Enhanced Performance of Fly Ash-Based Supports for Low-Cost Ceramic Membranes with the Addition of Bauxite
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Preparation of Fly Ash Support
2.3. Characterization
3. Results and Discussion
3.1. Characterization of Raw Materials
3.2. Effect of Bauxite Doping on the Micromorphologies of Ceramic Supports
3.3. Effect of Bauxite Doping on the Phase Composition of Ceramic Supports
3.4. Effect of Bauxite Doping on the Pore Structure of Ceramic Supports
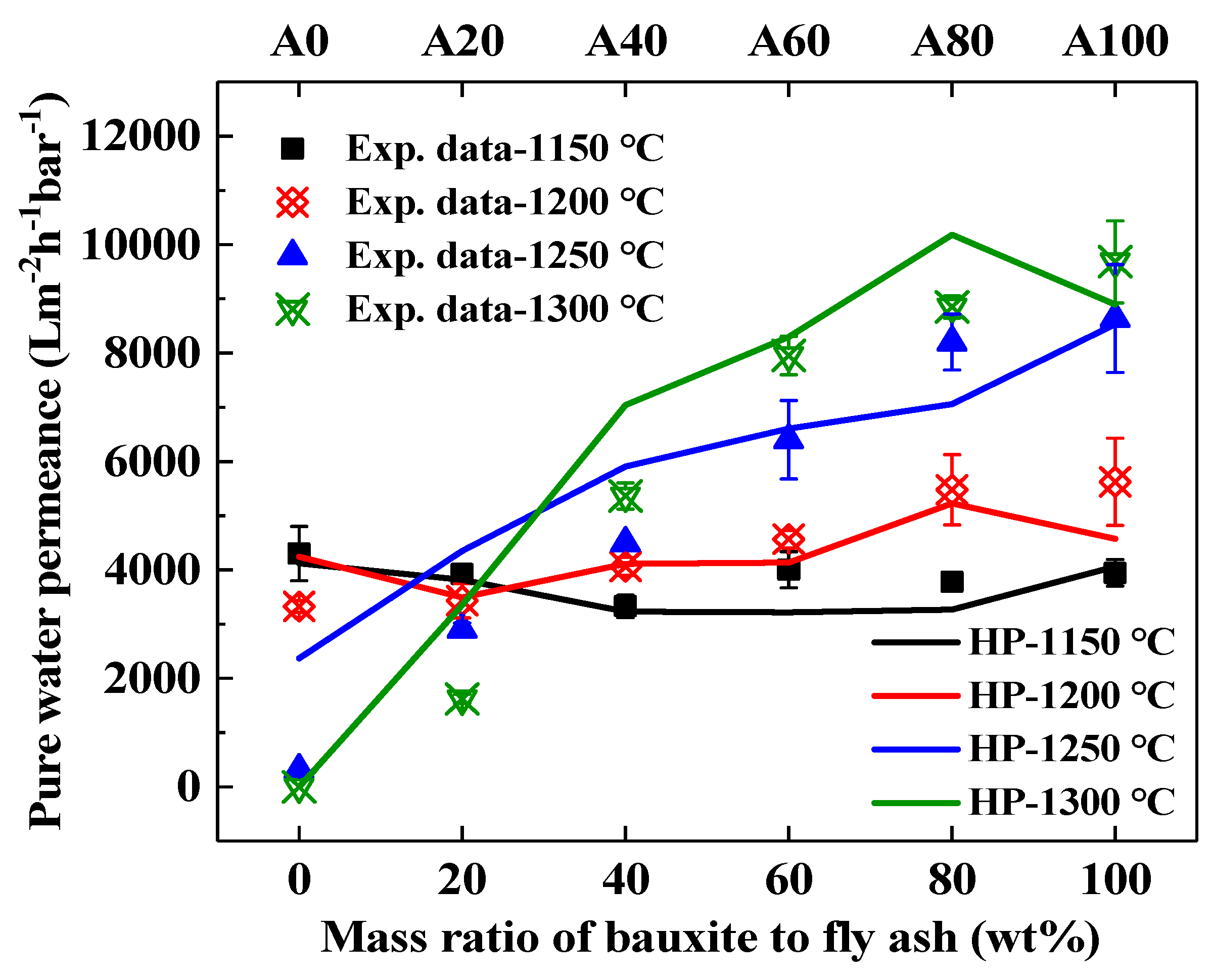
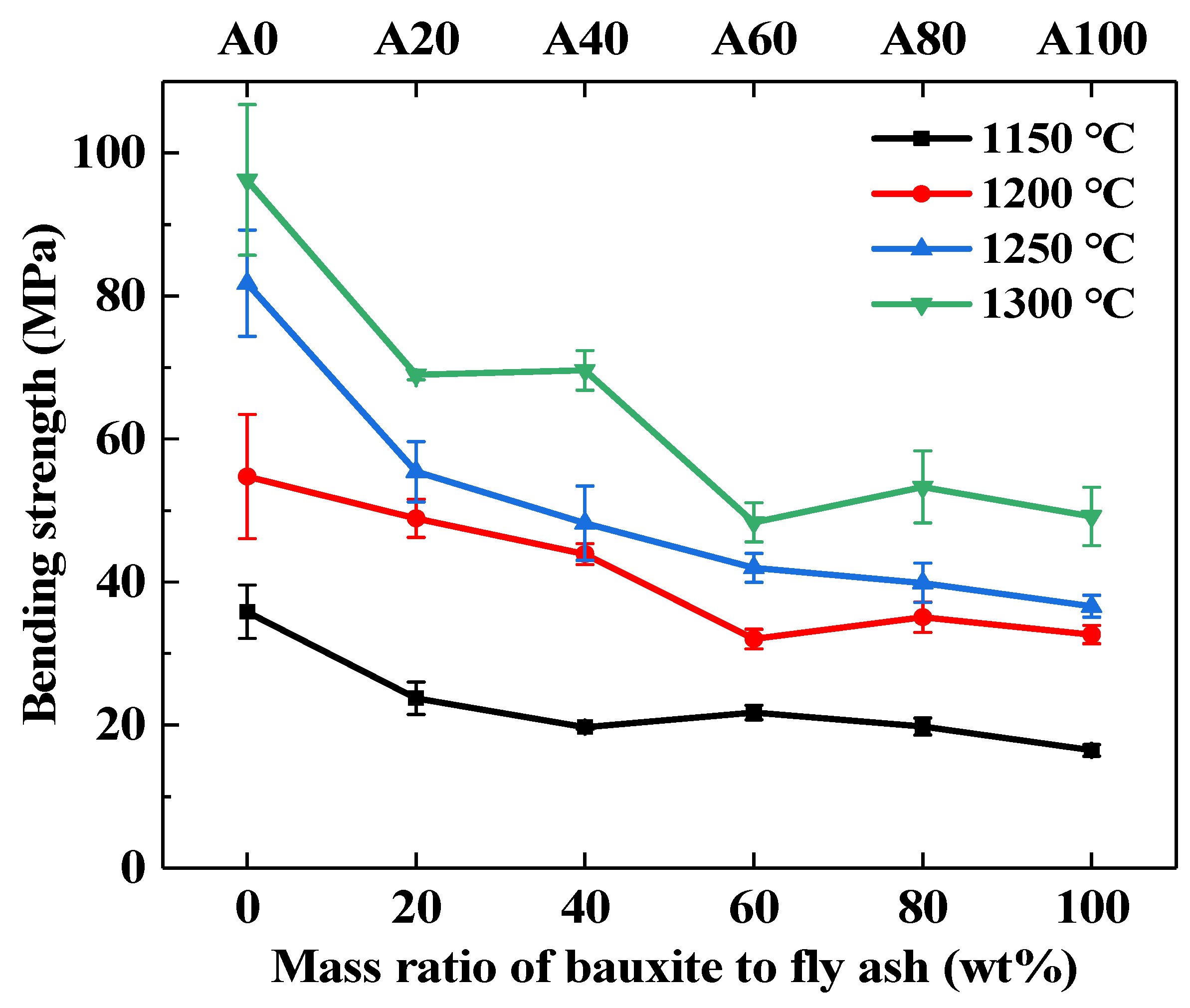
3.5. Effect of Bauxite Doping on the Performance of Ceramic Supports
3.6. Corrosion Resistance of Optimized Ceramic Supports
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters A review. Sep. Purif. Technol. 2018, 200, 198–220. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Ke, X.B.; Zhu, H.Y.; Gao, X.P.; Liu, J.W.; Zheng, Z.F. High-Performance Ceramic Membranes with a Separation Layer of Metal Oxide Nanofibers. Adv. Mater. 2007, 19, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Elaine Fung, Y.-L.; Wang, H. Investigation of reinforcement of porous alumina by nickel aluminate spinel for its use as ceramic membrane. J. Membr. Sci. 2013, 444, 252–258. [Google Scholar] [CrossRef]
- Zou, D.; Ke, X.; Qiu, M.; Chen, X.; Fan, Y. Design and fabrication of whisker hybrid ceramic membranes with narrow pore size distribution and high permeability via co-sintering process. Ceram. Int. 2018, 44, 21159–21169. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Harun, Z.; Othman, M.H.D.; Ismail, A.F.; Gani, P. Effect of kaolin particle size and loading on the characteristics of kaolin ceramic support prepared via phase inversion technique. J. Asian Ceram. Soc. 2018, 4, 164–177. [Google Scholar] [CrossRef]
