Study of Turbulence Promoters in Prolonging Membrane Life
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Simulated Secondary Treated Sewage
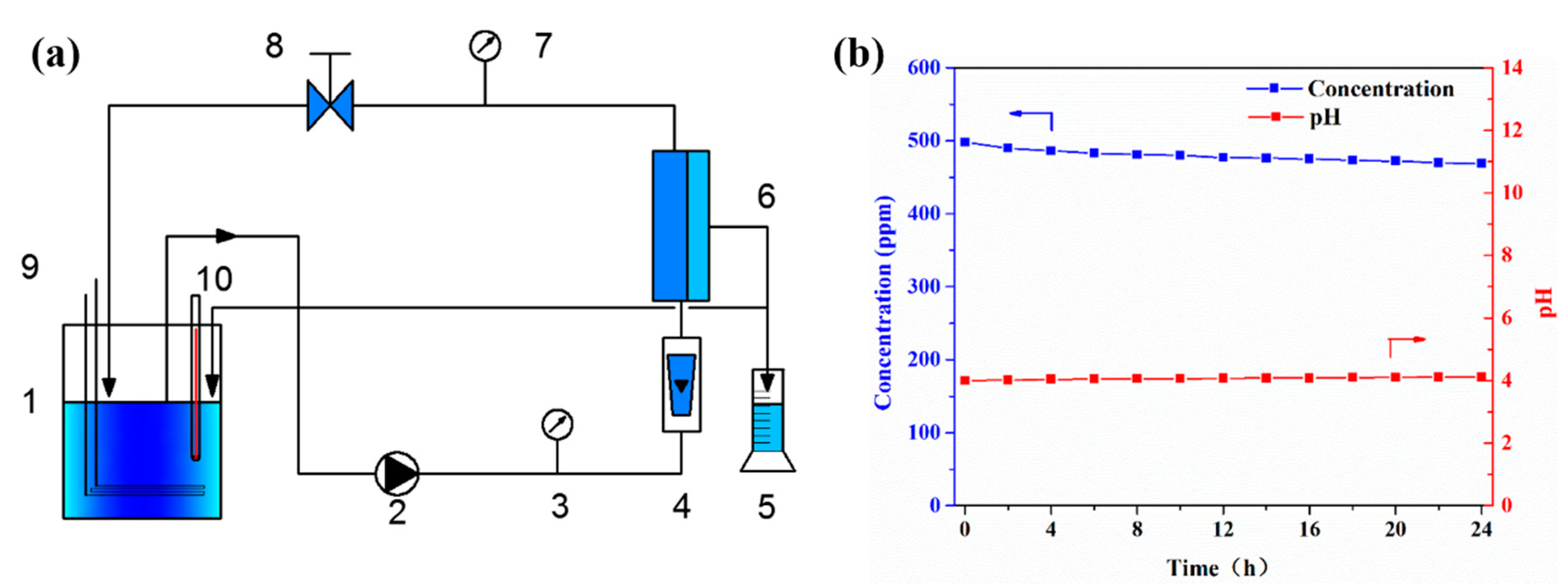
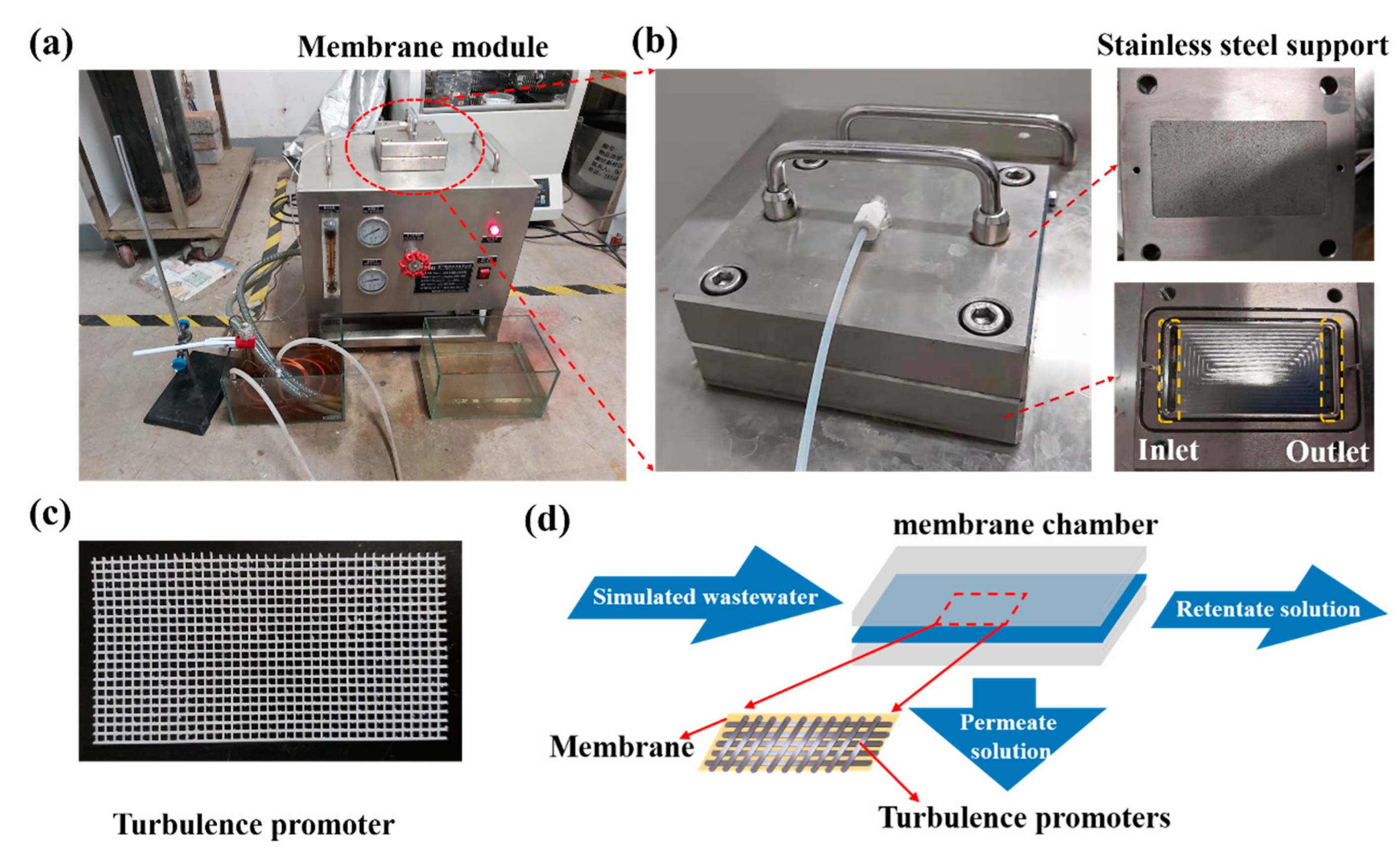
2.3. Experimental Equipment and Method
2.4. Variable Definitions
2.5. Analytical Methods
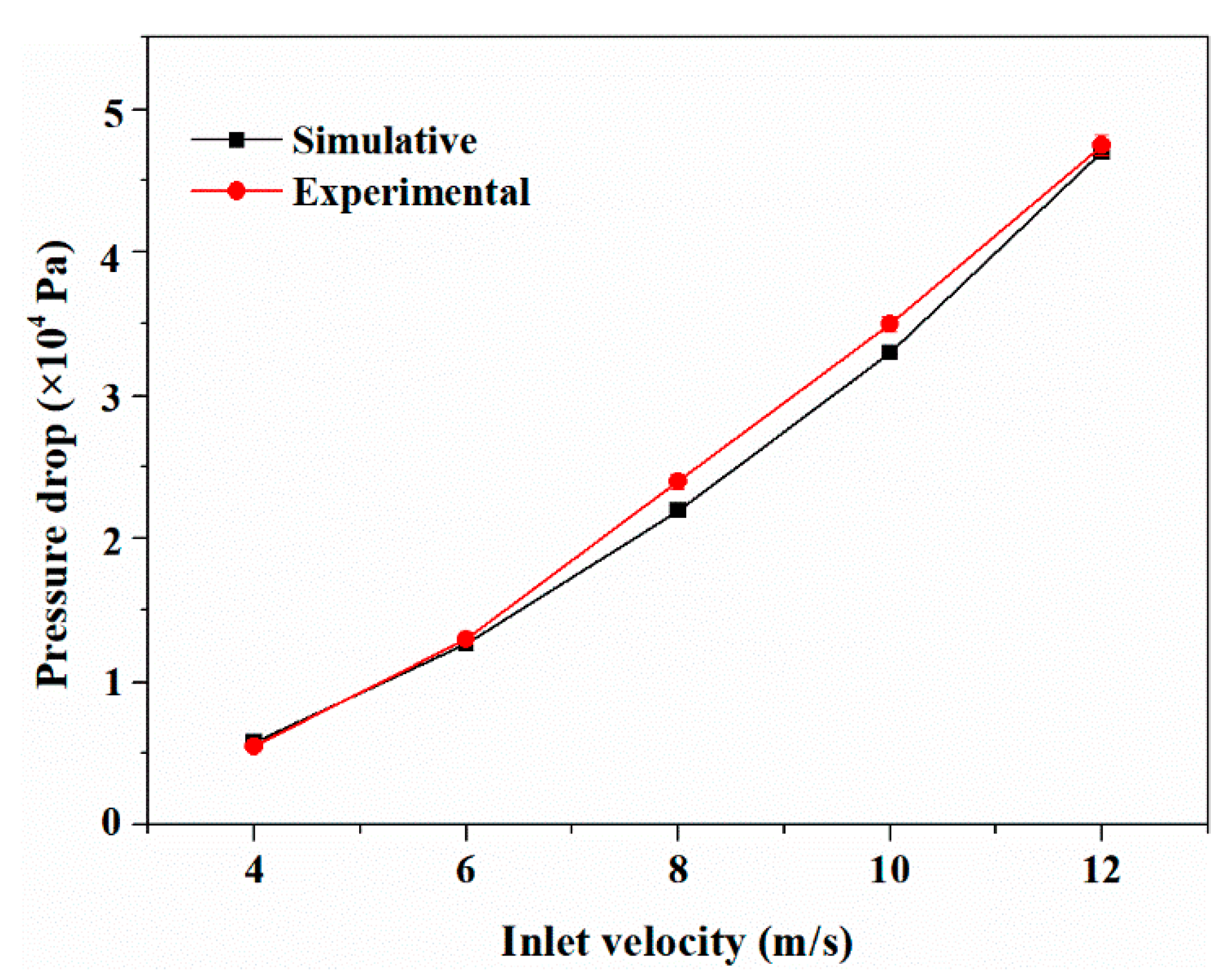
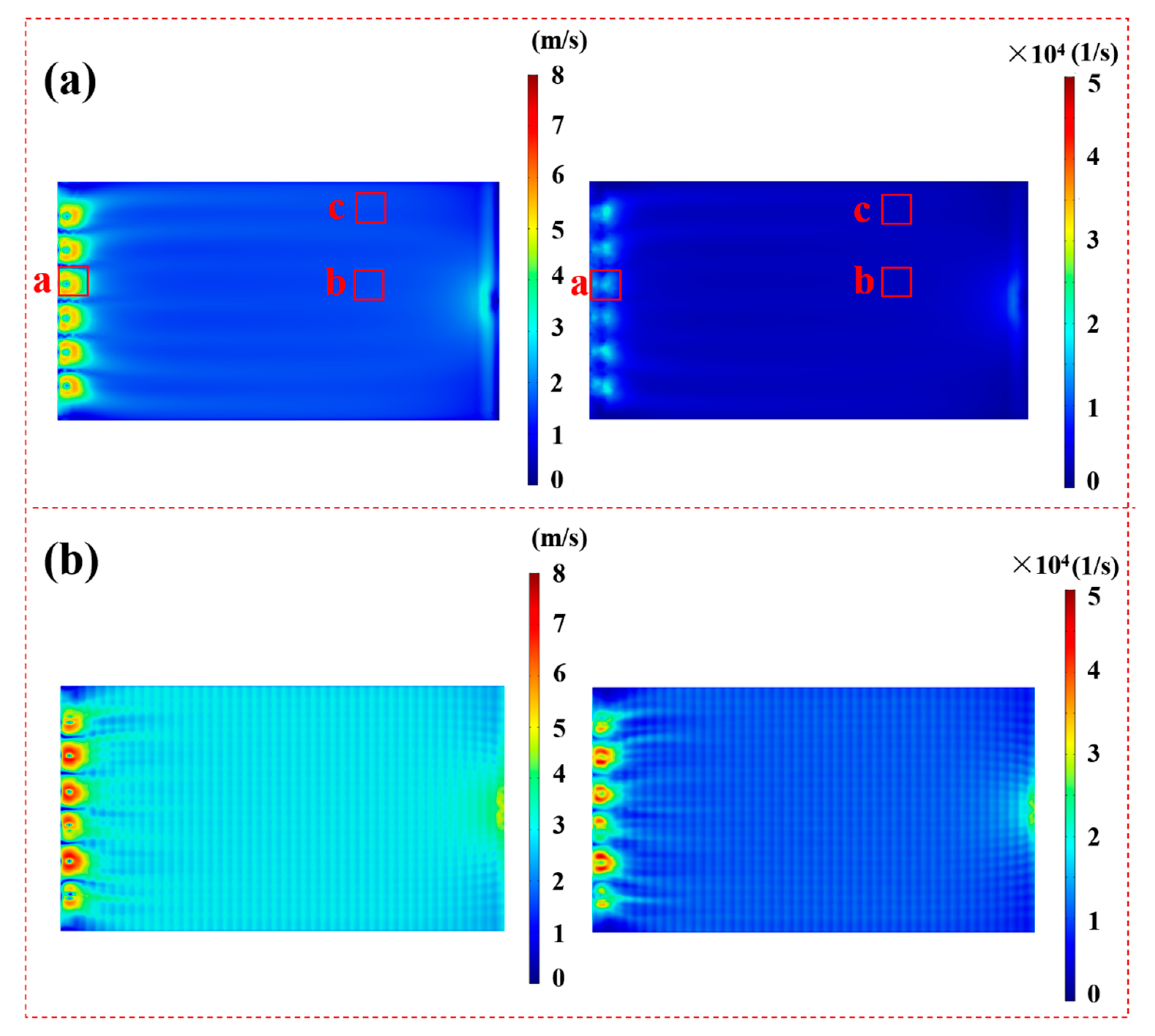
3. COMSOL Simulation
4. Results and Discussion
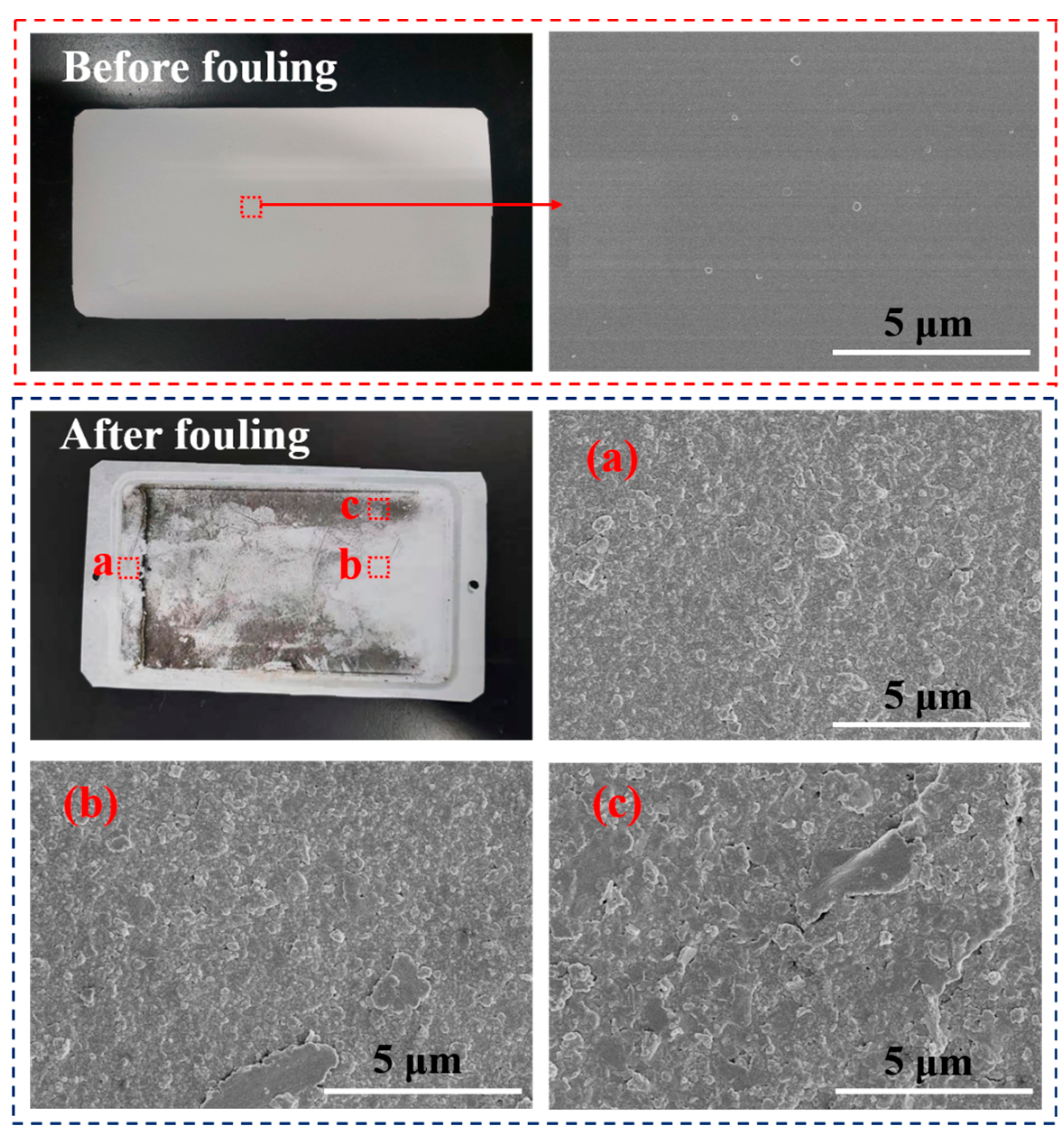
4.1. Effect of Turbulence Promoter on the Alleviation of Membrane Fouling
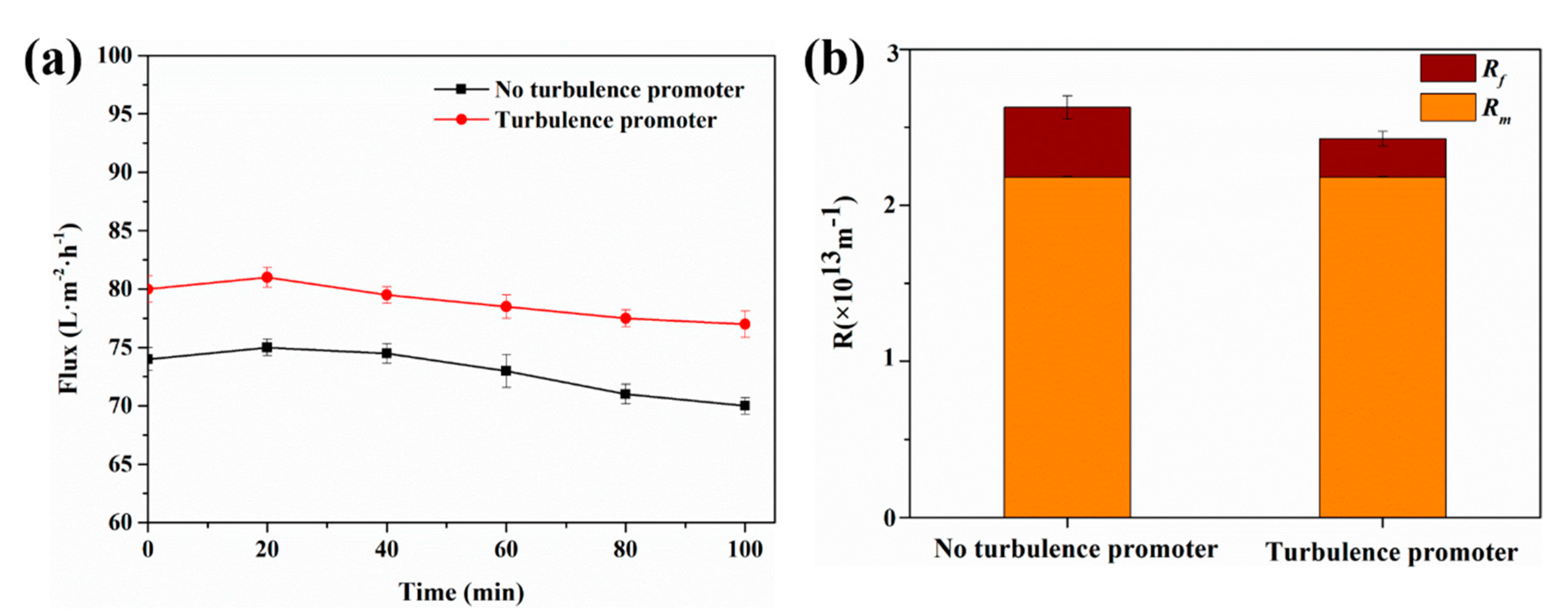
4.2. Effect of Turbulence Promoter on Flux and Membrane Resistance
4.2.1. 100-min Membrane Filtration Experiment
4.2.2. 24-h Membrane Filtration Experiment
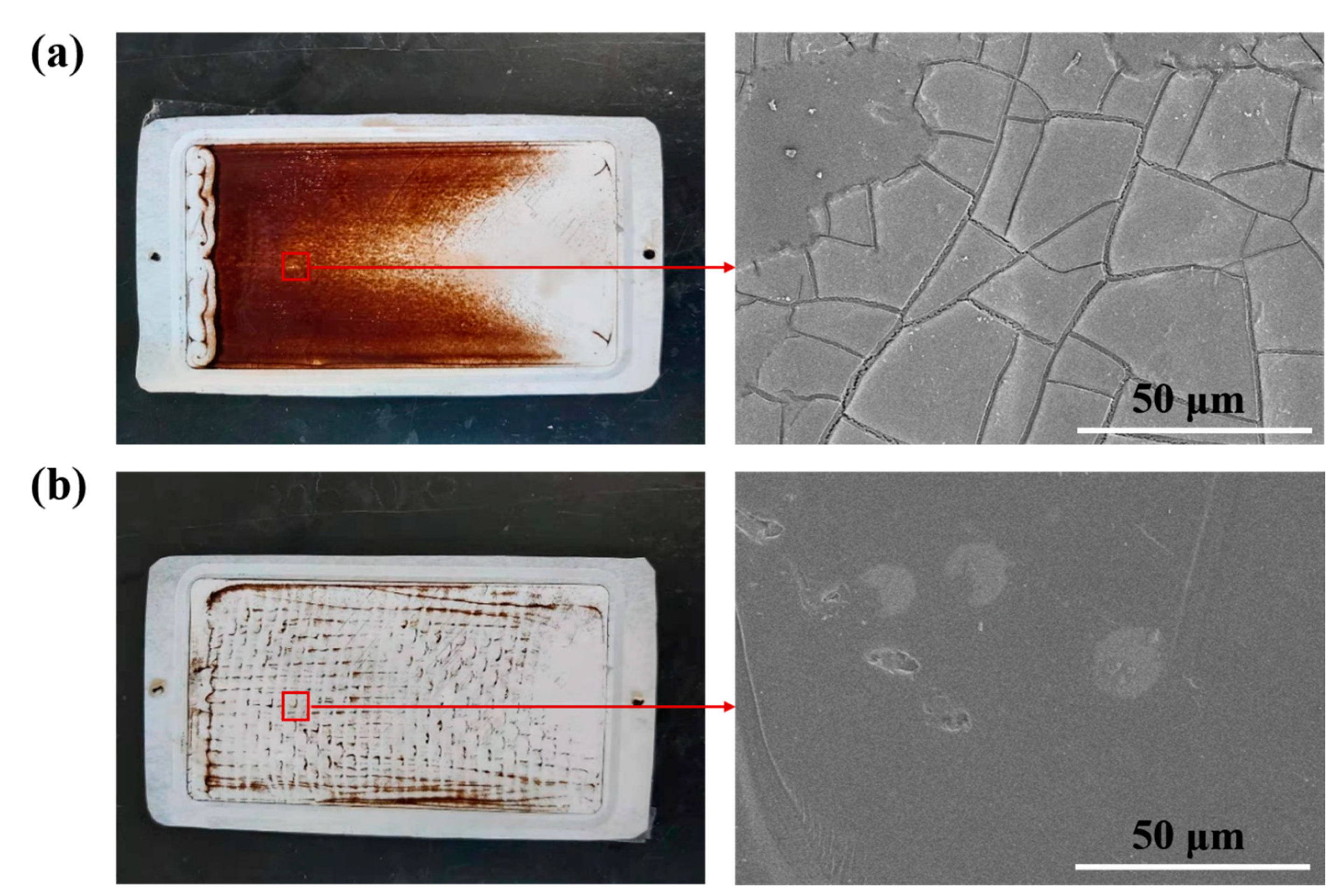
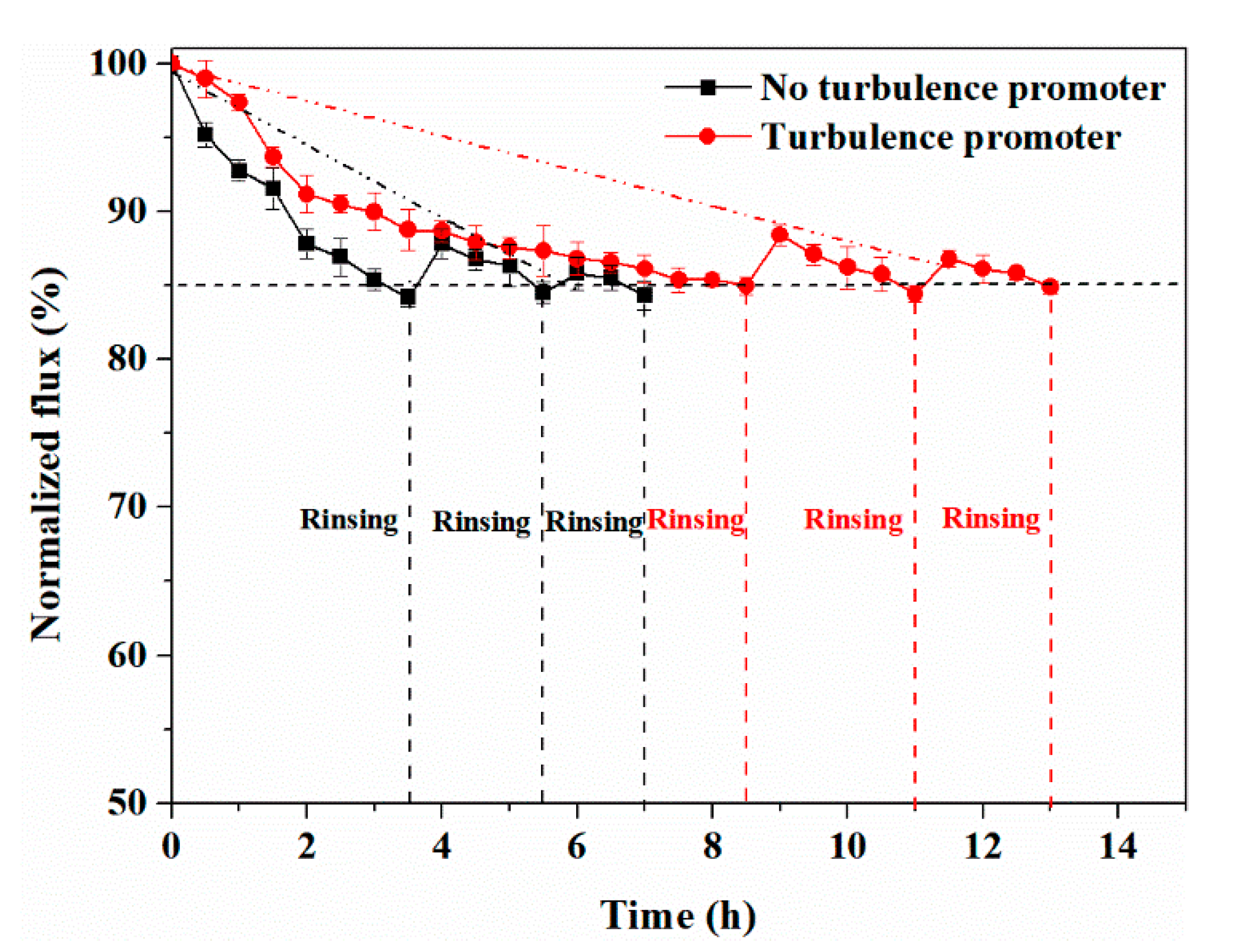
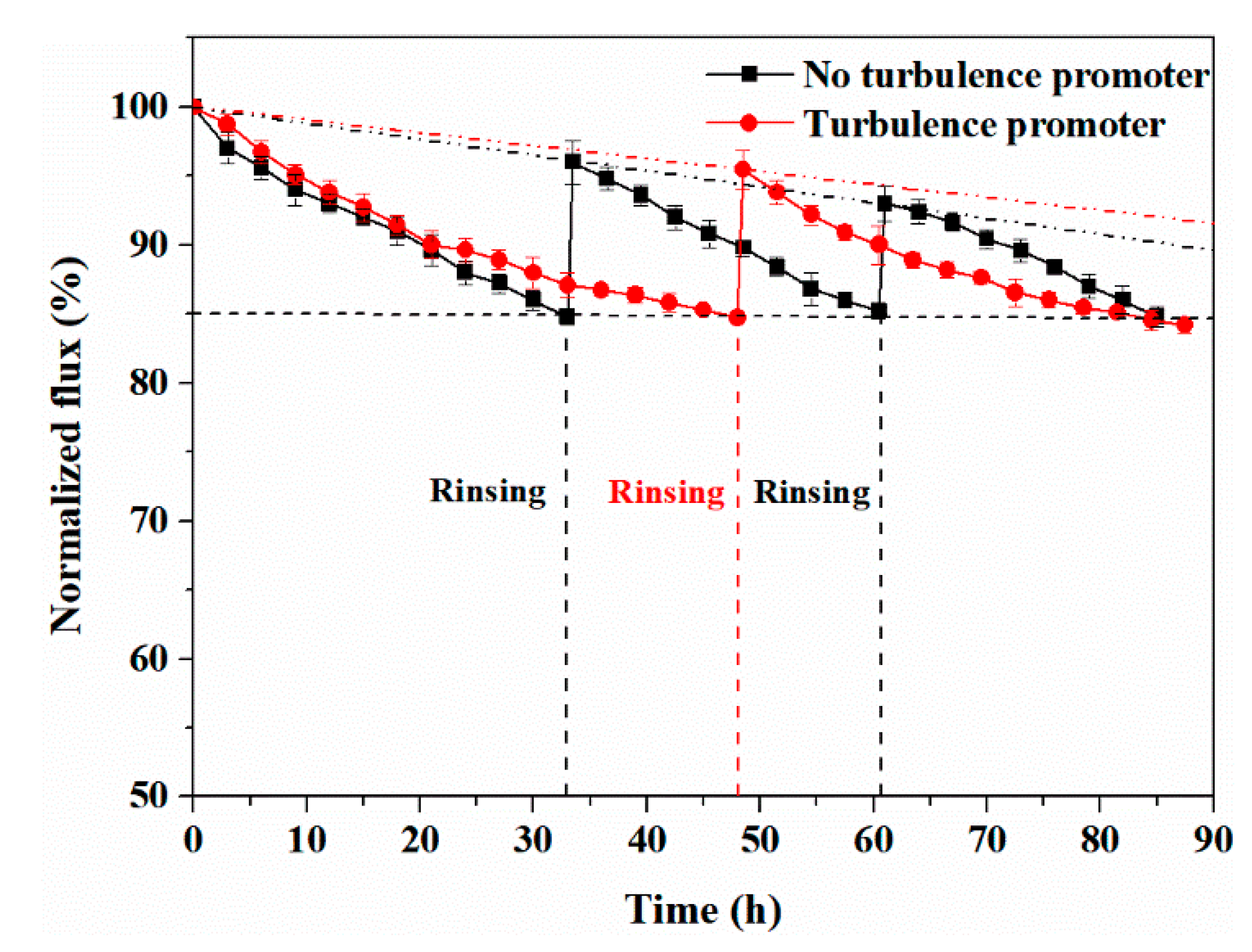
4.3. Effect of Turbulence Promoter on Membrane Life
4.3.1. Experiment with HA Concentration at 500 ppm
4.3.2. Experiment with HA Concentration at 250 ppm
4.3.3. Experiment with HA Concentration at 50 ppm
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jamil, S.; Loganathan, P.; Khan, S.J.; McDonald, J.A.; Kandasamy, J.; Vigneswaran, S. Enhanced nanofiltration rejection of inorganic and organic compounds from a wastewater-reclamation plant’s micro-filtered water using adsorption pre-treatment. Sep. Purif. Technol. 2021, 260, 118207. [Google Scholar] [CrossRef]
- Imbrogno, A.; Tiraferri, A.; Abbenante, S.; Weyand, S.; Schwaiger, R.; Luxbacher, T.; Schäfer, A.I. Organic fouling control through magnetic ion exchange-nanofiltration (MIEX-NF) in water treatment. J. Membr. Sci. 2018, 549, 474–485. [Google Scholar] [CrossRef]
- Chen, W.; Gu, Z.; Ran, G.; Li, Q. Application of membrane separation technology in the treatment of leachate in China: A review. Waste Manag. 2021, 121, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, X.; Xu, Y.; Cheng, X.; Li, G.; Liang, H. Hybrid UF/NF process treating secondary effluent of wastewater treatment plants for potable water reuse: Adsorption vs. coagulation for removal improvements and membrane fouling alleviation. Environ. Res. 2020, 188, 109833. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Zhang, J.; Dong, B. Pilot study on nanofiltration membrane in advanced treatment of drinking water. Water Supply 2020, 20, 2043. [Google Scholar] [CrossRef]
