydrophobic membranes filled with multilayer graphene platelets. Nanoscale 2019, 11, 11521–11529. [Google Scholar] [CrossRef]
- Macedonio, F.; Politano, A.; Drioli, E.; Gugliuzza, A. Bi2Se3-assisted membrane crystallization. Mater. Horiz. 2018, 5, 912–919. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, Q.; Zhang, K. Few-layers 2D O–MoS2 TFN nanofiltration membranes for future desalination. J. Membr. Sci. 2020, 604, 118052. [Google Scholar] [CrossRef]
- Rehman, F.; Thebo, K.H.; Aamir, M.; Akhtar, J. Nanomembranes for water treatment. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–240. [Google Scholar]
- Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition Metal Dichalcogenides for the Application of Pollution Reduction: A Review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E. The birth of topological insulators. Nature 2010, 464, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Kane, C. Colloquium: Topological insulators. Rev. Mod. Phys. 2010, 82, 3045–3067. [Google Scholar] [CrossRef]
- Manoharan, H.C. A romance with many dimensions. Nat. Nanotechnol. 2010, 5, 477–479. [Google Scholar] [CrossRef]
- Qi, X.-L.; Zhang, S.-C. The quantum spin Hall effect and topological insulators. Phys. Today 2010, 63, 33. [Google Scholar] [CrossRef]
- Hasan, M.Z.; Moore, J.E. Three-dimensional topological insulators. Annu. Rev. Condens. Matter. Phys. 2011, 2, 55–78. [Google Scholar] [CrossRef]