- Qin, W.; Peng, C.; Lv, M.; Wu, J. Preparation and properties of high-purity porous alumina support at low sintering temperature. Ceram. Int. 2014, 40, 13741–13746. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, Y.; Cerneaux, S.; Zhou, J.-e.; Zhang, X.; Wang, X.; Dong, Y. Preparation of microfiltration membrane supports using coarse alumina grains coated by nano TiO2 as raw materials. J. Eur. Ceram. Soc. 2014, 34, 4355–4361. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.H.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Zou, D.; Fan, Y. State-of-the-art developments in fabricating ceramic membranes with low energy consumption. Ceram. Int. 2021, 47, 14966–14987. [Google Scholar] [CrossRef]
- Hedfi, I.; Hamdi, N.; Rodriguez, M.A.; Srasra, E. Development of a low cost micro-porous ceramic membrane from kaolin and Alumina, using the lignite as porogen agent. Ceram. Int. 2016, 42, 5089–5093. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar] [CrossRef]
- Bouzerara, F.; Harabi, A.; Achour, S.; Larbot, A. Porous ceramic supports for membranes prepared from kaolin and doloma mixtures. J. Eur. Ceram. Soc. 2006, 26, 1663–1671. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Harun, Z.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Jamil, S.M.; Mohtor, N.H. Superhydrophilic, low cost kaolin-based hollow fibre membranes for efficient oily-wastewater separation. Mater. Lett. 2017, 191, 119–122. [Google Scholar] [CrossRef]