- Hafiz, M.; Hawari, A.H.; Alfahel, R.; Hassan, M.K.; Altaee, A. Comparison of Nanofiltration with Reverse Osmosis in Re-claiming Tertiary Treated Municipal Wastewater for Irrigation Purposes. Membranes 2021, 11, 32. [Google Scholar] [CrossRef]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure mem-brane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Tong, X.; Wu, Y.-H.; Wang, Y.-H.; Bai, Y.; Zhao, X.-H.; Luo, L.-W.; Mao, Y.; Ikuno, N.; Hu, H.-Y. Simulating and predicting the flux change of reverse osmosis membranes over time during wastewater reclamation caused by organic fouling. Environ. Int. 2020, 140, 105744. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total. Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purifica-tion: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Chong, T.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.; Leckie, J.O. The role of foulant–foulant electrostatic interaction on limiting flux for RO and NF membranes during humic acid fouling—Theoretical basis, experimental evidence, and AFM interaction force measure-ment. J. Membr. Sci. 2009, 326, 526–532. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.; Leckie, J.O. Fouling of reverse osmosis and nanofiltration membranes by humic acid—Effects of so-lution composition and hydrodynamic conditions. J. Membr. Sci. 2007, 290, 86–94. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Lee, C.-H.; Kim, K.-J.; Fane, A.G. Effect of calcium ion on the fouling of nanofilter by humic acid in drinking water production. Water Res. 1998, 32, 2180–2186. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.; Chang, H.; Zhou, Z.; Zhang, T.; Yang, Y.; Li, G.; Ji, H.; Cai, C.; Liang, H. Performance of hollow fiber ultrafil-tration membrane in a full-scale drinking water treatment plant in China: A systematic evaluation during 7-year opera-tion. J. Membr. Sci. 2020, 613, 118469. [Google Scholar] [CrossRef]
