Partial Desalination of Saline Groundwater: Comparison of Nanofiltration, Reverse Osmosis and Membrane Capacitive Deionisation
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigated Desalination Scenarios
2.2. Specific Energy Consumption and Water Recovery
2.3. Pressure-Driven Membrane Desalination
2.3.1. Specific Energy Consumption
2.3.2. Experimental Procedure
- DOW FILMTEC™ Flat Sheet NF90;
- Lewabrane® RO B085 HF;
- DOW FILMTEC™ SW30XLE.
2.4. Membrane Capacitive Deionisation
2.4.1. Specific Energy Consumption
2.4.2. Experimental Procedure
3. Results
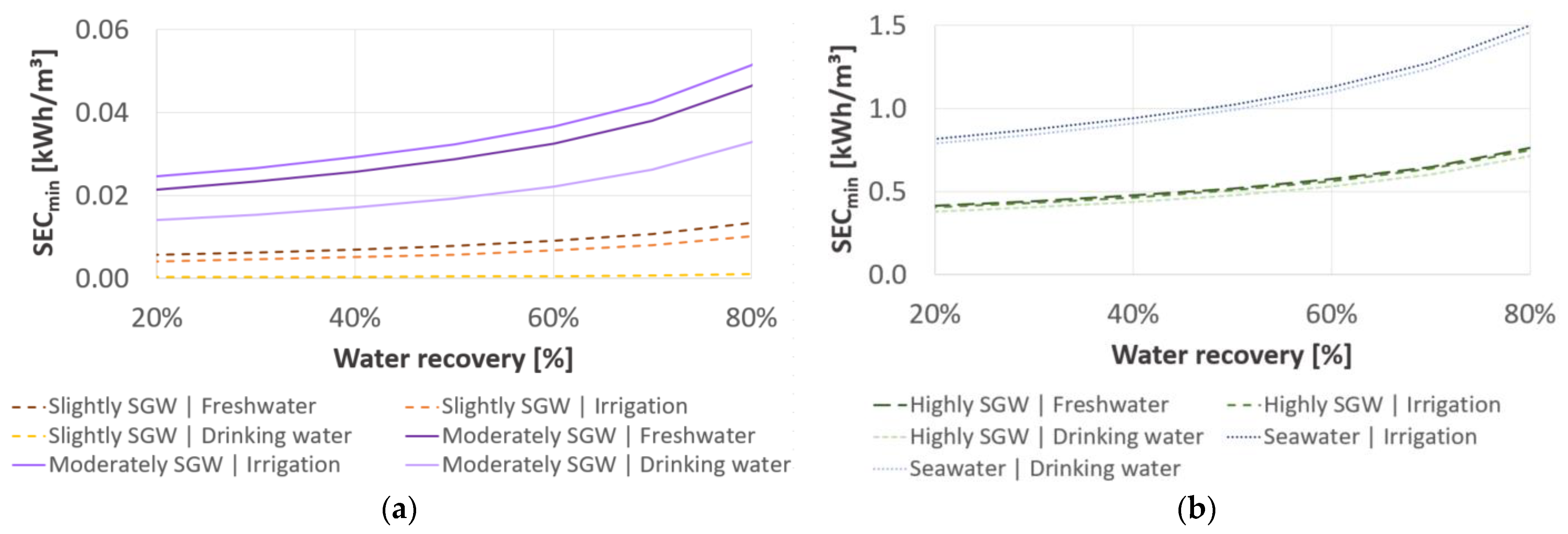
3.1. Minimum Specific Energy Consumption
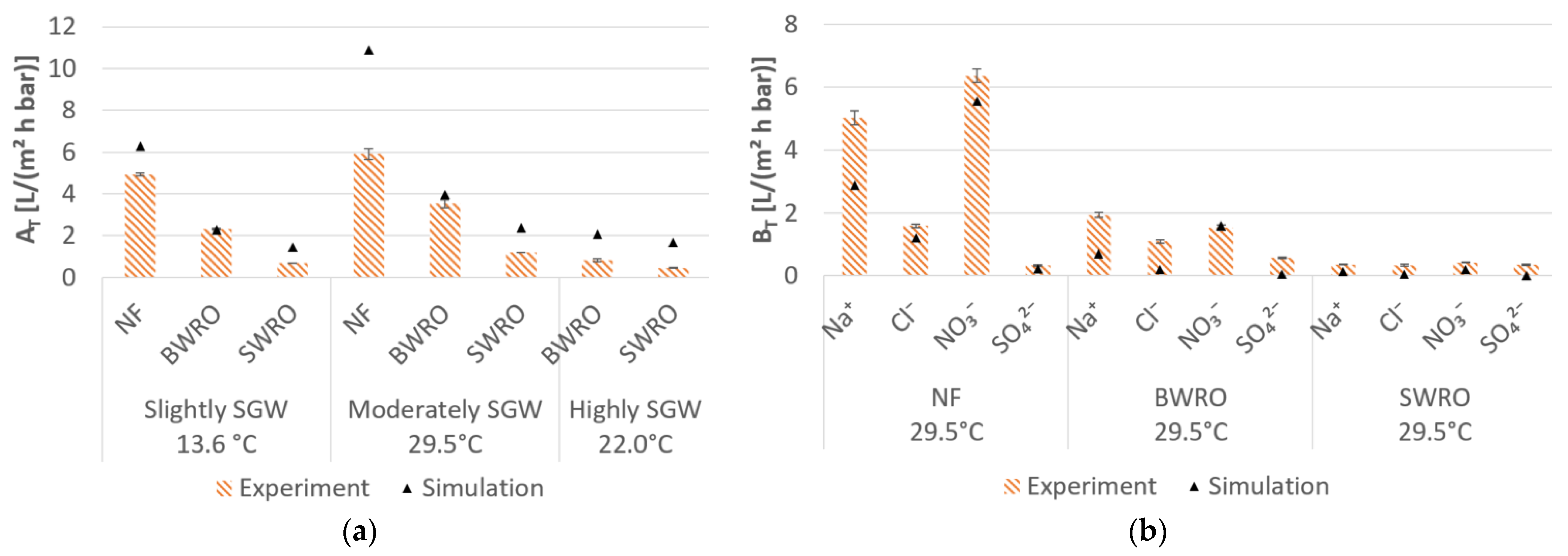
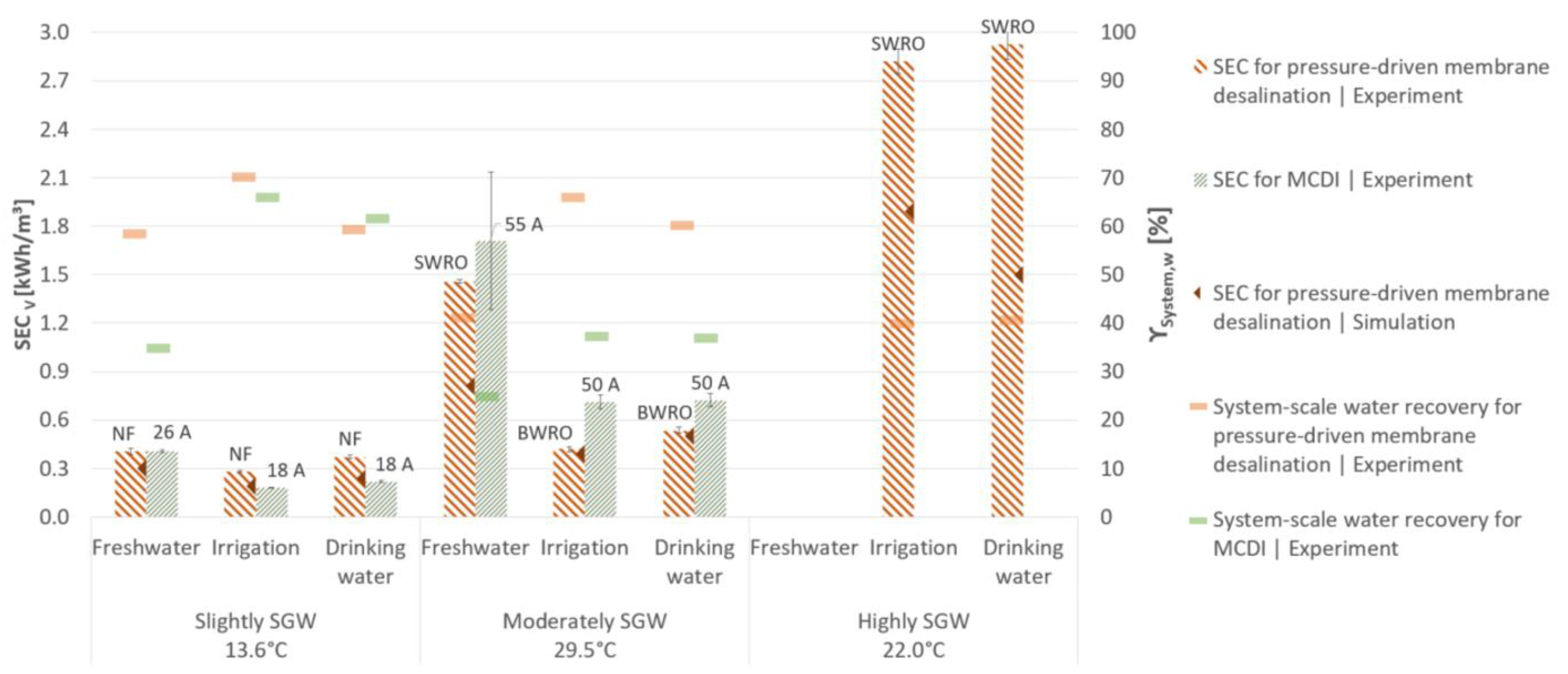
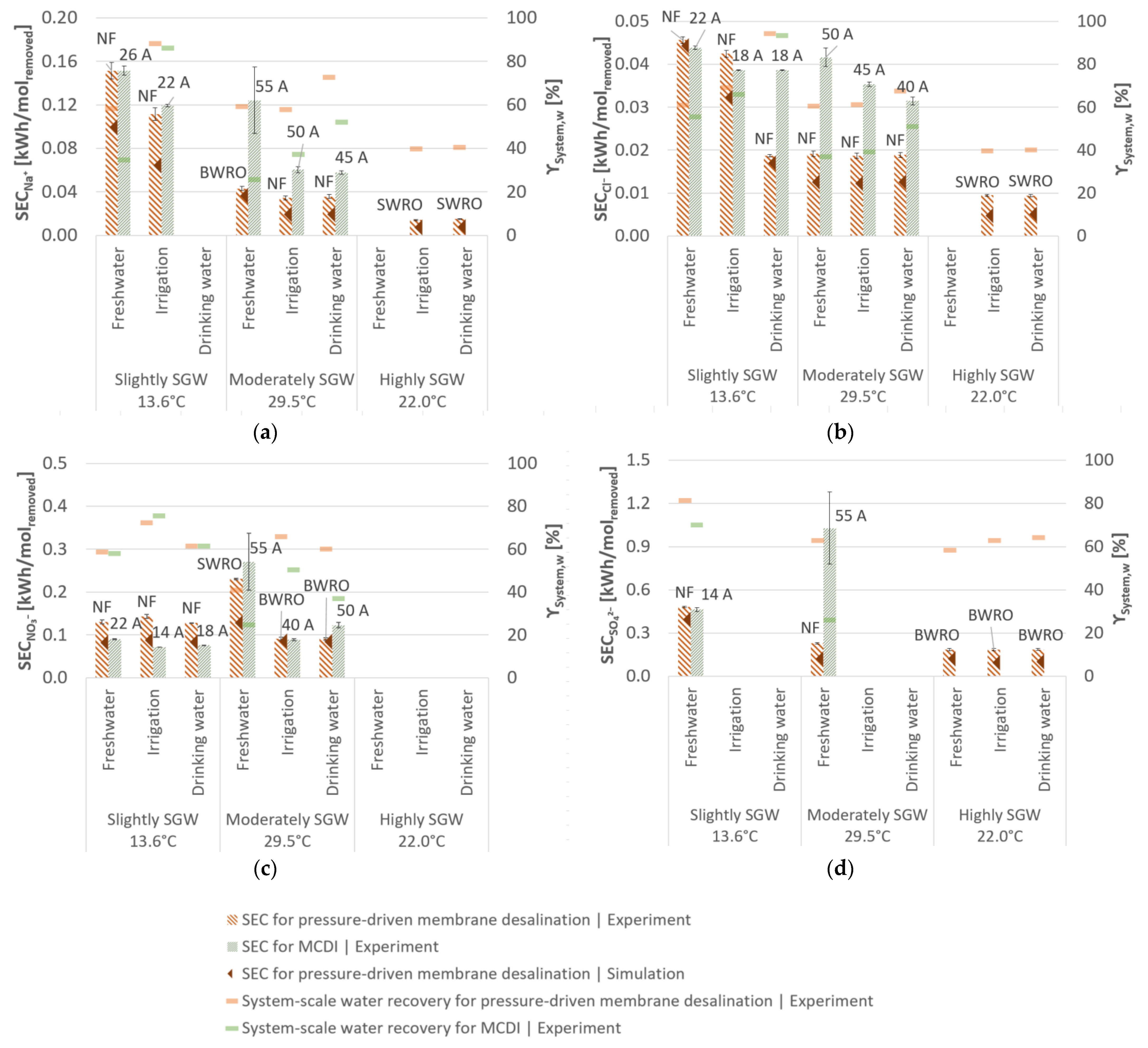
3.2. Desalination Performance of Pressure-Driven Membrane Desalination
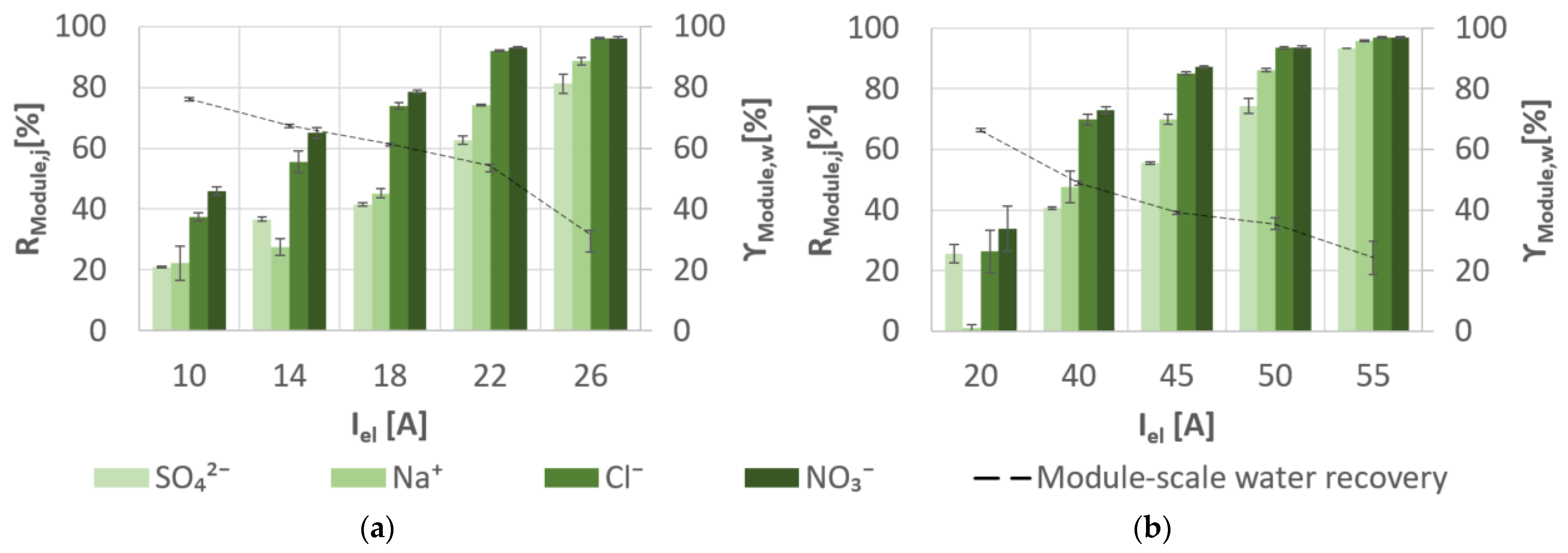
3.3. Desalination Performance of Membrane Capacitive Deionisation
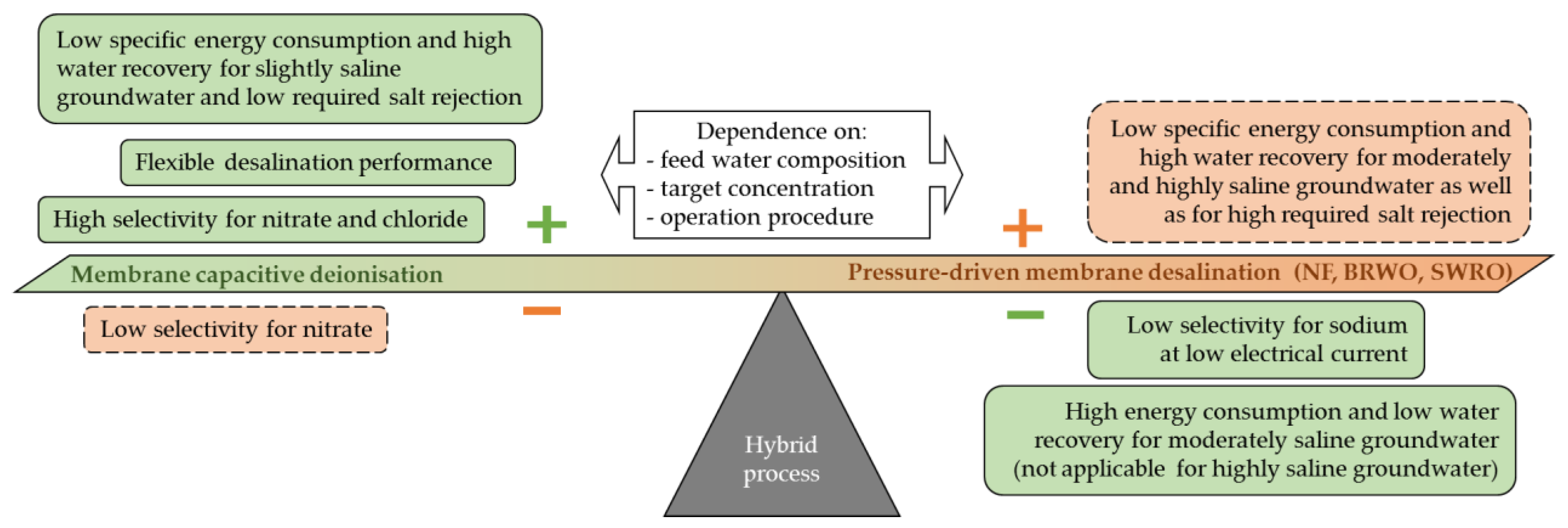
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNESCO. UN-Water United Nations World Water Development Report 2020: Water and Climate Change; UNESCO: Paris, France, 2020; ISBN 978-92-3-100371-4. [Google Scholar]
