Abstract
Mercury (Hg) is one of heavy metals with the highest toxicity and negative impact on the biological functions of living organisms. Therefore, many studies are devoted to solving the problem of Hg separation from wastewater. Membrane-based separation techniques have become more preferable in wastewater treatment area due to their ease of operation, mild conditions and also more resistant to toxic pollutants. This technique is also flexible and has a wide range of possibilities to be integrated with other techniques. Graphene oxide (GO) and derivatives are materials which have a nanostructure can be used as a thin and flexible membrane sheet with high chemical stability and high mechanical strength. In addition, GO-based membrane was used as a barrier for Hg vapor due to its nano-channels and nanopores. The nano-channels of GO membranes were also used to provide ion mobility and molecule filtration properties. Nowadays, this technology especially nanofiltration for Hg removal is massively explored. The aim of the review paper is to investigate Hg removal using functionalized graphene oxide nanofiltration. The main focus is the effectiveness of the Hg separation process.
1. Introduction
Mercury (Hg) is one of heavy metals with the highest toxicity and negative impact on the biological functions of living organisms (mainly humans) []. Mercury contamination in the environment pollutes water systems mainly due to atmospheric deposition (e.g., rainfall) and effluents from industrial processes primarily as Hg [,]. There are a lot of potential industries that contribute to mercury pollution such as chloroalkyl compound, vinyl chloride, plastics, electrical equipment, batteries, pulp and paper, and paint manufacturing [].
Hg pollution in waterways is a well-known problem and some countries such as U.S.A., Brazil, Indonesia, India, Iraq and China have detected mercury at harmful levels [,,]. Potentially harmful concentrations of Hg has also been observed in drinking water supplies [,] as well as reservoirs that could serve as drinking water sources [,,]. The World Health Organization has set 1 ppb as the maximum concentration of mercury in drinking water while the US EPA has set 2 ppb. The challenges associated with mercury removal are the generation of brine solutions or waste products that have to be disposed of or need regeneration, adding an additional process []. Therefore, Hg recovery in a more concentrated form is preferred and offers more benefits. Hg is typically present in very low concentrations along with other pollutants that will compete in e.g., chemical precipitation, ion exchange or adsorption processes, increasing the amount of material that must be processed to remove Hg. This introduces secondary pollution when chemical or biological treatment is used, lowering drinking water quality
Membrane technology has experienced rapid development of late, mainly due to its advantages and potential for various applications in many sectors [,,,,,,,]. In addition to providing a selective layer for one of the reaction components, the membrane can also act as a catalyst support and even be catalytically actively itself. Therefore, membrane technology is widely applied in the world of synthesis and waste treatment [,,,,,].
One of the membrane technology techniques considered to separate ions is nanostructured membrane technology. Graphene is one of the materials that has a nanostructure can be used as a thin and flexible membrane sheet with high chemical stability and high mechanical strength [,,,,,,,]. Monolayer graphene membranes are accepted to be able to remove metal ions very effectively and efficient for wastewater due to their nanopores [,]. Graphene membranes with functionalized nanopores have proven separation performance. The metal ion separation performance is promoted by nanopore size, temperature, driving pressure and carboxyl groups on the membrane surface, which increase the selectivity [,]. In addition, one of the graphene derivatives that is most applied to separate metal ions is graphene oxide (GO), which has a two-dimensional structure. Nowadays, GO membranes are a widely used kind of nanomaterial sheet for wastewater processing in industry due to their high selectivity properties for the separation the matrix ions of samples. Besides, many studies have modified the surface of the GO membranes, therefore their metal ion separation performance is increased significantly [,]. Furthermore, the laminated GO functions as a two-dimensional water channel due to its planar construction, good dispersity, and hydrophilicity [,]. GO membranes have nano-channels to provide ion mobility and molecule filtration properties [].
Mercury removal has been long term task for industries. There are various ways to remove mercury from wastewater so that it will not end up in our drinking water. This paper will briefly discuss general trends in mercury removal from aqueous solutions. Special attention will be given to nanofiltration using GO membrane materials. The effectivity of separation and also the benefits and drawbacks of GO-based nanofiltration will also be deeply discussed in the next sections.
2. The Toxicity of Mercury and Its Removal
Mercury is a neurotoxin that can cause damage to the central nervous system. High concentrations of mercury cause impairment of pulmonary and kidney function, chest pain and dyspnoea []. The classic example of mercury poisoning is the Minamata Bay incident in Japan []. Moreover, Hg accumulated in the body of organisms can attack the central nervous system, and excess exposure of the body for a long time can have a hard impact on human organs such as brain damage, gastrointestinal damage, and in extreme cases, death []. The dangers of mercury exposure have led to an increase of international restrictions on mercury levels in waterways []. Mercury can be present as elemental mercury (Hg0), oxidized mercury (Hg2+), and particulate mercury (Hgp) [,]. All orbitals of Hg0 are filled with electrons and it has no unoccupied orbitals (5d106s2 outer electron configuration) []. This makes Hg0 the most difficult species to remove due to its very high volatility, low water solubility, and relatively inertness [,,,]. On the other hand, Hg2+ is water soluble and can easily enter water bodies and be converted into methyl mercury (MeHg) and then accumulate in living organisms, including humans [,]. Hgp has a relatively short atmospheric lifetime and usually spreads along with flue gasses [], causing respiratory and chromosome damage [,].
Mitigation of mercury pollution of drinking water can be done by direct treatment of drinking water or treatment of pollution sources such as industrial wastewater streams where Hg concentrations are much higher. The common methods to remove mercury from wastewater are precipitation [], cementation [], ion exchange [], adsorption [], nanofiltration [], and solvent extraction []. Slow kinetics, low capacities due to heterogeneous reactions and interface transfer are the main limitations of said methods that make development of new techniques for mercury separation interesting [,]. In addition, adsorption of mercury can be carried out using several materials. Among sorbent materials, activated carbon is a commonly used sorbent because of its high removal capacity []. Moreover, addition of chlorine-, iodine- and sulfur-treated activated carbon boost its capacity to capture elemental mercury [], and the efficiency of mercury removal can also be enhanced by increasing the oxygen concentration []. Zhang et al. [] reported that a sulfur-functionalized polyamide-based nanofiltration membrane can effectively reduce Hg2+ concentrations in drinking water sample from 10 ppm to a low level of 0.18 ppb where the acceptable limit of Hg in drinking water is around 2 ppb. Single metals can also be used to adsorb mercury. Copper and some noble metals such as gold, silver, platinum and palladium have also been used for mercury removal. In particular, gold is preferred for Hg removal due to its efficiency. Moreover, gold is more immune to impurities such as organic substances or sulfur-containing species []. On the other hand, the efficiency of Hg removal is highly dependent on the temperature []. Metal oxides such as Fe2O3, CuO and CaO also exhibit significant mercury removal ability []. Specifically, MnO2-based materials have high efficiency for mercury removal, better regeneration, and high activity for a long time []. Other materials such as surfactants containing oxygen, nitrogen, and phosphorous also show promising mercury removal capacity [].
