Branched Sulfonimide-Based Proton Exchange Polymer Membranes from Poly(Phenylenebenzopheneone)s for Fuel Cell Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
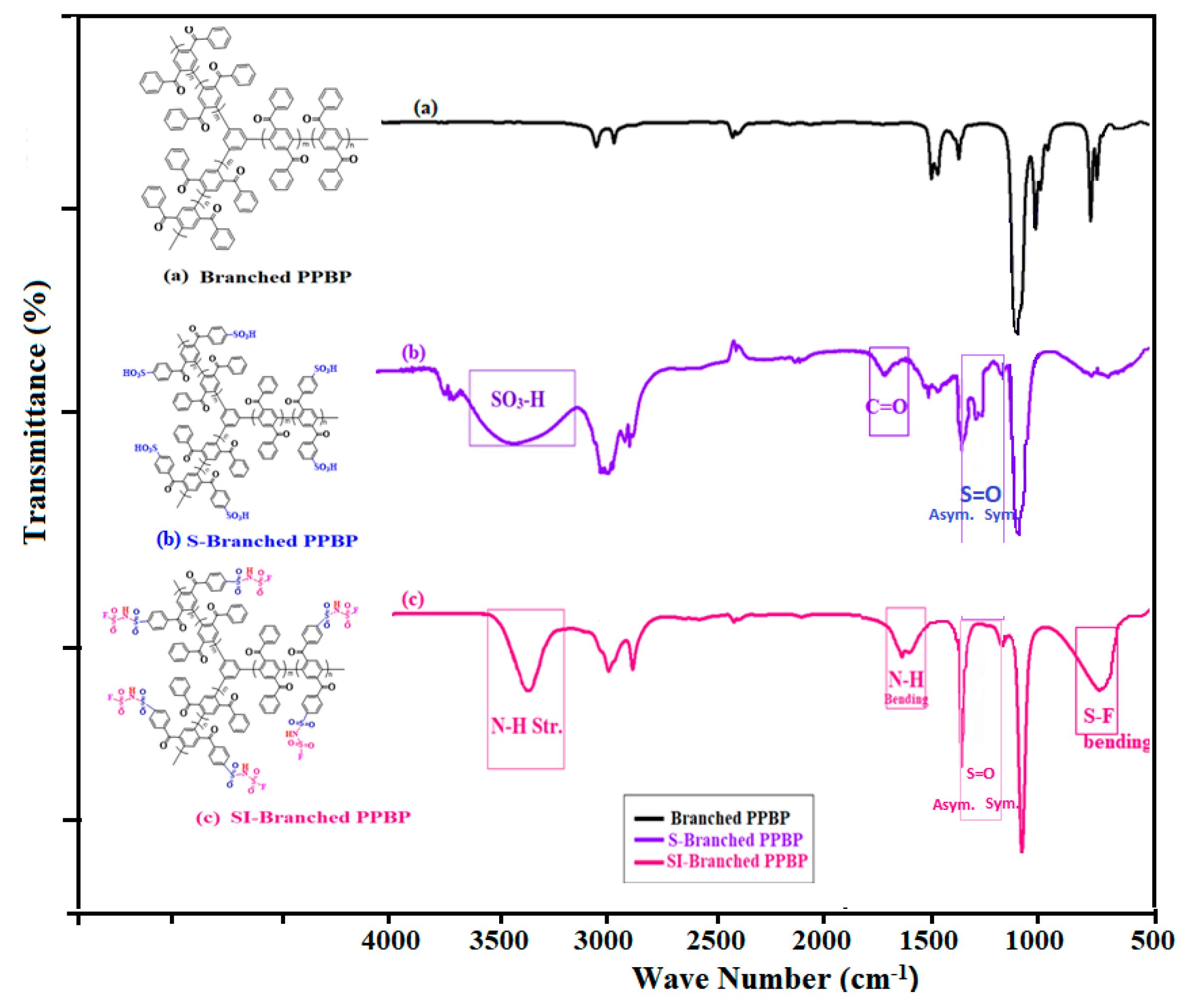
2.2. Synthesis of Branched Poly(phenylenebenzophenone) (PPBP)
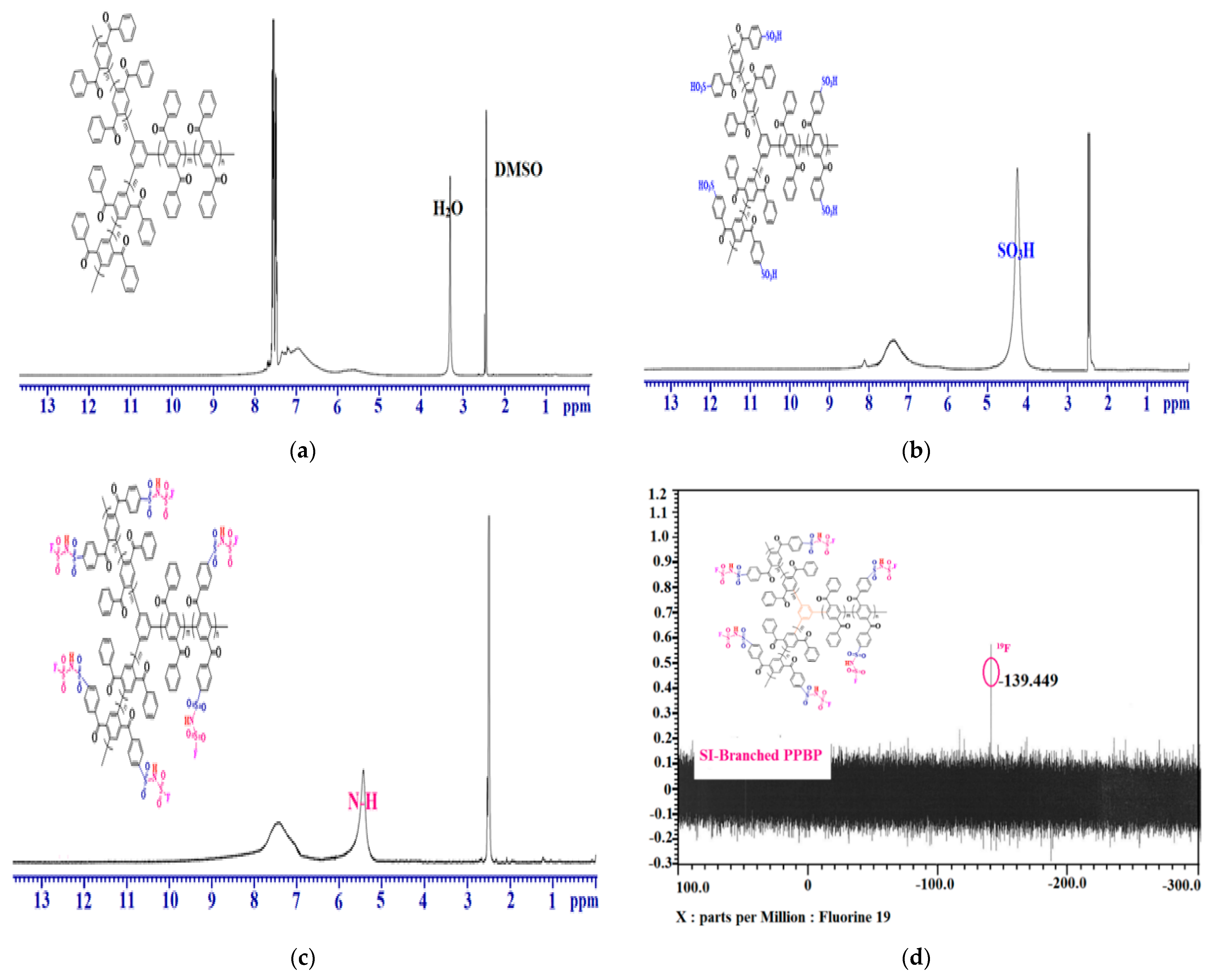
2.3. Sulfonation of the Branched Polymer (S-Branched PPBP)
2.4. Conversion into Sulfonimide form of the Sulfonated Branched Polymers (SI-Branched PPBP)
2.5. Characterizations and Measurement of Membranes Properties
3. Results and Discussion
3.1. Preparation of the Monomer
3.2. Preparation of the Sulfonimide Branched PPBP Polymers (SI-Branched PPBP)
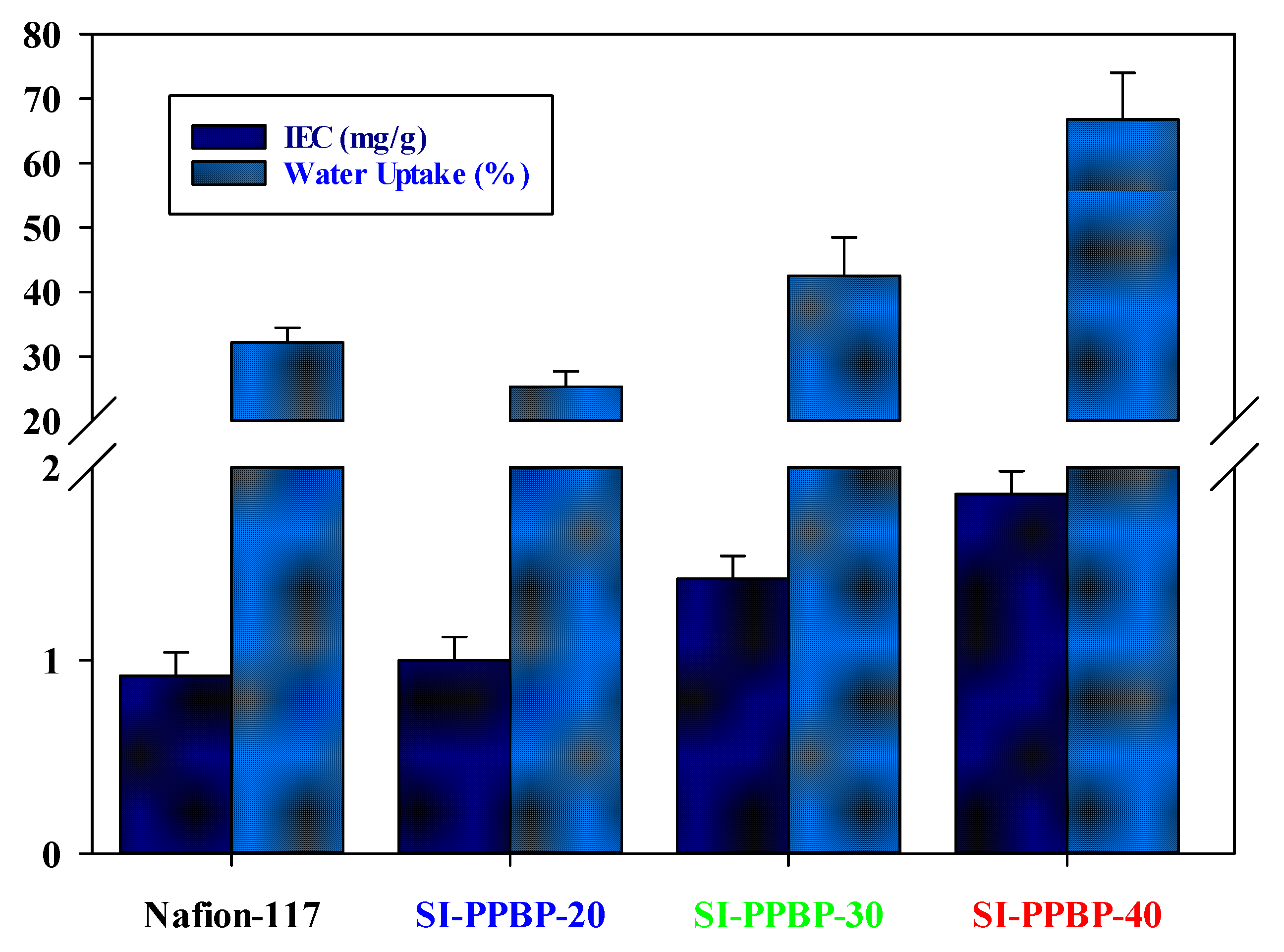
3.3. IEC, Water Uptake and Dimensional Stability of Membranes
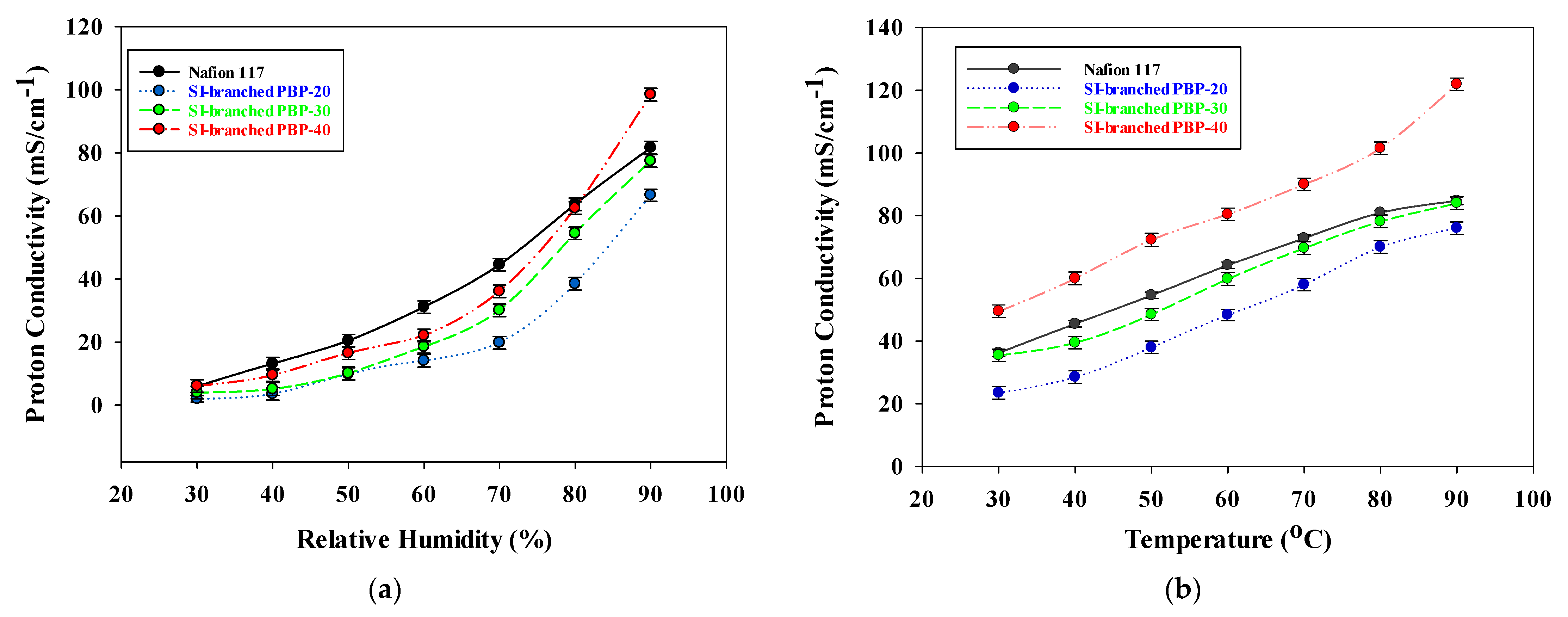
3.4. Proton Conductivity of the SI-Branched PPBP Membranes
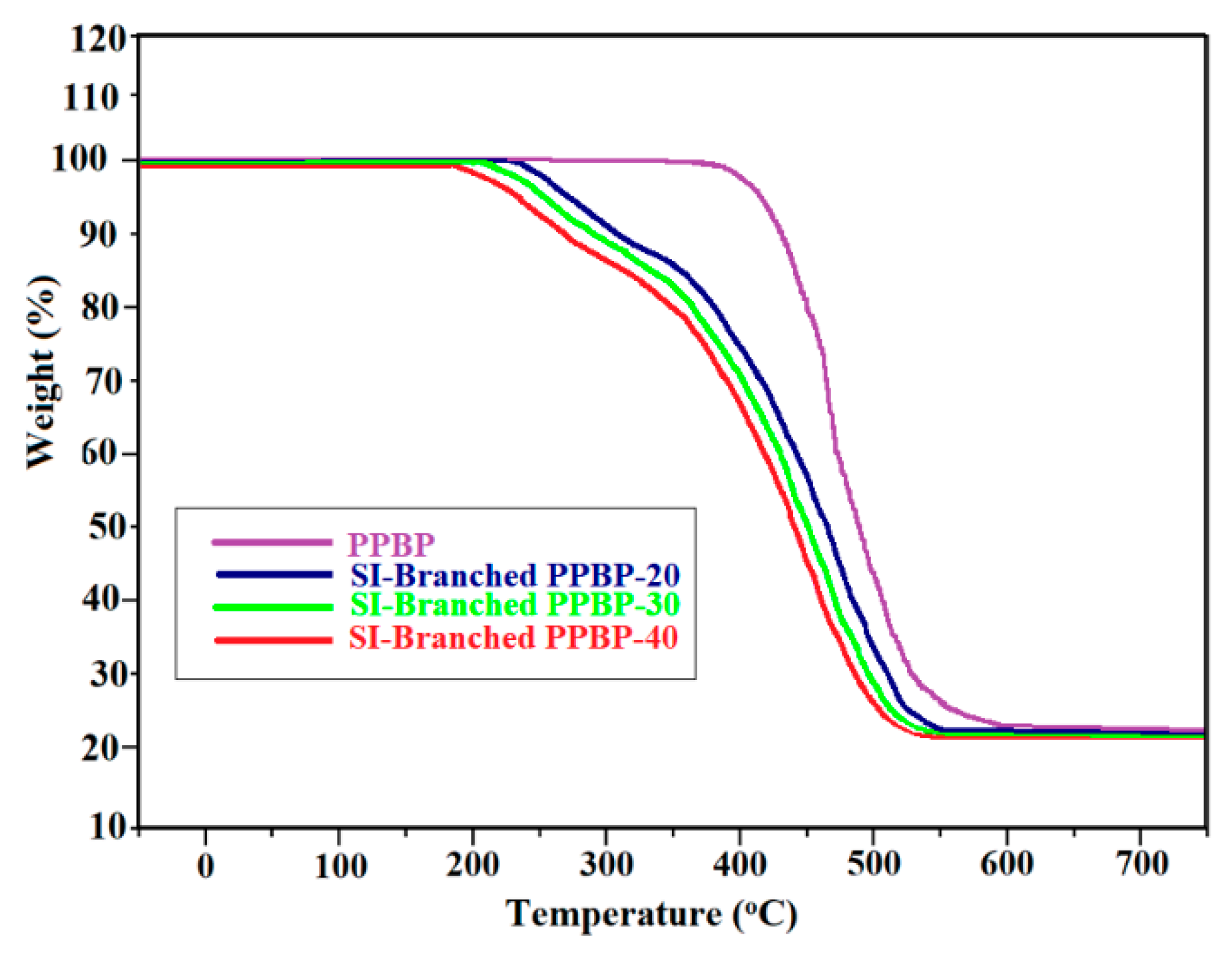
3.5. Thermo-Oxidative Stability of Membranes
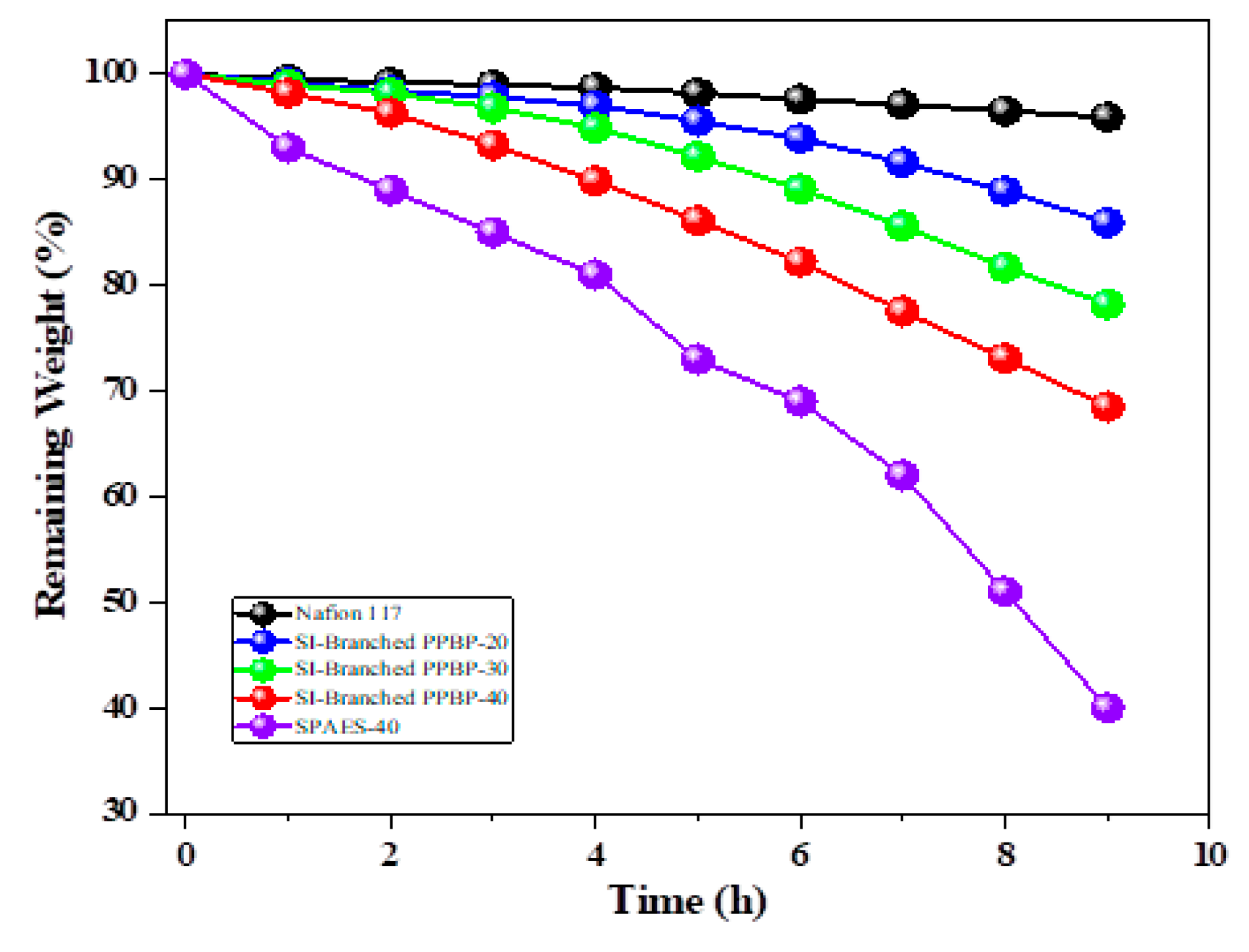
3.6. Chemical Stability of Membranes
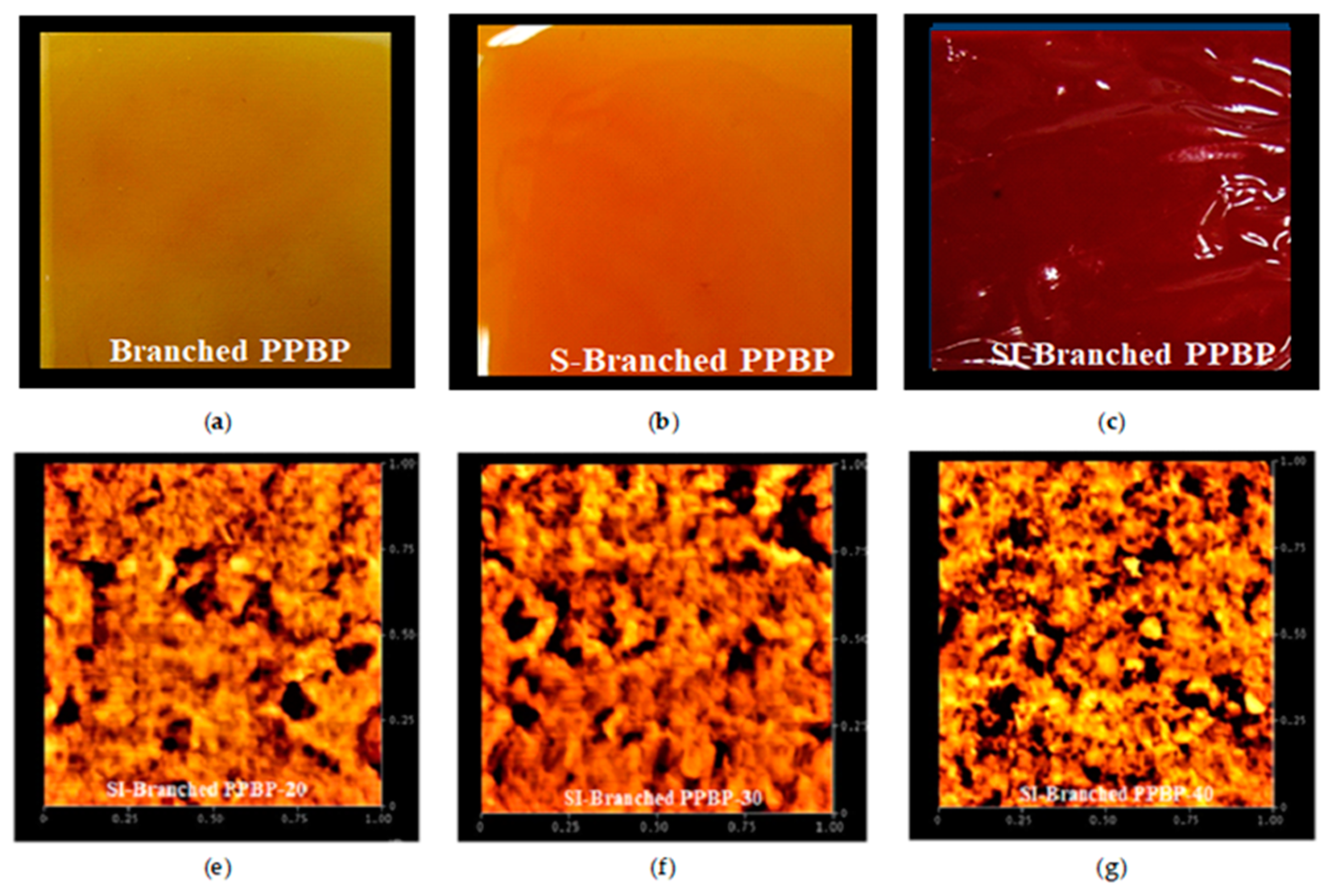
3.7. Morphology of the Membranes
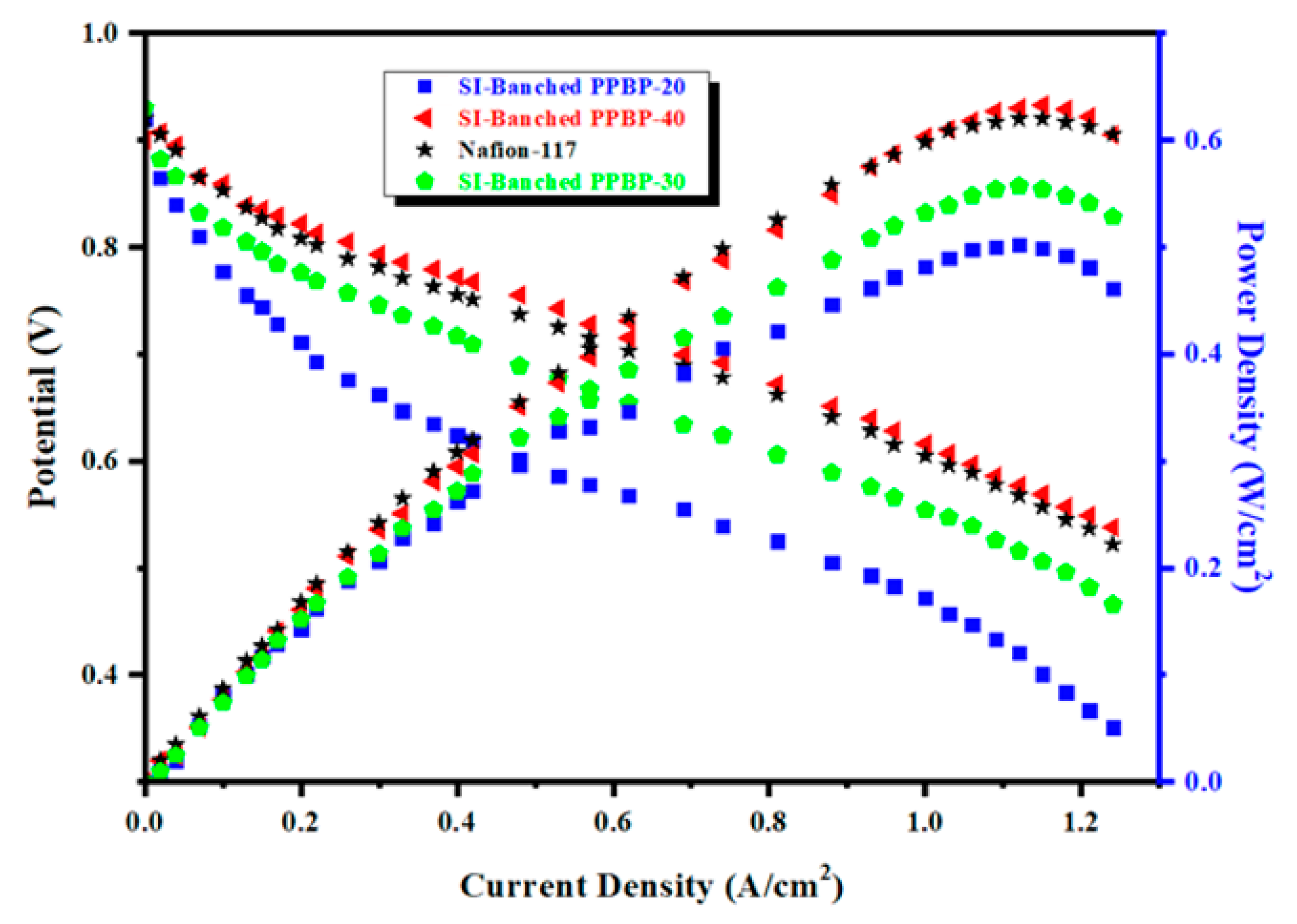
3.8. Cell Performance of the SI-Branched PPBP Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hensley, J.E.; Way, J.D. Synthesis and Characterization of Perfluorinated Carboxylate/ Sulfonate Ionomer Membranes for Separation and Solid Electrolyte Applications. Chem. Mater. 2007, 19, 4576–4584. [Google Scholar] [CrossRef]
- Li, N.; Shin, D.W.; Hwang, D.S.; Lee, Y.M.; Guiver, M.D. Polymer Electrolyte Membranes Derived from New Sulfone Monomers with Pendent Sulfonic Acid Groups. Macromolecules 2010, 43, 9810–9820. [Google Scholar] [CrossRef]
- Li, W.; Manthiram, A.; Guiver, M.D. Acid-Base Blend Membranes Consisting of Sulfonated Poly(Ether Ether Ketone) and 5-Amino-Benzotriazole Tethered Polysulfone for DMFC. J. Memb. Sci. 2010, 362, 289–297. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Du, N.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Ionomers for Proton Exchange Membrane Fuel Cells with Sulfonic Acid Groups on the End-Groups: Novel Branched Poly(Ether-Ketone)s with 3,6-Ditrityl-9H-Carbazole End-Groups. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3860–3868. [Google Scholar] [CrossRef]