- Longo, M.; De Santo, M.P.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Jansen, J.C. Force spectroscopy determination of Young’s modulus in mixed matrix membranes. Polymer 2018, 156, 22–29. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Bartolotta, A.; Coleman, J.N.; Backes, C. 2D-Crystal-Based Functional Inks. Adv. Mater. 2016, 28, 6136–6166. [Google Scholar] [CrossRef]
- Castillo, A.E.D.R.; Pellegrini, V.; Ansaldo, A.; Ricciardella, F.; Sun, H.; Marasco, L.; Buha, J.; Dang, Z.; Gagliani, L.; Lago, E.; et al. High-yield production of 2D crystals by wet-jet milling. Mater. Horiz. 2018, 5, 890–904. [Google Scholar] [CrossRef]
- Castillo, A.E.D.R.; Reyes-Vazquez, C.D.; Rojas-Martinez, L.E.; Thorat, S.B.; Serri, M.; Martinez-Hernandez, A.L.; Velasco-Santos, C.; Pellegrini, V.; Bonaccorso, F. Single-step exfoliation and functionalization of few-layers black phosphorus and its application for polymer composites. FlatChem 2019, 18, 100131. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Luque-Alled, J.M.; Leaper, S.; Alberto, M.; Fan, X.; Vijayaraghavan, A.; Gad-Allah, T.A.; El-Kalliny, A.S.; Szekely, G.; Ahmed, S.I.; et al. PVDF membranes containing reduced graphene oxide: Effect of degree of reduction on membrane distillation performance. Desalination 2019, 452, 196–207. [Google Scholar] [CrossRef]