- Lu, Q.; Dong, X.; Zhu, Z.; Dong, Y. Environment-oriented low-cost porous mullite ceramic membrane supports fabricated from coal gangue and bauxite. J. Hazard. Mater. 2014, 273, 136–145. [Google Scholar] [CrossRef]
- Wang, F.; Ye, J.; He, G.; Liu, G.; Xie, Z.; Li, J. Preparation and characterization of porous MgAl2O4 spinel ceramic supports from bauxite and magnesite. Ceram. Int. 2015, 41, 7374–7380. [Google Scholar] [CrossRef]
- Saja, S.; Bouazizi, A.; Achiou, B.; Ouaddari, H.; Karim, A.; Ouammou, M.; Aaddane, A.; Bennazha, J.; Alami Younssi, S. Fabrication of low-cost ceramic ultrafiltration membrane made from bentonite clay and its application for soluble dyes removal. J. Eur. Ceram. Soc. 2020, 40, 2453–2462. [Google Scholar] [CrossRef]
- Jana, S.; Purkait, M.K.; Mohanty, K. Preparation and characterization of low-cost ceramic microfiltration membranes for the removal of chromate from aqueous solutions. Appl. Clay Sci. 2010, 47, 317–324. [Google Scholar] [CrossRef]
- Achiou, B.; Elomari, H.; Bouazizi, A.; Karim, A.; Ouammou, M.; Albizane, A.; Bennazha, J.; Alami Younssi, S.; El Amrani, I.E. Manufacturing of tubular ceramic microfiltration membrane based on natural pozzolan for pretreatment of seawater desalination. Desalination 2017, 419, 181–187. [Google Scholar] [CrossRef]
- Zou, D.; Xu, J.; Chen, X.; Drioli, E.; Qiu, M.; Fan, Y. A novel thermal spraying technique to fabricate fly ash/alumina composite membranes for oily emulsion and spent tin wastewater treatment. Sep. Purif. Technol. 