- Haile, S.M.; Boysen, D.A.; Chisholm, C.R.I.; Merie, R.B. Solid Acids as Fuel Cell Electrolytes. Nature 2001, 410, 910–913. [Google Scholar] [CrossRef]
- Rozière, J.; Jones, D.J. Non-Fluorinated Polymer Materials for Proton Exchange Membrane Fuel Cells. Annu. Rev. Mater. Res. 2003, 33, 503–555. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative Polymer Systems for Proton Exchange Membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Lee, S.; Jang, H.; Hossain, M.A.; Gwak, G.; Ju, H.; Kim, D.; Kim, W. Sulfonated Poly(Ether Sulfone) Electrolytes Structured with Mesonaphthobifluorene Graphene Moiety for PEMFC. Int. J. Hydrog. Energy 2014, 39, 1532–1538. [Google Scholar] [CrossRef]
- Seo, D.W.; Lim, Y.D.; Lee, S.H.; Jeong, Y.G.; Hong, T.W.; Kim, W.G. Preparation and Characterization of Sulfonated Amine-Poly(Ether Sulfone)s for Proton Exchange Membrane Fuel Cell. Int. J. Hydrog. Energy 2010, 35, 13088–13095. [Google Scholar] [CrossRef]
- Kim, D.S.; Robertson, G.P.; Kim, Y.S.; Guiver, M.D. Copoly(Arylene Ether)s Containing Pendant Sulfonic Acid Groups as Proton Exchange Membranes † † NRCC Publication No. 50899. Macromolecules 2009, 42, 957–963. [Google Scholar] [CrossRef]
- Baijun, L.; Gilles, P.R.; Dae-Sik, K.; Michael, D.G.; Wei, H.; Zhenhua, J. Aromatic Poly(ether ketone)s with Pendant Sulfonic Acid Phenyl Groups Prepared by a Mild Sulfonation Method for Proton Exchange Membranes. Macromolecules 2007, 40, 1934–1944. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.H.; Guiver, M.D.; Lee, Y.M. Sulfonated Hydrocarbon Membranes for Medium-Temperature and Low-Humidity Proton Exchange Membrane Fuel Cells (PEMFCs). Prog. Polym. Sci. 2011, 36, 1443–1498. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, M.J.; Nam, S.Y. A Review of Polymer–Nanocomposite Electrolyte Membranes for Fuel Cell Application. J. Ind. Eng. Chem. 2015, 21, 36–52. [Google Scholar] [CrossRef]