Another method to remove mercury is selective catalytic reduction (SCR). By SCR, Hg0 is oxidized into Hg2+ that is easier to remove. Moreover, the catalyst will bind chemically with mercury so that the water effluent will be mercury-free. Gold is a promising catalyst for SCR by chlorine. Cl2 can easily chemisorb on the Au surface and will easily oxidize mercury []. V2O5 is another important SCR catalyst. The presence of HCl, strongly influence mercury adsorption and oxidation on vanadium catalysts []. H-ZSM-5-supported Fe and Cu have been synthesized for SCR of mercury as Fe/HZSM-5 and Cu/HZSM-5 have strong ability for Hg0 removal [,].
3. Membrane Separation for Mercury and Heavy Metals
Many researchers have developed various solutions in the area of mercury separation from wastewater [,]. Several methods are used to remove mercury such as adsorption, extraction, electrolysis, and modern ones with better performance, e.g., membrane technology [,,,]. Membrane technology (ultra-, micro- and nanofiltration) work based on the selectivity and the pore size of membranes. The separation mechanism firstly involves adsorption prior to extraction or rejection of chemicals from the permeate part [,,,]. Adsorption is frequently used for metal ion separation due to its low cost, simple design, and mild operation conditions.
In a membrane-based separation process, the membrane itself acts as a contactor layer through which ion complexes and particles pass via diffusion [,]. The nanopores of graphene membranes provide a significant pathway for ion penetration, therefore the ion selectivity facilitates the metal ion separation, Furthermore, the ion diffusion of porous graphene membranes can be enhanced by acid addition. To develop a metal ion separation performance, the pores of a graphene membrane can be modified by using oxide functional group derivatives [,,]. The membrane modules often used in separation of mercury and heavy metals are hollow fibers and sheet layers [,,]. Previous researchers have investigated various thickness and pore sizes of the membrane to enhance the mobilization of metal ions while using an immobilized solvent to achieve a more selective separation process [,,]. However, a thick membrane still has advantages i.e., in the form of a transverse flow contactor which is better than the parallel flow contactor that could be more unstable. Besides, a supported liquid membrane method has drawn attention as an alternative in extractive separation for metal ion removal or neutral molecules from dilute solution. Such a method is more simple and offers advantages compared to conventional extraction methods [,,]. Moreover, emulsion liquid membranes are also often used to separate mercury from wastewater and metal ion mixtures supported by trioctylamine (TOA) [,,] and bulk liquid membranes represent a potential method []. Furthermore, ion exchange membranes are another potential method for mercury removal [].
A lot of research has been done in the field of mercury removal and given good membrane utilization performance. Several materials can be used to separate of mercury and heavy metal ions via membrane filtration (e.g., using polyaniline, polypyrrole, cellulose triacetate, nylon, chitosan, polypyrrole, polyethersulfone, graphene-based membranes, zeolite-based membranes, polyvinylamine, etc [,,,,,]). Those kinds of polymers have the properties which are required in membrane-based separation for mercury removal such as low cost, high stability, high selectivity for mercury ions, thermal stability, high chemical resistance, good ion-exchange capacity, reproducibility, high selectivity for heavy metals, the possibility of forming coordinating ligands with mercury and adsorb anions through electrostatic interaction or hydrogen bonding [,,,,].
To get good separation results via a membrane-based process the separation process is designed based on the sample source of mercury and the characteristics of the sample as main factors. As an example, Koopman et al. [] used a hollow fiber membrane contactor for heavy metal separation in the phosphoric acid industry where its sample preparation and conditions were optimized. The examples of treatments for mercury come from the chlor-alkali industry, electrical and electronic industries (in the manufacture of mercury vapor lamps and fluorescent tubes, batteries, electric switchgears, etc.), plastics industry (in the manufacture of vinyl chloride), paper and pulp industry and pharmaceutical industries which each have suitable conditions for mercury separation.
Some authors have already reported membrane-based separation processes for mercury and heavy metals in various industries. Khan et al. [] have reported a polypyrrole polyantimonic membrane with acid-based ion exchange which is highly selective for mercury ion extraction. Some important divalent ions including Hg2+–Zn2+, Hg2+–Ni2+, Hg2+–Cu2+, Hg2+–Fe3+, Hg2+–Cd2+, Hg2+–Mg2+, etc. were separated using an organic-inorganic composite system []. In another study [] mercury was separated using a supported catalytic membrane, e.g., a Mn/Mo/Ru/Al2O3 membrane which achieved 95% Hg removal at 423 K. Moreover, Ura et al. [] used Nylon 6,6 as a support, trioctylamine (TOA) as a carrier and dichloroethane as the solvent to separate mercury and lignosulfonate. The results showed that the removal of mercury and LS from mixtures was about 52.6% and 50.2%, and even in pure solution an 81% removal was achieved. Huang and co-workers [] separated mercury using polyvinylamine as the mercury-binding polymer which achieved 99% removal. In their study, the ultrafiltration technique was used. The separation occurred on the surface of the amine polymer that created binding between mercury and the polymer. A graphene-based membrane that used graphene as nanostructured membrane with good mercury removal performance was reported by Jafar and co-workers []. A summary of the performance of different membrane separation techniques for mercury and heavy metals is shown in Table 1 and further discussion of graphene-based membrane (nanofiltration) will be provided in the next section.

Table 1.
Performance of Membrane Separation for Mercury and Heavy metals.
4. Graphene-Based Membranes
Graphene is a novel material that consists of a one layer honeycomb-like carbon structure (Figure 1). Thus, it is known as an ultrathin two-dimensional material regarding its one-molecule-thickness. Consequently, graphene has very unique properties as an ultra-thin, light, transparent [,] yet mechanically strong and thermally stable material [,]. Moreover, graphene is also reported to have a good optical [] and electrical [,] properties. Many researchers have functionalized graphene in order to improve its performance by introducing graphene-based derivatives, including graphene oxide (GO), reduced graphene oxide (rGO) and other composites. In composite forms, graphene may be strengthened by addition of other materials [,,,,] or strengthening a conventional material by incorporation of graphene [].
Figure 1.
Structural Illustration of a Graphene Sheet.
Edwards and Coleman [] categorized two types of graphene synthesis, namely bottom-up and top-down. In a bottom-up process, graphene is formed via reformation of some other component (mostly silicon carbide). On the contrary, in a top-down process graphite is exfoliated into a single layer graphene. Most researchers refer to the infamous Hummers [] method as the top-down graphene synthesis, especially for graphene oxide. The improvement of Hummers-based method has been of interest for some researchers [,,] to make it more feasible for massive production. More specifically, the synthesis method may affect the properties of the resulting graphene. Therefore the modifications of synthesis methods should consider the intended application of the graphene itself regarding its required properties [].
