1. Introduction
Binder-free self-supporting porous metal film possessing a large active surface area and good wettability could accelerate the electron transfer, ion transportation, and bubble detachment, which results in excellent electrochemical activity and stability in electrocatalysis, fuel cells, batteries and other electrochemical energy conversion fields [
1,
2,
3]. Among many self-supporting porous film preparation methods, the electrodeposition method is considered to be one of the most promising methods because it is convenient and easy to control [
4,
5]. Especially, the hydrogen bubble dynamic template (HBDT) method, which does not require subsequent removal of the template, has received more and more research attention in recent years [
1,
6,
7]. It is based on the hydrogen bubble channels generated by the strong hydrogen evolution side reaction at a high current density during the metal electrodeposition process as a dynamic template, and deposits grow along the bubble channel template. When the bubbles break away, a self-supporting porous film is formed (
Figure 1) [
8]. Various metal and alloy porous films with good electrochemical properties for electrochemical energy conversion, such as Cu [
9,
10], FeP [
11], CoP [
12,
13], NiCoP [
14], were prepared by using the HBDT method. However, it is difficult to control the porous structure of the film electrodeposited by HBDT method, and the mechanism is unclear.
Metal electrocrystallization and hydrogen evolution side reaction are the two main reactions during the process of electrodepositing porous materials by using the HBDT method. The characteristics of electroactive ion species for metal electrodeposition, such as molecular structure, exchange current density, and reduction overpotential, could affect the nucleation and growth of metal crystal, which in turn determines the microstructure [
15,
16]. Kim et al. [
17] found that PEG (polyethylene glycol) and MPSA (3-mercapto-1-propane sulfonic acid sodium salt) could form complexes with Cu
2+ to change the size, shape, and density of Cu deposits, which is beneficial to forming 3D interconnected porous Cu films. Nam et al. [
18] considered that BTA (benzotriazole, C
6H
4N
3H) and NH
4+ could also be complexed with Cu
2+ to change the electrocrytallization behavior. Consequently, porous Cu films with needle-like nanodendrites or grape-like particles were obtained. On the other hand, behaviors of bubbles as templates on the film surface, such as bubble adsorption, coalescence, growth, and detachment, could affect the porous structure [
2,
19]. Additionally, the stirring effect generated by the bubble detachment could change the electrocrystallization kinetic conditions to affect the film structure [
7,
20].
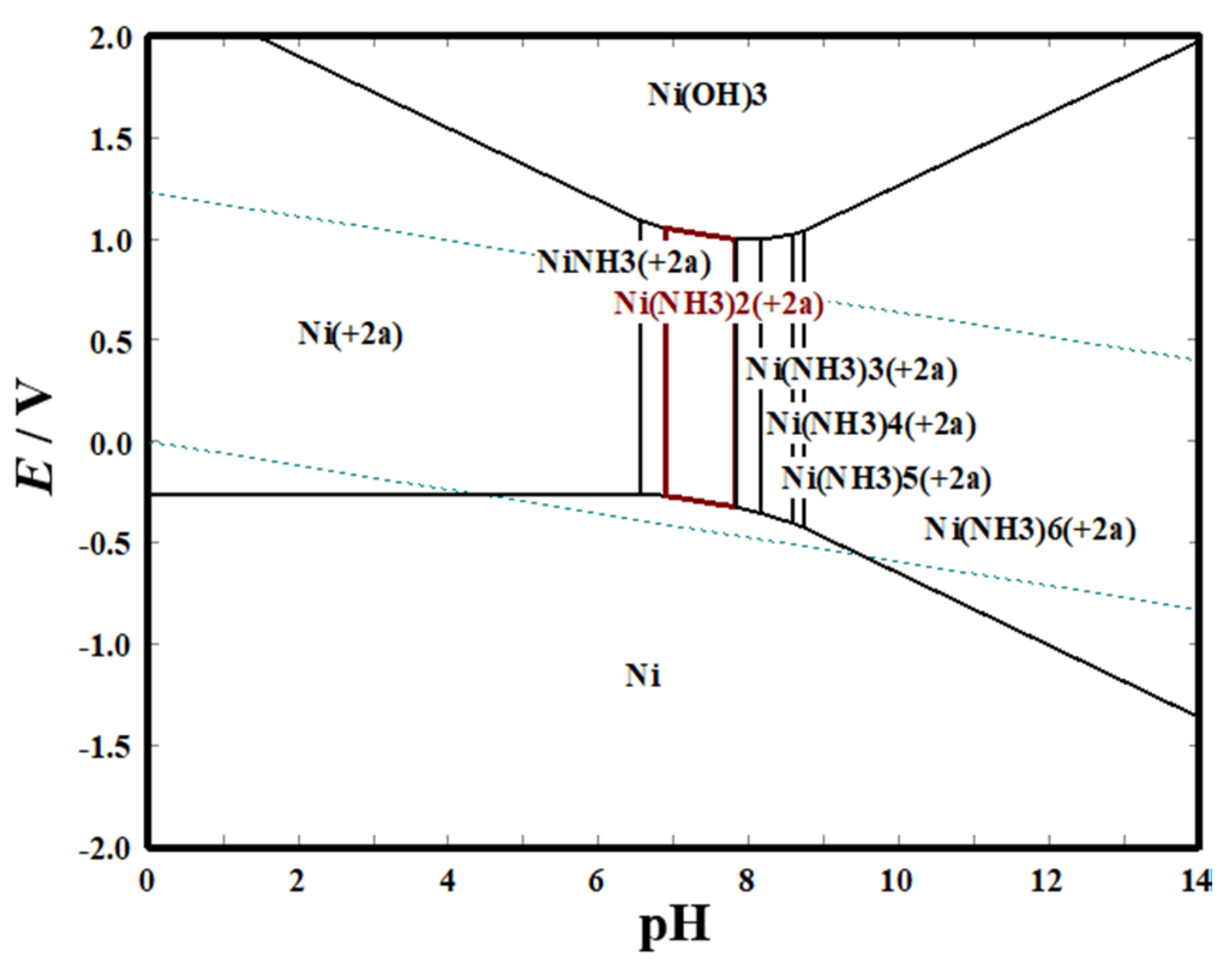
pH value is an important factor to adjust the form of complexes, especially in solutions containing NH
4+. M(NH
3)
n2+ complexes with different coordination number (n) are formed at different pH values [
16]. Moreover, the H
+ concentration in the solution that has a great impact on the hydrogen evolution side reaction is determined by the pH value. However, the dependence of pore structures of Ni films, which are a promising electrocatalyst for HER, on the pH value was not studied. On the other hand, EDTA could complex with metal ions to form a stable six-coordinate chelate, thereby changing the metal electrocrystallization behavior [
21]. Therefore, it is significant to study the influences of pH value and EDTA additive on the porous structure of Ni films electrodeposited by using the HBDT method.