- Chon, K.; Cho, J.; Shon, H.K. Fouling characteristics of a membrane bioreactor and nanofiltration hybrid system for mu-nicipal wastewater reclamation. Bioresour. Technol. 2013, 130, 239–247. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Kim, S.; Shin, J.; Rho, H.; Lee, Y.; Kim, Y.M.; Park, Y.; Oh, S.-E.; Cho, J.; Chon, K. Fouling behavior of marine organic matter in reverse osmosis membranes of a real-scale seawater desalination plant in South Korea. Desalination 2020, 485, 114305. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Characterization of Humic Acid Fouled Reverse Osmosis and Nanofiltration Membranes by Transmission Electron Microscopy and Streaming Potential Measurements. Environ. Sci. Technol. 2006, 41, 942–949. [Google Scholar] [CrossRef]
- Wang, S.; Lu, X.; Zhang, L.; Guo, J.; Zhang, H. Characterization of the Initial Fouling Layer on the Membrane Surface in a Membrane Bioreactor: Effects of Permeation Drag. Membranes 2019, 9, 121. [Google Scholar] [CrossRef]
- Koutsou, C.P.; Karabelas, A.J. A novel retentate spacer geometry for improved spiral wound membrane (SWM) module performance. J. Membr. Sci. 2015, 488, 129–142. [Google Scholar] [CrossRef]
- Wu, H.; Xie, J.; Mao, L. One-pot assembly tannic acid-titanium dual network coating for low-pressure nanofiltration mem-branes. Sep. Purif. Technol. 2020, 233, 116051. [Google Scholar] [CrossRef]
- Zou, D.; Chen, X.; Drioli, E.; Ke, X.; Qiu, M.; Fan, Y. Facile co-sintering process to fabricate sustainable antifouling silver nanoparticles (AgNPs)-enhanced tight ceramic ultrafiltration membranes for protein separation. J. Membr. Sci. 2020, 593, 117402. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Trilles, C.A.; De Guzman, M.R.; Pereira, J.M.; Aquino, R.R.; Huang, S.; Hu, C.; Lee, K.; Lai, J. Improved per-formance of thin-film nanocomposite nanofiltration membranes as induced by embedded polydopamine-coated silica na-noparticles. Sep. Purif. Technol. 2019, 224, 113–120. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, H.; Zhang, N.; Jiang, B.; Sun, Y.; Yang, N. A loose NF membrane by grafting TiO2-HMDI nanoparticles on PES/β-CD substrate for dye/salt separation. Sep. Purif. Technol. 2019, 218, 8–19. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Static turbulence promoters in cross-flow membrane filtration: A review. Chem. Eng. Commun. 2019, 207, 413–433. [Google Scholar] [CrossRef]
- Alqattan, J.; Kim, Y.; Kerdi, S.; Qamar, A.; Ghaffour, N. Hole-Type Spacers for More Stable Shale Gas-Produced Water Treatment by Forward Osmosis. Membranes 2021, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Hoiberg, B.; Shah, M.T. CFD study of multiphase flow in aerated grit tank. J. Water Process. Eng. 2021, 39, 101698. [Google Scholar] [CrossRef]