- Jiménez Cisneros, B.E.; Oki, T.; Arnell, N.W.; Benito, G.; Cogley, J.G.; Döll, P.; Jiang, T.; Mwakalila, S.S. Freshwater resources. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 229–269. ISBN 978-1-107-05807-1. [Google Scholar]
- Rodell, M.; Famiglietti, J.S.; Wiese, D.N.; Reager, J.T.; Beaudoing, H.K.; Landerer, F.W.; Lo, M.-H. Emerging trends in global freshwater availability. Nat. Cell Biol. 2018, 557, 651–659. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef]
- Vengosh, A. Salinization and Saline Environments. In Treatise on Geochemistry; Elsevier BV: Amsterdam, The Netherlands, 2003; pp. 1–35. [Google Scholar]
- Werner, A.D.; Bakker, M.; Post, V.E.; Vandenbohede, A.; Lu, C.; Ataie-Ashtiani, B.; Simmons, C.T.; Barry, D. Seawater intrusion processes, investigation and management: Recent advances and future challenges. Adv. Water Resour. 2013, 51, 3–26. [Google Scholar] [CrossRef]
- Klassen, J.; Allen, D. Assessing the risk of saltwater intrusion in coastal aquifers. J. Hydrol. 2017, 551, 730–745. [Google Scholar] [CrossRef]