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gómez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2010, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Sadasivuni, K.K.; Ponnamma, D.; Kim, J.; Thomas, S. (Eds.) Graphene-Based Polymer Nanocomposites in Electronics; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Cui, Z.; Pan, J.; Wang, Z.; Frappa, M.; Drioli, E.; Macedonio, F. Hyflon/PVDF membranes prepared by NIPS and TIPS: Comparison in MD performance. Sep. Purif. Technol. 2020, 247, 116992. [Google Scholar] [CrossRef]
- Mucha, M.; Jungwirth, P.J. Salt crystallization from an evaporating aqueous solution by molecular dynamics simulations. Phys. Chem. B 2003, 107, 8271–8274. [Google Scholar] [CrossRef]
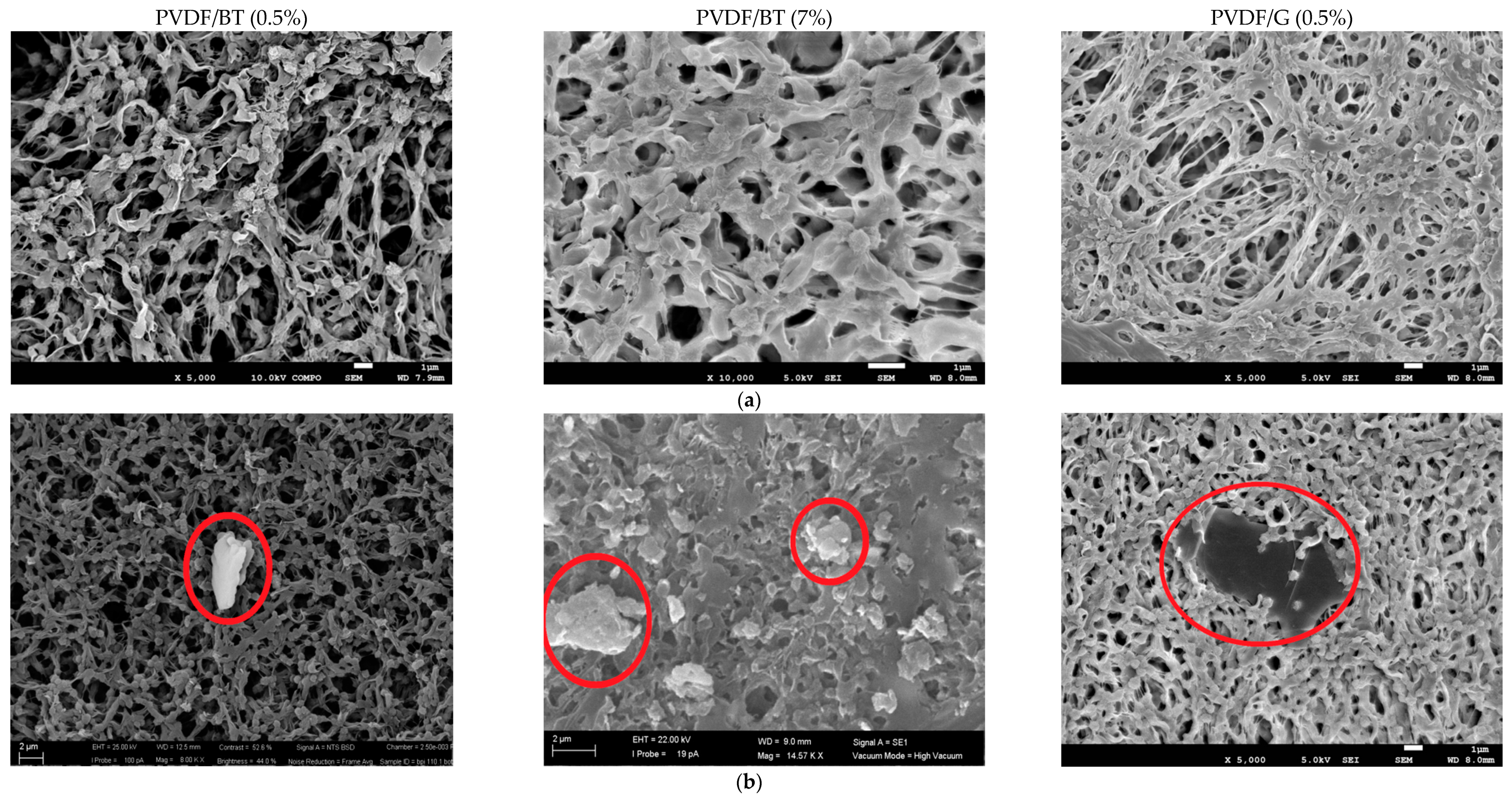


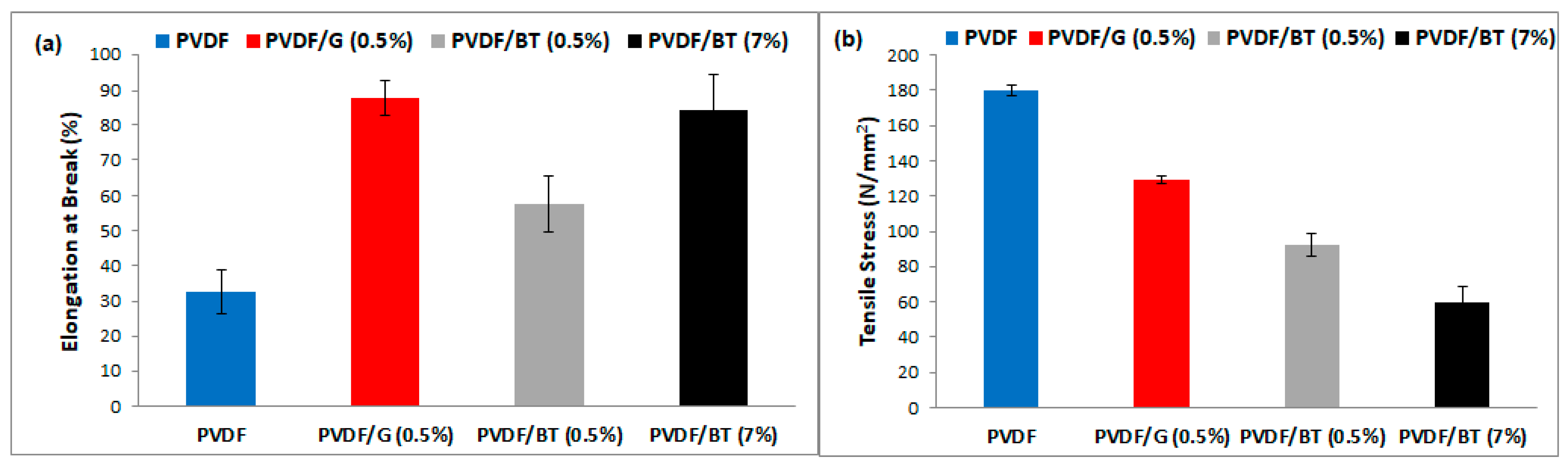
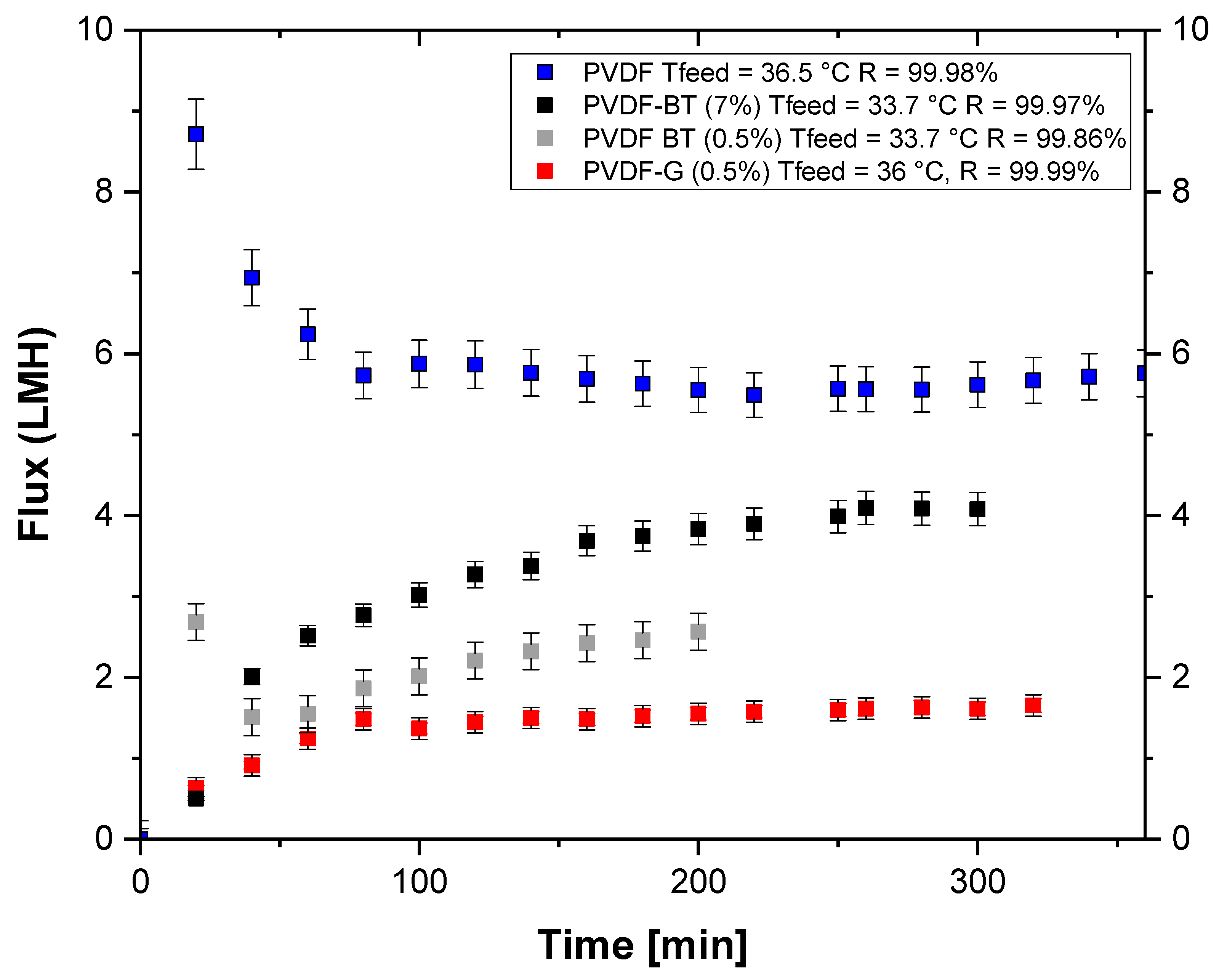

| Name of Membrane | Filler Dispersed in NMP |
|---|---|
| PVDF/BT (7%) | Bi2Te3 10 g∙L−1 |
| PVDF/BT (0.5%) | Bi2Te3 0.6 g∙L−1 |
| PVDF/G (0.5%) | Graphene 0.6 g∙L−1 |
| Membrane | Contact Angle (°) | Thickness (μm) | Mean Pore Size (μm) | Porosity (%) |
|---|---|---|---|---|
| PVDF | 139 ± 3 | 71 ± 2 | 0.52 ± 0.05 | 82 ± 4 |
| PVDF/G (0.5%) | 136 ± 1 | 62 ± 3 | 0.24 ± 0.05 | 56 ± 7 |
| PVDF/BT (0.5%) | 128 ± 8 | 68 ± 1 | 0.50 ± 0.2 | 75 ± 1 |
| PVDF/BT (7%) | 130 ± 2 | 100 ± 5 | 0.50 ± 0.08 | 77 ± 1 |
| PVDF | PVDF/G (0.5%) | |||||||
| CV | B0 | G (mm∙min−1) | dm (μm) | CV | B0 | G (mm∙min−1) | dm (μm) | |
| Sample 1 | 77.1 | 490,593 | 0.0000298 | 20.6 | 36.7 | 280,475 | 0.0000385 | 18.92 |
| Sample 2 | 48.4 | 257,598 | 0.0000524 | 42.5 | 43.8 | 199,025 | 0.0000456 | 23.25 |