- Kim, D.; Kim, D.; Kim, J.; Stoesser, T. Large Eddy Simulation of Flow and Tracer Transport in Multichamber Ozone Con-tactors. J. Environ. Eng. 2010, 136, 22–31. [Google Scholar] [CrossRef]
- Bruno, P.; Di Bella, G.; De Marchis, M. Perforated Baffles for the Optimization of Disinfection Treatment. Water 2020, 12, 3462. [Google Scholar] [CrossRef]
- Xu, Q.; Wan, Y.; Wu, Q.; Xiao, K.; Yu, W.; Liang, S.; Zhu, Y.; Hou, H.; Liu, B.; Hu, J.; et al. An efficient hydrody-namic-biokinetic model for the optimization of operational strategy applied in a full-scale oxidation ditch by CFD inte-grated with ASM2. Water Res. 2021, 193, 116888. [Google Scholar] [CrossRef]
- Duguay, J.; Lacey, J.; Massé, A. Evaluating the Euler-Euler approach for predicting a strongly 3D bubble-induced recircula-tory flow with OpenFOAM. Chem. Eng. Sci. 2021, 229, 115982. [Google Scholar] [CrossRef]
- Wang, J.; Ying, X.; Huang, Y.; Chen, Y.; Shen, D.; Zhang, X.; Feng, H.J. Numerical study of hydrodynamic characteristics in a moving bed biofilm reactor. Environ. Res. 2021, 194, 110614. [Google Scholar] [CrossRef] [PubMed]
- Elshaw, A.; Hassan, N.M.S.; Khan, M.M.K. Computational Fluid Dynamic Modelling and Optimisation of Wastewater Treatment Plant Bioreactor Mixer. Energies 2018, 11, 3530. [Google Scholar] [CrossRef]
- Horstmeyer, N.; Thies, C.; Lippert, T.; Drewes, J.E. A hydraulically optimized fluidized bed UF membrane reactor (FB-UF-MR) for direct treatment of raw municipal wastewater to enable water reclamation with integrated energy recov-ery. Sep. Purif. Technol. 2020, 235, 116165. [Google Scholar] [CrossRef]
- Hirche, D.; Chew, J.W.; Hinrichsen, O. CFD-DEM study of geometry changes in an AnFMBR towards particle momentum. Chem. Eng. J. 2020, 379, 122336. [Google Scholar] [CrossRef]
- Bopape, M.; Van Geel, T.; Dutta, A.; Van der Bruggen, B.; Onyango, M. Numerical Modelling Assisted Design of a Compact Ultrafiltration (UF) Flat Sheet Membrane Module. Membranes 2021, 11, 54. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; Li, F.; Shen, C.; Huang, M.; Wang, Z.; Cao, C.; Zhou, Q.; Sheng, Y.; Sand, W. CFD simulations of fiber-fiber interaction in a hollow fiber membrane bundle: Fiber distance and position matters. Sep. Purif. Technol. 2019, 209, 707–713. [Google Scholar] [CrossRef]