In this work, the influences of pH value and EDTA additive on the surface morphology and crystal structure of electrodeposited Ni films were studied. The structure evolution mechanism was analyzed based on the two aspects of hydrogen bubble evolution and metal electrocrystallization behaviors. Finally, the catalytic performance for HER of the electrodeposited Ni films with different structure was also evaluated.
3. Results and Discussion
The equilibrium potential of metal electrodeposition changes with the electroactive ion species (i.e., molecular structure) [
25], which affects the metal electrocrystallization behavior (including crystal nucleation and growth). In our previous study [
16], it was found that self-supporting porous Ni film could be electrodeposited only when the NH
4+ concentration reaches a certain value. According to the molecular structure characteristics of NH
4+, in addition to being used as a buffer and hydrogen source, it can also be used as a complexing agent to form complexes with Ni
2+ to change the electroactive ion species for Ni electrodeposition. As shown in the
E-pH diagram (
Figure 2), in solution containing 0.2 M NiCl
2, 2 M NH
4Cl, and 1 M NaCl, the molecular structure of Ni(NH
3)
n2+ (6 ≥
n ≥ 0) complex is different at various pH values. The number of NH
3 molecules bound by Ni
2+ in the formed Ni(NH
3)
n2+ (6 ≥
n ≥ 0) complexes gradually increases with the increase of the pH value, and the binding force of the coordination bond increases, thus the theoretical equilibrium potential shifts negatively (
Figure 2,
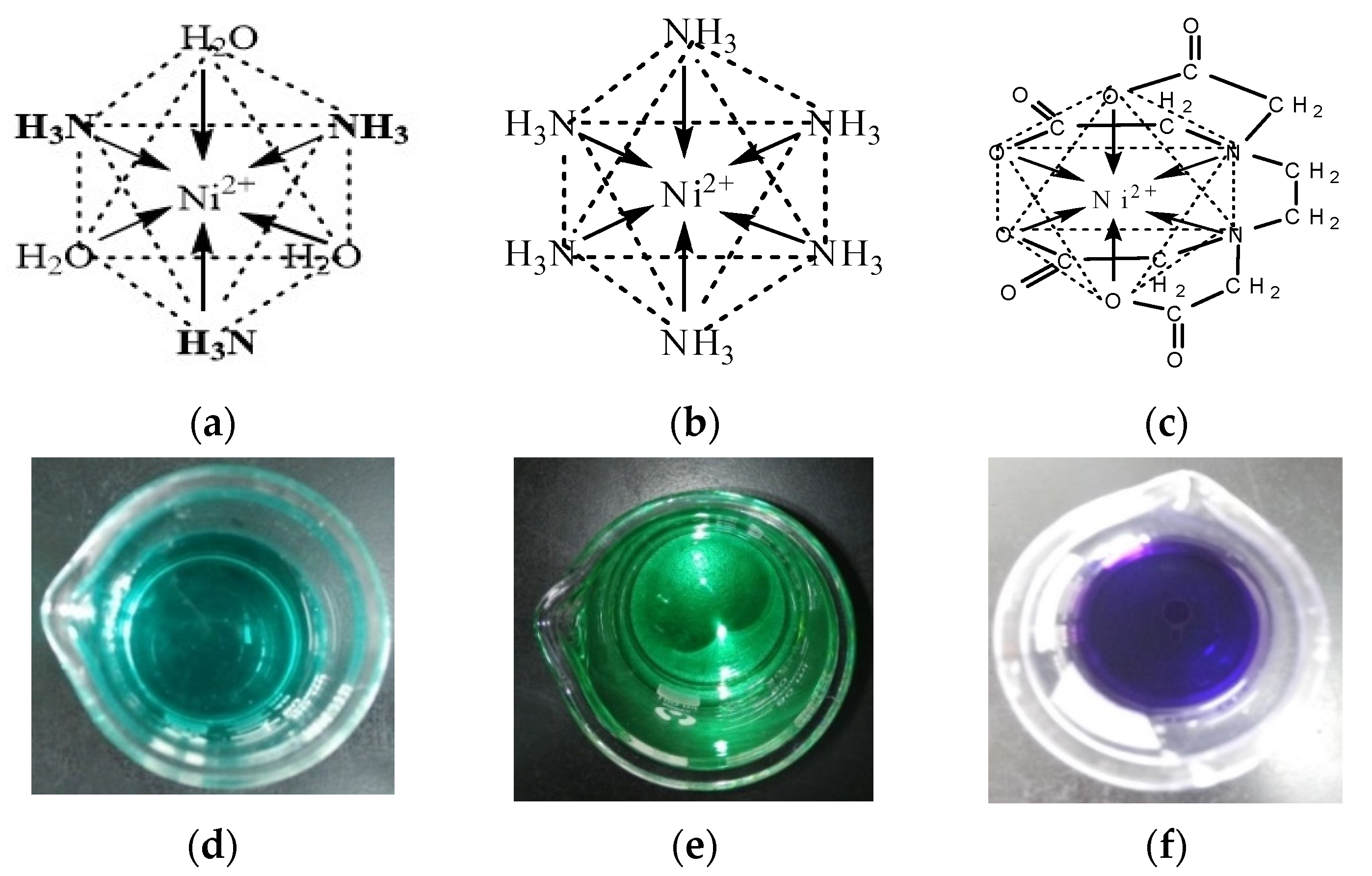
Figure 3a,b). Additionally, EDTA could chelate with Ni
2+ to form a cyclic chelate structure which is different from Ni(NH
3)
n2+ complexes (
Figure 3c). The six-coordinated chelate has a more stable thermodynamic structure than the Ni(NH
3)
n2+ complexes, so its theoretical equilibrium potential is more negative.