Due to its unique properties, graphene and its derivatives have been explored in a wide range of applications, including electrical devices [,], adsorbents [,,] and also as a separation membrane []. Considering its very small openings between the carbons, a perfect graphene sheet is impermeable to a lot of gases as small as helium []. However, researchers have modified graphene by creating holes to make it semipermeable to certain gases. The porous graphene sheet has been developed and reported to have a very high selectivity for hydrogen in the presence of many other gases including methane, nitrogen, carbon dioxide, oxygen, ammonia, and argon [,,,,].
In liquid applications, graphene-based membranes are mostly used in multilayer form [,,,]. The transport mechanism across the membrane utilizes the imperfections of the graphene sheet to create a channel for water (or another solvent). The defects include holes, wrinkles, inter-edge and inter-layer spaces [,,,,,]. The modification of graphene membranes in this application is related to widened water channels within the multi-layer membrane [,] which thus increase the flux while still considering the affected separation properties (e.g., rejection, selectivity). The modifications that have been reported includes the utilization of GO [,,,,,], creating holes within the sheet [], increasing the space between layers, which can be done via crosslinking or incorporating carbon nanotubes [,] and synthesizing the composites []. Compared to pure graphene, GO is reported to have more functional groups on the surface [,] hence widening the interlayer channel and increasing the flux. SEM photos (Figure 2a–d) show that the GO composite membrane is more dense due to layer by layer interactions. A schematic of a GO membrane for wastewater treatment is presented in Figure 2e.
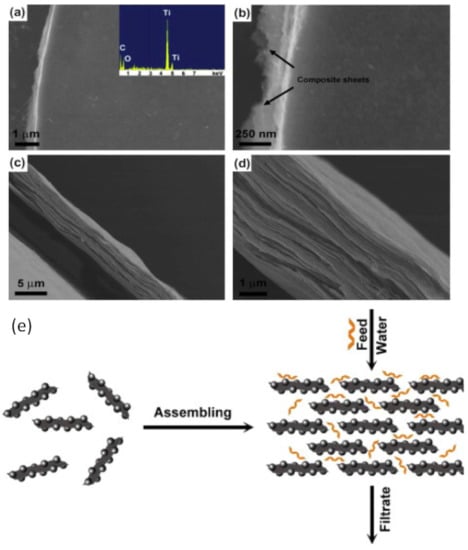
Figure 2.
(a–d) SEM photos of a GO composite membrane and (e) a schematic of a GO composite membrane for wastewater treatment. The figures are reproduced with permission from []. Copyright Elsevier, 2021.
Graphene membranes are reported to work within the nanofiltration range which is suitable for separation of ionic species [,,,], metals [,], and also dyes [,,,,]. In some references it was shown that nanofiltration process (the exclusion mechanism) depends on the steric, electric and dielectric properties of the metal ions [,,,]. Similar to most nanofiltration membranes, the separation process in a graphene nanofiltration membrane occurs by two types of mechanism, namely sieving and Donnan exclusion. In the sieving mechanism, the comparison of molecule size and the pore size does matter. The membrane will totally reject molecules which are bigger than the membrane pore size. In the Donnan exclusion mechanism, the separation considers the interactions between the membrane and the solutes related to their charges []. Unlike the original graphene, a GO membrane is charged, thus giving better performance in rejecting ionic species, including dyes [].
5. Graphene Oxide-Based Nanofiltration Membrane Preparation
In general, the main route for making GO is chemical oxidation and exfoliation of graphite using the Brodie, Staudenmaier, or Hummers methods. Brodie reported that an oxidizing KClO4 solution with fuming HNO3 can form GO only with graphite carbon containing a graphite-structured region. Staudenmaier showed that GO formation occured when graphite was reacted with strong acids (i.e., H2SO4, HNO3, and KClO4). Hummers and Offeman found a very practical method to prepare GO using H2SO4 and KMnO4 [,]. At present, GO is usually synthesized according to the modified Hummer method in which the rate of reaction is carefully controlled to keep the reaction temperature below 20 °C. The appearance of GO and graphene can be analyzed by SEM, as seen in the example results shown in Figure 3. The GO profile is more rigid and thick than that of graphene, therefore GO is very promising as a high selectivity membrane to separate Hg from wastewater.
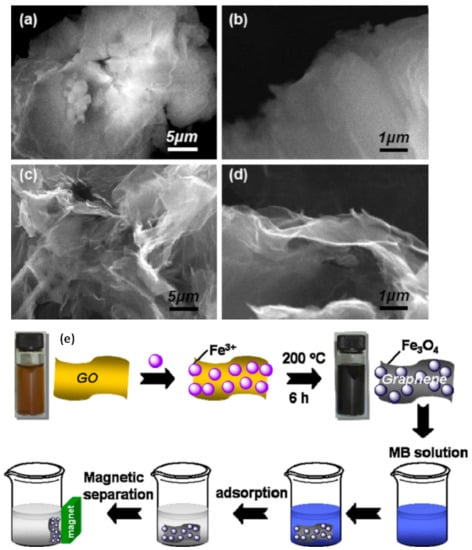
Figure 3.
The SEM photos of GO (a,b) and graphene (c,d), and a schematic of GO membrane preparation (e). The figures are reproduced with permission from [] Copyright Elsevier, 2021.
Several review papers have described the preparation of Fe3O4/GO nanocomposites by two different routes: impregnation (denoted as mGOi) and coprecipitation (denoted as mGOp). In particular, Fe3O4 nanoparticles can be synthesized by the Massart method with mixtures of FeCl3·6H2O and FeCl2·4H2O heated to 60 °C. The clear yellow solution product is separated under vigorous agitation. Then, aqueous ammonia solution is added to the solution until the pH of the solution reaches 10. The reaction was maintained for an additional 30 min under vigorous stirring. Nitrogen was used as the protective gas throughout the experiment. After completion of the reaction, the resulting black precipitate was collected by an external magnetic field, followed by washing several times with water and ethanol. Finally, the Fe3O4 nanoparticles were freeze-dried. The next step is GO membrane preparation by using a coating method. A GO aqueous solution was made by dissolving GO powder in deionized water. To form the GO nanosheets, the GO solution was subjected to ultrasonic irradiation several times. Usually, a ceramic hollow fiber membrane is used as GO membrane material due to its properties like being easily stacked on the surface with pressure driving. Then the as-prepared GO membrane was dried in a vacuum chamber at 40 °C and is ready to be used for Hg separation [,,].
6. Utilization of Graphene Oxide-Based Nanofiltration for Mercury Removal
The method of mercury separation via membrane filtration has been discussed in the previous section. Besides the mentioned sieving and Donnan exclusion mechanisms, the separation process in a membrane system can be enhanced by introducing external forces or modifying the component of interest. Some researchers introduce other agents such as polyethyleneimine (PEI) [] or iron sulfide [] to form a complex thus promoting the separation process. These complexes may either be bigger in size or have a special interaction with the membrane surface hence promoting the separation. A complex with bigger size allows the process to be performed in the ultrafiltration range thus the utility requirements are less.
