ECMO Retrieval over the Mediterranean Sea: Extending Hospital Arms
Abstract
1. Introduction
2. Materials and Methods
2.1. Historical Perspective
2.2. Transport Protocol
- Cannulas of different size: HLS arterial cannulas (15 cm in length) for jugular cannulation, 13, 15, 17, 19, and 21 Fr; femoral venous cannulas (38 cm and 55 cm) 19, 21, 23, and 25 Fr. (Maquet Getinge Group, Germany);
- J-tip guidewires 100 cm and 150 cm in length, currently upgraded with a 180 cm guidewire;
- Various percutaneous insertion kits: a PIK with four multistep dilators 10/12 Fr, 12/14 Fr, 14/16 Fr., 16/18 Fr. (Maquet Getinge Group, Germany); a set of PIK dilator L cannula accessories 18/20 Fr., 20/22 Fr., 22/24 Fr. (Maquet–Getinge Group, Germany); the Opus vascular access kit with a stepped vessel dilator 8/10 Fr., 13 Fr., 16 Fr., 20 Fr., 24 Fr., 26 Fr., and 28 Fr. is also currently available (Medtronic, Minneapolis, MN, USA).
2.3. Modes of Transportation
2.4. Patient Cohort
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
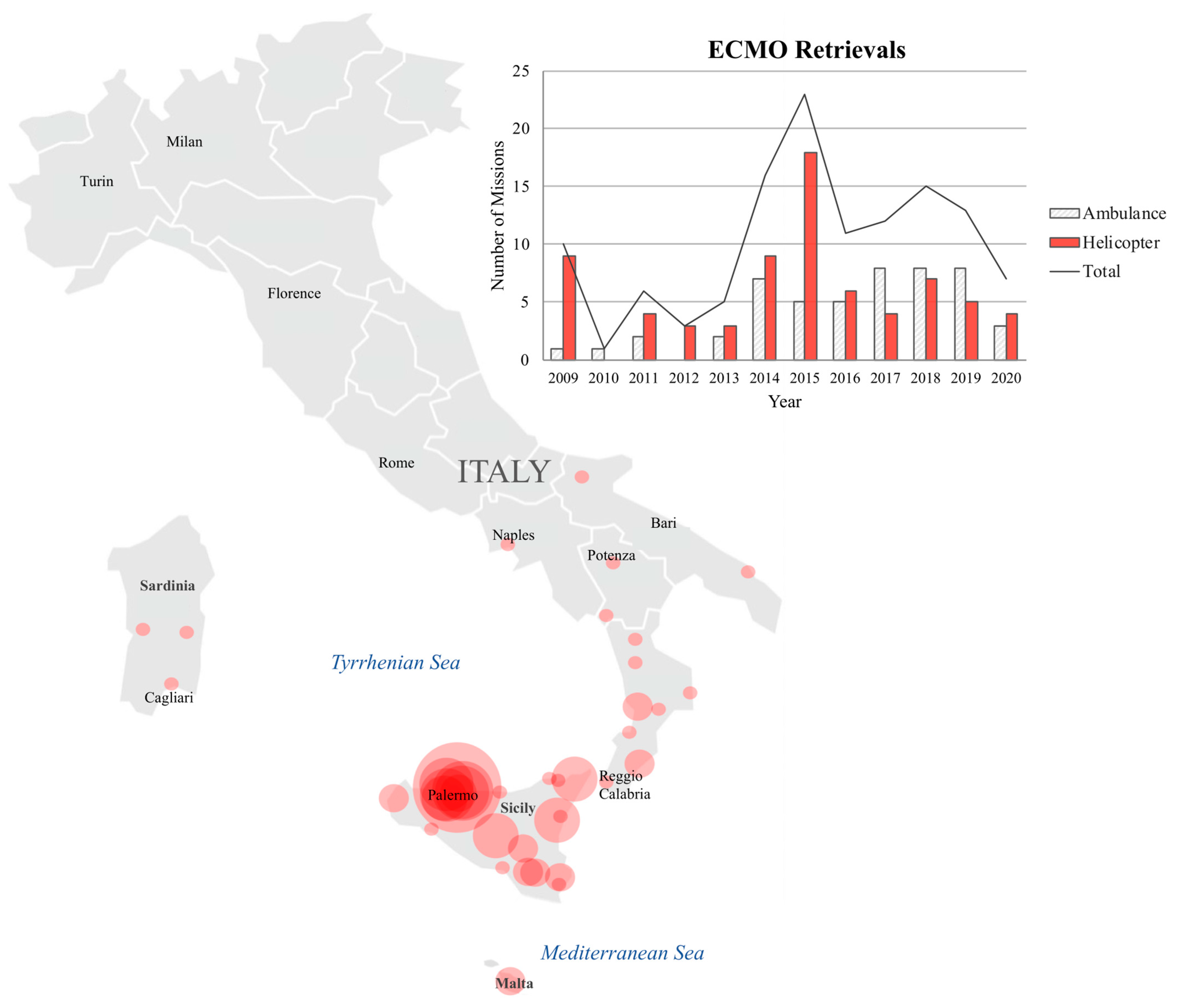
3.2. Transport
3.3. Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalewski, M.; Zieliński, K.; Gozdek, M.; Raffa, G.M.; Pilato, M.; Alanazi, M.; Gilbers, M.; Heuts, S.; Natour, E.; Bidar, E.; et al. Veno-Arterial Extracorporeal Life Support in Heart Transplant and Ventricle Assist Device Centres. Meta-Analysis. ESC Heart Fail. 2020. Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Whitman, G.; Milojevic, M.; Raffa, G.; McMullan, D.M.; Boeken, U.; Haft, J.; Bermudez, C.; Shah, A.; D’Alessandro, D.A. 2020 EACTS/ELSO/STS/AATS expert consensus on post-cardiotomy extracorporeal life support in adult patients. Eur. J. Thorac. Cardiovasc. Surg. 2021, 59, 12–53. [Google Scholar] [CrossRef]
- Combes, A.; Schmidt, M.; Hodgson, C.L.; Fan, E.; Ferguson, N.D.; Fraser, J.F.; Jaber, S.; Pesenti, A.; Ranieri, M.; Rowan, K.; et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 2020, 46, 2464–2476. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Peek, G.J.; Hajage, D.; Hardy, P.; Abrams, D.; Schmidt, M.; Dechartres, A.; Elbourne, D. ECMO for severe ARDS: Systematic review and individual patient data meta-analysis. Intensive Care Med. 2020, 46, 2048–2057. [Google Scholar] [CrossRef]
- Makdisi, G.; Wang, I.W. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J. Thorac. Dis. 2015, 7, E166–E176. [Google Scholar]
- Abrams, D.; Garan, A.R.; Abdelbary, A.; Bacchetta, M.; Bartlett, R.H.; Beck, J.; Belohlavek, J.; Chen, Y.S.; Fan, E.; Ferguson, N.D.; et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018, 44, 717–729. [Google Scholar] [CrossRef]
- Combes, A.; Brodie, D.; Bartlett, R.; Brochard, L.; Brower, R.; Conrad, S.; De Backer, D.; Fan, E.; Ferguson, N.; Fortenberry, J.; et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am. J. Respir. Crit. Care Med. 2014, 190, 488–496. [Google Scholar] [CrossRef]