Based on the above theoretical analysis, different forms of electroactive ion species for Ni electrodeposition can be formed by adjusting the pH value of the basic solution (
Table 1). To obtain Ni chelate, 0.1 M EDTA is added to the basic solution. As
Figure 3d–f shows, the solution changes from dark green to light blue as the pH value increases from 3 to 7.7, and the solution containing 0.1 M EDTA is dark purple. The above results indicate that different electroactive ion species for Ni electrodeposition are formed under different solution conditions.
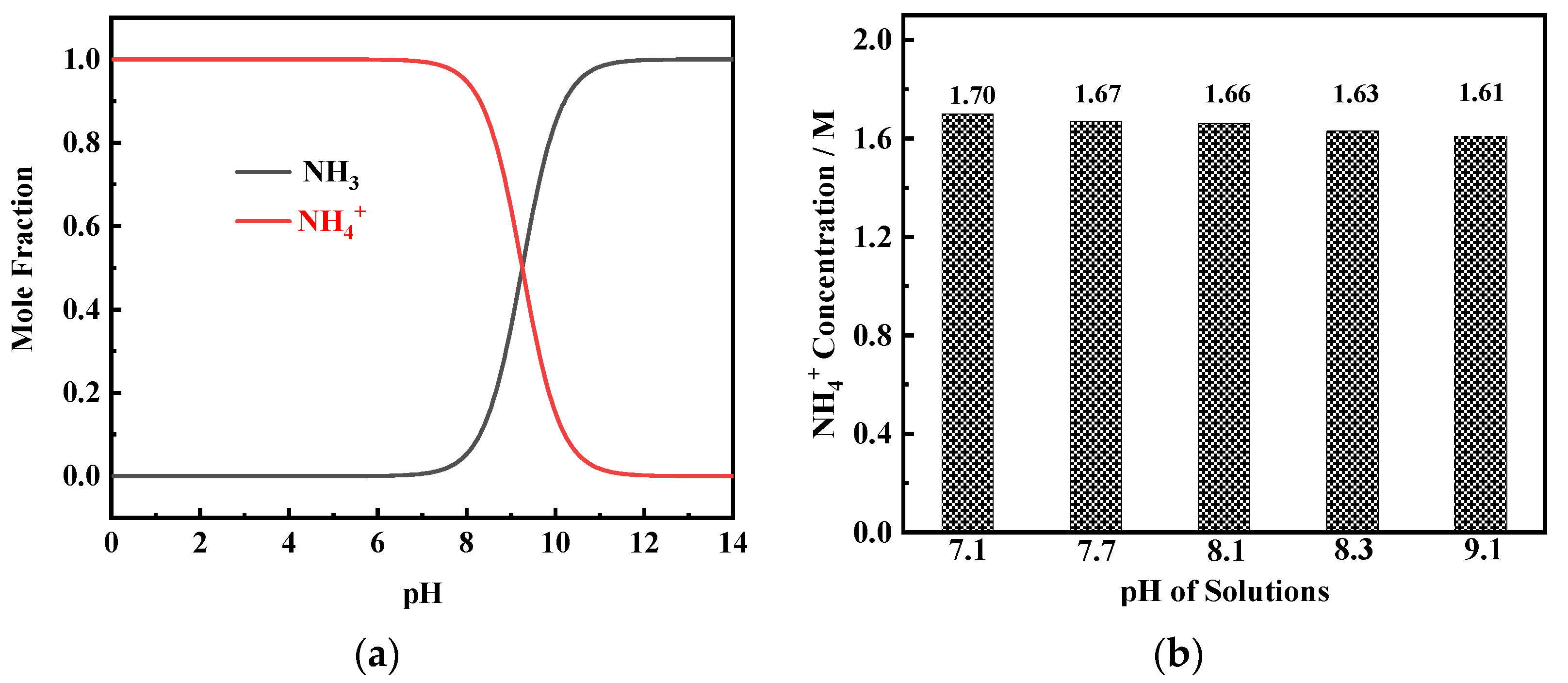
To obtain the high pH value, a large amount of dilute NaOH solution is added to the basic solution to react with NH
4+ (reaction (2)). The product NH
3.H
2O is decomposed into NH
3 and H
2O (reaction (3)). Ni
2+ complexes react with NH
3 to form Ni(NH
3)
n2+ complexes (reaction (4)) which are reduced to metal Ni (reaction (5)) during the electrodeposition process. From the mole fraction–pH diagram of NH
3–NH
4+ species (
Figure 4a), it can be found that, when pH value is greater than 7, the mole fraction of NH
3 begins to increase while the mole fraction of NH
4+ begins to decrease. The results of the ion chromatography measurement show that in solutions containing 0.2 M NiCl
2, 2 M NH
4Cl, and 1 M NaCl, when the pH value reaches between 7.1 and 9.1, the NH
4+ concentration is in the range of 1.6–1.7 M (
Figure 4b). This result indicates that even in the solution with a high pH value (9.1), very little NH
3 volatilizes, and the NH
4+ concentration in the solution can reach the critical content required for porous Ni film formation [
16].