2019, 219, 127–136. [Google Scholar] [CrossRef]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Zou, D.; Fan, W.; Xu, J.; Drioli, E.; Chen, X.; Qiu, M.; Fan, Y. One-step engineering of low-cost kaolin/fly ash ceramic membranes for efficient separation of oil-water emulsions. J. Membr. Sci. 2021, 621, 118954. [Google Scholar] [CrossRef]
- Zong, Y.; Wan, Q.; Cang, D. Preparation of anorthite-based porous ceramics using high-alumina fly ash microbeads and steel slag. Ceram. Int. 2019, 45, 22445–22451. [Google Scholar] [CrossRef]
- Jedidi, I.; Khemakhem, S.; Larbot, A.; Ben Amar, R. Elaboration and characterisation of fly ash based mineral supports for microfiltration and ultrafiltration membranes. Ceram. Int. 2009, 35, 2747–2753. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements—A review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dong, Y.; Dong, X.; Hampshire, S.; Zhu, L.; Zhu, Z.; Li, L. Feasible recycling of industrial waste coal fly ash for preparation of anorthite-cordierite based porous ceramic membrane supports with addition of dolomite. J. Eur. Ceram. Soc. 2016, 36, 1059–1071. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Tian, Y.; Zhao, Y.; Wang, K.; Li, G.; Chai, Y. Preparation and characterization of mullite whisker reinforced ceramics made from coal fly ash. Ceram. Int. 2019, 45, 5613–5616. [Google Scholar] [CrossRef]
- Zou, D.; Qiu, M.; Chen, X.; Drioli, E.; Fan, Y. One step co-sintering process for low-cost fly ash based ceramic microfiltration membrane in oil-in-water emulsion treatment. Sep. Purif. Technol. 2019, 210, 511–520. [Google Scholar] [CrossRef]