Besides modifying the aqueous mercury into a complex species which has special surface properties, it is also possible to modify the membrane thus enhancing the separation process. Some researchers [,,,] have introduced functionalized graphene sheets constructed by holes in a graphene sheet which is modified by some agents that improve the surface properties. Figure 4 illustrates a functionalized graphene where the holes are modified by other functional groups. The functional groups introduced in the graphene holes include chlorine [], xanthate [] and thiol groups []. Besides the holes, the surface of the graphene membrane itself can be modified forming a composites, such as an iron-graphene composite [].
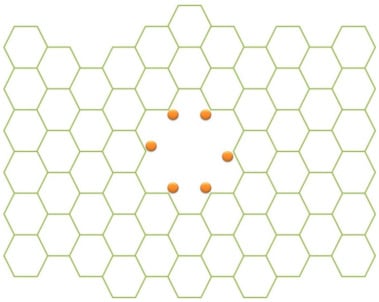
Figure 4.
Illustration of a functionalized graphene sheet. This Figure is adopted and reproduced with permission from []. Copyright Elsevier, 2021.
Even though functionalized graphene sheets offer special affinity for aqueous mercury (or its complexes), the functional groups themselves may form a barrier that hinders the passage of the mercury. Azamat [] reported that an electrical force was required in order to support the mercury transfer across the membrane. In another case, Cui [] used a magnetic force to enhance the separation. Besides those external factors, some process parameters such as pH and ion concentration also affect the separation performance. The performance of graphene oxide-based membranes for Hg removal is summarized in Table 2.

Table 2.
The performances graphene oxide-based membranes for mercury Removal.
Utilization of membrane materials in order to modify the interaction properties between the solute and membrane surface is closely related to adsorption processes. In other cases, a graphene-based material showed good affinity for adsorption processes for organic dyes [,,]. Furthermore, similar materials also give good performance in nanofiltration processes [,,,]. Studies of mercury adsorption using graphene and its derivatives have been carried out by some researchers. Modifications have also been performed thus an effective removal higher than 99% was achieved [,,,,].
Recently, research in mercury removal via graphene oxide nanofiltration has been limited to functionalized graphene sheets [,]. However, there is huge potential to utilize graphene oxide-based membranes in other configuration which are discussed in the previous section. Other studies of adsorption techniques have already presented good results. Referring to graphene oxide membrane applications in organic dye removal, many studies were conducted following good results in adsorption processes. Thus, mercury separation using graphene oxide-based membranes seems to have great potential.
7. Conclusions
Hg is one of the highest toxic substances that should be removed from any wastewater prior to environmental discharge. Several techniques can be used to perform the removal, including adsorption, liquid extraction, electrolysis, and membrane separation processes (i.e., more efficient and effective techniques). The membrane separation process can be improved by adding complexes or selecting a proper membrane material, including oxide derivatives. Graphene oxide-based membrane has presented excellent performances in nanofiltration processes for Hg removal. Recent utilization of graphene oxide-based membranes in Hg separation is only limited to functionalized graphene sheets, therefore it needs widely more improvement. Several graphene membrane types can be developed and have big potential for Hg removal such as layered membranes, intercalated membranes, crosslinked, and also composites.
Author Contributions
Conceptualization, M.Z.; formal analysis, M.Z.; writing—original draft preparation, M.Z.; writing—review and editing, M.Z.; visualization, M.Z.; project administration, M.Z.; funding acquisition, M.Z. The author has read and agreed to the published version of the manuscript.
Funding
The authors thank to PPMI, ITB, 2020 has funded this research.
Conflicts of Interest
The author declares no conflict of interest.
References
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Wagner-Döbler, I. Pilot plant for bioremediation of mercury-containing industrial wastewater. Appl. Microbiol. Biotechnol. 2003, 62, 124–133. [Google Scholar] [CrossRef]
- Barron-Zambrano, J.; Laborie, S.; Viers, P.; Rakib, M. Mercury removal from aqueous solutions by complexation—Ultrafiltration. Desalination 2002, 144, 201–206. [Google Scholar] [CrossRef]
- Jiang, G.-B.; Shi, J.-B.; Feng, X.-B. Mercury pollution in China. Environ. Sci. Technol. 2006, 40, 3672–3678. [Google Scholar] [CrossRef]
- Barringer, J.L.; Szabo, Z. Overview of investigations into mercury in ground water, soils, and septage. N. J. Coast. Plain Water Air Soil Pollut. 2006, 175, 193–221. [Google Scholar] [CrossRef]
- Lisha, K.; Pradeep, A.; Pradeep, T. Towards a practical solution for removing inorganic mercury from drinking water using gold nanoparticles. Gold Bull. 2009, 42, 144–152. [Google Scholar] [CrossRef]
- Heaven, S.; Ilyushchenko, M.; Tanton, T.; Ullrich, S. Mercury in the River Nura and its floodplain, Central Kazakhstan: I. River sediments and water. Sci. Total Environ. 2000, 260, 35–44. [Google Scholar] [CrossRef]
- Yan, H.; Feng, X.; Shang, L.; Qiu, G. The variations of mercury in sediment profiles from a historically mercury-contaminated reservoir, Guizhou province, China. Sci. Total Environ. 2008, 407, 497–506. [Google Scholar] [CrossRef]
- Liu, B.; Yan, H.; Wang, C.; Li, Q. Insights into low fish mercury bioaccumulation in a mercury-contaminated reservoir, Guizhou, China. Environ. Pollut. 2012, 160, 109–117. [Google Scholar] [CrossRef]
- Oehmen, A.; Vergel, D.; Fradinho, J.; Reis, M.A.M. Mercury removal from water streams through the ion exchange membrane bioreactor concept. J. Hazard. Mater. 2014, 264, 65–70. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification—An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Strathmann, H.; Grabowski, A.; Eigenberger, G. Ion-exchange membranes in the chemical process industry. Ind. Eng. Chem. Res. 2013, 52, 10364–10379. [Google Scholar] [CrossRef]
- Wenten, I. Reverse osmosis applications: Prospect and challenges. Desalination 2016, 391, 112–125. [Google Scholar] [CrossRef]