- Zimmerer, C.; Kottke, V. Effects of spacer geometry on pressure drop, mass transfer, mixing behavior, and residence time distribution. Desalination 1996, 104, 129–134. [Google Scholar] [CrossRef]
- Da Costa, A.; Fane, A.; Fell, C.; Franken, A. Optimal channel spacer design for ultrafiltration. J. Membr. Sci. 1991, 62, 275–291. [Google Scholar] [CrossRef]
- Fimbres-Weihs, G.; Wiley, D. Numerical study of mass transfer in three-dimensional spacer-filled narrow channels with steady flow. J. Membr. Sci. 2007, 306, 228–243. [Google Scholar] [CrossRef]
- Gao, Y.; Haavisto, S.; Tang, C.Y.; Salmela, J.; Li, W. Characterization of fluid dynamics in spacer-filled channels for mem-brane filtration using Doppler optical coherence tomography. J. Membr. Sci. 2013, 448, 198–208. [Google Scholar] [CrossRef]
- Bucs, S.S.; Linares, R.V.; Marston, J.O.; Radu, A.I.; Vrouwenvelder, J.S.; Picioreanu, C. Experimental and numerical characterization of the water flow in spacer-filled channels of spiral-wound membranes. Water Res. 2015, 87, 299–310. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Xu, X.; Liu, F. Saw-tooth spacer for membrane filtration: Hydrodynamic investigation by PIV and filtration experiment validation. Chem. Eng. Process. Process. Intensif. 2015, 91, 23–34. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Bucs, S.S.; Li, Z.; Abughdeeb, M.; Amy, G.; Vrouwenvelder, J.S. Impact of spacer thickness on bio-fouling in forward osmosis. Water Res. 2014, 57, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Willems, P.; Deen, N.G.; Kemperman, A.; Lammertink, R.; Wessling, M.; Annaland, M.V.S.; Kuipers, J.; Van Der Meer, W. Use of Particle Imaging Velocimetry to measure liquid velocity profiles in liquid and liquid/gas flows through spacer filled channels. J. Membr. Sci. 2010, 362, 143–153. [Google Scholar] [CrossRef]
- De Marchis, M.; Milici, B.; Napoli, E. Numerical observations of turbulence structure modification in channel flow over 2D and 3D rough walls. Int. J. Heat Fluid Flow 2015, 56, 108–123. [Google Scholar] [CrossRef]