- Foo, C.T.; Salleh, M.A.M.; Ying, K.K.; Matori, K.A. Mineralogy and thermal expansion study of mullite-based ceramics synthesized from coal fly ash and aluminum dross industrial wastes. Ceram. Int. 2019, 45, 7488–7494. [Google Scholar] [CrossRef]
- Schneider, H.; Fischer, R.X.; Schreuer, J. Mullite: Crystal Structure and Related Properties. J. Am. Ceram. Soc. 2015, 98, 2948–2967. [Google Scholar] [CrossRef]
- Lerdprom, W.; Bhowmik, A.; Grasso, S.; Zapata-Solvas, E.; Jayaseelan, D.D.; Reece, M.J.; Lee, W.E. Impact of spark plasma sintering (SPS) on mullite formation in porcelains. J. Am. Ceram. Soc. 2018, 101, 525–535. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, Z.; Sun, W.; Hou, J.; He, B.; Dong, Y. Cost-effective utilization of mineral-based raw materials for preparation of porous mullite ceramic membranes via in-situ reaction method. Appl. Clay Sci. 2016, 120, 135–141. [Google Scholar] [CrossRef]
- Wei, Z.; Hou, J.; Zhu, Z. High-aluminum fly ash recycling for fabrication of cost-effective ceramic membrane supports. J. Alloy. Compd. 2016, 683, 474–480. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, X.; Ma, Q.; Meng, G. Preparation of cordierite-based porous ceramic micro-filtration membranes using waste fly ash as the main raw materials. J. Membr. Sci. 2006, 285, 173–181. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, Y.; Li, L.; Liu, J.; You, S.-J. Coal fly ash industrial waste recycling for fabrication of mullite-whisker-structured porous ceramic membrane supports. RSC Adv. 2015, 5, 11163–11174. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lan, G.S.; Tuan, W.H. Preparation of mullite by the reaction sintering of kaolinite and alumina. J. Eur. Ceram. Soc. 2000, 20, 2519–2525. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, W.; Li, J.; Qiao, L.; Zheng, J.; Sheng, J. Utilization of coal fly ash in the glass-ceramic production. J. Hazard. Mater. 2007, 149, 523–526. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, S.; Zhang, X.; Yang, J.; Liu, X.; Meng, G. Fabrication and characterization of low cost tubular mineral-based ceramic membranes for micro-filtration from natural zeolite. J. Membr. Sci. 2006, 281, 592–599. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, X.; Dong, D.; Wang, S.; Yang, J.; Gao, J.; Liu, X.; Meng, G. Elaboration and chemical corrosion resistance of tubular macro-porous cordierite ceramic membrane supports. J. Membr. Sci. 2007, 304, 65–75. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, B.; Zhou, J.-E.; Zhang, X.; Ling, Y.; Liu, X.; Meng, G.; Hampshire, S. Corrosion resistance characterization of porous alumina membrane supports. Mater. Charact. 2011, 62, 409–418. [Google Scholar] [CrossRef]
- Dele-Afolabi, T.T.; Hanim, M.A.A.; Norkhairunnisa, M.; Sobri, S.; Calin, R. Investigating the effect of porosity level and pore former type on the mechanical and corrosion resistance properties of agro-waste shaped porous alumina ceramics. Ceram. Int. 2017, 43, 8743–8754. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, Y.; Peng, C.; Wu, J. Preparation and characterization of high flux alumina membrane supports by composite pore former method. Ceram. Int. 2020, 46, 11297–11303. [Google Scholar] [CrossRef]