- Himma, N.F.; Anisah, S.; Prasetya, N.; Wenten, I.G. Advances in preparation, modification, and application of polypropylene membrane. J. Polym. Eng. 2016, 36, 329–362. [Google Scholar] [CrossRef]
- Khoiruddin, K.; Hakim, A.; Wenten, I. Advances in electrodeionization technology for ionic separation—A review. Membr. Water Treat. 2014, 5, 87–108. [Google Scholar] [CrossRef]
- Wenten, I.G.; Victoria, A.V.; Tanukusuma, G.; Khoiruddin, K. Simultaneous clarification and dehydration of crude palm oil using superhydrophobic polypropylene membrane. J. Food Eng. 2019, 248, 23–27. [Google Scholar] [CrossRef]
- Makertihartha, I.; Dharmawijaya, P.T.; Zunita, M.; Wenten, I.G. Hydrogen Selective Layer for Dehydrogenation Membrane Reactor. Adv. Sci. Lett. 2017, 23, 5726–5728. [Google Scholar] [CrossRef]
- Wenten, I.G.; Syaifi, Y.S.; Saputra, F.A.; Zunita, M. Preparation of antibacterial and antifouling PSF/ZnO/eugenol membrane for peat water ultrafiltration. Water Supply 2019, 19, 2248–2255. [Google Scholar] [CrossRef]
- Criscuoli, A.; Basile, A.; Drioli, E.; Loiacono, O. An economic feasibility study for water gas shift membrane reactor. J. Membr. Sci. 2001, 181, 21–27. [Google Scholar] [CrossRef]
- Li, K. Ceramic Membranes for Separation and Reaction; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zunita, M.; Makertihartha, I.; Saputra, F.; Syaifi, Y. Metal oxide based antibacterial membrane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 395, 012021. [Google Scholar] [CrossRef]
- Makertihartha, I.; Zunita, M.; Rizki, Z.; Dharmawijaya, P. Solvent extraction of gold using ionic liquid based process. AIP Conf. Proc. 2017, 1805. [Google Scholar] [CrossRef]
- Makertihartha, I.; Zunita, M.; Dharmawijaya, P.; Wenten, I. Supported ionic liquid membrane in membrane reactor. AIP Conf. Proc. 2017, 1788. [Google Scholar] [CrossRef]
- Makertihartha, I.; Rizki, Z.; Zunita, M.; Dharmawijaya, P. Dyes removal from textile wastewater using graphene based nanofiltration. AIP Conf. Proc. 2017, 1840. [Google Scholar] [CrossRef]
- Tsetseris, L.; Pantelides, S.T. Graphene: An impermeable or selectively permeable membrane for atomic species? Carbon 2014, 67, 58–63. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Zunita, M.; Irawanti, R.; Koesmawati, T.A.; Lugito, G. Graphene Oxide (Go) Membrane in Removing Heavy Metals From Wastewater: A Review. Chem. Eng. Trans. 2020, 82, 415–420. [Google Scholar]
- Makertihartha, I.; Rizki, Z.; Zunita, M.; Dharmawijaya, P.T. Graphene Based Nanofiltration for Mercury Removal from Aqueous Solutions. Adv. Sci. Lett. 2017, 23, 5684–5686. [Google Scholar] [CrossRef]
- Makertiharta, I.; Dharmawijaya, P.; Zunita, M.; Wenten, I. Rare earth element enrichment using membrane based solvent extraction. AIP Conf. Proc. 2017, 1805. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zhao, Y.; Zhang, X. Selective separation of metal ions via monolayer nanoporous graphene with carboxyl groups. Anal. Chem. 2016, 88, 10002–10010. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, X.; Li, F.; Zhang, N. Influence of nanopore density on ethylene/acetylene separation by monolayer graphene. Phys. Chem. Chem. Phys. 2019, 21, 6126–6132. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Chae, H.-R.; Lee, J.; Lee, C.-H.; Kim, I.-C. Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Shao, X.; Liu, S. Dual fluorescence of graphene oxide: A time-resolved study. J. Phys. Chem. A 2012, 116, 7308–7313. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Gao, X.; Yuan, Y. Declining flux and narrowing nanochannels under wrinkles of compacted graphene oxide nanofiltration membranes. Carbon 2016, 108, 568–575. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kadirvelu, K. Uptake of mercury (II) from wastewater by activated carbon from an unwanted agricultural solid by-product: Coirpith. Carbon 1999, 37, 79–84. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Galbreath, K.C.; Zygarlicke, C.J. Mercury speciation in coal combustion and gasification flue gases. Environ. Sci. Technol. 1996, 30, 2421–2426. [Google Scholar] [CrossRef]
- Presto, A.A.; Granite, E.J. Survey of catalysts for oxidation of mercury in flue gas. Environ. Sci. Technol. 2006, 40, 5601–5609. [Google Scholar] [CrossRef]
- Lee, W.; Bae, G.-N. Removal of elemental mercury (Hg (0)) by nanosized V2O5/TiO2 catalysts. Environ. Sci. Technol. 2009, 43, 1522–1527. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Yan, N.; Wu, D. Nanosized Cation-Deficient Fe− Ti Spinel: A Novel Magnetic Sorbent for Elemental Mercury Capture from Flue Gas. ACS Appl. Mater. Interfaces 2011, 3, 209–217. [Google Scholar] [CrossRef]
- Kellie, S.; Cao, Y.; Duan, Y.; Li, L. Factors affecting mercury speciation in a 100-MW coal-fired boiler with low-NO x burners. Energy Fuels 2005, 19, 800–806. [Google Scholar] [CrossRef]
- Eom, Y.; Jeon, S.H.; Ngo, T.A.; Kim, J. Heterogeneous mercury reaction on a selective catalytic reduction (SCR) catalyst. Catal. Lett. 2008, 121, 219–225. [Google Scholar] [CrossRef]
- Hu, C.-X.; Zhou, J.S.; Luo, Z.Y.; Sheng, H. Effect of oxidation treatment on the adsorption and the stability of mercury on activated carbon. J. Environ. Sci. 2006, 18, 1161–1166. [Google Scholar] [CrossRef]
- Mergler, D.; Anderson, H.A.; Chan, L.H.M.; Mahaffey, K.R. Methylmercury exposure and health effects in humans: A worldwide concern. AMBIO 2007, 36, 3–11. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, L.; Li, Z.; Liu, J.M. Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a Chinese population. Reprod. Toxicol. 2013, 35, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.-C.; Yang, I.-L.; Liu, C.-K. Measure and modeling the ambient air particles and particle bound mercury Hg (p) at a traffic sampling site. Atmos. Res. 2010, 97, 97–105. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lo, C.-T.; Fang, G.-C.; Huang, C.-S. Particulate-bound mercury (Hg [p]) size distributions in Central Taiwan. Environ. Forensics 2012, 13, 98–104. [Google Scholar] [CrossRef]
- Silvo, K.; Melanen, M.; Honkasalo, A.; Ruonala, S. Integrated pollution prevention and control—the Finnish approach. Resour. Conserv. Recycl. 2002, 35, 45–60. [Google Scholar] [CrossRef]