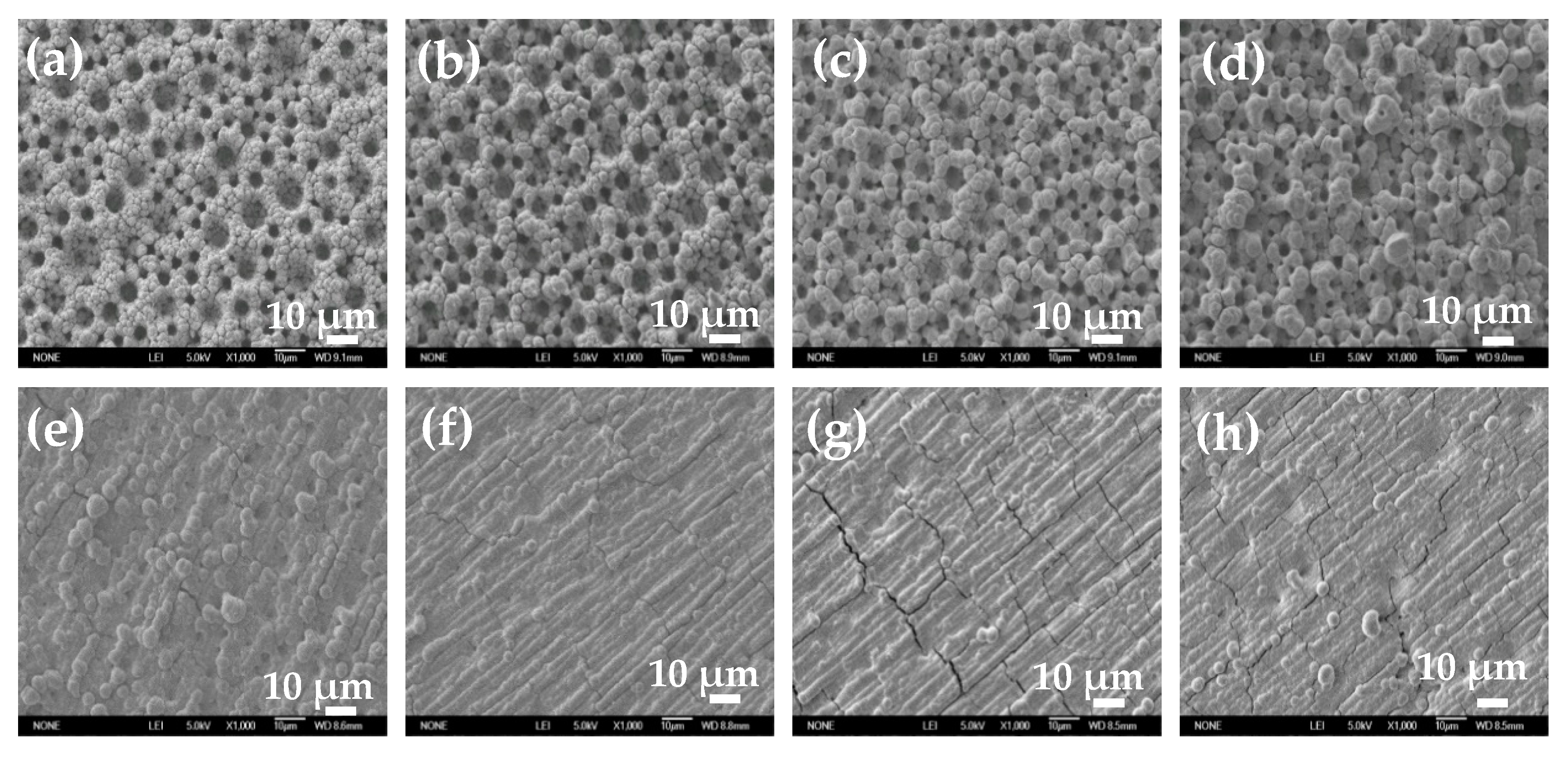
To study the influence of electroactive ion species on the surface structure of electrodeposited Ni film, Ni films were electrodeposited in solutions with different pH values or containing 0.1 M EDTA at −1 A cm
−2 for 30 s. SEM images of electrodeposited Ni films at two different magnifications are shown in
Figure 5 and
Figure 6. When the pH value is less than 7.7, self-supporting Ni films with uniform porous structure can be electrodeposited (
Figure 5a–d). With the increase of the pH value, the diameter of the pore decreases, and the pore wall becomes thicker. When the pH value reaches 8.1, the porous structure degenerates and disappears, and a rough Ni film composed of agglomerated particles appears on the electrode surface (
Figure 5e). When the pH value further increases (pH = 8.3), the number of the agglomerated particle decreases and the size of the agglomerated particle becomes smaller (
Figure 5f). When the pH value reaches 9.1, the agglomerated particles disappear, and a flat and compact Ni film is formed. It is worth noting that Ni film obtained by reducing the chelate in basic solution with 0.1 M EDTA is flatter and more compact, without obvious agglomerated particles on the surface (
Figure 5h).
Figure 6 is the magnified image of the corresponding SEM image in
Figure 5. When the pH value is 3 or 6.5, smaller size Ni particles with dispersed arrangement are obtained. When the pH value is greater than 7.1, Ni particles are tightly agglomerated together. As the pH value further increases, Ni particles gradually become larger. When the pH value is higher than 8.3, the surface of the Ni film is flat and compact, without obvious particle boundaries. The Ni film obtained by reducing the chelate is flatter and more compact, without obvious particle boundaries. At the same time, it should be noted that there are cracks on the surface of flat and compact Ni films (
Figure 5g,h,
Figure 6f,h).
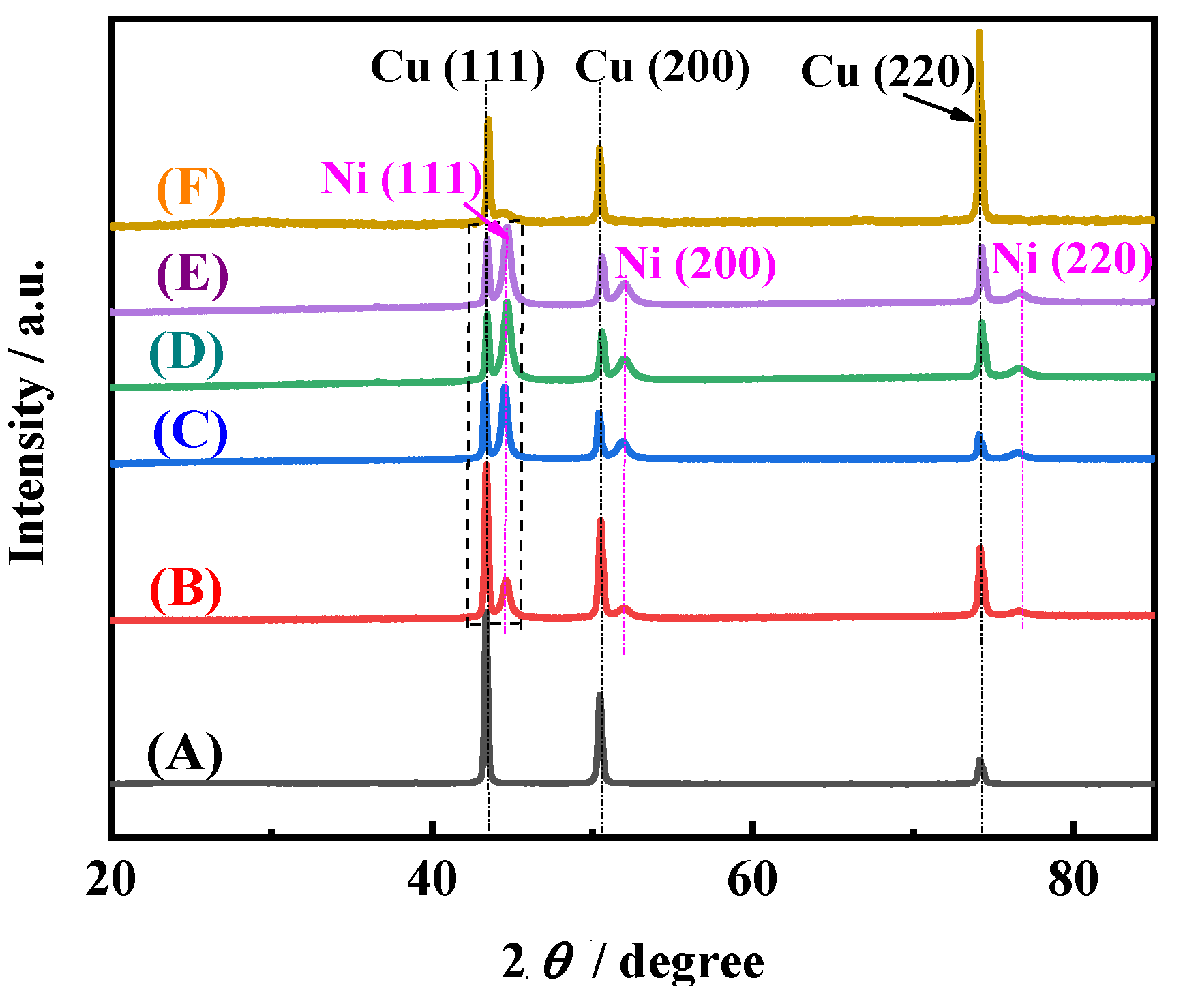
Figure 7 shows the typical XRD patterns of electrodeposited Ni films. It can be seen that for the Ni films electrodeposited in EDTA-free solutions with different pH values, except for the three peaks Cu(111), Cu(200), and Cu(220) of the Cu substrate [
26], three peaks Ni(111), Ni(200), and Ni(220) of electrodeposited Ni films appear [
2], which is consistent with the previous results of the electrodeposition Ni [
2]. The crystal plane Ni(111) is the preferred orientation, and the intensity of three characteristic peaks for Ni films increases with the increase of the pH value. It is worth noting that, for Ni film electrodeposited in solution with 0.1 M EDTA, only a broad and less sharp peak appears in the range of 44°–45°. It shows that amorphous Ni is obtained [
7], which may be because EDTA changes the electrocrytallization mechanism. The above results indicate that pH value and EDTA not only affect the surface morphology but also the crystal structure of the electrodeposited Ni film.
The current efficiency of hydrogen evolution (i.e., bubble evolution amount) can affect the electrocrystallization behaviors (especially crystal growth) by changing the electrodeposition dynamics condition near the electrode surface, which can determine the morphology and size of the electrodeposited metal particle [
20]. Additionally, the porous structure of the electrodeposited Ni film is related to the behavior (such as bubble nucleation, coalescence, growth and detachment) of the bubble on the substrate surface [
2,
19]. Therefore, during the Ni electrodeposition process, the current efficiency of hydrogen evolution in different solutions is calculated based on the mass of electrodeposited Ni. As shown in
Figure 8a, it can be found that in acid region (3 ≤ pH ≤7.1), the current efficiency of hydrogen evolution slightly decreases with the increase of the pH value. Yet in the alkaline region (7.1 ≤ pH ≤ 9.1), the current efficiency of hydrogen evolution slightly increases with the increase of the pH value. For the solution with 0.1 M EDTA, the maximum current efficiency of hydrogen evolution is obtained. However, for different solutions, the current efficiency of hydrogen evolution is about 60%. That is, the form of electroactive ion species used for Ni electrodeposition hardly affects the current efficiency of hydrogen evolution.
Furthermore, bubble size and bubble residence time on the substrate surface ultimately rely on the break-off diameter (
d) which is expressed by Formula (6) [
2,
19,
27]:
where
Φ is the contact angle (°),
g is the gravity acceleration (9.8 m s
−1),
γ is the surface tension (mN m
−1),
i is the current density (A cm
−2),
ρl and
ρg are solution density (kg dm
−3) and gas density (kg dm
−3), respectively.
It can be seen from Formula (6) that at a high current density, the bubble break-off diameter is mainly determined by the interface wettability characteristic, such as contact angle (
Φ) and surface tension (
γ). To accurately calculate the real-time break-off diameter of the adsorption bubble during the electrodeposition process, the real-time contact angle is measured, which is related to the surface condition of the electrode. As
Figure 8b shows, contact angles of all electrodeposited Ni films decrease significantly in the first 10 s, and then tend to level off. It also can be found that the contact angle decreases with the increase of the pH value, and the solution with 0.1 M EDTA possesses the smallest contact angle. The surface tension of different solutions is also measured and shown in
Table 2. The surface tension values are all around 76.5 mN m
−1, with little difference. According to Formula (6), the bubble break-off diameter is calculated based on the measurement parameter values in
Figure 8b and
Table 2. As
Table 2 shows, the bubble break-off diameter decreases with the increase of the pH value. For example, it decreases from 154.34 μm at pH = 3 to 117.29 μm at pH = 8.3. In solution with 0.1 M EDTA, the minimum bubble break-off diameter is obtained, which is about 109.91 μm. As the insulating hydrogen bubbles adsorbed on the film surface are used as a porous structure template, the pore diameter depends on the size of the bubble. So, the pore diameter of Ni film decreases with the increase of the pH value (
Figure 5).
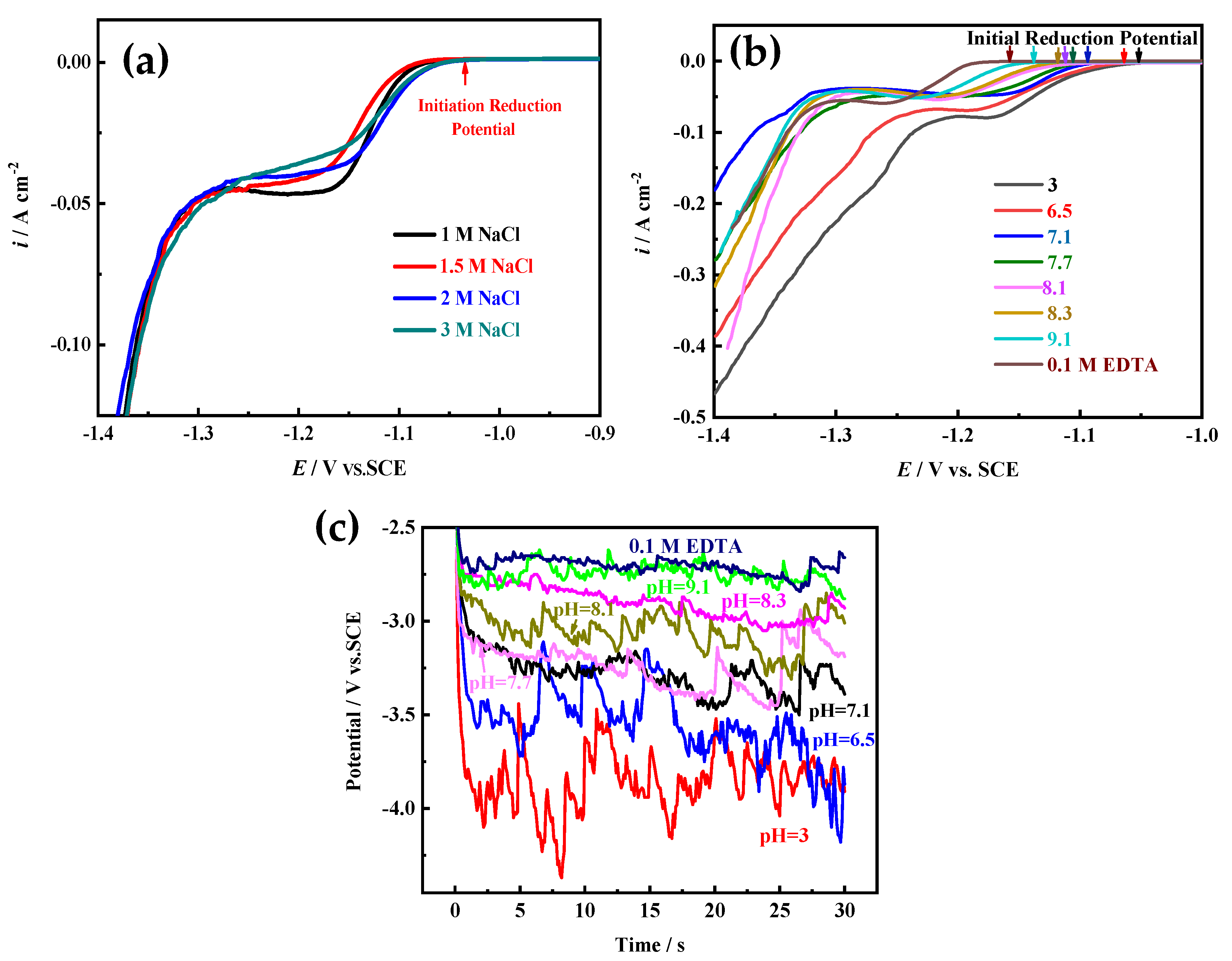
On the other hand, electrocrystallization behavior (including crystal nucleation and growth) is also an important factor for determining the structure of electrodeposited film, which greatly relies on the electroactive ion species for metal electrodeposition and the electrode reaction kinetic conditions [
15,
16]. According to the above research, different Ni electroactive ion species are formed in solutions with different pH values or components (
Figure 2,
Figure 3 and
Figure 4). To reveal the microstructure evolution mechanism of Ni films electrodeposited in solutions with different pH values or 0.1 M EDTA, the polarization curves of Ni electrodeposition in different solutions are measured at 10 mV s
−1. Moreover, the influence of NaCl concentration on the polarization curve of Ni electrodeposition is also investigated, because by using NaOH for pH adjustment, a large amount of NaCl would be produced to obtain a higher pH value (pH ≥ 7.1). As
Figure 9a shows, the location of the initial reduction potential and peak potential of Ni electrodeposition has hardly changed in solutions containing 0.2 M NiCl
2 and 2 M NH
4Cl and different NaCl concentrations, indicating that NaCl concentration has hardly any effect on Ni electrocrystallization behavior. From the results of
Figure 9b, it can be found that the initial reduction potentials and peak potentials of Ni electrodeposition shift toward a negative direction with the increase of the pH value, and in solution with 0.1 M EDTA, the most negative initial reduction potential for Ni electrodeposition is used. The results indicate that with the increase of the pH value, the Ni electroactive ion species change from Ni
2+ to Ni(NH
3)
n2+ (6 ≥ n ≥ 0) complexes, and as the number of NH
3 molecules (i.e., n) bound in the Ni(NH
3)
n2+ complexes increases, the reduction equilibrium potential gradually shifts more negative. Ni electrodeposited in solution containing 0.1 M EDTA needs the most negative equilibrium potential.
According to the metal electrocrystallization nucleation theory, the relationships of nucleation formation energy
A (J mol
−1), nucleation probability
W, and overpotential
η (V) are expressed as [
28]:
where
σ is interfacial free energy (J m
−2),
V is molar volume (m
3 mol
−1),
z is the number of transferred electrons,
F is the Faraday constant (96485 C mol
−1),
B and
b are constants,
hi is critical nucleation radius (m). According to Equations (7)–(9), with the decrease of overpotential (i.e., the increase of the pH or adding EDTA), the nucleation formation energy and critical nucleation radius increase, and nucleation probability decreases. Therefore, the growth probability and size of electrodeposited Ni crystal increases. This conclusion is also confirmed by the results of SEM and XRD measurements in
Figure 5,
Figure 6 and
Figure 7. That is, in EDTA-free solutions with low pH value, due to the high overpotential of Ni electroactive ion species, the particles with small size are easy to generate, leading to the formation of a rough porous structure which exhibits a large surface area and good wettability. In solutions with high pH value or 0.1 M EDTA, the Ni(NH
3)
n2+ complexes or Ni-EDTA chelate are formed, which can inhibit the nucleation of Ni electrocrystallization, and enhance the leveling effect of isotropic growth of the electrodeposited film. Consequently, the electrodeposited Ni film grows uniformly without forming protrusion particles.
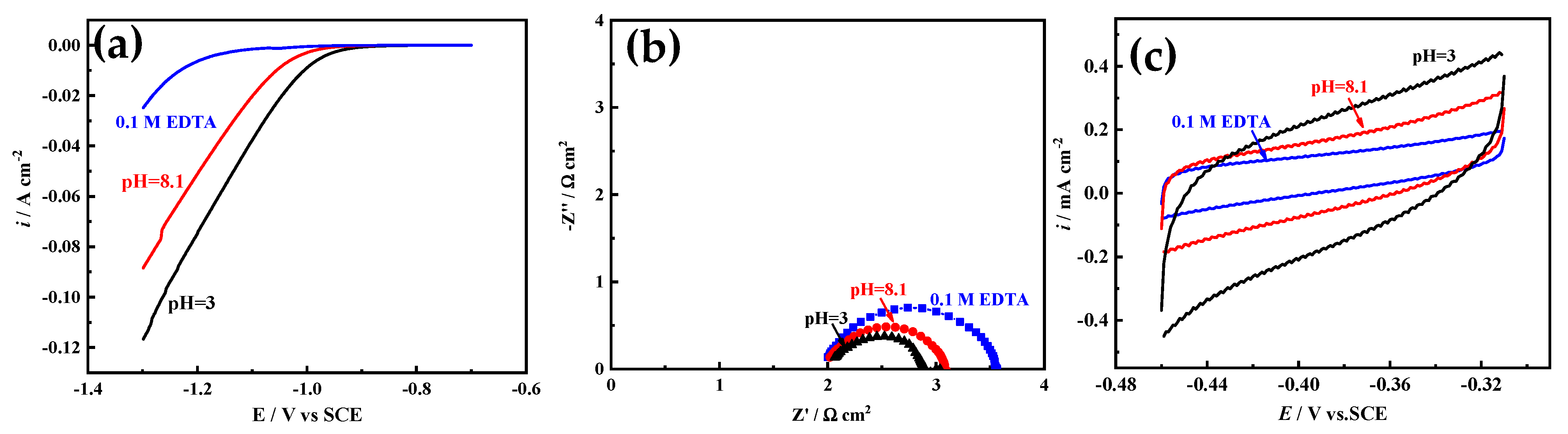
Ni film is usually used as a catalytic electrode or current collector for hydrogen evolution reaction (HER) [
3]. The electrocatalytic activity area is an important factor affecting the HER activity. Herein, Ni films electrodeposited in acid solution (pH = 3), alkaline solution (pH = 8.1), and solution with 0.1 M EDTA are selected to study the HER electrocatalytic activity, and the electrocatalytic activity area is simply evaluated based on EIS and CV measurements [
29,
30]. As
Figure 10a shows, with the increase of pH value, the HER electrocatalytic activity of Ni film decreases, and for Ni film electrodeposited in solution with 0.1 M EDTA, the worst HER electrocatalytic activity appears. It is well known that the electrocatalytic activity area of the Ni film is inversely proportional to the semicircle diameter of the Nyquist plot [
29] (that is, proportional to the electrochemical surface roughness), and proportional to the area covered by the CV curve [
30] (that is, proportional to the electric double layer capacitance). As shown in
Figure 10b,c, with the increase of pH value, the semicircle diameter increases, and the area covered by the CV curve decreases, indicating that the catalytic activity area of the film decreases. In solution with 0.1 M EDTA, the semicircle diameter is the largest and the area covered by the CV curve is the smallest, exhibiting that the catalytic activity area of the film is the smallest, which is consistent with the surface structure of Ni films (
Figure 5 and
Figure 6). The BET measurement also shows that the particle of the Ni film electrodeposited in acid solution (pH = 3) possesses the largest special surface area (about 125 m
2 g
−1), which are much larger than those of Ni films electrodeposited in alkaline solution (pH = 8.1) (about 91 m
2 g
−1) and solution with 0.1 M EDTA (about 47 m
2 g
−1).
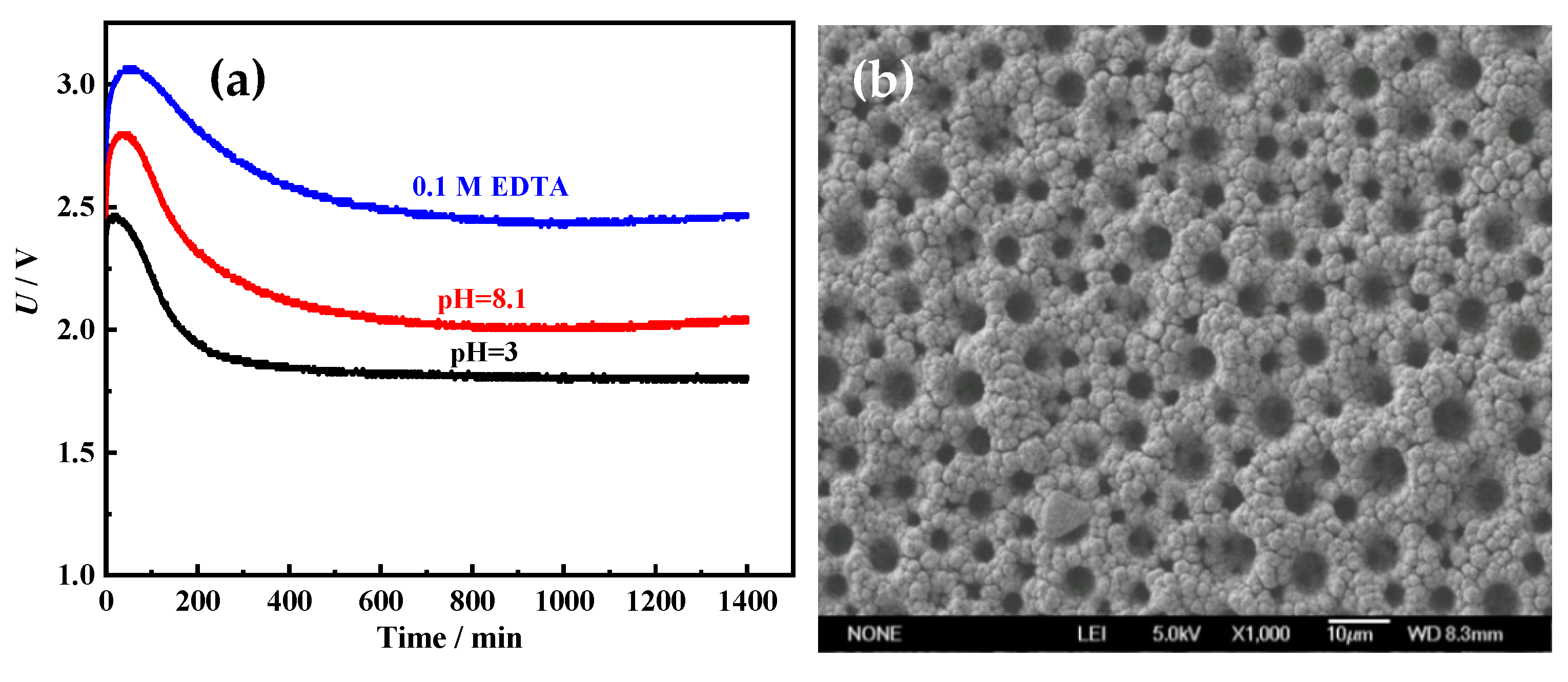
To investigate the stability of the Ni films for HER, the long-term electrolysis was performed in 1 M NaOH at 100 mA cm
−2 for 1400 min. The cell voltage–time (U–t) curves were recorded and are shown in
Figure 11a. Cell voltages of all Ni films increase firstly, and then drop. After about 300 min, cell voltages are almost unchanged. It means that all porous metal films possess good long-term stability. In addition, it is also found that the cell voltages of porous Ni film electrodeposited in acid solution (pH = 3) (about 1.85 V) are much lower than those of Ni films electrodeposited alkaline solution (pH = 8.1) (about 2.15 V) and solution with 0.1 M EDTA (about 2.55 V). It proves once again that the Ni film electrodeposited in acid solution (pH = 3) possesses the best HER performance. From the SEM image (
Figure 11b) and XRD pattern (
Figure 11c) of the Ni film electrodeposited in acid solution (pH = 3) after the long-term electrolysis, it can be found that the morphology and crystal structure are almost unchanged. Through the comparison of the XPS spectra for the fresh Ni film electrodeposited in acid solution (pH = 3) and the Ni film after the long-term electrolysis, it also can be found that the surface chemical compositions and states of the Ni film before and after use are almost the same (
Figure 11d). The Ni film surface is mainly composed of metallic Ni and a small amount of Ni oxide (Ni
2+) [
29]. The above results indicate that the Ni film exhibits excellent long-term catalytic stability for HER.