- Bonadonna, D.; Barac, Y.D.; Ranney, D.N.; Rackley, C.R.; Mumma, K.; Schroder, J.N.; Milano, C.A.; Daneshmand, M.A. Interhospital ECMO Transport: Regional Focus. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 327–334. [Google Scholar] [CrossRef]
- Ericsson, A.; Frenckner, B.; Broman, L.M. Adverse Events during Inter-Hospital Transports on Extracorporeal Membrane Oxygenation. Prehosp. Emerg. Care 2017, 21, 448–455. [Google Scholar] [CrossRef]
- Krzak, A.M.; Fowles, J.A.; Vuylsteke, A. Mobile extracorporeal membrane oxygenation service for severe acute respiratory failure—A review of five years of experience. J. Intensive Care Soc. 2020, 21, 134–139. [Google Scholar] [CrossRef]
- Gattinoni, L.; Carlesso, E.; Langer, T. Clinical review: Extracorporeal membrane oxygenation. Crit. Care 2011, 15, 243. [Google Scholar] [CrossRef]
- Roger, D.; Dudouit, J.M.; Résière, D.; Mehdaoui, H.; Courcier, D.; Villain, L.; Léonard, C.; Roques, F.; Lebreton, G. Interhospital transfer of ECMO-assisted patients in Martinique. Ann. Fr. Anesth. Reanim. 2013, 32, 307–314. [Google Scholar] [CrossRef]
- Broman, L.M.; Frenckner, B. Transportation of Critically Ill Patients on Extracorporeal Membrane Oxygenation. Front. Pediatr. 2016, 13, 63. [Google Scholar] [CrossRef]
- Heuer, J.F.; Mirschel, M.; Bleckmann, A.; Quintel, M.; Moerer, O. Interhospital transport of ARDS patients on extracorporeal membrane oxygenation. J. Artif. Organs 2019, 22, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Tipograf, Y.; Liou, P.; Oommen, R.; Agerstrand, C.; Abrams, D.; Brodie, D.; Bacchetta, M. A decade of interfacility extracorporeal membrane oxygenation transport. J. Thorac. Cardiovasc. Surg. 2019, 157, 1696–1706. [Google Scholar] [CrossRef]
- Broman, L.M.; Dirnberger, D.R.; Malfertheiner, M.V.; Aokage, T.; Morberg, P.; Næsheim, T.; Pappalardo, F.; Di Nardo, M.; Preston, T.; Burrell, A.J.C.; et al. International Survey on Extracorporeal Membrane Oxygenation Transport. ASAIO J. 2020, 66, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Leow, L.; Papadimas, E.; Subbian, S.K.; MacLaren, G.; Ramanathan, K. Organization of extracorporeal membrane oxygenation services for COVID-19. Asian Cardiovasc. Thorac. Ann. 2020, 29, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Combes, A.; Lo Coco, V.; DePiero, M.E.; Belohlavek, J.; Euro ECMO COVID-19 Working Group; Euro-ELSO Steering Committee. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021, 47, 344–348. [Google Scholar] [CrossRef]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Iwashyna, T.J.; Slutsky, A.S.; Fan, E.; Bartlett, R.H.; Tonna, J.E.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020, 396, 1071–1078. [Google Scholar] [CrossRef]
- Read, M.D.; Nam, J.J.; Biscotti, M.; Piper, L.C.; Thomas, S.B.; Sams, V.G.; Elliott, B.S.; Negaard, K.A.; Lantry, J.H.; DellaVolpe, J.D.; et al. Evolution of the United States Military Extracorporeal Membrane Oxygenation Transport Team. Mil. Med. 2020, 185, e2055–e2060. [Google Scholar] [CrossRef]
- D’Ancona, G.; Capitanio, G.; Chiaramonte, G.; Serretta, R.; Turrisi, M.; Pilato, M.; Arcadipane, A. Extracorporeal membrane oxygenator rescue and airborne transportation of patients with influenza a (h1n1) acute respiratory distress syndrome in a mediterranean underserved area. Interact Cardiovasc. Thorac. Surg. 2011, 12, 935–937. [Google Scholar] [CrossRef][Green Version]
- Sauer, C.M.; Yuh, D.D.; Bonde, P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015, 61, 31–36. [Google Scholar] [CrossRef]
- Martucci, G.; Panarello, G.; Occhipinti, G.; Raffa, G.; Tuzzolino, F.; Capitanio, G.; Carollo, T.; Lino, G.; Bertani, A.; Vitulo, P.; et al. Impact of cannula design on packed red blood cell transfusions: Technical advancement to improve outcomes in extracorporeal membrane oxygenation. J. Thorac. Dis. 2018, 10, 5813–5821. [Google Scholar] [CrossRef]
- Vitulo, P.; Beretta, M.; Martucci, G.; Baravoglia, C.M.H.; Romano, G.; Bertani, A.; Martino, L.; Callari, A.; Panarello, G.; Pilato, M.; et al. Challenge of Pregnancy in Patients with Pre-Capillary Pulmonary Hypertension: Veno-Arterial Extracorporeal Membrane Oxygenation as an Innovative Support for Delivery. J. Cardiothorac. Vasc. Anesth. 2017, 31, 2152–2155. [Google Scholar] [CrossRef][Green Version]
- Martucci, G.; Burgio, G.; Lullo, F.; Panarello, G.; Arcadipane, A. Veno-arterial extracorporeal membrane oxygenation as an intraoperative rescue option in case of portopulmonary hypertension recognized during liver transplantation. Minerva Anestesiol. 2017, 83, 1336–1337. [Google Scholar] [CrossRef][Green Version]
- Martucci, G.; Panarello, G.; Occhipinti, G.; Ferrazza, V.; Tuzzolino, F.; Bellavia, D.; Sanfilippo, F.; Santonocito, C.; Bertani, A.; Vitulo, P.; et al. Anticoagulation and Transfusions Management in Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome: Assessment of Factors Associated with Transfusion Requirements and Mortality. J. Intensive Care Med. 2019, 34, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Martucci, G.; Panarello, G.; Bertani, A.; Occhipinti, G.; Pintaudi, S.; Arcadipane, A. Veno-venous ECMO in ARDS after post-traumatic pneumonectomy. Intensive Care Med. 2013, 39, 2235–2236. [Google Scholar] [CrossRef]
- Pappalardo, F.; Pieri, M.; Greco, T.; Patroniti, N.; Pesenti, A.; Arcadipane, A.; Ranieri, M.; Gattinoni, L.; Landoni, G.; Holzgraefe, B.; et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: The ECMOnet score. Intensive Care Med. 2013, 39, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Broman, L.M.; Wittberg, L.P.; Westlund, C.J.; Gilbers, M.; Perry da Câmara, L.; Swol, J.; Taccone, F.S.; Malfertheiner, M.V.; Di Nardo, M.; Vercaemst, L.; et al. Pressure and flow properties of cannulae for extracorporeal membrane oxygenation I: Return (arterial) cannulae. Perfusion 2019, 34 (Suppl. S1), 58–64. [Google Scholar] [CrossRef]
- Broman, L.M.; Wittberg, L.P.; Westlund, C.J.; Gilbers, M.; Perry da Câmara, L.; Westin, J.; Taccone, F.S.; Malfertheiner, M.V.; Di Nardo, M.; Swol, J.; et al. Pressure and flow properties of cannulae for extracorporeal membrane oxygenation II: Drainage (venous) cannulae. Perfusion 2019, 34 (Suppl. S1), 65–73. [Google Scholar] [CrossRef]
- Martucci, G.; Grasselli, G.; Tanaka, K.; Tuzzolino, F.; Panarello, G.; Schmidt, M.; Bellani, G.; Arcadipane, A. Hemoglobin trigger and approach to red blood cell transfusions during veno-venous extracorporeal membrane oxygenation: The international TRAIN-ECMO survey. Perfusion 2019, 34 (Suppl. S1), 39–48. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.; Wells, M.T.; Ullman, R.; King, F.; Shmukler, C. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PLoS ONE 2014, 9, e112479. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Zogheib, E.; Rozé, H.; Repesse, X.; Lebreton, G.; Luyt, C.E.; Trouillet, J.L.; Bréchot, N.; Nieszkowska, A.; Dupont, H.; et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013, 39, 1704–1713. [Google Scholar] [CrossRef]
- Murray, J.F.; Matthay, M.A.; Luce, J.M.; Flick, M.R. An expanded definition of the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1988, 138, 720–723. [Google Scholar] [CrossRef]
- Schmidt, M.; Bailey, M.; Sheldrake, J.; Hodgson, C.; Aubron, C.; Rycus, P.T.; Scheinkestel, C.; Cooper, D.J.; Brodie, D.; Pellegrino, V.; et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am. J. Respir. Crit. Care Med. 2014, 189, 1374–1382. [Google Scholar] [CrossRef]
- Ehrenwerth, J.; Sorbo, S.; Hackel, A. Transport of critically ill adults. Crit. Care Med. 1986, 14, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Pantridge, J.F.; Geddes, J.S. A mobile intensive-care unit in the management of myocardial infarction. Lancet 1967, 2, 271–273. [Google Scholar] [CrossRef]
- Bartlett, R.H. Esperanza: The First Neonatal ECMO Patient. ASAIO J. 2017, 63, 832–843. [Google Scholar] [CrossRef] [PubMed]
- American College of Surgeons. Resources for Optimal Care of the Injured Patient, 1st ed.; American College of Surgeons: Chicago, IL, USA, 2007. [Google Scholar]
- Champion, H.R.; Copes, W.S.; Sacco, W.J.; Lawnick, M.M.; Keast, S.L.; Bain, L.W.; Flanagan, M.E.; Frey, C.F. The Major Trauma Outcome Study: Establishing national norms for trauma care. J. Trauma 1990, 30, 1356–1365. [Google Scholar] [CrossRef]
- Labib, A.; Alinier, G. Transport and Retrieval on Extracorporeal Membrane Oxygenation (ECMO): Setup and Activities of an Immersive Transport and Retrieval on ECMO Workshop. J. Cardiothorac. Vasc. Anesth. 2020. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Eng. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.T.; Braby, J.; Scanlon, M.C. Using Failure Mode and Effects Analysis to Design a Mobile Extracorporeal MembraneOxygenation Team. Air Med. J. 2011, 30, 201–207. [Google Scholar] [CrossRef]
- Salas de Armas, I.A.; Akkanti, B.H.; Janowiak, L.; Banjac, I.; Dinh, K.; Hussain, R.; Cabrera, R.; Herrera, T.; Sanger, D.; Akay, M.H.; et al. Inter-hospital COVID ECMO air transportation. Perfusion 2020. Advance online publication. [Google Scholar] [CrossRef]
- Riera, J.; Argudo, E.; Martínez-Martínez, M.; García, S.; García-de-Acilu, M.; Santafé, M.; Díaz, C.; Contreras, S.; Cortina, A.; Bonilla, C.; et al. Extracorporeal Membrane Oxygenation Retrieval in Coronavirus Disease 2019: A Case-Series of 19 Patients Supported at a High-Volume Extracorporeal Membrane Oxygenation Center. Crit. Care Explor. 2020, 2, e0228. [Google Scholar] [CrossRef]

| Patient Characteristics | |
| Age (years) | 43 (36–54) |
| Male gender | 96 (78.69) |
| Weight (kg) | 82 (70–96) |
| Height (cm) | 170 (165–175) |
| BMI (kg/m2) | 28.0 (24.4–33.0) |
| Pre-ECMO Profile | |
| Hospital length of stay (days) | 6.2 (3.0–12.1) |
| ICU length of stay (days) | 4.0 (2.0–9.0) |
| Days on mechanical ventilation | 3.0 (2.0–8.0) |
| Prone positioning | 17 (14.05) |
| Nitric oxide | 17 (14.05) |
| PaO2/FiO2 | 60 (52–67) |
| SAPS II | 39 (31–46) |
| SOFA score | 8 (6–10) |
| Murray score | 3.5 (3.5–3.75) |
| PRESERVE score | 4 (3–5) |
| ECMOnet | 5.5 (5.0–7.0) |
| RESP Score | 2 (0–4) |
| Charlson comorbidity index | 1 (0–2) |
| Treatment Data | |
| Diagnosis | |
| Viral pneumonia | 63 (51.63) |
| Bacterial pneumonia | 34 (27.87) |
| Trauma | 15 (12.30) |
| Other acute respiratory diagnosis | 7 (5.74) |
| Other chronic respiratory diagnosis | 2 (1.64) |
| Graft failure | 1 (0.82) |
| Drainage | 24 (23–25) |
| RBC volume (mL) | 1793 (750–3672) |
| RBC units | 7 (3–15) |
| FFP | 900 (500–1750) |
| Platelets | 880 (300–2071) |
| Hospital length of stay (days) | 33.0 (20.0–52.0) |
| ICU length of stay (days) | 28.0 (17.0–41.0) |
| Rescue Mission | |
| Transport distance km (miles) | 140.4 (87.2) (3.4–200.2) (2.11–124.3) |
| Transport duration (hours) | 6.0 (3.0–7.5) |
| Helicopter | 72 (59.01%) |
| Ambulance | 50 (40.98%) |
| Complications | |
| Delays due to helicopter unavailability | 4 (3.3%) |
| Femoral artery lesion | 2 (1.6%) |
| Transport on V–A ECMO due to difficult cannulation | 1 (0.8%) |
| Oxygen flow error | 1 (0.8%) |
| Pump failure | 1 (0.8%) |
| Oxygenator failure | 1 (0.8%) |
| Power outage | 1 (0.8%) |
| Mortality during transport | 1 (0.8%) |
| 95% | CI | |||
|---|---|---|---|---|
| OR | Lower | Upper | p Value | |
| Age (years) | 1.001 | 0.97 | 1.033 | 0.9547 |
| Sex (female) | 1.729 | 0.593 | 5.042 | 0.3157 |
| Weight (kg) | 0.993 | 0.975 | 1.011 | 0.4128 |
| Height (cm) | 1.016 | 0.974 | 1.059 | 0.4656 |
| BMI (kg/m2) | 0.959 | 0.9 | 1.022 | 0.1988 |
| Prone | 5.031 | 1.724 | 14.684 | 0.0003 |
| Nitric oxide | 3.75 | 1.304 | 10.78 | 0.0142 |
| Length of hospital stay pre-ECMO | 1.05 | 1.005 | 1.096 | 0.0295 |
| Length of ICU stay pre-ECMO | 1.064 | 1.004 | 1.128 | 0.037 |
| MV days pre-ECMO | 1.077 | 1.013 | 1.146 | 0.0179 |
| P/F | 1.006 | 0.975 | 1.037 | 0.7173 |
| Drainage | 0.857 | 0.675 | 1.087 | 0.2037 |
| SAPS II | 1.037 | 1 | 1.076 | 0.488 |
| SOFA | 1.056 | 0.928 | 1.203 | 0.4057 |
| Murray | 0.261 | 0.0049 | 1.375 | 0.1131 |
| PRESERVE | 1.153 | 0.935 | 1.421 | 0.1842 |
| ECMOnet | 1.205 | 0.832 | 1.744 | 0.3231 |
| RESP score | 0.9 | 0.79 | 1.026 | 0.1142 |
| Charlson | 1.146 | 0.874 | 1.502 | 0.3235 |
| RBC units | 1.035 | 1.006 | 1.064 | 0.0164 |
| FFP | 1.411 | 0.444 | 4.486 | 0.5598 |
| Total FFP (yes or no) | 1 | 0.998 | 1.001 | 0.5781 |
| Platelets | 5 | 2.123 | 11.775 | 0.0002 |
| Total platelets (yes or no) | 1.001 | 1 | 1.001 | 0.0387 |
| Transport distance (km) | 0.995 | 0.991 | 0.999 | 0.0108 |
| Transport duration (hours) | 0.83 | 0.704 | 0.978 | 0.0265 |
| AKI | 7.8 | 2.216 | 27.457 | 0.0014 |
| Furosemide | 1.755 | 0.725 | 4.248 | 0.2124 |
| CRRT | 10.673 | 3.455 | 32.97 | <0.001 |
| Creatinine | 1.048 | 0.833 | 1.318 | 0.6902 |
| MVD post | 0.861 | 0.791 | 0.938 | 0.0006 |
| Duration of ECMO support (days) | 1.008 | 1.004 | 1.012 | 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hildreth, B.A.; Panarello, G.; Martucci, G.; Tuzzolino, F.; Piacentini, A.; Occhipinti, G.; Giunta, A.; Genco, F.; Raffa, G.M.; Pilato, M.; et al. ECMO Retrieval over the Mediterranean Sea: Extending Hospital Arms. Membranes 2021, 11, 210. https://doi.org/10.3390/membranes11030210
Hildreth BA, Panarello G, Martucci G, Tuzzolino F, Piacentini A, Occhipinti G, Giunta A, Genco F, Raffa GM, Pilato M, et al. ECMO Retrieval over the Mediterranean Sea: Extending Hospital Arms. Membranes. 2021; 11(3):210. https://doi.org/10.3390/membranes11030210
Chicago/Turabian StyleHildreth, Brianna A., Giovanna Panarello, Gennaro Martucci, Fabio Tuzzolino, Alberto Piacentini, Giovanna Occhipinti, Andrea Giunta, Fabio Genco, Giuseppe M. Raffa, Michele Pilato, and et al. 2021. "ECMO Retrieval over the Mediterranean Sea: Extending Hospital Arms" Membranes 11, no. 3: 210. https://doi.org/10.3390/membranes11030210
APA StyleHildreth, B. A., Panarello, G., Martucci, G., Tuzzolino, F., Piacentini, A., Occhipinti, G., Giunta, A., Genco, F., Raffa, G. M., Pilato, M., Capitanio, G., & Arcadipane, A. (2021). ECMO Retrieval over the Mediterranean Sea: Extending Hospital Arms. Membranes, 11(3), 210. https://doi.org/10.3390/membranes11030210