- Ritter, J.A.; Bibler, J. Removal of mercury from waste water: Large-scale performance of an ion exchange process. Water Sci. Technol. 1992, 25, 165–172. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Cai, H.; Xia, Y.; Zhang, P. An l-cystine/l-cysteine impregnated nanofiltration membrane with the superior performance of an anchoring heavy metal in wastewater. RSC Adv. 2020, 10, 3438–3449. [Google Scholar] [CrossRef]
- Fiskum, S.K.; Rapko, B.M.; Lumetta, G.J. Partitioning of mercury from actinides in the TRUEX process. Solvent Extr. Ion Exch. 2001, 19, 643–657. [Google Scholar] [CrossRef]
- Volchek, K.; Krentsel, E.; Zhilin, Y.; Shtereva, G. Polymer binding/ultrafiltration as a method for concentration and separation of metals. J. Membr. Sci. 1993, 79, 253–272. [Google Scholar] [CrossRef]
- Chaufer, B.; Deratani, A. Removal of metal ions by complexation-ultrafiltration using water-soluble macromolecules: Perspective of application to wastewater treatment. Nucl. Chem. Waste Manag. 1988, 8, 175–187. [Google Scholar] [CrossRef]
- Olson, E.; Miller, S.; Sharma, R.; Dunham, G. Catalytic effects of carbon sorbents for mercury capture. J. Hazard. Mater. 2000, 74, 61–79. [Google Scholar] [CrossRef]
- Granite, E.J.; Pennline, H.W.; Hargis, R.A. Novel sorbents for mercury removal from flue gas. Ind. Eng. Chem. Res. 2000, 39, 1020–1029. [Google Scholar] [CrossRef]
- Hall, B.; Schager, P.; Weesmaa, J. The homogeneous gas phase reaction of mercury with oxygen, and the corresponding heterogeneous reactions in the presence of activated carbon and fly ash. Chemosphere 1995, 30, 611–627. [Google Scholar] [CrossRef]
- Baldeck, C.M.; Kalb, G.W.; Crist, H.L. Determination of elemental mercury in an emission source having a high sulfur dioxide concentration by amalgamation with gold and ultraviolet spectrophotometry. Anal. Chem. 1974, 46, 1500–1505. [Google Scholar] [CrossRef]
- Aeschliman, D.B.; Norton, G.A. Collection and thermal evolution behaviors of different mercury species captured with gold. Environ. Sci. Technol. 1999, 33, 2278–2283. [Google Scholar] [CrossRef]
- Ghorishi, S.B.; Lee, C.W.; Jozewicz, W.S.; Kilgroe, J.D. Effects of fly ash transition metal content and flue gas HCl/SO2 ratio on mercury speciation in waste combustion. Environ. Eng. Sci. 2005, 22, 221–231. [Google Scholar] [CrossRef]
- Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.-H. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol. 2012, 90, 4–20. [Google Scholar] [CrossRef]
- Ling, L.; Fan, M.; Wang, B.; Zhang, R. Application of computational chemistry in understanding the mechanisms of mercury removal technologies: A review. Energy Environ. Sci. 2015, 8, 3109–3133. [Google Scholar] [CrossRef]
- Spencer, N.; Lambert, R. Chlorine chemisorption and surface chloride formation on Au (111). Surf. Sci. 1981, 107, 237–248. [Google Scholar] [CrossRef]
- Xu, W.; Tong, L.; Qi, H.; Zhou, X. Effect of Flue Gas Components on Hg0 Oxidation over Fe/HZSM-5 Catalyst. Ind. Eng. Chem. Res. 2014, 54, 146–152. [Google Scholar] [CrossRef]
- Fan, X.; Li, C.; Zeng, G.; Zhang, X. The effects of Cu/HZSM-5 on combined removal of Hg 0 and NO from flue gas. Fuel Process. Technol. 2012, 104, 325–331. [Google Scholar] [CrossRef]
- Li, Q.; Sun, L.; Zhang, Y.; Qian, Y. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II) and Cr(VI) by polyaniline/humic acid composite. Desalination 2011, 266, 188–194. [Google Scholar] [CrossRef]
- Wang, J.; Deng, B.; Chen, H.; Wang, X. Removal of Aqueous Hg(II) by Polyaniline: Sorption Characteristics and Mechanisms. Environ. Sci. Technol. 2009, 43, 5223–5228. [Google Scholar] [CrossRef]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D. Heavy metal separation with polymer inclusion membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Saha, P.; Ghoshal, A.K. Simultaneous separation of mercury and lignosulfonate from aqueous solution using supported liquid membrane. J. Membr. Sci. 2010, 346, 37–44. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Yang, F. Removal of aqueous Hg(II) and Cr(VI) using phytic acid doped polyaniline/cellulose acetate composite membrane. J. Hazard. Mater. 2014, 280, 20–30. [Google Scholar] [CrossRef]
- Pancharoen, U.; Somboonpanya, S.; Chaturabul, S.; Lothongkum, A.W. Selective removal of mercury as HgCl 4 2− from natural gas well produced water by TOA via HFSLM. J. Alloy. Compd. 2010, 489, 72–79. [Google Scholar] [CrossRef]
- Shamsipur, M.; Hashemi, O.R.; Lippolis, V. A supported liquid membrane system for simultaneous separation of silver (I) and mercury (II) from dilute feed solutions. J. Membr. Sci. 2006, 282, 322–327. [Google Scholar] [CrossRef]
- Zulfikar, M.; Maulina, D.; Nasir, M.; Alni, A. Poly (acrylic acid)/SiO2 composite nanofiber functionalized with mercapto groups for the removal of humic acid from aqueous solution. Desalin. Water Treat. 2019, 141, 115–123. [Google Scholar] [CrossRef]
- Boricha, A.G.; Murthy, Z. Acrylonitrile butadiene styrene/chitosan blend membranes: Preparation, characterization and performance for the separation of heavy metals. J. Membr. Sci. 2009, 339, 239–249. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Tan, H.; Qi, W. Combustion fabrication of nanoporous graphene for ionic separation membranes. Adv. Funct. Mater. 2018, 28, 1805026. [Google Scholar] [CrossRef]
- Tan, H.; Liu, T.; Zhang, X.; Shan, Q. Preparation of vortex porous graphene chiral membrane for enantioselective separation. Anal. Chem. 2020, 92, 13630–13633. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, X.; Li, Z.; Liang, Q. Nitrogen-doped nanoporous graphene induced by a multiple confinement strategy for membrane separation of rare earth. Iscience 2021, 24, 101920. [Google Scholar] [CrossRef]
- Azamat, J.; Khataee, A.; Joo, S.W. Functionalized graphene as a nanostructured membrane for removal of copper and mercury from aqueous solution: A molecular dynamics simulation study. J. Mol. Graph. Model. 2014, 53, 112–117. [Google Scholar] [CrossRef]
- Mercader-Trejo, F.E.; Rodríguez de San Miguel, E.; de Gyves, J. Mercury (II) removal using polymer inclusion membranes containing Cyanex 471X. J. Chem. Technol. Biotechnol. 2009, 84, 1323–1330. [Google Scholar] [CrossRef]
- Brinchi, L.; Germani, R.; Mancini, M.V.; Savelli, G. Carrier-Mediated Transport of Toxic Heavy Metal Ions in Bulk Liquid Membranes. Eur. J. Org. Chem. 2004, 2004, 1330–1335. [Google Scholar] [CrossRef]