- Subramani, A.; Kim, S.; Hoek, E.M.V. Pressure, flow, and concentration profiles in open and spacer-filled membrane chan-nels. J. Membr. Sci. 2006, 277, 7–17. [Google Scholar] [CrossRef]
- Guillen, G.; Hoek, E.M. Modeling the impacts of feed spacer geometry on reverse osmosis and nanofiltration processes. Chem. Eng. J. 2009, 149, 221–231. [Google Scholar] [CrossRef]
- Shakaib, M.; Haque, M.E.-U. Numerical simulations for fluid dynamics and temperature patterns in membrane distillation channels. Heat Mass Transf. 2019, 55, 3509–3522. [Google Scholar] [CrossRef]
- Santos, J.L.C.; Geraldes, V.; Velizarov, S.; Crespo, J.G. Investigation of flow patterns and mass transfer in membrane mod-ule channels filled with flow-aligned spacers using computational fluid dynamics (CFD). J. Membr. Sci. 2007, 305, 103–117. [Google Scholar] [CrossRef]
- Radu, A.; van Steen, M.; Vrouwenvelder, J.; van Loosdrecht, M.; Picioreanu, C. Spacer geometry and particle deposition in spiral wound membrane feed channels. Water Res. 2014, 64, 160–176. [Google Scholar] [CrossRef]
- Schwinge, J.; Wiley, D.; Fane, A. Novel spacer design improves observed flux. J. Membr. Sci. 2004, 229, 53–61. [Google Scholar] [CrossRef]
- Saeed, A.; Vuthaluru, R.; Vuthaluru, H.B. Impact of Feed Spacer Filament Spacing on Mass Transport and Fouling Propensi-ties of RO Membrane Surfaces. Chem. Eng. Commun. 2015, 202, 634–646. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Huang, A.; Soesanto, J.F.; Luo, Y.-L.; Hsu, T.-Y.; Chen, C.-H.; Hwang, K.-J.; Ho, C.-D.; Tung, K.-L.; Susanto, J.F. 3D printing design of turbulence promoters in a cross-flow microfiltration system for fine particles removal. J. Membr. Sci. 2019, 573, 647–656. [Google Scholar] [CrossRef]
- Pal, S.; Bharihoke, R.; Chakraborty, S.; Ghatak, S.K.; De, S.; Dasgupta, S. An experimental and theoretical analysis of turbu-lence promoter assisted ultrafiltration of synthetic fruit juice. Sep. Purif. Technol. 2008, 62, 659–667. [Google Scholar] [CrossRef]
- Pal, S.; Ambastha, S.; Ghosh, T.B.; De, S.; Dasgupta, S. Optical evaluation of deposition thickness and measurement of per-meate flux enhancement of simulated fruit juice in presence of turbulence promoters. J. Membr. Sci. 2008, 315, 58–66. [Google Scholar] [CrossRef]