- Jones, B.F.; Vengosh, A.; Rosenthal, E.; Yechieli, Y. Geochemical Investigations. In Seawater Intrusion in Coastal Aquifers — Concepts, Methods and Practices; Bear, J., Cheng, A.H.-D., Sorek, S., Ouazar, D., Herrera, I., Eds.; Theory and Applications of Transport in Porous Media; Springer Netherlands: Dordrecht, The Netherlands, 1999; pp. 51–71. ISBN 978-94-017-2969-7. [Google Scholar]
- Alfarrah, N.; Walraevens, K. Groundwater Overexploitation and Seawater Intrusion in Coastal Areas of Arid and Semi-Arid Regions. Water 2018, 10, 143. [Google Scholar] [CrossRef]
- Rhoades, J.D.; Kandiah, A.; Mashali, A.M. The Use of Saline Waters for Crop Production; FAO Irrigation and Drainage Paper: Rome, Italy, 1992; ISBN 978-92-5-103237-4. [Google Scholar]
- Konikow, L.F.; Reilly, T.E. Seawater Intrusion in the United States. In Seawater Intrusion in Coastal Aquifers—Concepts, Methods and Practices; Theory and Applications of Transport in Porous Media; Bear, J., Cheng, A.H.-D., Sorek, S., Ouazar, D., Herrera, I., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 463–506. ISBN 978-94-017-2969-7. [Google Scholar]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; ISBN 978-92-5-102263-4. [Google Scholar]