- Ma, J.; Du, B.; He, C.; Zeng, S.; Hua, K.; Xi, X.; Luo, B.; Shui, A.; Tian, W. Corrosion Resistance Properties of Porous Alumina–Mullite Ceramic Membrane Supports. Adv. Eng. Mater. 2020, 22, 1901442. [Google Scholar] [CrossRef]
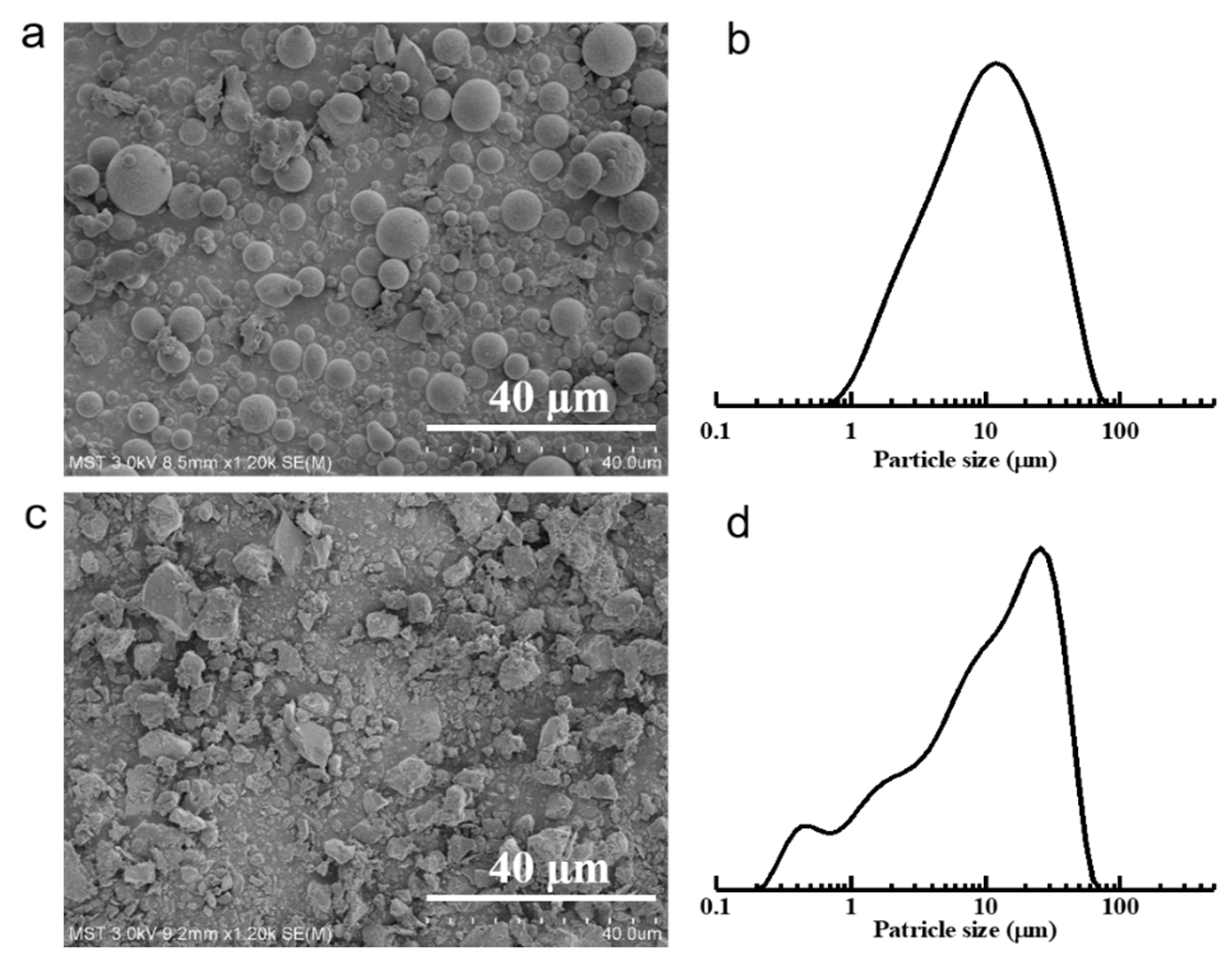
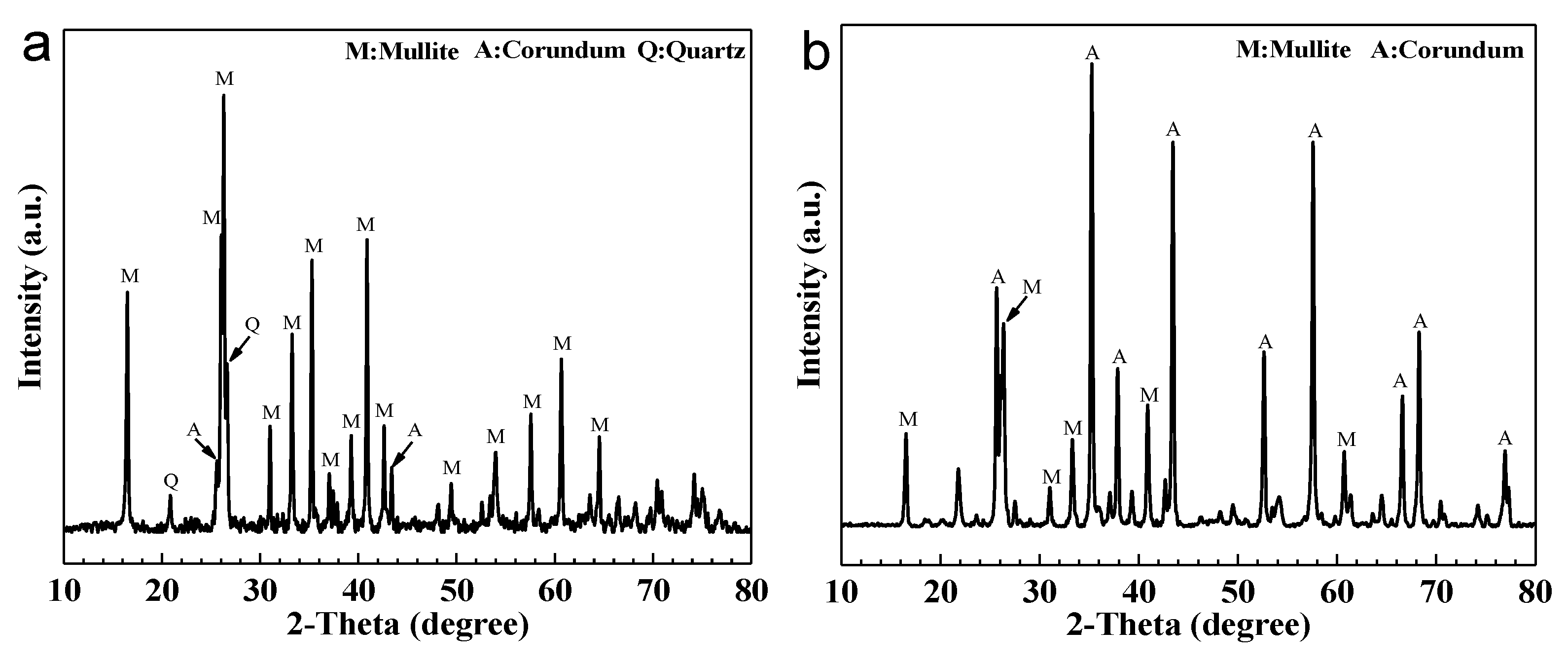
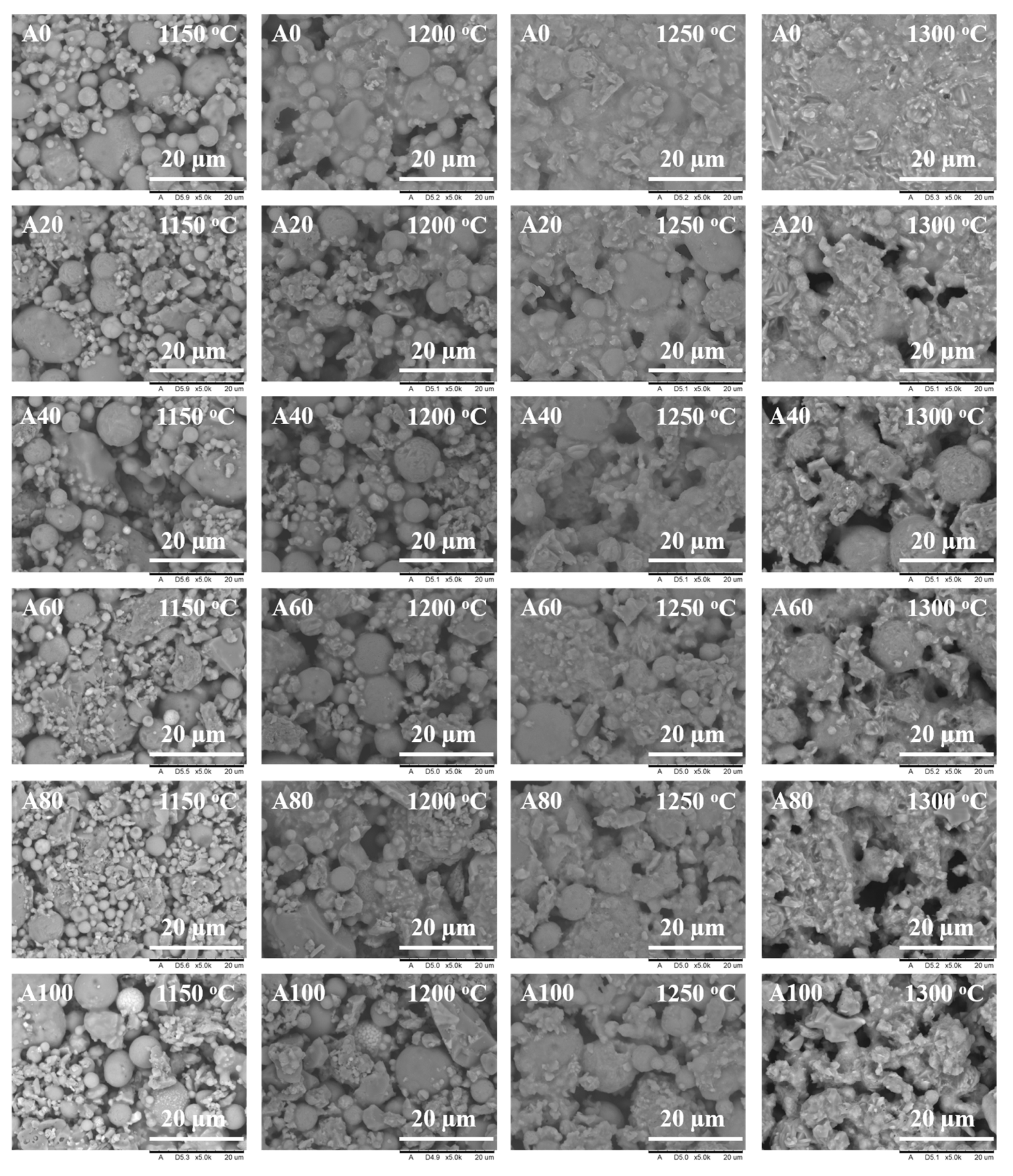


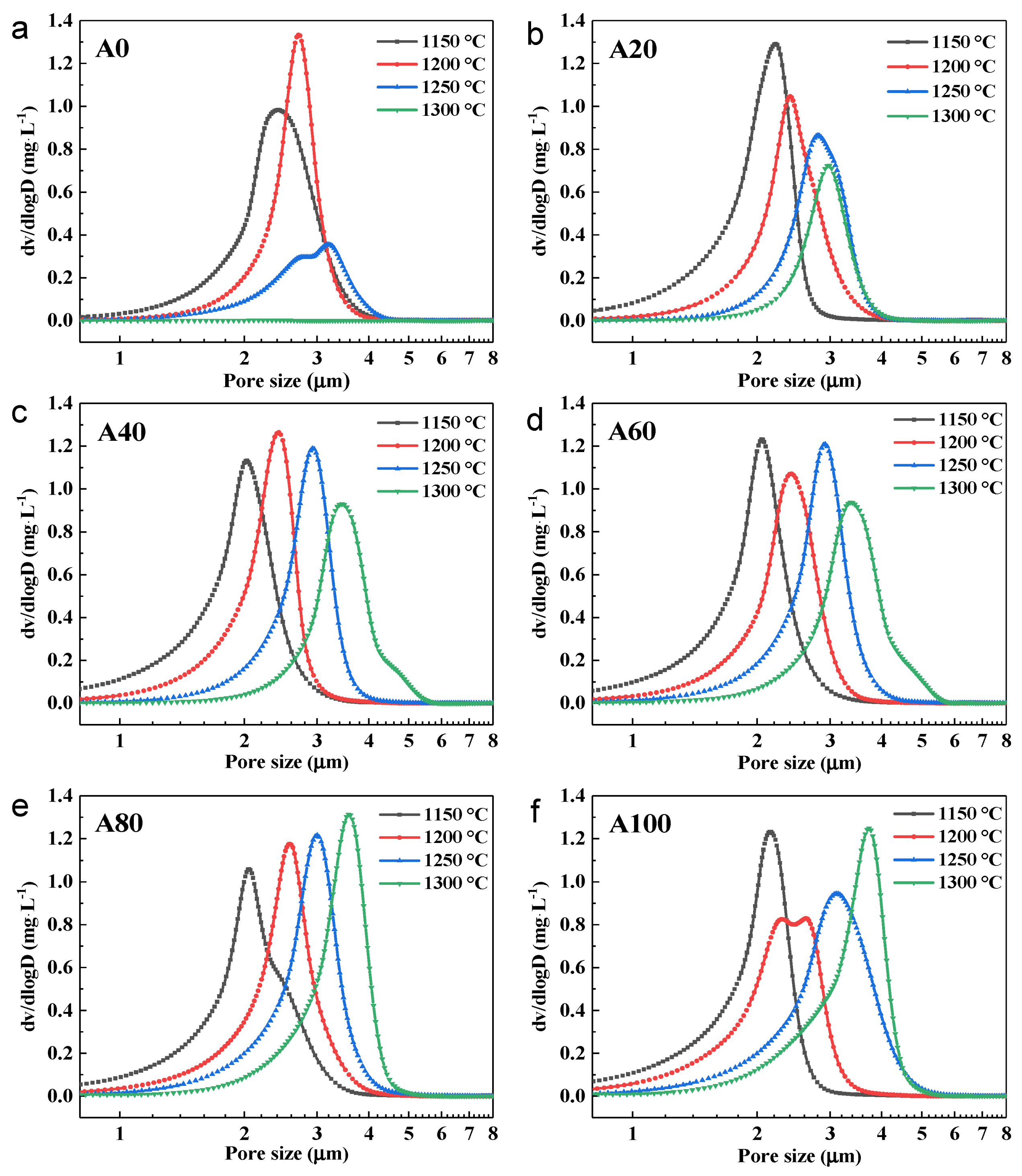

| Sample Code | Fly Ash (g) | Bauxite (g) | Glycerol (g) | PVA (g) 1 |
|---|---|---|---|---|
| A0 | 100 | 0 | 4.0 | 3.0 |
| A20 | 100 | 20 | 4.8 | 3.6 |
| A40 | 100 | 40 | 5.6 | 4.2 |
| A60 | 100 | 60 | 6.4 | 4.8 |
| A80 | 100 | 80 | 7.2 | 5.4 |
| A100 | 100 | 100 | 8.0 | 6.0 |
| Al2O3 | SiO2 | CaO | C | Fe2O3 | TiO2 | K2O | MgO | P2O5 | Na2O | Other * | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fly ash | 39.35 | 42.88 | 4.09 | 3.84 | 3.63 | 1.55 | 1.22 | 0.61 | 0.54 | 0.29 | 2.00 |
| Bauxite | 71.89 | 18.98 | 0.34 | 0.69 | 3.02 | 3.21 | 0.48 | 0.12 | 0.22 | 0.04 | 1.01 |
| Raw Materials | Sintering Temperature | Bending Strength | Pore Size | Corrosion Test | Mass Loss/Bending Strength after 8 h Acid Corrosion | Mass Loss/Bending Strength after 8 h Alkali Corrosion | Ref. | |
|---|---|---|---|---|---|---|---|---|
| (℃) | (MPa) | (μm) | Acid | Alkali | %/MPa | %/MPa | ||
| Cordierite, kaolin | 1380 | 31.03 | 8.66 | 20 wt% H2SO4, 105–107 °C | 10 wt% NaOH, 105–106 °C | 17 N/A | 12 27 | [40] |
| Alumina, kaolin | 1620 | 87.02 | 2.96 | 20 wt% H2SO4, 105–107 °C | 10 wt% NaOH, 105–107 °C | 1 78 | 23 15 | [41] |
| Alumina, rice husk | 1450 | N/A | 138 | 20 wt% H2SO4, 110 °C | 10 wt% NaOH, 110 °C | 0.95 N/A | 2 N/A | [42] |
| Alumina, sugarcane bagasse | 1450 | N/A | 84 | 20 wt% H2SO4, 110 °C | 10 wt% NaOH, 110 °C | 1.6 N/A | 1 N/A | |
| Alumina, carbon black, sawdust, HEC, TiO2 | 1500 | 46.2 | 2.42 | 20 wt% H2SO4, 80 °C | 10 wt% NaOH, 80 °C | 0.44 N/A | 0.39 N/A | [43] |
| Alumina, silica | 1550 | 51.1 | 3.1 | 20 wt% H2SO4, 80 °C | 10 wt% NaOH, 80 °C | 0.20 48 | 2.0 47 | [44] |
| 1550 | 49.7 | 3.1 | 20 wt% H2SO4, 80 °C | 10 wt% NaOH, 80 °C | 0.30 47 | 1.9 43 | ||
| Fly ash, bauxite | 1300 | 69.6 | 3.4 | 20 wt% H2SO4 100 °C | 10 wt% NaOH, 100 °C | 0.56 56 | 2.54 65 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Zou, D.; Xu, J.; Chen, X.; Qiu, M.; Fan, Y. Enhanced Performance of Fly Ash-Based Supports for Low-Cost Ceramic Membranes with the Addition of Bauxite. Membranes 2021, 11, 711. https://doi.org/10.3390/membranes11090711
Fan W, Zou D, Xu J, Chen X, Qiu M, Fan Y. Enhanced Performance of Fly Ash-Based Supports for Low-Cost Ceramic Membranes with the Addition of Bauxite. Membranes. 2021; 11(9):711. https://doi.org/10.3390/membranes11090711
Chicago/Turabian StyleFan, Wan, Dong Zou, Jingrui Xu, Xianfu Chen, Minghui Qiu, and Yiqun Fan. 2021. "Enhanced Performance of Fly Ash-Based Supports for Low-Cost Ceramic Membranes with the Addition of Bauxite" Membranes 11, no. 9: 711. https://doi.org/10.3390/membranes11090711
APA StyleFan, W., Zou, D., Xu, J., Chen, X., Qiu, M., & Fan, Y. (2021). Enhanced Performance of Fly Ash-Based Supports for Low-Cost Ceramic Membranes with the Addition of Bauxite. Membranes, 11(9), 711. https://doi.org/10.3390/membranes11090711