- Ferreira, F.B.; Ullmann, G.; Vieira, L.G.M.; Cardoso, V.L.; Reis, M.H.M. Hydrodynamic performance of 3D printed turbu-lence promoters in cross-flow ultrafiltrations of Psidium myrtoides extract. Chem. Eng. Process 2020, 154, 108005. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Li, K.; Zhang, H.; Yang, M. Molecular characterization of effluent organic matter in secondary effluent and reclaimed water: Comparison to natural organic matter in source water. J. Environ. Sci. 2018, 63, 140–146. [Google Scholar] [CrossRef]
- Aryal, A.; Sathasivan, A.; Heitz, A.; Zheng, G.; Nikraz, H.; Ginige, M.P. Combined BAC and MIEX pre-treatment of second-ary wastewater effluent to reduce fouling of nanofiltration membranes. Water Res. 2015, 70, 214–223. [Google Scholar] [CrossRef]
- Xu, P.; Bellona, C.; Drewes, J.E. Fouling of nanofiltration and reverse osmosis membranes during municipal wastewater reclamation: Membrane autopsy results from pilot-scale investigations. J. Membr. Sci. 2010, 353, 111–121. [Google Scholar] [CrossRef]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration mem-branes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Ye, W.; Bernstein, N.J.; Lin, J.; Jordens, J.; Zhao, S.; Tang, C.Y.; Van der Bruggen, B. Theoretical and experimental study of organic fouling of loose nanofiltration membrane. J. Taiwan Inst. Chem. Eng. 2018, 93, 509–518. [Google Scholar] [CrossRef]
- Maartens, A.; Swart, P.; Jacobs, E.P. Removal of Natural Organic Matter by Ultrafiltration: Characterisation, Fouling and Cleaning. Water Sci. Technol. 1999, 40, 113–120. [Google Scholar] [CrossRef]
- Fenu, A.; De Wilde, W.; Gaertner, M.; Weemaes, M.; De Gueldre, G.; Van De Steene, B. Elaborating the membrane life concept in a full scale hollow-fibers MBR. J. Membr. Sci. 2012, 421-422, 349–354. [Google Scholar] [CrossRef]
- Bréhant, A.; Glucina, K.; Chevrel, M.; Boulanger, G.; Gorisse, H.; Laine, J.M. A toolbox to secure the choice and operation of membrane technologies. J. Membr. Sci. 2011, 421–422, 349–354. [Google Scholar] [CrossRef]
- Hajibabania, S.; Le-Clech, P. Ageing of porous membranes in water and wastewater treatment. In Chemeca 2011: Engineering a Better World: Sydney Hilton Hotel, Sydney, NSW, Australia, 18–21 September 2011; Engineers Australia: Canberra, Australia, 2011. [Google Scholar]