- Pan, S.-Y.; Haddad, A.Z.; Kumar, A.; Wang, S.-W. Brackish water desalination using reverse osmosis and capacitive deionization at the water-energy nexus. Water Res. 2020, 183, 116064. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Bazargan, A.; Salgado, B. Fundamentals of Desalination Technology. In A Multidisciplinary Introduction to Desalination; Bazargan, A., Ed.; IWA Publishing: London, UK, 2018; pp. 41–92. ISBN 978-1-78040-915-3. [Google Scholar]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2012; ISBN 9780470743720. [Google Scholar]
- Voutchkov, N. Desalination Project Cost Estimating and Management; Taylor & Francis: Boca Raton, FL, USA, 2018; ISBN 978-0-8153-7414-5. [Google Scholar]
- Dach, H. Comparison of Nanofiltration and Reverse Osmosis Processes for a Selective Desalination of Brackish Water Feeds. Ph.D. Thesis (Engineering Sciences), Université d’Angers, Angers, France, 2008. [Google Scholar]
- Schäfer, A.I.; Richards, B.S. Testing of a hybrid membrane system for groundwater desalination in an Australian national park. Desalination 2005, 183, 55–62. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- Folaranmi, G.; Bechelany, M.; Sistat, P.; Cretin, M.; Zaviska, F. Towards Electrochemical Water Desalination Techniques: A Review on Capacitive Deionization, Membrane Capacitive Deionization and Flow Capacitive Deionization. Membranes 2020, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Honarparvar, S.; Zhang, X.; Chen, T.; Na, C.; Reible, D. Modeling technologies for desalination of brackish water—Toward a sustainable water supply. Curr. Opin. Chem. Eng. 2019, 26, 104–111. [Google Scholar] [CrossRef]
- Sillanpää, M.E.T.; Shestakova, M. Electrochemical Water Treatment Methods: Fundamentals, Methods and Full Scale Applications; Butterworth-Heinemann, an imprint of Elsevier: Oxford, UK, 2017; ISBN 978-0-12-811462-9. [Google Scholar]
- Welgemoed, T.; Schutte, C. Capacitive Deionization Technology™: An alternative desalination solution. Desalination 2005, 183, 327–340. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive deionization (CDI) for desalination and water treatment—Past, present and future (a review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Van Der Wal, A. Membrane capacitive deionization. J. Membr. Sci. 2010, 346, 256–262. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.M.; Van Der Wal, A. Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ. Sci. 2012, 5, 9520–9527. [Google Scholar] [CrossRef]
- Hassanvand, A.; Wei, K.; Talebi, S.; Chen, G.Q.; Kentish, S.E. The Role of Ion Exchange Membranes in Membrane Capacitive Deionisation. Membranes 2017, 7, 54. [Google Scholar] [CrossRef]
- Qin, M.; Deshmukh, A.; Epsztein, R.; Patel, S.K.; Owoseni, O.M.; Walker, W.S.; Elimelech, M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 2019, 455, 100–114. [Google Scholar] [CrossRef]
- Zhao, R.; Porada, S.; Biesheuvel, P.; Van Der Wal, A. Energy consumption in membrane capacitive deionization for different water recoveries and flow rates, and comparison with reverse osmosis. Desalination 2013, 330, 35–41. [Google Scholar] [CrossRef]
- Patel, S.K.; Qin, M.; Walker, W.S.; Elimelech, M. Energy Efficiency of Electro-Driven Brackish Water Desalination: Electrodialysis Significantly Outperforms Membrane Capacitive Deionization. Environ. Sci. Technol. 2020, 54, 3663–3677. [Google Scholar] [CrossRef] [PubMed]
- Porada, S.; Zhang, L.; Dykstra, J.E. Energy consumption in membrane capacitive deionization and comparison with reverse osmosis. Desalination 2020, 488, 114383. [Google Scholar] [CrossRef]
- Tomaszkiewicz, M.; Najm, M.A.; El-Fadel, M. Development of a groundwater quality index for seawater intrusion in coastal aquifers. Environ. Model. Softw. 2014, 57, 13–26. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2005; ISBN 978-0-415-36421-8. [Google Scholar]
- Abd-Elhamid, H.F.; Javadi, A.A. A Cost-Effective Method to Control Seawater Intrusion in Coastal Aquifers. Water Resour. Manag. 2011, 25, 2755–2780. [Google Scholar] [CrossRef]
- Ebeling, P.; Händel, F.; Walther, M. Potential of mixed hydraulic barriers to remediate seawater intrusion. Sci. Total. Environ. 2019, 693, 133478. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bruggen, B.; Milis, R.; Vandecasteele, C.; Bielen, P.; Van San, E.; Huysman, K. Electrodialysis and nanofiltration of surface water for subsequent use as infiltration water. Water Res. 2003, 37, 3867–3874. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- German Drinking Water Ordinance: Verordnung über die Qualität von Wasser für den menschlichen Gebrauch (Trinkwasserverordnung—TrinkwV 2001), Trinkwasserverordnung in der Fassung der Bekanntmachung vom 10. März 2016 (BGBl. I S. 459), die zuletzt durch Artikel 99 der Verordnung vom 19. Juni 2020 (BGBl. I S. 1328) geändert worden ist. 2020.