- Gupta, S.; Chakraborty, M.; Murthy, Z. Removal of mercury by emulsion liquid membranes: Studies on emulsion stability and scale up. J. Dispers. Sci. Technol. 2013, 34, 1733–1741. [Google Scholar] [CrossRef]
- Visser, H.C.; Reinhoudt, D.N.; de Jong, F. Carrier-mediated transport through liquid membranes. Chem. Soc. Rev. 1994, 23, 75–81. [Google Scholar] [CrossRef]
- Sirlin, C.; Burgard, M.; Leroy, M.; Prevost, M. Silver nitrate refining using supported liquid membranes. J. Membr. Sci. 1990, 54, 299–305. [Google Scholar] [CrossRef]
- Cahn, R.; Li, N. Separation of phenol from waste water by the liquid membrane technique. Sep. Sci. 1974, 9, 505–519. [Google Scholar] [CrossRef]
- Rajasimman, M.; Sangeetha, R.; Karthik, P. Statistical optimization of process parameters for the extraction of chromium (VI) from pharmaceutical wastewater by emulsion liquid membrane. Chem. Eng. J. 2009, 150, 275–279. [Google Scholar] [CrossRef]
- Matos, C.T.; Velizarov, S.; Crespo, J.G.; Reis, M.A.M. Simultaneous removal of perchlorate and nitrate from drinking water using the ion exchange membrane bioreactor concept. Water Res. 2006, 40, 231–240. [Google Scholar] [CrossRef]
- Hanif, Z.; Lee, S.; Qasim, G.H.; Ardiningsih, I. Polypyrrole multilayer-laminated cellulose for large-scale repeatable mercury ion removal. J. Mater. Chem. A 2016, 4, 12425–12433. [Google Scholar] [CrossRef]
- Khan, A.A.; Alam, M.M. New and novel organic–inorganic type crystalline ‘polypyrrolel/polyantimonic acid’composite system: Preparation, characterization and analytical applications as a cation-exchange material and Hg (II) ion-selective membrane electrode. Anal. Chim. Acta 2004, 504, 253–264. [Google Scholar] [CrossRef]
- Nakagawa, R.; Yumita, Y. Change and behavior of residual mercury in paddy soils and rice of Japan. Chemosphere 1998, 37, 1483–1487. [Google Scholar] [CrossRef]
- Han, D.S.; Orillano, M.; Khodary, A.; Duan, Y. Reactive iron sulfide (FeS)-supported ultrafiltration for removal of mercury (Hg(II)) from water. Water Res. 2014, 53, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Sudharsan, S.; Kannan, R.S. Synthesis, characterization and ion-exchange properties of novel hybrid polymer nanocomposites for selective and effective mercury (ii) removal. RSC Adv. 2015, 5, 79665–79678. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Streets, D.G.; Hao, J. Trends in Anthropogenic Mercury Emissions in China from 1995 to 2003. Environ. Sci. Technol. 2006, 40, 5312–5318. [Google Scholar] [CrossRef]
- Koopman, C.; Witkamp, G. Extraction of heavy metals from industrial phosphoric acid in a transverse flow hollow fiber membrane contactor. Sep. Sci. Technol. 2002, 37, 1273–1290. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, N.; Liu, P.; Yang, S. Removal of elemental mercury with Mn/Mo/Ru/Al2O3 membrane catalytic system. Front. Environ. Sci. Eng. 2013, 7, 464–473. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.R.; Zhang, Y.; Lawless, D. Removal of mercury (II) from wastewater by polyvinylamine-enhanced ultrafiltration. Sep. Purif. Technol. 2015, 154, 1–10. [Google Scholar] [CrossRef]
- Müslehiddinoğlu, J.; Uludağ, Y.; Özbelge, H.Ö.; Yilmaz, L. Effect of operating parameters on selective separation of heavy metals from binary mixtures via polymer enhanced ultrafiltration. J. Membr. Sci. 1998, 140, 251–266. [Google Scholar] [CrossRef]
- Spreti, N.; Brinchi, L.; Germani, R.; Mancini, M.V. A new carrier for selective removal of heavy metal ions from aqueous solutions through bulk liquid membranes. Eur. J. Org. Chem. 2004, 2004, 3865–3871. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Huang, W. Preparation and recovery of polysulfone affinity membrane with mercapto as chelating group for Hg2+ cations. J. Appl. Polym. Sci. 2007, 103, 2514–2522. [Google Scholar] [CrossRef]
- Meeks, N.D.; Davis, E.; Jain, M.; Skandan, G. Mercury removal by thiol-functionalized metal oxide–carbon black sorbent and mixed-matrix membranes. Environ. Prog. Sustain. Energy 2013, 32, 705–714. [Google Scholar] [CrossRef]
- Urgun-Demirtas, M.; Benda, P.L.; Gillenwater, P.S.; Negri, M.C. Achieving very low mercury levels in refinery wastewater by membrane filtration. J. Hazard. Mater. 2012, 215-216, 98–107. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef]
- Jankovský, O.; Marvan, P.; Nováček, M.; Luxa, J. Synthesis procedure and type of graphite oxide strongly influence resulting graphene properties. Appl. Mater. Today 2016, 4, 45–53. [Google Scholar] [CrossRef]
- Frank, I.W.; Tanenbaum, D.M.; Van Der Zande, A.M.; McEuen, P.L. Mechanical properties of suspended graphene sheets. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2007, 25, 2558–2561. [Google Scholar] [CrossRef]
- Falkovsky, L.A. Optical properties of graphene. J. Phys. Conf. Ser. 2008, 129, 012004. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Petridis, C.; Konios, D.; Stylianakis, M.M.; Kakavelakis, G. Solution processed reduced graphene oxide electrodes for organic photovoltaics. Nanoscale Horiz. 2016, 1, 375–382. [Google Scholar] [CrossRef]
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide—TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef]
- Galán-vidal, C.A.; Romero-romo, M.; Palomar-pardave, M. Mercury Ions Removal from Aqueous Solution Using an Activated Composite Membrane Mercury Ions Removal from Aqueous Solution Using an Activated Composite Membrane. Environ. Sci. Technol. 2005. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W. Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene Oxide Synthesized by using Modified Hummers Approach. Int. J. Renew. Energy Environ. Eng. 2014, 02, 58–63. [Google Scholar]
- Kaniyoor, A.; Baby, T.T.; Ramaprabhu, S. Graphene synthesis via hydrogen induced low temperature exfoliation of graphite oxide. J. Mater. Chem. 2010, 20, 8467. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene—carbon nanotube hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Jiao, T.; Guo, H.; Zhang, Q.; Peng, Q. Reduced Graphene Oxide-Based Silver Nanoparticle-Containing Composite Hydrogel as Highly Efficient Dye Catalysts for Wastewater Treatment. Nat. Publ. Group 2015. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-Based Membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, J.; Zhang, J.; Su, G. Separation of Hydrogen and Nitrogen Gases with Porous Graphene Membrane Separation of Hydrogen and Nitrogen Gases with Porous Graphene Membrane. J. Phys. Chem. C 2011, 115, 23261–23266. [Google Scholar] [CrossRef]