- Lin, W.-C.; Shao, R.-P.; Wang, X.-M.; Huang, X. Impacts of non-uniform filament feed spacers characteristics on the hydraulic and anti-fouling performances in the spacer-filled membrane channels: Experiment and numerical simulation. Water Res. 2020, 185, 116251. [Google Scholar] [CrossRef] [PubMed]
- Yakhot, V.; Orszag, S.A. Renormalization group analysis of turbulence. I. Basic theory. J. Sci. Comput. 1986, 1, 3–51. [Google Scholar] [CrossRef]
- Im, I.; Gwak, G.D.; Kim, S.M.; Park, Y.K. A Numerical Study of the Flow Characteristics and Separation Efficiency of a Hy-drocyclone. Ksce. J. Civ. Eng. 2018, 22, 4272–4281. [Google Scholar] [CrossRef]
- Field, R.; Wu, D.; Howell, J.; Gupta, B. Critical flux concept for microfiltration fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Neal, P.R.; Li, H.; Fane, A.G.; Wiley, D.E. The effect of filament orientation on critical flux and particle deposition in spac-er-filled channels. J. Membr. Sci. 2003, 214, 165–178. [Google Scholar] [CrossRef]
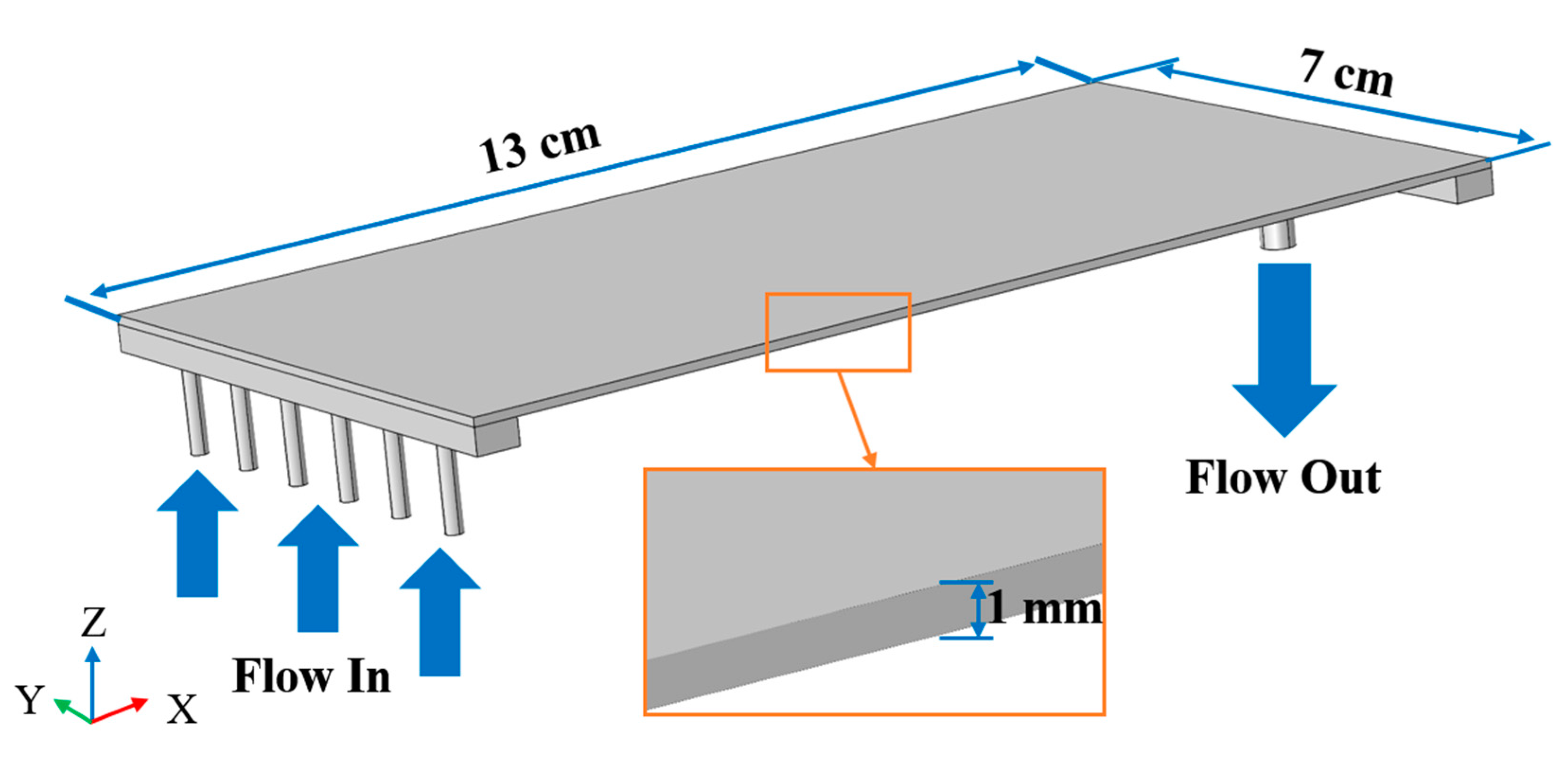
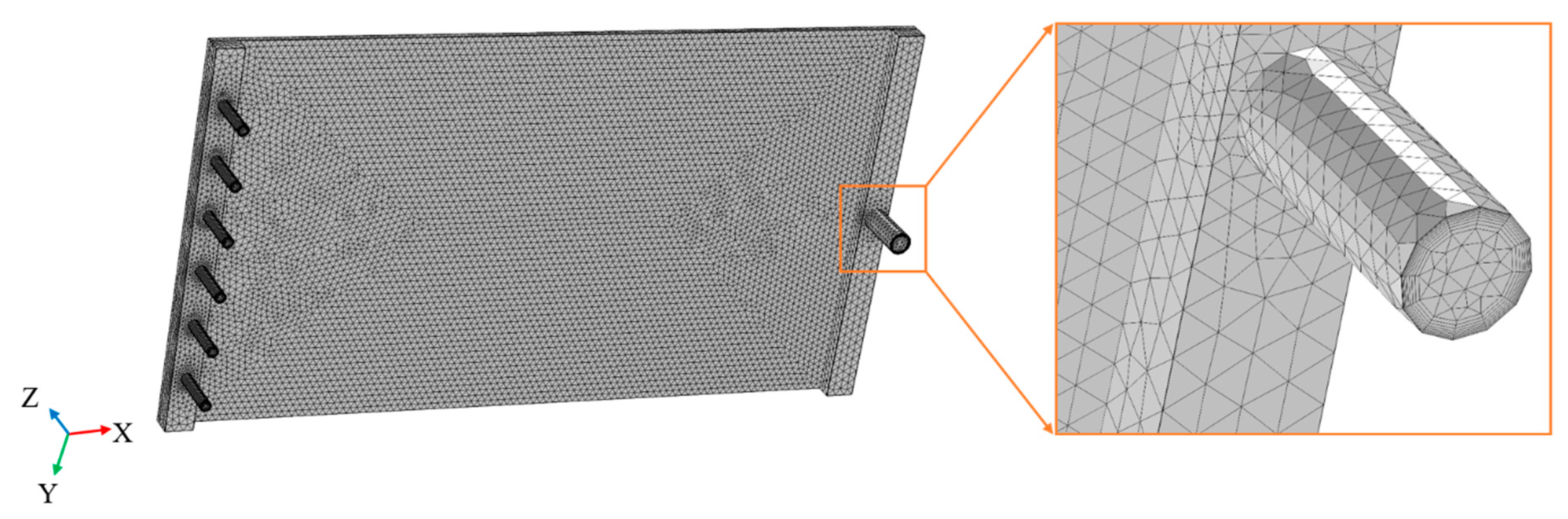




| Parameter | Value | Unit |
|---|---|---|
| Model geometry | ||
| Chamber length | 130 | mm |
| Chamber width | 70 | mm |
| Chamber height | 1 | mm |
| Inlet pipe diameter | 2 | mm |
| Inlet pipe length | 10 | mm |
| Outlet pipe diameter | 4 | mm |
| Outlet pipe length | 10 | mm |
| Model parameters | ||
| Inlet flow velocity | 8 | m/s |
| Outlet pressure | 0 | Pa |
| Water density | 997 | kg/m3 |
| Water viscosity | 0.89 × 10−3 | Pa·s |
| Temperature | 25 | °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Hu, B.; Yang, N.; Zhang, L.; Sun, Y.; Xiao, X. Study of Turbulence Promoters in Prolonging Membrane Life. Membranes 2021, 11, 268. https://doi.org/10.3390/membranes11040268
Jiang B, Hu B, Yang N, Zhang L, Sun Y, Xiao X. Study of Turbulence Promoters in Prolonging Membrane Life. Membranes. 2021; 11(4):268. https://doi.org/10.3390/membranes11040268
Chicago/Turabian StyleJiang, Bin, Binxing Hu, Na Yang, Luhong Zhang, Yongli Sun, and Xiaoming Xiao. 2021. "Study of Turbulence Promoters in Prolonging Membrane Life" Membranes 11, no. 4: 268. https://doi.org/10.3390/membranes11040268
APA StyleJiang, B., Hu, B., Yang, N., Zhang, L., Sun, Y., & Xiao, X. (2021). Study of Turbulence Promoters in Prolonging Membrane Life. Membranes, 11(4), 268. https://doi.org/10.3390/membranes11040268