| Sample 3 | 53.8 | 149,088 | 0.0000795 | 65.1 | 44.2 | 374,721 | 0.0000251 | 17.27 |
| PVDF/BT (0.5%) | PVDF/BT (7%) | |||||||
| Sample 1 | 54.2 | 1,060,979 | 0.0000317 | 9.56 | 46.0 | 337,756 | 0.0000239 | 13.38 |
| Sample 2 | 44.4 | 1,633,024 | 0.0000315 | 12.08 | 29.5 | 343,006 | 0.0000245 | 13.93 |
| Sample 3 | 43.1 | 811,706 | 0.0000388 | 16.82 | 65.4 | 392,031 | 0.0000212 | 16.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frappa, M.; Macedonio, F.; Gugliuzza, A.; Jin, W.; Drioli, E. Performance of PVDF Based Membranes with 2D Materials for Membrane Assisted-Crystallization Process. Membranes 2021, 11, 302. https://doi.org/10.3390/membranes11050302
Frappa M, Macedonio F, Gugliuzza A, Jin W, Drioli E. Performance of PVDF Based Membranes with 2D Materials for Membrane Assisted-Crystallization Process. Membranes. 2021; 11(5):302. https://doi.org/10.3390/membranes11050302
Chicago/Turabian StyleFrappa, Mirko, Francesca Macedonio, Annarosa Gugliuzza, Wanqin Jin, and Enrico Drioli. 2021. "Performance of PVDF Based Membranes with 2D Materials for Membrane Assisted-Crystallization Process" Membranes 11, no. 5: 302. https://doi.org/10.3390/membranes11050302
APA StyleFrappa, M., Macedonio, F., Gugliuzza, A., Jin, W., & Drioli, E. (2021). Performance of PVDF Based Membranes with 2D Materials for Membrane Assisted-Crystallization Process. Membranes, 11(5), 302. https://doi.org/10.3390/membranes11050302