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Pilson, M.E.Q. An Introduction to the Chemistry of the Sea; Cambridge University Press (CUP): Cambridge, UK, 2012. [Google Scholar]
- Han, D.; Post, V.E.; Song, X. Groundwater salinization processes and reversibility of seawater intrusion in coastal carbonate aquifers. J. Hydrol. 2015, 531, 1067–1080. [Google Scholar] [CrossRef]
- Askri, B.; Ahmed, A.T.; Al-Shanfari, R.A.; Bouhlila, R.; Al-Farisi, K.B.K. Isotopic and geochemical identifications of groundwater salinisation processes in Salalah coastal plain, Sultanate of Oman. Geochemistry 2016, 76, 243–255. [Google Scholar] [CrossRef]
- Askri, B.; Al-Shanfari, R.A. Assessment of Hydro-chemical Processes Inducing the Groundwater Salinisation in Coastal Regions: Case Study of the Salalah Plain, Sultanate of Oman. In The Economics of Water; Springer Nature: Berlin/Heidelberg, Germany, 2017; pp. 351–368. [Google Scholar]
- Elewa, H.H.; Shohaib, R.E.; Qaddah, A.A.; Nousir, A.M. Determining groundwater protection zones for the Quaternary aquifer of northeastern Nile Delta using GIS-based vulnerability mapping. Environ. Earth Sci. 2012, 68, 313–331. [Google Scholar] [CrossRef]
- Salalah Climate: Average Temperature, Weather by Month, Salalah Water Temperature—Climate-Data.Org. Available online: https://en.climate-data.org/asien/oman/dhofar/salala-2087/#climate-table (accessed on 19 October 2018).
- Port Said Climate: Average Temperature, Weather by Month, Port Said Water Temperature—Climate-Data.Org. Available online: https://en.climate-data.org/africa/egypt/port-said-governorate/port-said-504/#climate-table (accessed on 19 October 2018).
- Zhao, R.; Van Soestbergen, M.; Rijnaarts, H.H.M.; Van Der Wal, A.; Bazant, M.Z.; Biesheuvel, P.M. Time-dependent ion selectivity in capacitive charging of porous electrodes. J. Colloid Interface Sci. 2012, 384, 38–44. [Google Scholar] [CrossRef]
- Spiegler, K.; El-Sayed, Y. The energetics of desalination processes. Desalination 2001, 134, 109–128. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J. Elements of Physical Chemistry, 7th ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2017; ISBN 978-1-4292-1813-9. [Google Scholar]
- Yip, N.Y.; Elimelech, M. Comparison of Energy Efficiency and Power Density in Pressure Retarded Osmosis and Reverse Electrodialysis. Environ. Sci. Technol. 2014, 48, 11002–11012. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Water Application Value Engine (WAVE)—Software 1.58; The Dow Chemical Company: Midland, MI, USA, 2018.
- LewaPlus®-Software 2.1.1; LANXESS Deutschland GmbH: Cologne, Germany, 2020.
- Lipnizki, J.; Dinges, B.; Schaberger, H. Simulation und Auslegung mehrstufiger Wasser-Entsalzungsprozesse unter Einsatz von Umkehrosmose und Ionenaustausch. VGB Powertech 2013, 7, 87–93. [Google Scholar]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The Critical Need for Increased Selectivity, Not Increased Water Permeability, for Desalination Membranes. Environ. Sci. Technol. Lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- LANXESS Deutschland GmbH Lewabrane—Technical Service Bulletins. 2013. Available online: https://lpt.lanxess.com/media-events/lewabrane-downloads/4/ (accessed on 12 June 2020).
- DuPontTM FILMTECTM. Reverse Osmosis Membranes—Technical Manual; DuPont Water Solutions: Wilmington, DE, USA, 2020; Volume 3. [Google Scholar]
- Kadaj, E.; Bosleman, R. