Amphiphilic Poly(dimethylsiloxane-ethylene-propylene oxide)-polyisocyanurate Cross-Linked Block Copolymers in a Membrane Gas Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
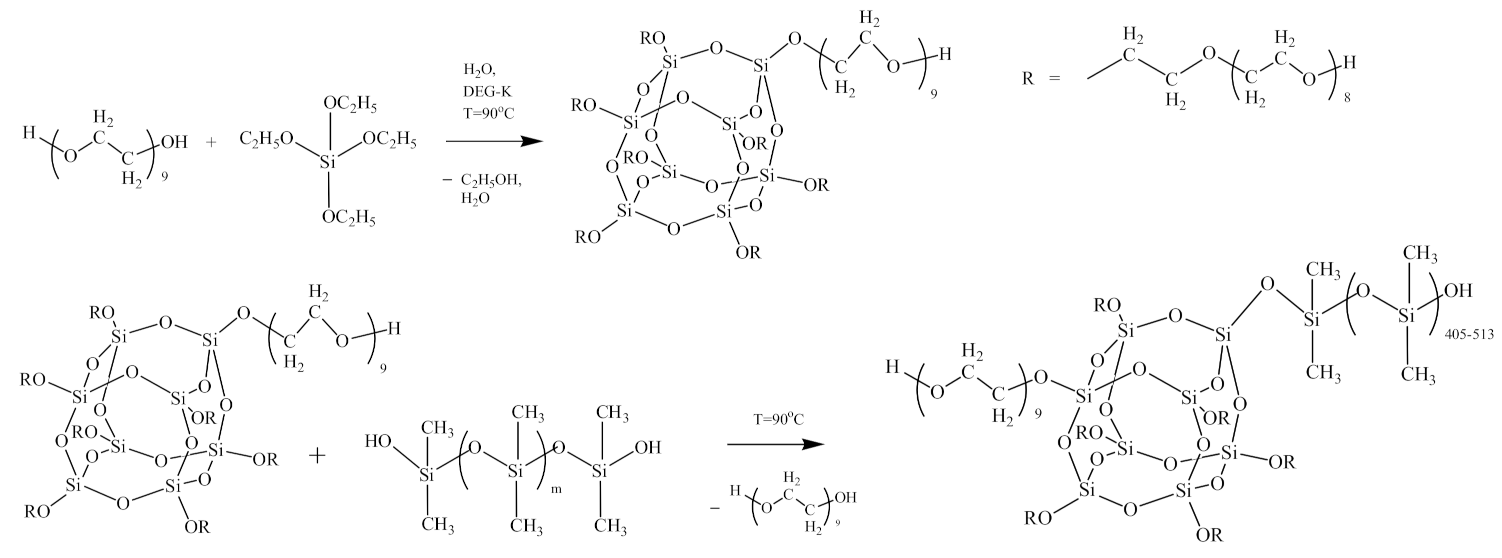
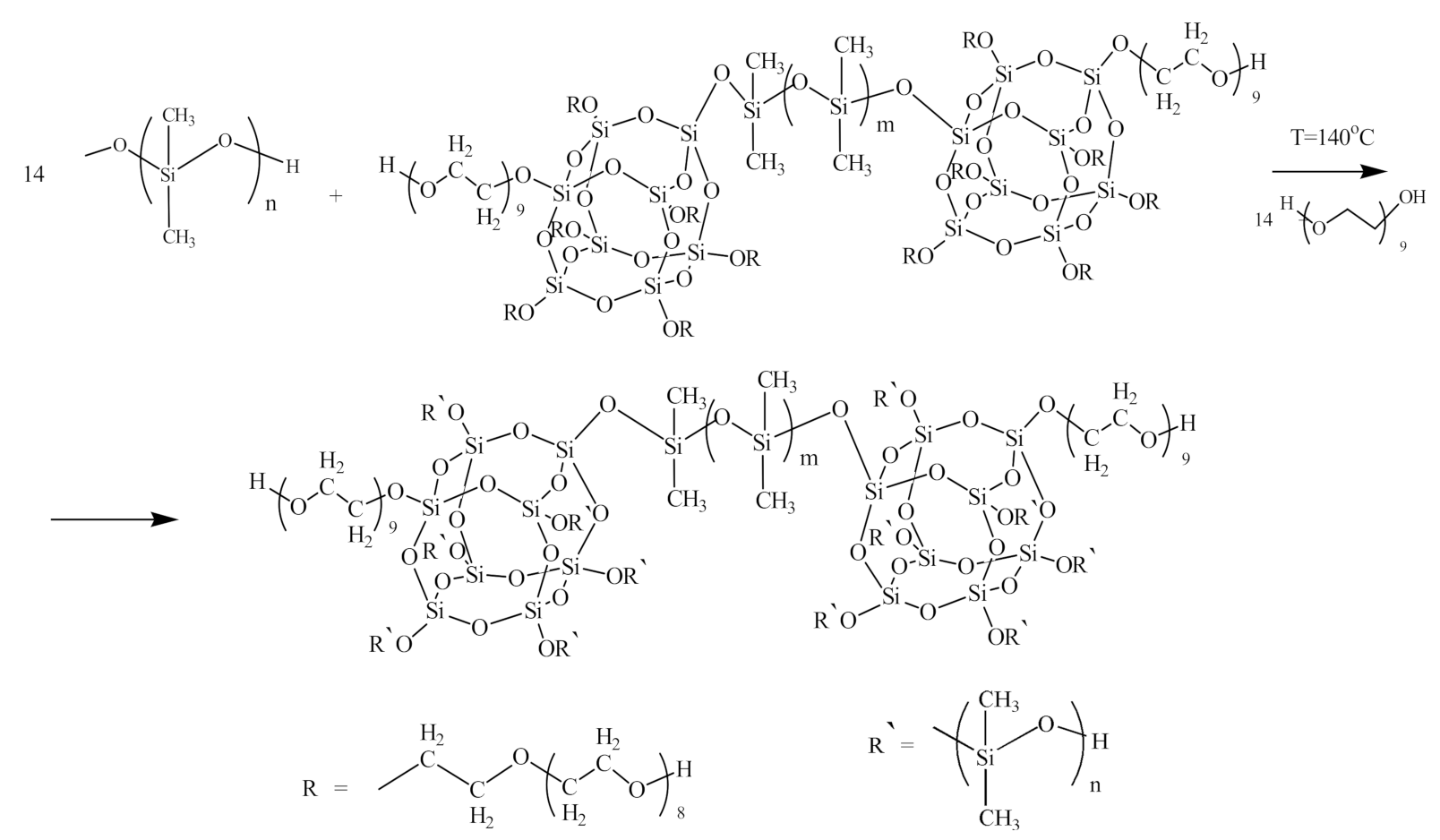
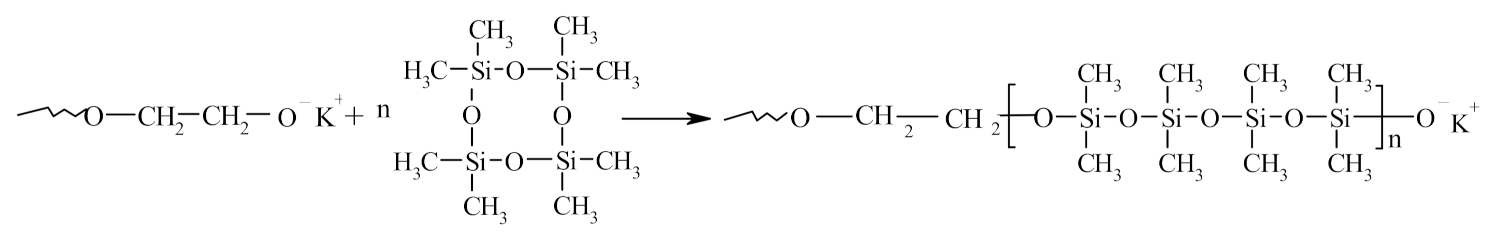
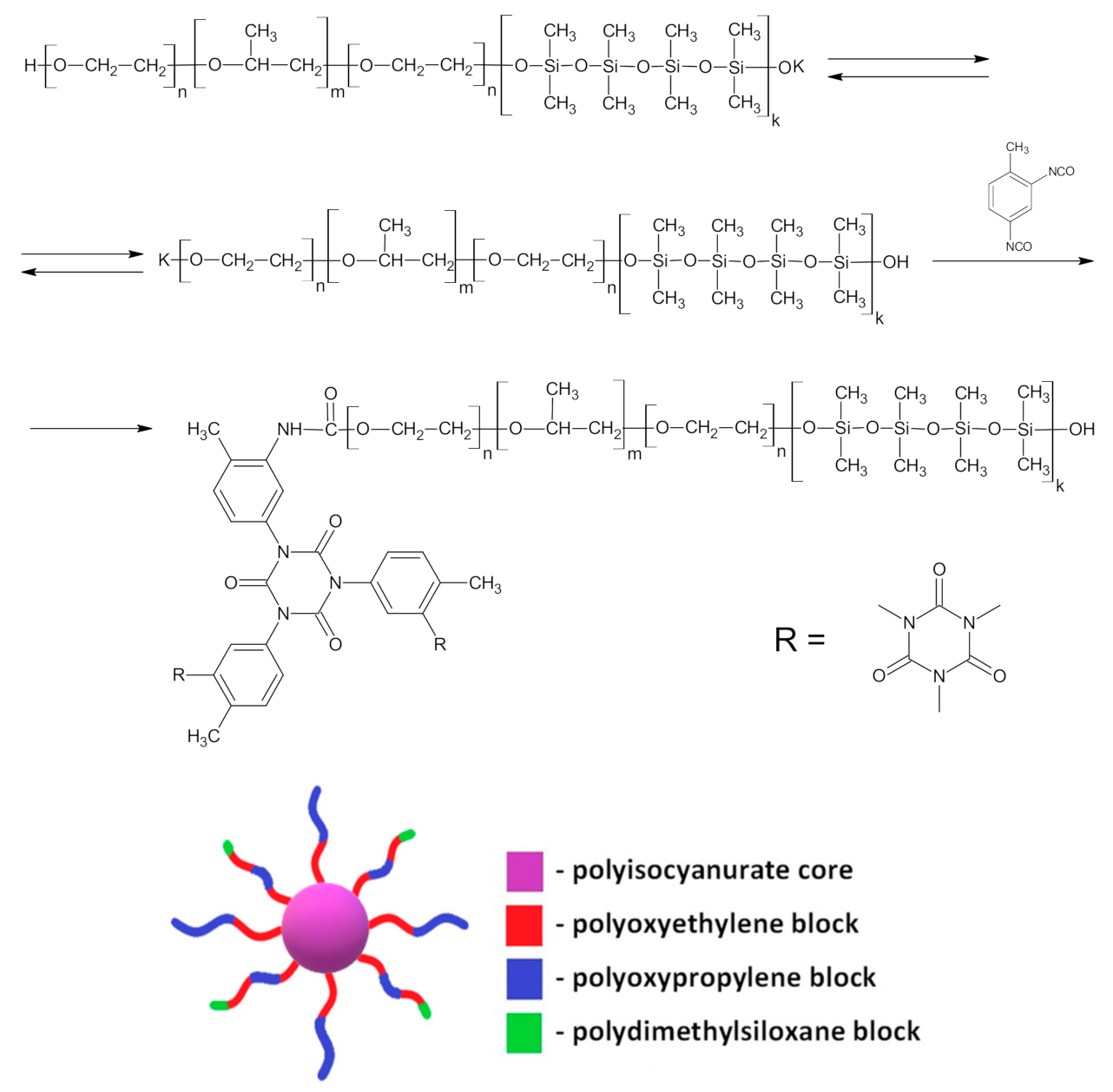
2.2. Synthesis of Polymers Based on PPEG, D4, TDI
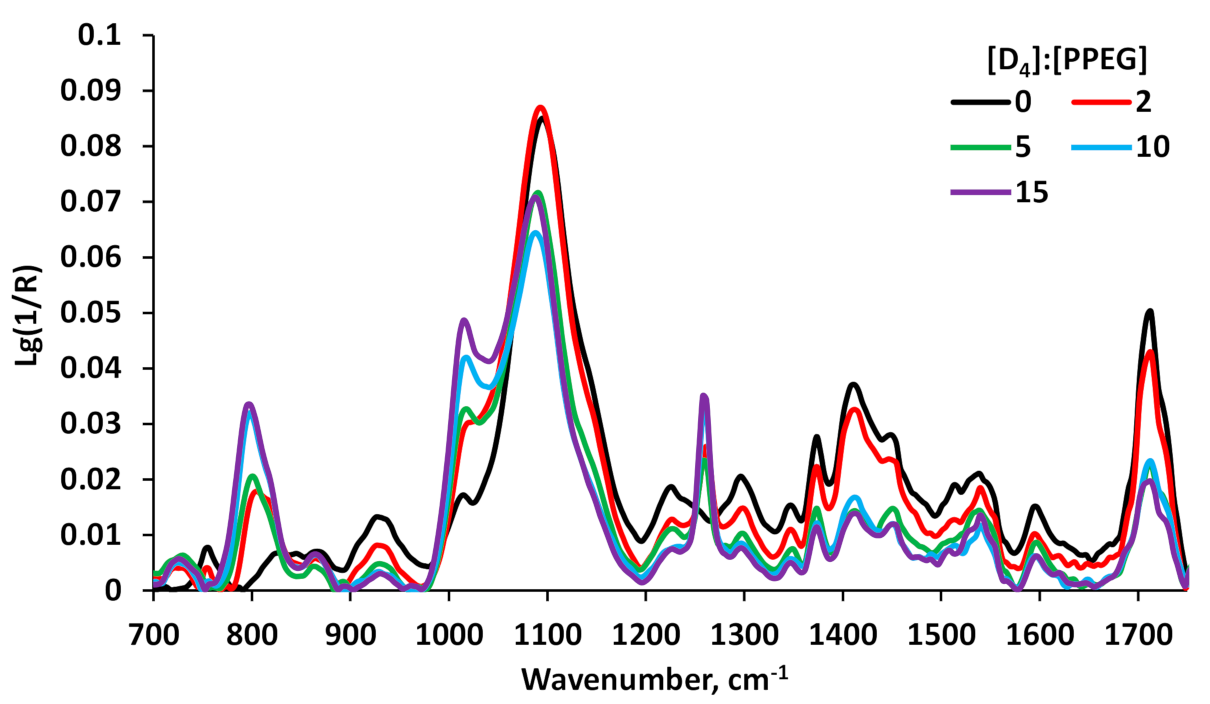
2.3. Fourier Transform Infrared Spectroscopy Analysis (FTIR)
2.4. Measurements of the Surface Tension
2.5. Light-Scattering
2.6. Measurement of Temperature Dependence of Dielectric Loss Tangent
2.7. Thermomechanical Analysis
2.8. Mechanical Loss Tangent Measurements (MLT)
2.9. Atomic Force Microscopy Analysis (AFM)
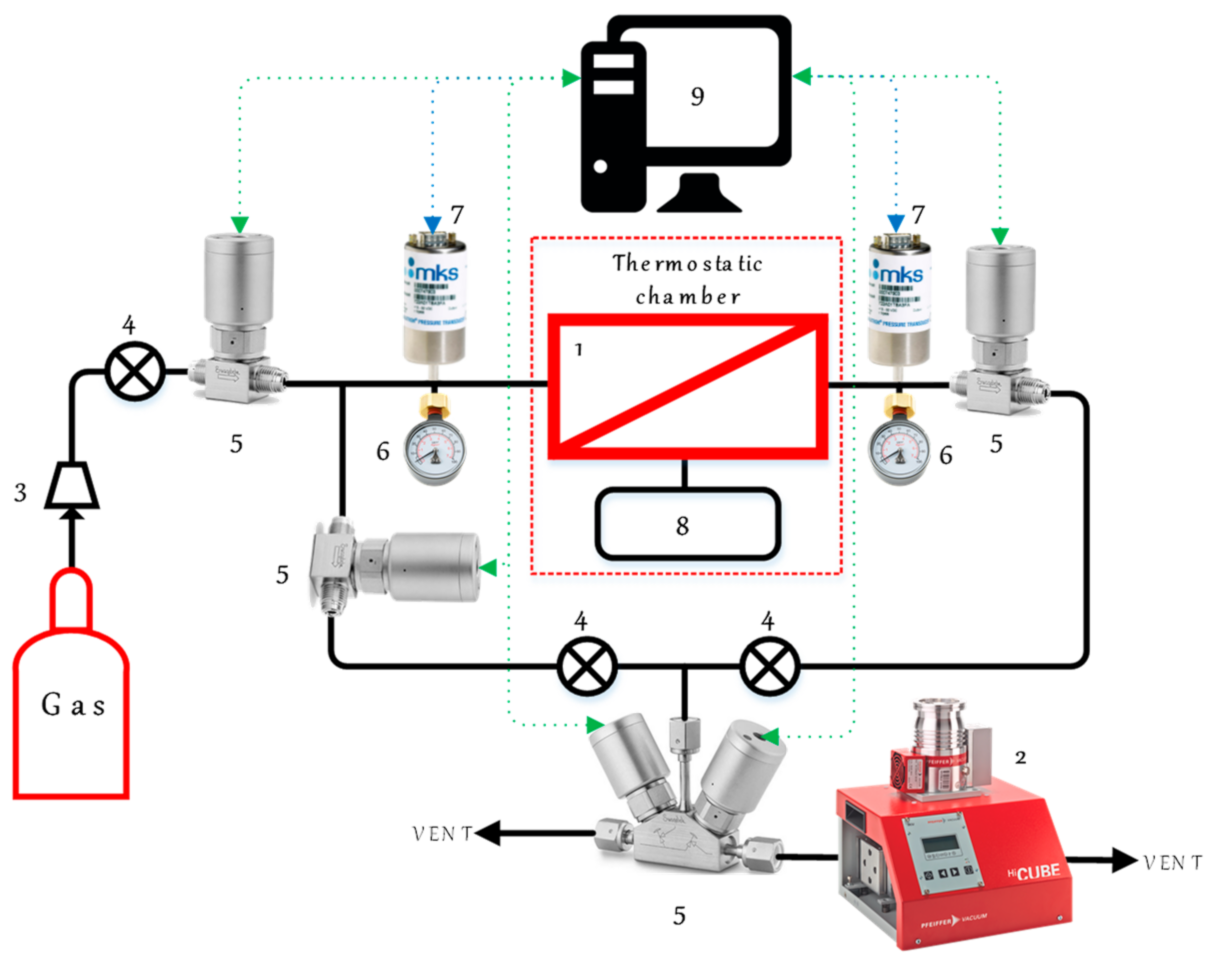
2.10. Permeability Measurements
3. Results and Discussion
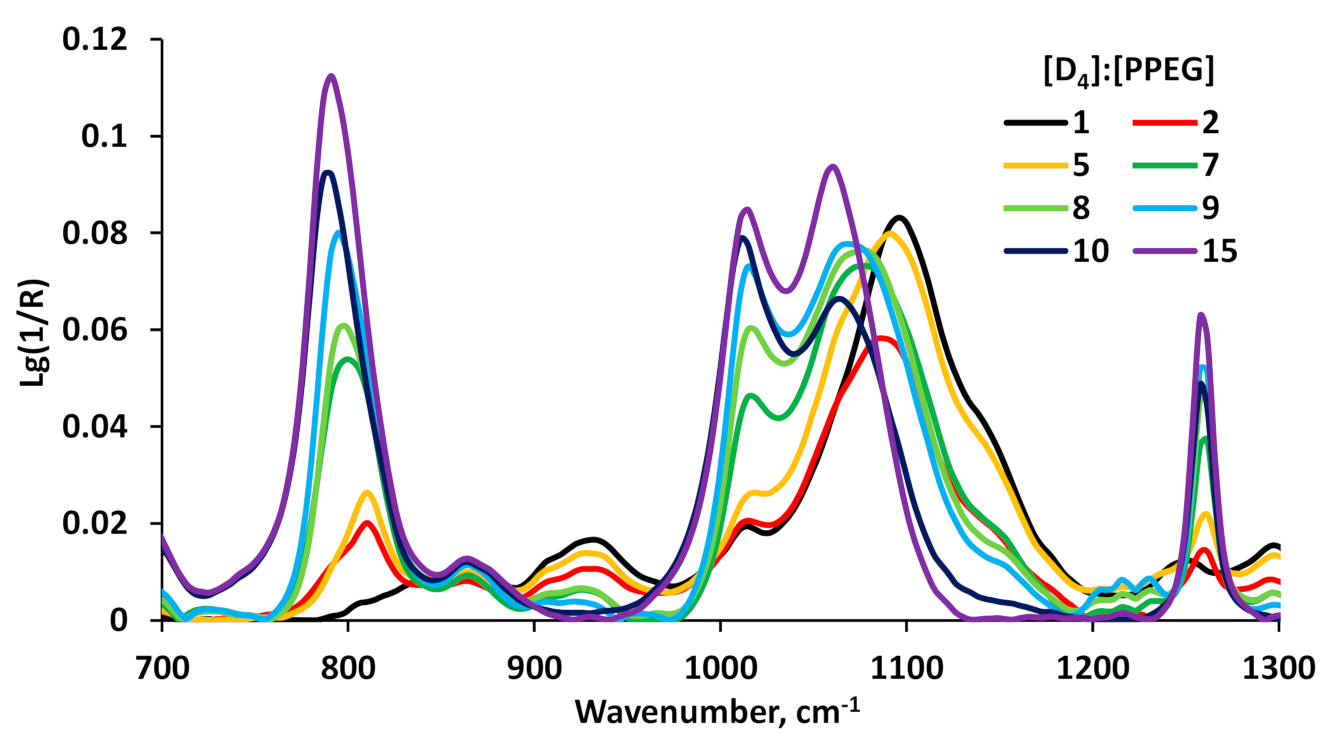
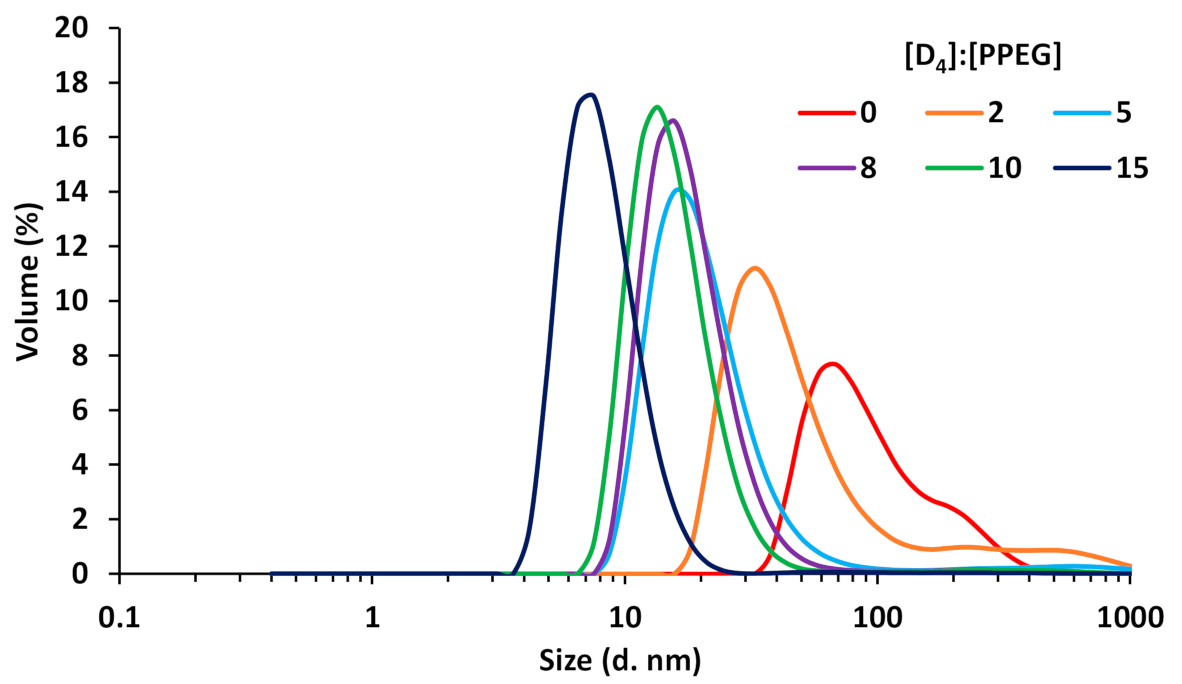
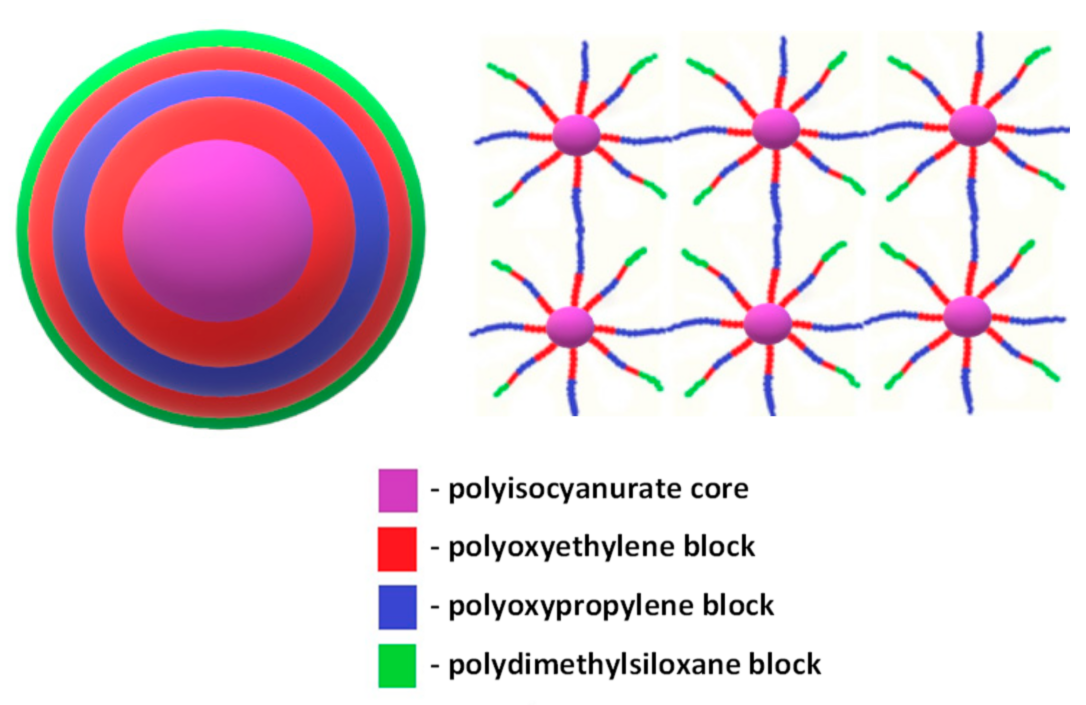
3.1. Synthesis and Physico-Chemical Characterization of MBC Based on PPEG and D4
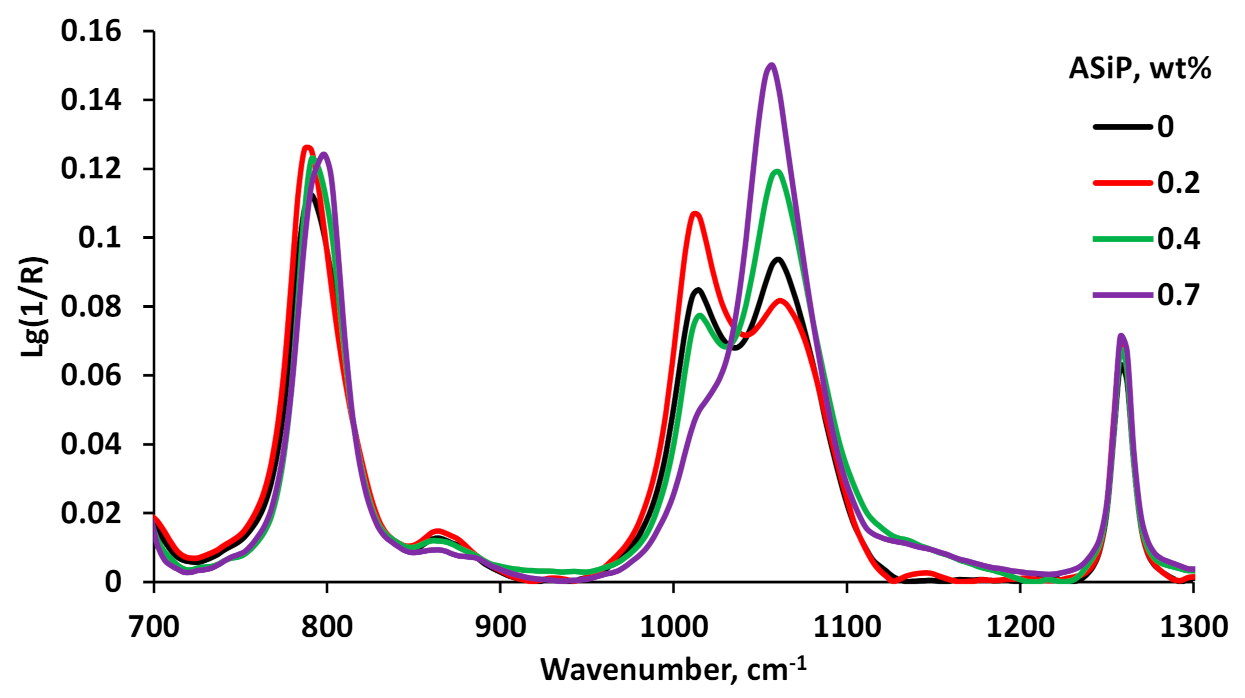
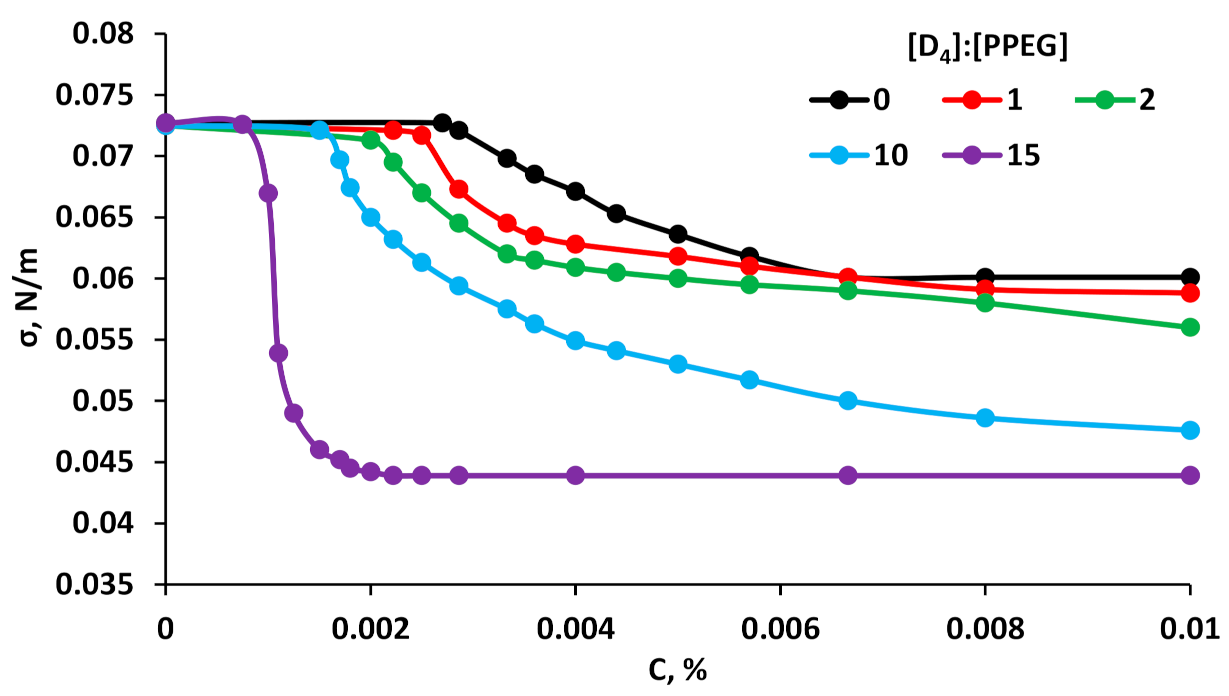
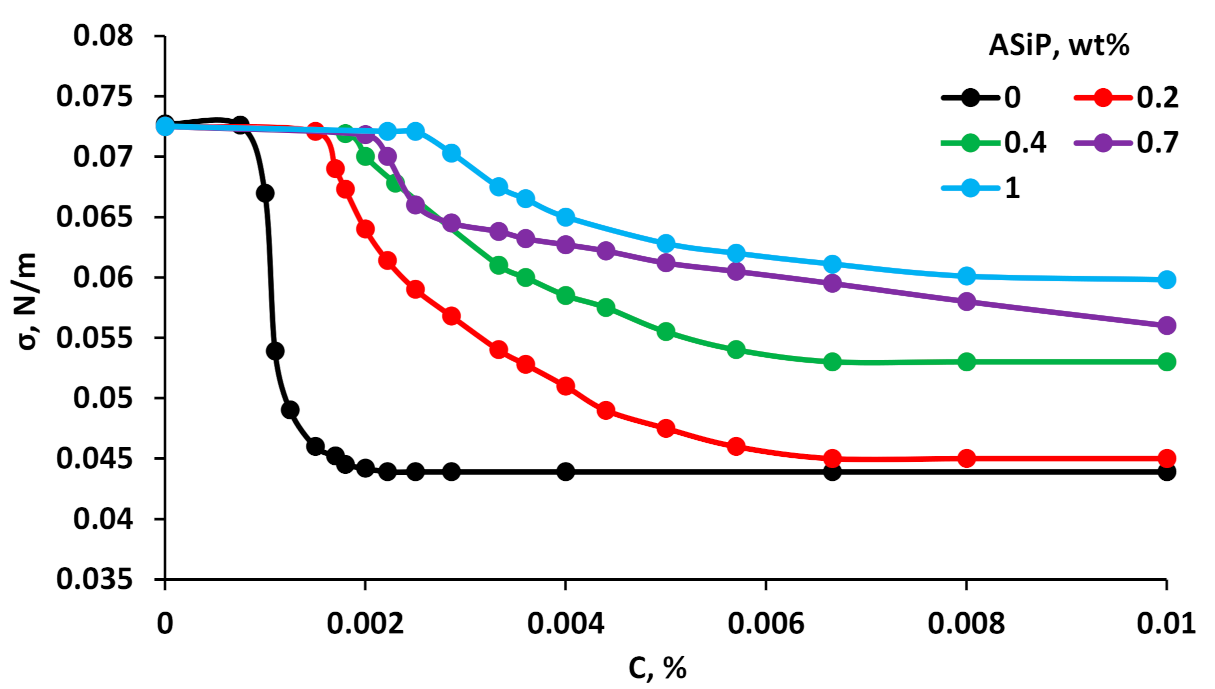
3.2. Surface Active Properties of MBC
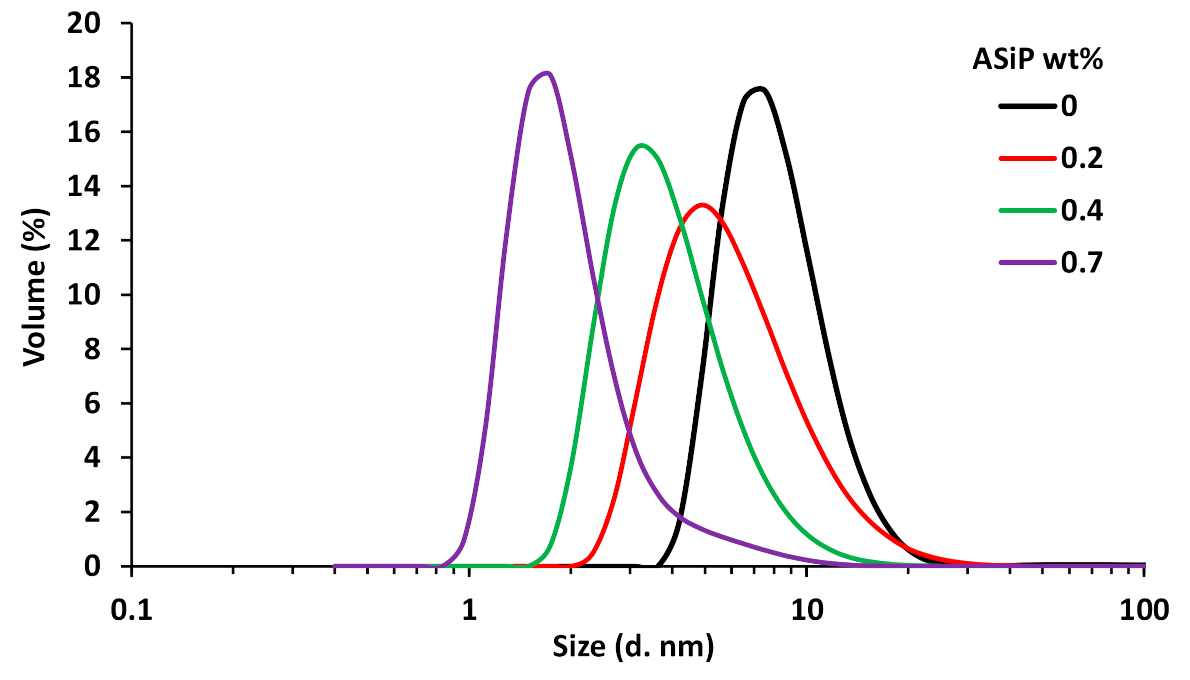
3.3. Polymers Characterization
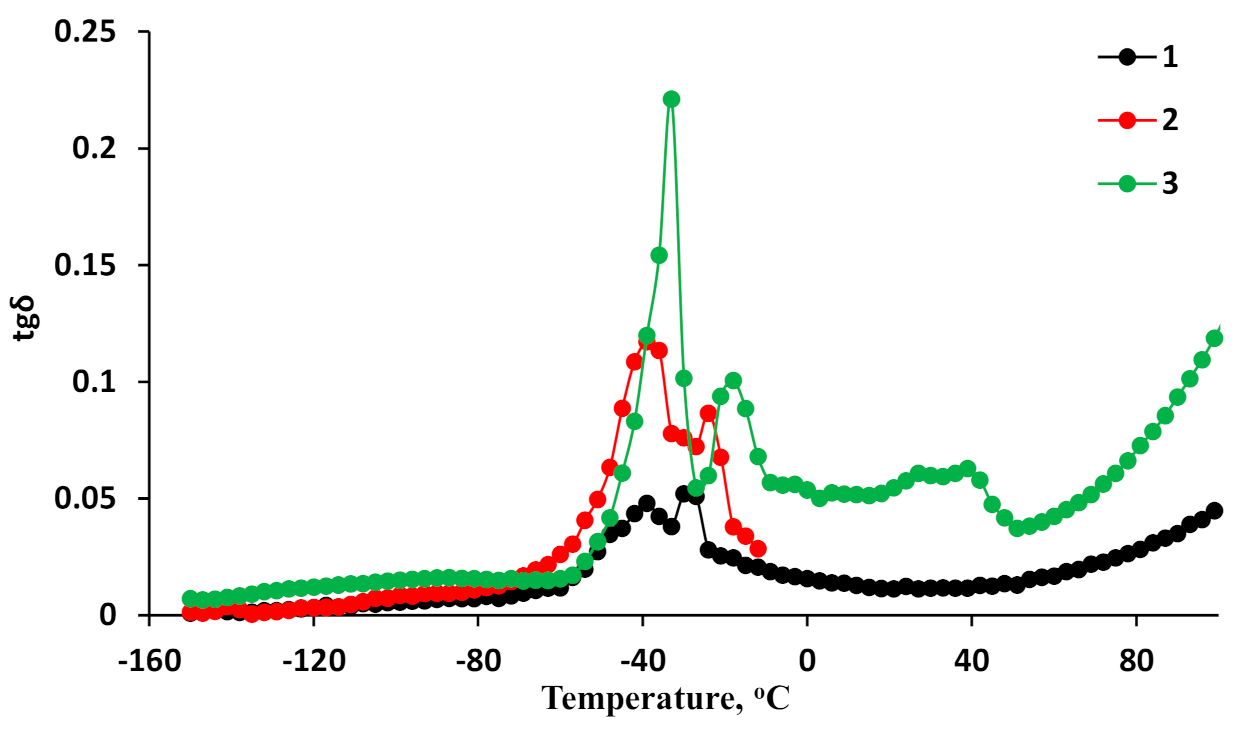
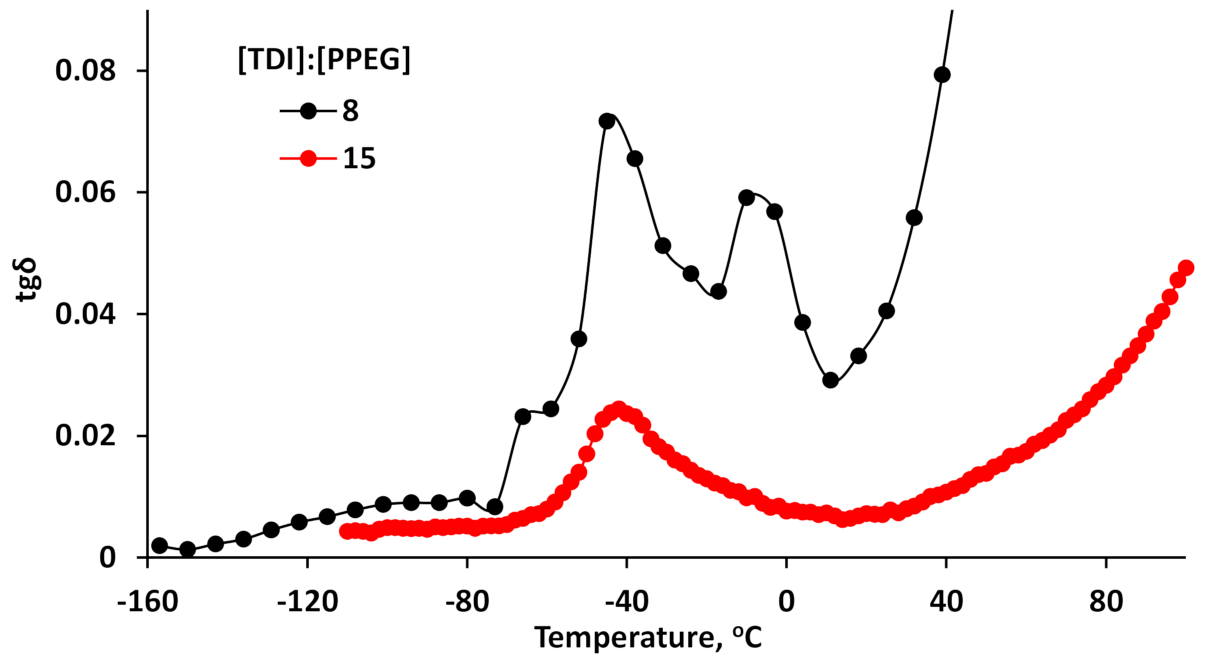
3.4. Dielectric Loss Tangents
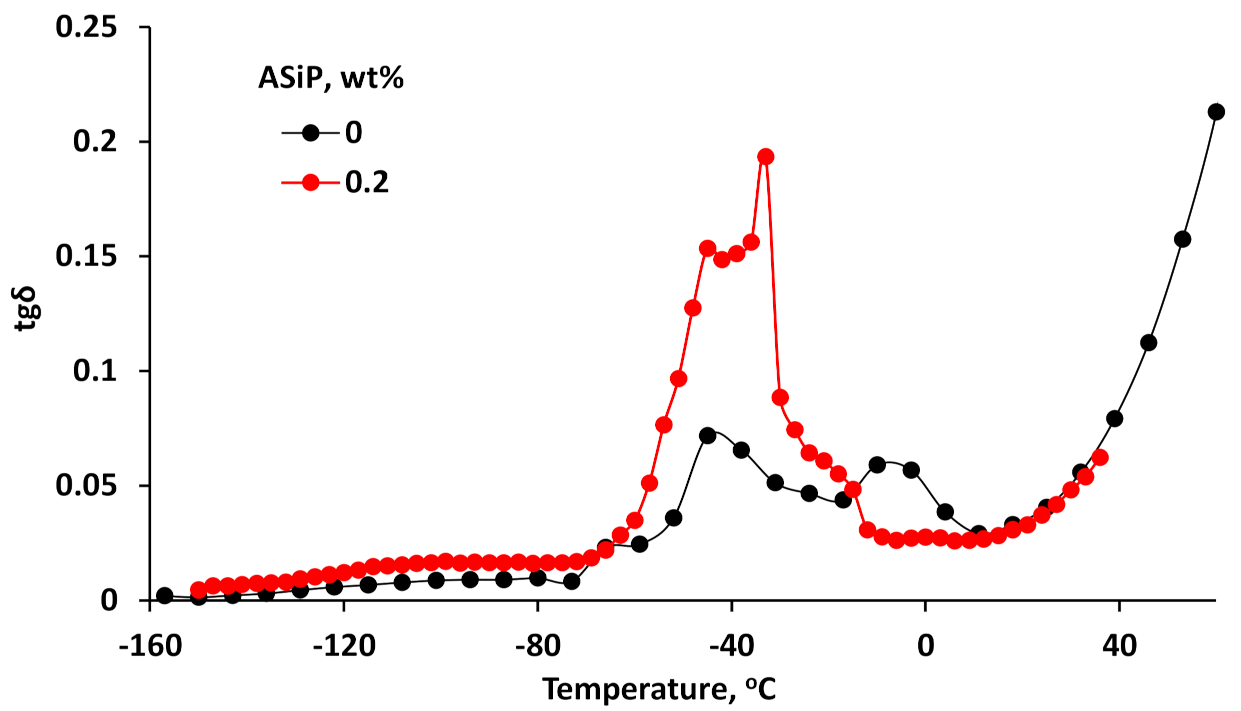
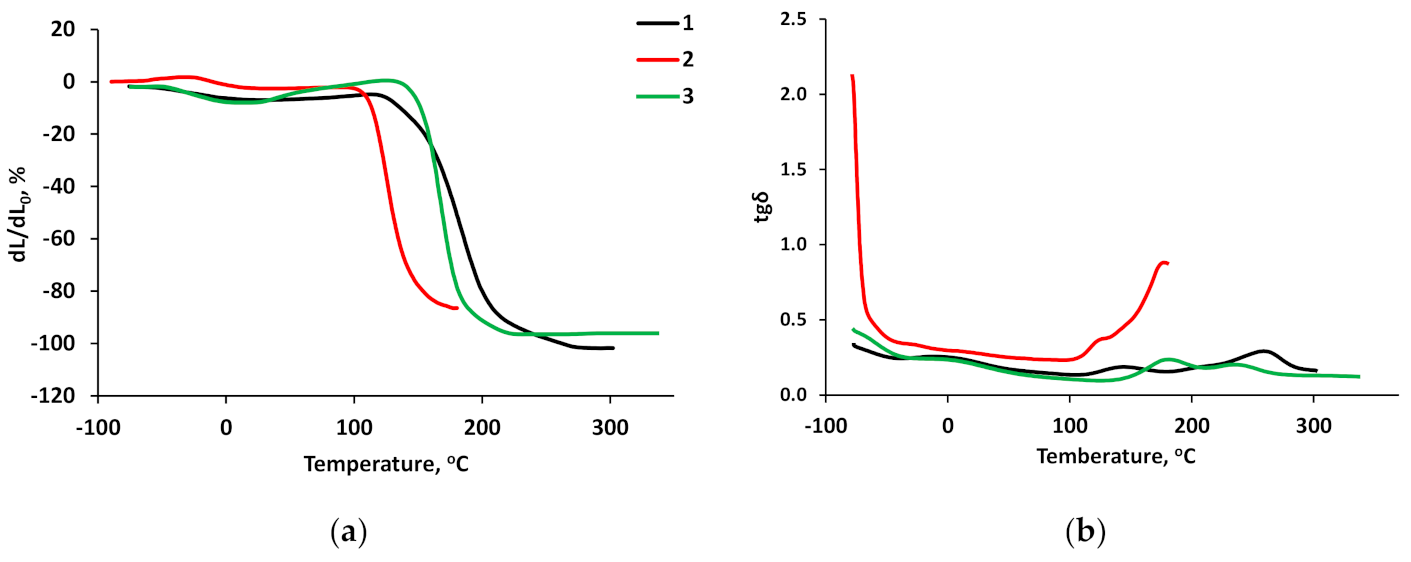
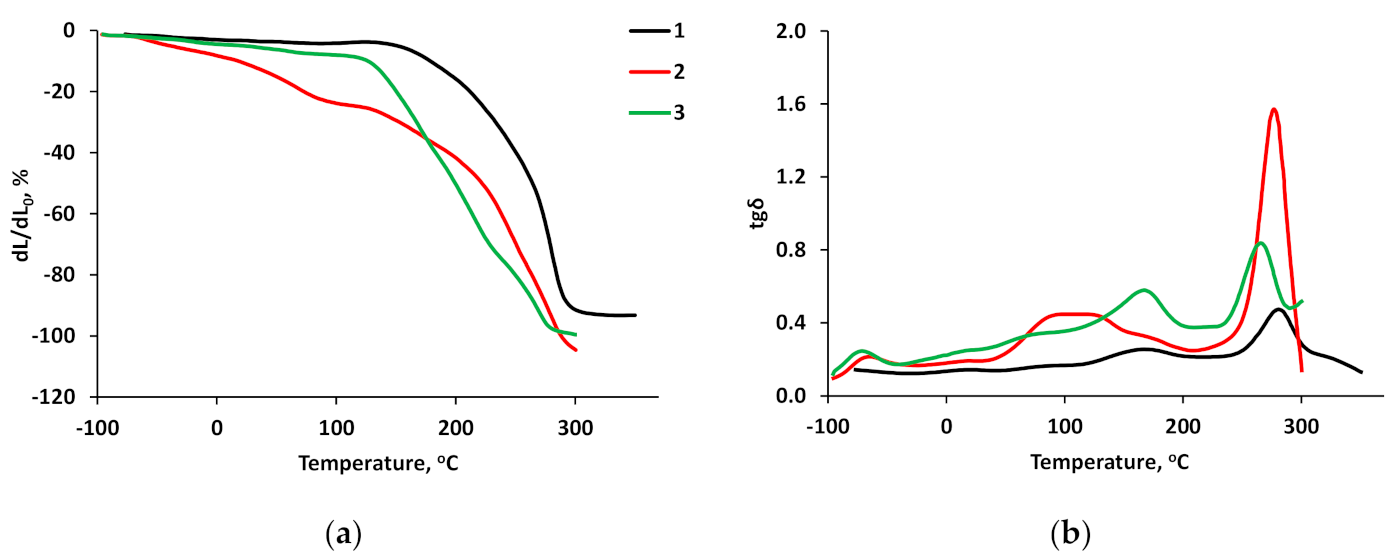
3.5. Thermomechanical Studies
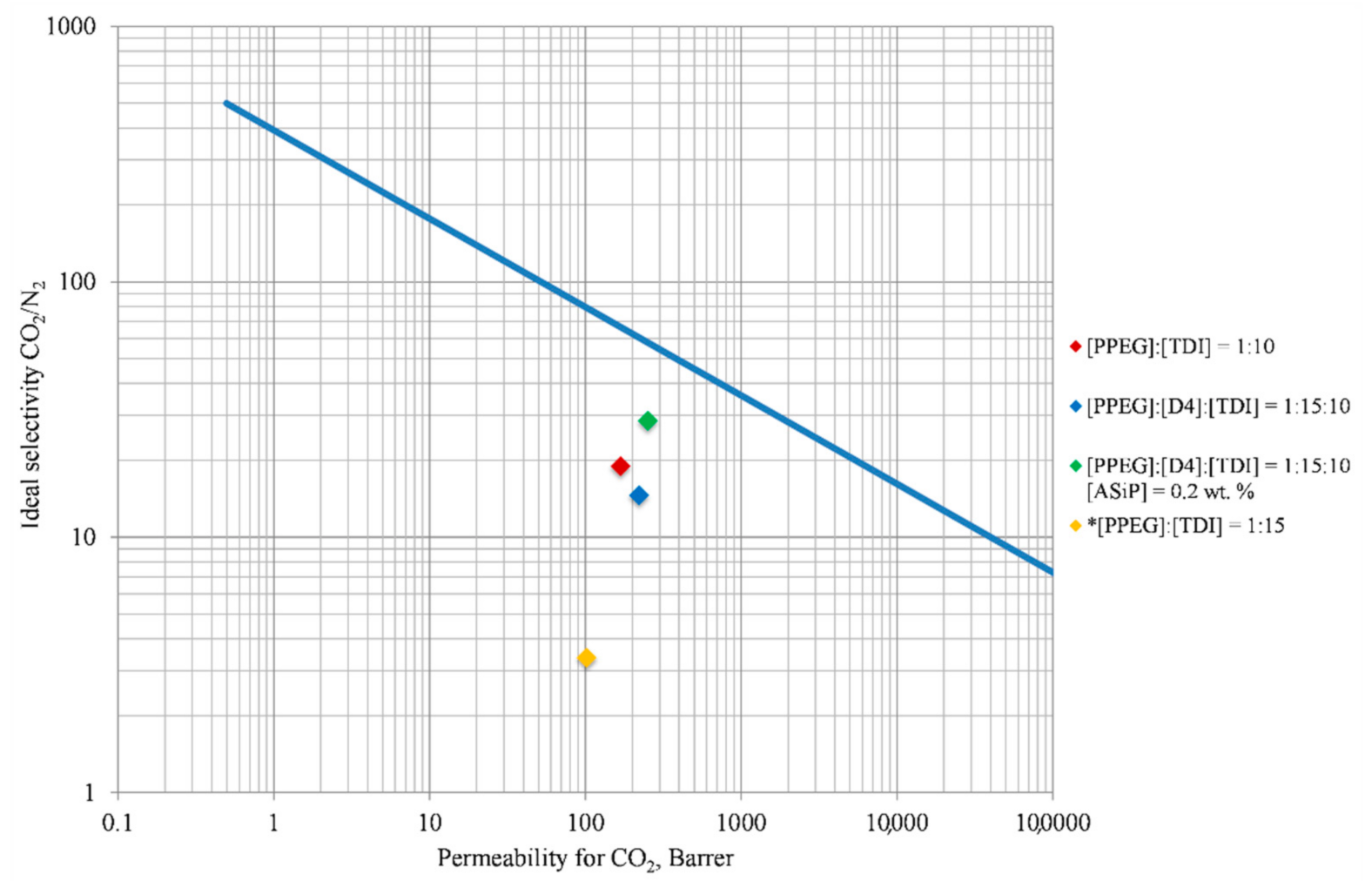
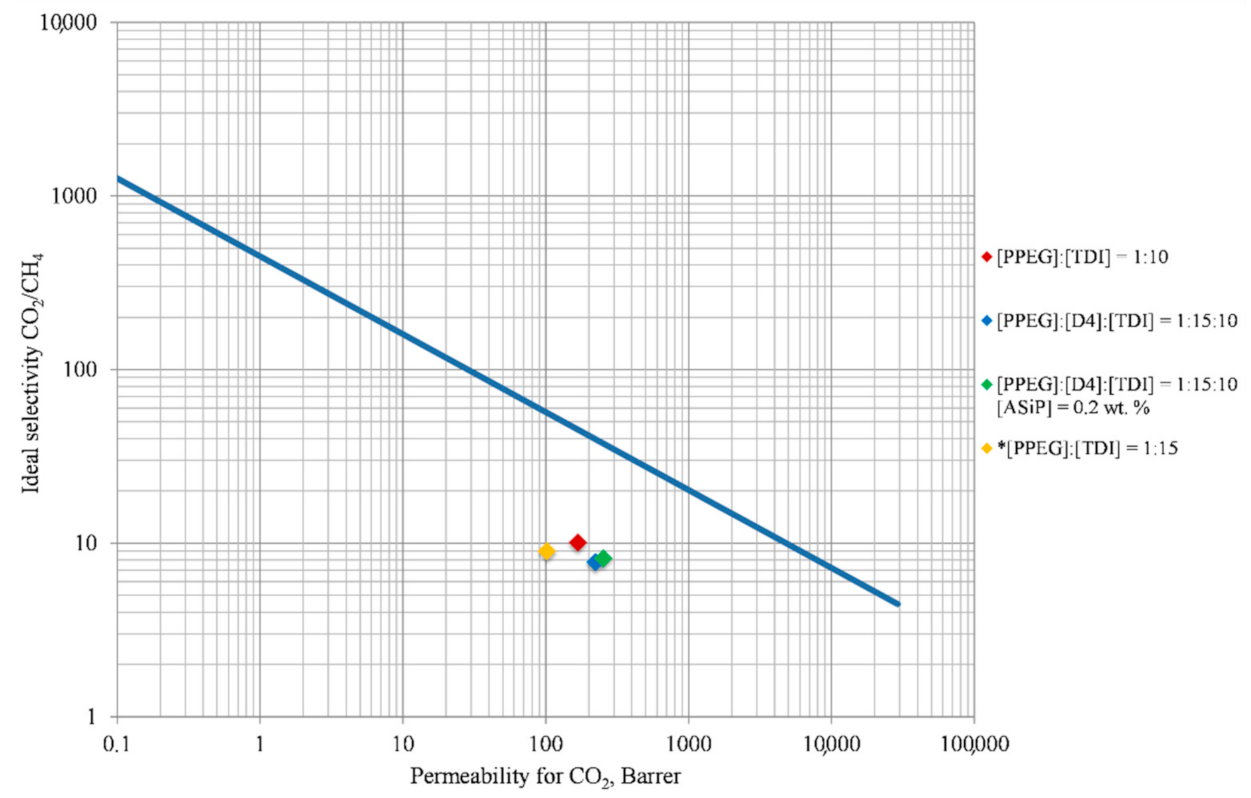
3.6. Gas Transport Properties of Obtained Polymer Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | block copolymer |
| MBC | multiblock copolymer |
| PPEG | potassium-substituted block copolymer of propylene and ethylene oxide |
| POE | polyoxyethylene |
| POP | polyoxypropylene |
| TDI | 2,4-toluene diisocyanate |
| D4 | octamethylcyclotetrasiloxane |
| ASiP | amphiphilic branched silica derivatives associated with oligomeric medium |
| FTIR | Fourier transform infrared spectroscopy analysis |
| CMC | critical micelle concentration |
| TMA | thermomechanical analysis |
| PDMS | polydimethylsiloxane |
References
- Wang, F.; Sylvia, J.M.; Jacob, M.M.; Peramunage, D. Amphiphilic Block Copolymer Membrane for Vanadium Redox Flow Battery. J. Power Sources 2013, 242, 575–580. [Google Scholar] [CrossRef]
- Xu, J.; Luan, S.; Qin, B.; Wang, Y.; Wang, K.; Qi, P.; Song, S. Backbone-Hydrazone-Containing Biodegradable Copolymeric Micelles for Anticancer Drug Delivery. J. Nanoparticle Res. 2016, 18, 316. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Vishnevetskii, D.V.; Garina, E.S.; Plutalova, A.V.; Litmanovich, E.A.; Korolev, B.A.; Shlyakhtin, A.V.; Kostina, Y.V.; Bondarenko, G.N. Controlled Synthesis of Multiblock Copolymers by Pseudoliving Radical Polymerization via the Reversible Addition-Fragmentation Chain-Transfer Mechanism. Polym. Sci. Ser. B 2012, 54, 127–141. [Google Scholar]
- Kabachii, Y.A.; Kochev, S.Y.; Valetskii, P.M. Synthesis of Amphiphilic Block Copolymer from Optically Active N-Benzylprolinol Acrylate. Polym. Sci. Ser. B 2006, 48, 240–243. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, F.; Huang, S.; Yan, C. From Toroidal to Rod-like Nanostructure, a Mechanism Study for the Reversible Morphological Control on Amphiphilic Triblock Copolymer Micelles. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1450–1457. [Google Scholar]
- Barrie, J.A.; Munday, K. Gas Transport in Heterogeneous Polymer Blends. J. Membr. Sci. 1983, 13, 175–195. [Google Scholar] [CrossRef]
- Barrie, J.A.; Williams, M.J.L.; Spenser, H.G. Gas Transport in Heterogeneous Polymer Blends: III. Alternating Block Copolymers of Poly(Bisphenol-a Carbonate) and Polydimethylsiloxane. J. Membr. Sci. 1984, 21, 185–202. [Google Scholar] [CrossRef]
- Robeson, L.M.; Noshay, A.; Matzner, M.; Merriam, C.N. Physical Property Characteristics of Polysulfone/Poly-(Dimethylsiloxane) Block Copolymers. Die Angew. Makromol. Chem. 1973, 29–30, 47–51. [Google Scholar] [CrossRef]
- Huin, C.; Gall, T.L.; Barteau, B.; Pitard, B.; Montier, T.; Lehn, P.; Cheradame, H.; Guégan, P. Evidence of DNA Transfer across a Model Membrane by a Neutral Amphiphilic Block Copolymer. J. Gene Med. 2011, 13, 538–548. [Google Scholar] [CrossRef]
- Serkhacheva, N.; Plutalova, A.; Kozhunova, E.; Prokopov, N.; Chernikova, E. Amphiphilic Triblock Copolymers Based on Acrylic Acid and Alkyl Acrylates Synthesized via RAFT Polymerization-Induced Self-Assembly and RAFT Miniemulsion Polymerization. Polym. Sci. Ser. B 2018, 60, 204–217. [Google Scholar] [CrossRef]
- Romanyuk, A.V.; Melik-Nubarov, N.S. Micelles of Amphiphilic Copolymers as a Medium for Peroxyoxalate Chemiluminescent Reaction in Water Environment. Polym. Sci. Ser. B 2015, 57, 360–369. [Google Scholar] [CrossRef]
- Estrina, G.A.; Gur’eva, L.L.; Komarov, B.A.; Bogdanova, L.M.; Kurochkin, S.A.; Estrin, Y.I. The Study of Formation of Amphiphilic Network Block Copolymer of N-Isopropylacrylamide. Polym. Sci. Ser. B 2018, 60, 1–8. [Google Scholar] [CrossRef]
- Istratov, V.V.; Gomzyak, V.I.; Krupina, T.V.; Vasnev, V.A.; Chvalun, S.N. Amphiphilic Linear-Branched Copolylactides and Disperse Systems on Their Basis. Polym. Sci. Ser. B 2017, 59, 730–736. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Plutalova, A.V.; Mineeva, K.O.; Vishnevetskii, D.V.; Lysenko, E.A.; Serkhacheva, N.S.; Prokopov, N.I. Ternary Copolymers of Acrylic Acid, N-Isopropylacrylamide, and Butyl Acrylate: Synthesis and Aggregative Behavior in Dilute Solutions. Polym. Sci. Ser. B 2016, 58, 564–573. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Akhmetshina, A.I.; Davletbaev, R.S.; Zaripov, I.I.; Gumerov, A.M.; Sharifullin, R.R. Optically Transparent Mesoporous Polymers Based on Anionic Macroinitiators and 2,4-Toluene Diisocyanate. Polym. Sci. Ser. B 2014, 56, 814–821. [Google Scholar] [CrossRef]
- Istratov, V.V.; Milushkova, E.V.; Gritskova, I.A.; Vasnev, V.A. Synthesis, Properties, and Application of Surface-Active Block Copolymers Based on Poly(Ethylene Oxide) and Polyorganosiloxanes in the Heterogeneous Polymerization of Styrene. Polym. Sci. Ser. B 2014, 56, 721–727. [Google Scholar]
- Maassen, H.-P.; Yang, J.L.; Wegner, G. The Structure of Poly(Ethylene Oxide)-Poly(Dimethyl-Siloxane) Triblock Copolymers in Solution. In Makromolekulare Chemie. Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, Switzerland, 1990; Volume 39, pp. 215–228. [Google Scholar]
- Ballet, F.; Candau, F. Investigation of the Structure of Polymeric Microemulsions by Proton Magnetic Resonance. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 155–163. [Google Scholar] [CrossRef]
- Rogovina, L.Z.; Slonimskii, G.L. The Structure and Properties of Block Copolymers and Their Solutions. Russ. Chem. Rev. 1977, 46, 988–1006. [Google Scholar] [CrossRef]
- Rogovina, L.Z.; Chalykh, A.Y.; Adamova, L.V.; Aliyev, A.D.; Nekhayenko, Y.A.; Valetskii, P.M.; Slonimskii, G.L.; Tager, A.A. Structure and Thermodynamic Stability of Polyblock Copolymers of Poly-(Arylate-Dimethyl Siloxane). Polym. Sci. USSR 1980, 22, 476–485. [Google Scholar] [CrossRef]
- Nagasaki, Y.; Matsukura, F.; Kato, M.; Aoki, H.; Tokuda, T. New Thermosensitive Rubbery Polymers. Synthesis of Poly(Siloxyethylene Glycol) and Its Aqueous Solution Properties. Macromolecules 1996, 29, 5859–5863. [Google Scholar] [CrossRef]
- Neugebauer, D.; Zhang, Y.; Pakula, T.; Matyjaszewski, K. PDMS−PEO Densely Grafted Copolymers. Macromolecules 2005, 38, 8687–8693. [Google Scholar] [CrossRef]
- Abetz, V.; Simon, P.F.W. Phase Behaviour and Morphologies of Block Copolymers. Adv. Polym. Sci. 2005, 189, 125–212. [Google Scholar]
- Yang, J.; Wegner, G.; Koningsveld, R. Phase Behavior of Ethylene Oxide-Dimethylsiloxane PEO-PDMS-PEO Triblock Copolymers with Water. Colloid Polym. Sci. 1992, 270, 1080–1084. [Google Scholar] [CrossRef]
- Castillo, R.V.; Müller, A.J. Crystallization and Morphology of Biodegradable or Biostable Single and Double Crystalline Block Copolymers. Prog. Polym. Sci. 2009, 34, 516–560. [Google Scholar] [CrossRef]
- Lapienis, G. Star-Shaped Polymers Having PEO Arms. Prog. Polym. Sci. 2009, 34, 852–892. [Google Scholar] [CrossRef]
- Zuckerman, J.J. Synthesis of Organosilicon Monomers. By A.D. Petrov, V.F. Mironov, V.A. Ponomarenko, and E.A. Chernyshev. Inorg. Chem. 1964, 3, 1473. [Google Scholar] [CrossRef]
- Raigorodskii, I.M.; Gol’dberg, E.S. Polyorgano-Polysiloxane Block Copolymers. Russ. Chem. Rev. 1987, 56, 1079–1095. [Google Scholar] [CrossRef]
- Makarova, L.I.; Filimonova, L.V.; Dubrovina, L.V.; Buzin, M.I.; Nikiforova, G.G.; Zavin, B.G.; Papkov, V.S. Synthesis and Properties of Siloxane(Ethylene Oxide)Urethane Block Copolymers. Polym. Sci. Ser. B 2010, 52, 346–352. [Google Scholar] [CrossRef]
- Chatterjee, G.; Houde, A.A.; Stern, S.A. Poly(Ether Urethane) and Poly(Ether Urethane Urea) Membranes with High H2S/CH4 Selectivity. J. Membr. Sci. 1997, 135, 99–106. [Google Scholar] [CrossRef]
- Li, H.; Freeman, B.D.; Ekiner, O.M. Gas Permeation Properties of Poly(Urethane-Urea)s Containing Different Polyethers. J. Membr. Sci. 2011, 369, 49–58. [Google Scholar]
- Talakesh, M.M.; Sadeghi, M.; Chenar, M.P.; Khosravi, A. Gas Separation Properties of Poly(Ethylene Glycol)/Poly(Tetramethylene Glycol) Based Polyurethane Membranes. J. Membr. Sci. 2012, 415–416, 469–477. [Google Scholar] [CrossRef]
- Nebipasagil, A.; Park, J.; Lane, O.R.; Sundell, B.J.; Mecham, S.J.; Freeman, B.D.; Riffle, J.S.; McGrath, J.E. Polyurethanes Containing Poly(Arylene Ether Sulfone) and Poly(Ethylene Oxide) Segments for Gas Separation Membranes. Polymer 2017, 118, 256–267. [Google Scholar]
- Yoshino, M.; Ito, K.; Kita, H.; Okamoto, K.-I. Effects of Hard-Segment Polymers on CO2/N2 Gas-Separation Properties of Poly(Ethylene Oxide)-Segmented Copolymers. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1707–1715. [Google Scholar] [CrossRef]
- Park, S.; Lee, A.S.; Do, Y.S.; Kim, J.F.; Hwang, S.S.; Lee, Y.M.; Lee, J.-H.; Lee, J.S. Side-Chain Engineering of Ladder-Structured Polysilsesquioxane Membranes for Gas Separations. J. Membr. Sci. 2016, 516, 202–214. [Google Scholar] [CrossRef]
- Kang, W.R.; Lee, A.S.; Park, S.; Park, S.-H.; Baek, K.-Y.; Lee, K.B.; Lee, S.-H.; Lee, J.-H.; Hwang, S.S.; Lee, J.S. Free-Standing, Polysilsesquioxane-Based Inorganic/Organic Hybrid Membranes for Gas Separations. J. Membr. Sci. 2015, 475, 384–394. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Mazil’nikov, A.I.; Zaripov, I.I.; Davletbaev, R.S.; Gumerov, A.M.; Parfenov, V.V. Synthesis of Block Copolymers Based on a Macroinitiator and 2,4-Toluene Diisocyanate. Polym. Sci. Ser. B 2018, 60, 51–57. [Google Scholar] [CrossRef]
- Davletbaev, R.; Akhmetshina, A.; Gumerov, A.; Davletbaeva, I. Supramolecular Architecture of Polymers as the Basis of Obtaining Mesoporous Polymers. Compos. Interfaces 2014, 21, 611–621. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Nurgaliyeva, G.R.; Akhmetshina, A.I.; Davletbaev, R.S.; Atlaskin, A.A.; Sazanova, T.S.; Efimov, S.V.; Klochkov, V.V.; Vorotyntsev, I.V. Porous Polyurethanes Based on Hyperbranched Amino Ethers of Boric Acid. RSC Adv. 2016, 6, 111109–111119. [Google Scholar]
- Davletbaeva, I.M.; Gumerov, A.M.; Galyautdinova, A.F.; Shkodich, V.F. Synthesis of Polysiloxaneurethane Polymers by the Aromatic Isocyanate-Activated Opening of the Octamethyltetrasiloxane Ring via an Anionic Mechanism. Theor. Found. Chem. Eng. 2010, 44, 150–161. [Google Scholar]
- Davletbaeva, I.M.; Akhmetshina, A.I.; Gumerov, A.M.; Kopylova, T.N.; Samsonova, L.G.; Zulina, N.A. Spectral Luminescent and Laser Properties of Rhodamine 6G in Poly(Urethane-Co-Siloxanes). Polym. Sci. Ser. A 2011, 53, 578–582. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Gumerov, A.M.; Galyautdinova, A.F.; Davletbaev, R.S.; Krasil’nikova, O.K. Polymers Derived from a Polyether, 2,4-Toluene Diisocyanate, and Octamethylcyclotetrasiloxane. Russ. J. Appl. Chem. 2011, 84, 1587–1590. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Davletbaeva, I.; Grebenschikova, E.; Sazanova, T.; Petukhov, A.; Atlaskin, A.; Razov, E.; Zaripov, I.; Martins, C.; Neves, L.; et al. The Effect of Microporous Polymeric Support Modification on Surface and Gas Transport Properties of Supported Ionic Liquid Membranes. Membranes 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Zaripov, I.I.; Davletbaeva, I.M.; Faizulina, Z.Z.; Davletbaev, R.S.; Gubaidullin, A.T.; Atlaskin, A.A.; Vorotyntsev, I.V. Synthesis and Characterization of Novel Nanoporous Gl-POSS-Branched Polymeric Gas Separation Membranes. Membranes 2020, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Davletbaeva, I.M.; Zaripov, I.I.; Mazilnikov, A.I.; Davletbaev, R.S.; Sharifullin, R.R.; Atlaskin, A.A.; Sazanova, T.S.; Vorotyntsev, I.V. Synthesis and Study of Gas Transport Properties of Polymers Based on Macroinitiators and 2,4-Toluene Diisocyanate. Membranes 2019, 9, 42. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Emelina, O.Y.; Vorotyntsev, I.V.; Davletbaev, R.S.; Grebennikova, E.S.; Petukhov, A.N.; Akhmetshina, A.I.; Sazanova, T.S.; Loskutov, V.V. Synthesis and Properties of Novel Polyurethanes Based on Amino Ethers of Boric Acid for Gas Separation Membranes. RSC Adv. 2015, 5, 65674–65683. [Google Scholar] [CrossRef]
- Davletbaev, R.S.; Zaripov, I.I.; Faizulina, Z.Z.; Davletbaeva, I.M.; Domrachova, D.S.; Gumerov, A.M. Synthesis and Characterization of Amphiphilic Branched Silica Derivatives Associated with Oligomeric Medium. RSC Adv. 2019, 9, 21233–21242. [Google Scholar]
- Daynes, H.A. The Process of Diffusion through a Rubber Membrane. Proc. R. Soc. Lond. 1920, 97, 286–307. [Google Scholar]
- Barrer, R.M.; Rideal, E.K. Permeation, Diffusion and Solution of Gases in Organic Polymers. Trans. Faraday Soc. 1939, 35, 628–643. [Google Scholar]
- Vorotyntsev, V.M.; Drozdov, P.N.; Vorotyntsev, I.V.; Smirnov, K.Y. Germane High Purification by Membrane Gas Separation. Desalination 2006, 200, 232–233. [Google Scholar] [CrossRef]
- Trubyanov, M.M.; Drozdov, P.N.; Atlaskin, A.A.; Battalov, S.V.; Puzanov, E.S.; Vorotyntsev, A.V.; Petukhov, A.N.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Unsteady-State Membrane Gas Separation by Novel Pulsed Retentate Mode for Improved Membrane Module Performance: Modelling and Experimental Verification. J. Membr. Sci. 2017, 530, 53–64. [Google Scholar] [CrossRef]
- Atlaskin, A.A.; Trubyanov, M.M.; Yanbikov, N.R.; Vorotyntsev, A.V.; Drozdov, P.N.; Vorotyntsev, V.M.; Vorotyntsev, I.V. Comprehensive Experimental Study of Membrane Cascades Type of “Continuous Membrane Column” for Gases High-Purification. J. Membr. Sci. 2019, 572, 92–101. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisted. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]


| Sample | Ra, nm | Rz, nm |
|---|---|---|
| [PPEG]:[TDI] = 1:10 | 12.36 | 46.84 |
| [PPEG]:[D4]:[TDI] = 1:15:10 | 90.18 | 402.27 |
| [PPEG]:[D4]:[TDI] = 1:15:10 [ASiP] = 0.2 wt.% | 4.32 | 19.75 |
| [PPEG]:[D4]:[TDI] = 1:15:10 [ASiP] = 0.4 wt.% | 6.51 | 32.81 |
| Sample | P, Barrer | ||
|---|---|---|---|
| N2 | CH4 | CO2 | |
| [PPEG]:[TDI] = 1:10 | 8.8 | 16.6 | 167.4 |
| [PPEG]:[D4]:[TDI] = 1:15:10 | 9.0 | 28.5 | 221.5 |
| [PPEG]:[D4]:[TDI] = 1:15:10 [ASiP] = 0.2 wt.% | 8.8 | 30.7 | 251.4 |
| [PPEG]:[TDI] = 1:15 Obtained in the presence of cocatalysts [45] | 30.1 | 11.3 | 101.8 |
| Sample | Gas System | |
|---|---|---|
| CO2/N2 | CO2/CH4 | |
| [PPEG]:[TDI] = 1:10 | 19.0 | 10.1 |
| [PPEG]:[D4]:[TDI] = 1:15:10 | 14.6 | 7.8 |
| [PPEG]:[D4]:[TDI] = 1:15:10 [ASiP] = 0.2 wt.% | 28.6 | 8.2 |
| [PPEG]:[TDI] = 1:15 Obtained in the presence of cocatalysts [45] | 3.38 | 9.01 |
| Sample | D × 1010, m2 s−1 | ||
|---|---|---|---|
| N2 | CH4 | CO2 | |
| [PPEG]:[TDI] = 1:10 | 2.63 | 1.01 | 4.00 |
| [PPEG]:[D4]:[TDI] = 1:15:10 | 2.70 | 1.20 | 4.10 |
| [PPEG]:[D4]:[TDI] = 1:15:10 [ASiP] = 0.2 wt.% | 2.64 | 1.28 | 4.40 |
| [PPEG]:[TDI] = 1:15 Obtained in the presence of cocatalysts [45] | 3.20 | 0.90 | 3.70 |
| Sample | S × 105, mol m−3 Pa−1 | ||
|---|---|---|---|
| N2 | CH4 | CO2 | |
| [PPEG]:[TDI] = 1:10 | 1.12 | 5.50 | 14.01 |
| [PPEG]:[D4]:[TDI] = 1:15:10 | 1.12 | 7.95 | 18.09 |
| [PPEG]:[D4]:[TDI] = 1:15:10 [ASiP] = 0.2 wt.% | 1.12 | 8.03 | 19.13 |
| [PPEG]:[TDI] = 1:15 Obtained in the presence of cocatalysts [45] | 3.15 | 4.20 | 9.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davletbaeva, I.M.; Dzhabbarov, I.M.; Gumerov, A.M.; Zaripov, I.I.; Davletbaev, R.S.; Atlaskin, A.A.; Sazanova, T.S.; Vorotyntsev, I.V. Amphiphilic Poly(dimethylsiloxane-ethylene-propylene oxide)-polyisocyanurate Cross-Linked Block Copolymers in a Membrane Gas Separation. Membranes 2021, 11, 94. https://doi.org/10.3390/membranes11020094
Davletbaeva IM, Dzhabbarov IM, Gumerov AM, Zaripov II, Davletbaev RS, Atlaskin AA, Sazanova TS, Vorotyntsev IV. Amphiphilic Poly(dimethylsiloxane-ethylene-propylene oxide)-polyisocyanurate Cross-Linked Block Copolymers in a Membrane Gas Separation. Membranes. 2021; 11(2):94. https://doi.org/10.3390/membranes11020094
Chicago/Turabian StyleDavletbaeva, Ilsiya M., Ilgiz M. Dzhabbarov, Askhat M. Gumerov, Ilnaz I. Zaripov, Ruslan S. Davletbaev, Artem A. Atlaskin, Tatyana S. Sazanova, and Ilya V. Vorotyntsev. 2021. "Amphiphilic Poly(dimethylsiloxane-ethylene-propylene oxide)-polyisocyanurate Cross-Linked Block Copolymers in a Membrane Gas Separation" Membranes 11, no. 2: 94. https://doi.org/10.3390/membranes11020094
APA StyleDavletbaeva, I. M., Dzhabbarov, I. M., Gumerov, A. M., Zaripov, I. I., Davletbaev, R. S., Atlaskin, A. A., Sazanova, T. S., & Vorotyntsev, I. V. (2021). Amphiphilic Poly(dimethylsiloxane-ethylene-propylene oxide)-polyisocyanurate Cross-Linked Block Copolymers in a Membrane Gas Separation. Membranes, 11(2), 94. https://doi.org/10.3390/membranes11020094