- Schrier, J. Helium separation using porous graphene membranes. J. Phys. Chem. Lett. 2010, 1, 2284–2287. [Google Scholar] [CrossRef]
- Blankenburg, S.; Bieri, M.; Fasel, R.; Müllen, K. Porous graphene as an atmospheric nanofilter. Nanoporous Mater. 2010, 6, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.E.; Cooper, V.R.; Dai, S. Porous gaphene as the ultimate membrane for gas separation. Nano Lett. 2009, 9, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Gao, J.; Chung, T.S. Layer-by-layer construction of graphene oxide (GO) framework composite membranes for highly ef fi cient heavy metal removal. J. Membr. Sci. 2016, 515, 230–237. [Google Scholar] [CrossRef]
- Schaepe, S. Engineering Graphene Oxide Membranes for Contaminant Removal and Bacterial Inactivation. Master’s Thesis, University of Nebraska Lincoln, Lincoln, NE, USA, 2015. [Google Scholar]
- Wang, J.; Tsuzuki, T.; Tang, B.; Sun, L.; Dai, X.J.; Rajmohan, G.D.; Li, J.; Wang, X. Recyclable textiles functionalized with reduced graphene oxide@ ZnO for removal of oil spills and dye pollutants. Aust. J. Chem. 2014, 67, 71–77. [Google Scholar] [CrossRef]
- Wei, N.; Peng, X.; Xu, Z. Understanding Water Permeation in Graphene Oxide Membranes. ACS Appl. Mater. Interfaces 2014, 6, 5877–5883. [Google Scholar] [CrossRef]
- Chong, J.Y.; Wang, B.; Li, K. Graphene oxide membranes in fluid separations. Sep. Eng. 2016, 12, 98–105. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Li, Q.; Ma, J. Preparation, characterization, and application of mesoporous silica-grafted graphene oxide for highly selective lead adsorption. Chem. Eng. J. 2015, 273, 630–637. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Huang, H.; Ying, Y.; Peng, X. Graphene oxide nanosheet: An emerging star material for novel separation membranes. J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 13772–13782. [Google Scholar] [CrossRef]
- Chong, J.Y.; Aba, N.F.D.; Wang, B.; Mattevi, C. UV-Enhanced Sacrificial Layer Stabilised Graphene Oxide Hollow Fibre Membranes for Nanofiltration. Nature 2015. [Google Scholar] [CrossRef]
- Aba, N.F.D.; Yi, J.; Wang, B.; Mattevi, C. Graphene oxide membranes on ceramic hollow fibers—Microstructural stability and nano fi ltration performance. J. Membr. Sci. 2015, 484, 87–94. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofi ltration Membrane for Water Purifi cation. Anvanced Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Gao, C. High-Flux Graphene Oxide Nano fi ltration Membrane Intercalated by Carbon Nanotubes. Appl. Mater. Interfaces 2015, 7, 8147–8155. [Google Scholar] [CrossRef]
- Nan, Q.; Li, P.; Cao, B. Applied Surface Science Fabrication of positively charged nanofiltration membrane via the layer-by-layer assembly of graphene oxide and polyethylenimine for desalination. Appl. Surf. Sci. 2016, 387, 521–528. [Google Scholar] [CrossRef]
- Aghigh, A.; Alizadeh, V.; Wong, H.Y.; Islam, S. Recent advances in utilization of graphene for fi ltration and desalination of water: A review. Desalination 2015, 365, 389–397. [Google Scholar] [CrossRef]
- Azamat, J.; Shirforush, B.; Khataee, A.; Woo, S. Removal of a hazardous heavy metal from aqueous solution using functionalized graphene and boron nitride nanosheets: Insights from simulations. J. Mol. Graph. Model. 2015, 61, 13–20. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mansoor, B.; Mansour, A.; Khraisheh, M. Functional graphene nanosheets: The next generation membranes for water desalination. DES 2015, 356, 208–225. [Google Scholar] [CrossRef]
- Liu, F.; Chung, S.; Oh, G.; Seo, T.S. Three-Dimensional Graphene Oxide Nanostructure for Fast and Efficient Water-Soluble Dye Removal. Appl. Mater. Interfaces 2012, 4, 922–927. [Google Scholar] [CrossRef]
- Kumar, V.S.; Hariharan, K.S.; Mayya, K.S.; Han, S. Volume averaged reduced order Donnan Steric Pore Model for nano fi ltration membranes. DES 2013, 322, 21–28. [Google Scholar] [CrossRef]
- Jiao, T.; Liu, Y.; Wu, Y.; Zhang, Q. Facile and Scalable Preparation of Graphene Oxide-Based Magnetic Hybrids for Fast and Highly Efficient Removal of Organic Dyes. Nat. Publ. Group 2015. [Google Scholar] [CrossRef]
- Zunita, M.; Makertiharta, I.; Irawanti, R.; Prasetya, N. Graphene oxide-inorganic composite membrane: A review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Barron-Zambrano, J.; Laborie, S.; Viers, P.; Rakib, M. Mercury removal and recovery from aqueous solutions by coupled complexation-ultrafiltration and electrolysis. J. Membr. Sci. 2004, 229, 179–186. [Google Scholar] [CrossRef]
- Babu, C.M.; Vinodh, R.; Abidov, A. Removal of heavy metals using Amine crosslinked Reduced Graphene Oxide. Adv. Sci. Technol. Lett. 2015, 120, 430–433. [Google Scholar]
- Cui, L.; Guo, X.; Wei, Q.; Wang, Y. Removal of mercury and methylene blue from aqueous solution by xanthate functionalized magnetic graphene oxide: Sorption kinetic and uptake mechanism. J. Colloid Interface Sci. 2015, 439, 112–120. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.H.; Cole, M.A.; Losic, D. Functionalized three-dimensional (3D) graphene composite for high efficiency removal of mercury. Environ. Sci. Water Res. Technol. 2016, 2, 390–402. [Google Scholar] [CrossRef]
- Hartanto, Y.; Yaswari, Y.; Zunita, M.; Soerawidjaja, T.H. Decolorization of crude terpineol by adsorption. Sep. Sci. Technol. 2017, 52, 1967–1972. [Google Scholar] [CrossRef]
- Ziaei, E.; Mehdinia, A.; Jabbari, A. A novel hierarchical nanobiocomposite of graphene oxide-magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal. Chim. Acta 2014, 850, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.; Goncalves, G.; Emami, N.; Pereira, E. Optimized graphene oxide foam with enhanced performance and high selectivity for mercury removal from water. J. Hazard. Mater. 2016, 301, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kong, L.; Liu, J. Removal of mercury and fluoride from aqueous solutions by three-dimensional reduced-graphene oxide aerogel. Res. Chem. Intermed. 2016, 42, 4513–4530. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).