- Branco, C.M.; Sharma, S.; de Camargo Forte, M.M.; Steinberger-Wilckens, R. New Approaches towards Novel Composite and Multilayer Membranes for Intermediate Temperature-Polymer Electrolyte Fuel Cells and Direct Methanol Fuel Cells. J. Power Sources 2016, 316, 139–159. [Google Scholar] [CrossRef]
- Cho, C.G.; Kim, Y.S.; Yu, X.; Hill, M.; McGrath, J.E. Synthesis and Characterization of Poly(Arylene Ether Sulfone) Copolymers with Sulfonimide Side Groups for a Proton-Exchange Membrane. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6007–6014. [Google Scholar] [CrossRef]
- Harrison, W.L.; Hickner, M.A.; Kim, Y.S.; McGrath, J.E. Poly(Arylene Ether Sulfone) Copolymers and Related Systems from Disulfonated Monomer Building Blocks: Synthesis, Characterization, and Performance—A Topical Review. Fuel Cells 2005, 5, 201–212. [Google Scholar] [CrossRef]
- Wang, F.; Hickner, M.; Kim, Y.S.; Zawodzinski, T.A.; McGrath, J.E. Direct Polymerization of Sulfonated Poly(Arylene Ether Sulfone) Random (Statistical) Copolymers: Candidates for New Proton Exchange Membranes. J. Memb. Sci. 2002, 197, 231–242. [Google Scholar] [CrossRef]
- Miyahara, T.; Hayano, T.; Matsuno, S.; Watanabe, M.; Miyatake, K. Sulfonated Polybenzophenone/Poly(Arylene Ether) Block Copolymer Membranes for Fuel Cell Applications. ACS Appl. Mater. Interfaces 2012, 4, 2881–2884. [Google Scholar] [CrossRef]
- Baijun, L.; Yu, S.K.; Wei, H.; Gilles, P.R.; Bryan, S.P.; Michael, D.G. Homopolymer-like sulfonated phenyl- and diphenyl-poly(arylene ether ketone)s for fuel cell applications. J. Power Sources 2008, 185, 899–903. [Google Scholar] [CrossRef]
- Xie, H.; Tao, D.; Xiang, X.; Ou, Y.; Bai, X.; Wang, L. Synthesis and Properties of Highly Branched Star-Shaped Sulfonated Block Poly(Arylene Ether)s as Proton Exchange Membranes. J. Membr. Sci. 2015, 473, 226–236. [Google Scholar] [CrossRef]
- Rowlett, J.R.; Chen, Y.; Shaver, A.T.; Lane, O.; Mittelsteadt, C.; Xu, H.; Zhang, M.; Moore, R.B.; Mecham, S.; McGrath, J.E. Multiblock Poly(Arylene Ether Nitrile) Disulfonated Poly(Arylene Ether Sulfone) Copolymers for Proton Exchange Membranes: Part 1 Synthesis and Characterization. Polymer 2013, 54, 6305–6313. [Google Scholar] [CrossRef]
- Cataldo, S.; Ernestino, L.; Adele, B.; Giuseppe, B.; Isabella, N. Highly performing and low-cost nanostructured membranes based on Polysulfone and layered doubled hydroxide for high-temperature proton exchange membrane fuel cells. J. Power Sources 2020, 471, 228440. [Google Scholar] [CrossRef]
- Yılser, D.; Hu, S.D.; Inci, E. Polybenzimidazole/SiO2 hybrid membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2016, 41, 10044–10052. [Google Scholar] [CrossRef]
- Ravi, K.; Mohamed, M.; Keith, S. Sulfonated polyether ether ketone–sulfonated graphene oxide composite membranes for polymer electrolyte fuel cells. RSC Adv. 2014, 4, 617. [Google Scholar] [CrossRef]
- Kerres, J.A. Blended and Cross-Linked Ionomer Membranes for Application in Membrane Fuel Cells. Fuel Cells 2005, 5, 230–247. [Google Scholar] [CrossRef]
- Ding, F.C.; Wang, S.J.; Xiao, M.; Meng, Y.Z. Cross-Linked Sulfonated Poly(Phathalazinone Ether Ketone)s for PEM Fuel Cell Application as Proton-Exchange Membrane. J. Power Sources 2007, 164, 488–495. [Google Scholar] [CrossRef]
- Lee, K.S.; Jeong, M.H.; Lee, J.P.; Lee, J.S. End-Group Cross-Linked Poly(Arylene Ether) for Proton Exchange Membranes. Macromolecules 2009, 42, 584–590. [Google Scholar] [CrossRef]
- Calandra, P.; Turco Liveri, V.; Ruggirello, A.M.; Licciardi, M.; Lombardo, D.; Mandanici, A. Anti-Arrhenian Behaviour of Conductivity in Octanoic Acid-Bis(2-Ethylhexyl)Amine Systems: A Physico-Chemical Study. J. Mater. Chem. C 2015, 3, 3198–3210. [Google Scholar] [CrossRef]
- Wang, L.; Li, K.; Zhu, G.; Li, J. Preparation and Properties of Highly Branched Sulfonated Poly(Ether Ether Ketone)s Doped with Antioxidant 1010 as Proton Exchange Membranes. J. Memb. Sci. 2011, 379, 440–448. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, H.; Li, X.; Wang, L.; Liu, B.; Jiang, Z. Synthesis and Characterization of Sulfonated Poly(Arylene Ether)s with Sulfoalkyl Pendant Groups for Proton Exchange Membranes. J. Memb. Sci. 2008, 318, 271–279. [Google Scholar] [CrossRef]
- Xie, H.; Wang, D.; Tao, D.; Wang, L. Synthesis of Highly Branched Sulfonated Polymers and the Effects of Degree of Branching on Properties of Branched Sulfonated Polymers as Proton Exchange Membranes. J. Power Sources 2014, 262, 328–337. [Google Scholar] [CrossRef]
- Li, Y.; Xie, M.; Wang, X.; Chao, D.; Liu, X.; Wang, C. Novel Branched Sulfonated Poly(Ether Ether Ketone)s Membranes for Direct Methanol Fuel Cells. Int. J. Hydrog. Energy 2013, 38, 12051–12059. [Google Scholar] [CrossRef]
- Seo, D.W.; Park, H.S.; Choi, S.W.; Jeong, Y.G.; Hong, T.W.; Kim, W.G. Synthesis and Characterization of Branched Sulfonated Poly(Ether Sulfone Ketone) as Proton Exchange Membrane. Polym. J. 2008, 40, 979–985. [Google Scholar] [CrossRef]
- Matsumoto, K.; Higashihara, T.; Ueda, M. Star-Shaped Sulfonated Block Copoly(Ether Ketone)s as Proton Exchange Membranes. Macromolecules 2008, 41, 7560–7565. [Google Scholar] [CrossRef]
- Hlil, A.R.; Matsumura, S.; Hay, A.S. Polymers Containing Di(1 H -Benzo[d]Imidazol-2-Yl)Arene Moieties: Polymerization via N−C Coupling Reactions. Macromolecules 2008, 41, 1912–1914. [Google Scholar] [CrossRef]
- Kim, T.; Choi, Y.W.; Kim, C.S.; Yang, T.H.; Kim, M.N. Sulfonated Poly(Arylene Ether Sulfone) Membrane Containing Sulfated Zirconia for High-Temperature Operation of PEMFCs. J. Mater. Chem. 2011, 21, 7612–7621. [Google Scholar] [CrossRef]
- Park, H.S.; Seo, D.W.; Choi, S.W.; Jeong, Y.G.; Lee, J.H.; Kim, D.I.; Kim, W.G. Preparation and Characterization of Branched and Linear Sulfonated Poly(Ether Ketone Sulfone) Proton Exchange Membranes for Fuel Cell Applications. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1792–1799. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zhu, G.; Li, J. Synthesis and Properties of Highly Branched Sulfonated Poly(Arylene Ether)s as Proton Exchange Membranes. Eur. Polym. J. 2011, 47, 1985–1993. [Google Scholar] [CrossRef]
- Sato, O.; Kasai, T.; Sato, M.; Sakajiri, K.; Tsujii, Y.; Kang, S.; Watanabe, J.; Tokita, M. High-Density Poly(Hexyl Methacrylate) Brushes Offering a Surface for near-Zero Azimuthal Anchoring of Liquid Crystals at Room Temperature. J. Mater. Chem. C 2013, 1, 7992–7995. [Google Scholar] [CrossRef]
- Wang, C.; Young Lee, S.; Won Shin, D.; Rae Kang, N.; Lee, Y.M.; Guiver, M.D. Proton-conducting Membranes from Poly(Ether Sulfone)s Grafted with Sulfoalkylamine. J. Memb. Sci. 2013, 427, 443–450. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Zhao, C.; Shao, K.; Zhang, G.; Li, H.; Lin, H.; Na, H. Novel Side-Chain-Type Sulfonated Poly(Arylene Ether Ketone) with Pendant Sulfoalkyl Groups for Direct Methanol Fuel Cells. Polymer 2009, 50, 4471–4478. [Google Scholar] [CrossRef]
- Kim, D.S.; Robertson, G.P.; Guiver, M.D. Comb-Shaped Poly(Arylene Ether Sulfone)s as Proton Exchange Membranes. Macromolecules 2008, 41, 2126–2134. [Google Scholar] [CrossRef]
- Patel, R.; Im, S.J.; Ko, Y.T.; Kim, J.H.; Min, B.R. Preparation and Characterization of Proton Conducting Polysulfone Grafted Poly(Styrene Sulfonic Acid) Polyelectrolyte Membranes. J. Ind. Eng. Chem. 2009, 15, 299–303. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, H.; Li, X.; Liu, B.; Jiang, Z. Poly(Arylene Ether)s with Pendant Sulfoalkoxy Groups Prepared by Direct Copolymerization Method for Proton Exchange Membranes. J. Power Sources 2008, 184, 1–8. [Google Scholar] [CrossRef]
- Yuan, S.; Guo, X.; Aili, D.; Pan, C.; Li, Q.; Fang, J. Poly(Imide Benzimidazole)s for High-Temperature Polymer Electrolyte Membrane Fuel Cells. J. Memb. Sci. 2014, 454, 351–358. [Google Scholar] [CrossRef]
- Sutradhar, S.C.; Jang, H.; Banik, N.; Yoo, J.; Ryu, T.; Yang, H.; Yoon, S.; Kim, W. Synthesis and Characterization of Proton Exchange Poly (Phenylenebenzophenone)s Membranes Grafted with Propane Sulfonic Acid on Pendant Phenyl Groups. Int. J. Hydrog. Energy 2017, 42, 12749–12758. [Google Scholar] [CrossRef]
- Sutradhar, S.C.; Rahman, M.M.; Ahmed, F.; Ryu, T.; Yoon, S.; Lee, S.; Kim, J.; Lee, Y.; Jin, Y.; Kim, W. Synthesis of Nickel-Catalyzed Sulfonated Poly (Phenylenebenzophenone)s from Primarily Sulfonated Monomer for Proton Exchange Membranes. Int. J. Hydrog. Energy 2019, 44, 11311–11320. [Google Scholar] [CrossRef]
- Sutradhar, S.C.; Rahman, M.M.; Ahmed, F.; Ryu, T.; Yoon, S.; Lee, S.; Kim, J.; Lee, Y.; Jin, Y.; Kim, W. Thermally and Chemically Stable Poly(Phenylenebenzophenone) Membranes for Proton Exchange Membrane Fuel Cells by Ni (0) Catalyst. J. Ind. Eng. Chem. 2019, 76, 233–239. [Google Scholar] [CrossRef]
- Sutradhar, S.C.; Rahman, M.M.; Ahmed, F.; Ryu, T.; Yoon, S.; Lee, S.; Jin, Y.; Kim, W. Improved Proton Conductive Membranes from Poly(Phenylenebenzophenone)s with Pendant Sulfonimide Acid Groups for Fuel Cells. J. Power Sources 2019, 442, 227233. [Google Scholar] [CrossRef]
- Ahmed, F.; Sutradhar, S.C.; Ryu, T.; Jang, H.; Choi, K.; Yang, H.; Yoon, S.; Rahman, M.M.; Kim, W. Comparative Study of Sulfonated Branched and Linear Poly(Phenylene)s Polymer Electrolyte Membranes for Fuel Cells. Int. J. Hydrog. Energy 2018, 43, 5374–5385. [Google Scholar] [CrossRef]
- Ha, Y.H.; Scott, C.E.; Thomas, E.L. Miscible Blends of Poly(Benzoyl Paraphenylene) and Polycarbonate. Polymer 2001, 42, 6463–6472. [Google Scholar] [CrossRef]
- Ninivin, C.L.; Balland-Longeau, A.; Demattei, D.; Coutanceau, C.; Lamy, C.; Léger, J.M. Sulfonated Derivatives of Polyparaphenylene as Proton Conducting Membranes for Direct Methanol Fuel Cell Application. J. Appl. Electrochem. 2004, 34, 1159–1170. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, L.; Higashihara, T.; Ueda, M. Polymer Electrolyte Membranes Based on Poly(m-Phenylene)s with Sulfonic Acid via Long Alkyl Side Chains. Polym. Chem. 2013, 4, 1235–1242. [Google Scholar] [CrossRef]
- Singh, R.; Hay, A.S. Synthesis and Physical Properties of Soluble, Amorphous Poly(Ether Ketone)s Containing the o-Dibenzoylbenzene Moiety. Macromolecules 1992, 25, 1017–1024. [Google Scholar] [CrossRef]
- Rehahn, M.; Schlüter, A.D.; Wegner, G.; Feast, W.J. Soluble Poly(Para-Phenylene)s. 2. Improved Synthesis of Poly(Para-2,5-Di-n-Hexylphenylene) via Pd-Catalysed Coupling of 4-Bromo-2,5-Di-n-Hexylbenzeneboronic Acid. Polymer 1989, 30, 1060–1062. [Google Scholar] [CrossRef]
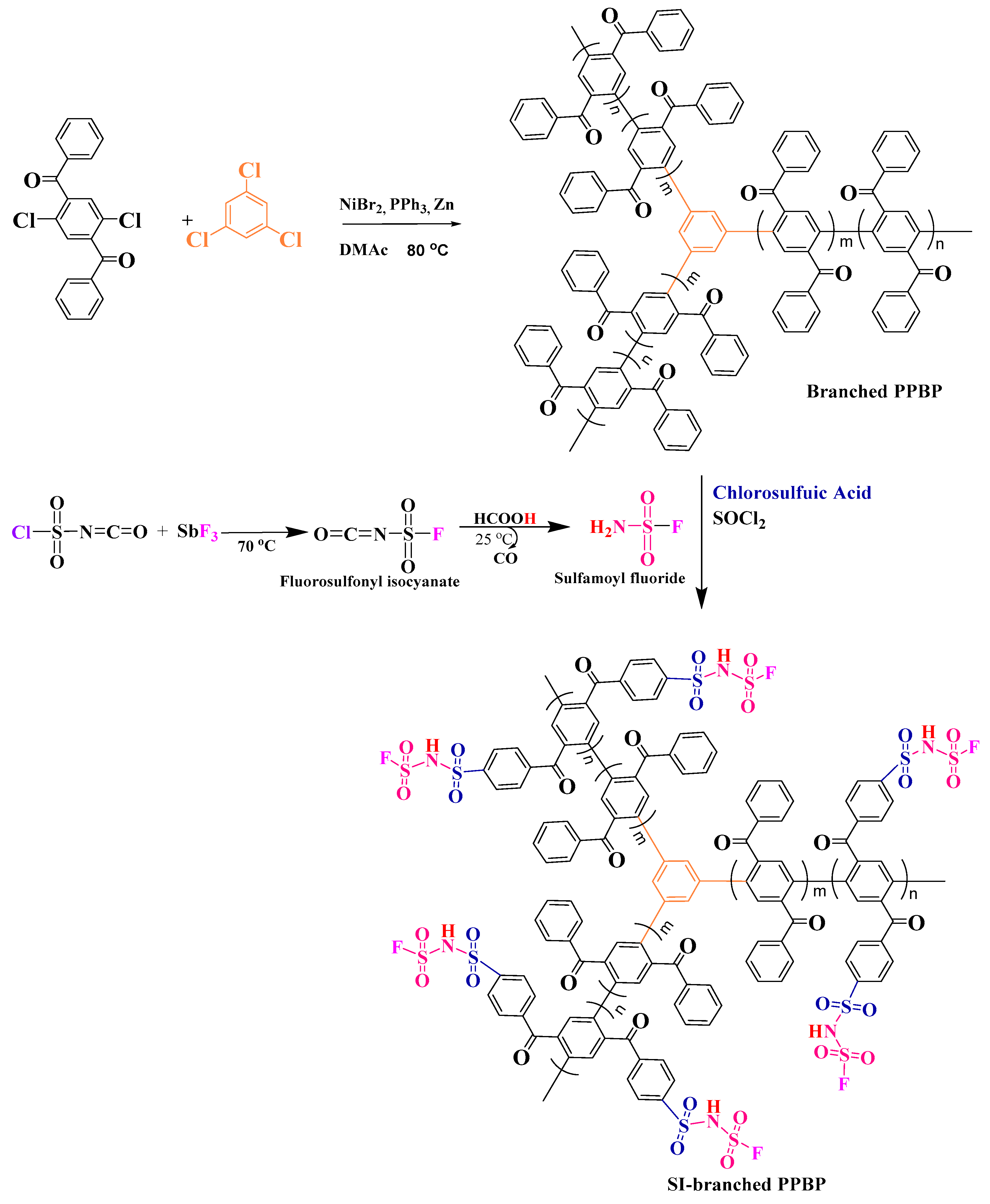
| Polymers | Viscosity a ηint (dl/g) | IEC (meq/g) | Water Uptake b, (%) | Dimensional Changes c | Hydration Number, Per Sulfonimide Group c, λ | Membrane Properties | Conductivity Measurement d, σ (mS/cm) | Young’s Modulus e, (MPa) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Δt (%) | Δl (%) | Shape, cm | Thickness, L μm | |||||||
| SI-branched PPBP-20 | 0.73 | 1.00 | 25.3 | 3.1 | 5.2 | 14.0 | 2 × 3 | 75 | 75.9 | 1030 |
| SI-branched PPBP-30 | 0.82 | 1.42 | 42.5 | 4.7 | 6.3 | 16.59 | 2 × 3 | 75 | 83.1 | 1065 |
| SI-branched PPBP-40 | 0.93 | 1.86 | 66.8 | 7.6 | 10.8 | 19.95 | 2 × 3 | 75 | 121.887 | 1120 |
| Nafion-117® | - | 0.91 | 32.17 | 31.21 | 14.10 | 19.6 | 2 × 3 | 175 | 84.74 | 165.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, S.C.; Yoon, S.; Ryu, T.; Jin, L.; Zhang, W.; Kim, W.; Jang, H. Branched Sulfonimide-Based Proton Exchange Polymer Membranes from Poly(Phenylenebenzopheneone)s for Fuel Cell Applications. Membranes 2021, 11, 168. https://doi.org/10.3390/membranes11030168
Sutradhar SC, Yoon S, Ryu T, Jin L, Zhang W, Kim W, Jang H. Branched Sulfonimide-Based Proton Exchange Polymer Membranes from Poly(Phenylenebenzopheneone)s for Fuel Cell Applications. Membranes. 2021; 11(3):168. https://doi.org/10.3390/membranes11030168
Chicago/Turabian StyleSutradhar, Sabuj Chandra, Sujin Yoon, Taewook Ryu, Lei Jin, Wei Zhang, Whangi Kim, and Hohyoun Jang. 2021. "Branched Sulfonimide-Based Proton Exchange Polymer Membranes from Poly(Phenylenebenzopheneone)s for Fuel Cell Applications" Membranes 11, no. 3: 168. https://doi.org/10.3390/membranes11030168
APA StyleSutradhar, S. C., Yoon, S., Ryu, T., Jin, L., Zhang, W., Kim, W., & Jang, H. (2021). Branched Sulfonimide-Based Proton Exchange Polymer Membranes from Poly(Phenylenebenzopheneone)s for Fuel Cell Applications. Membranes, 11(3), 168. https://doi.org/10.3390/membranes11030168









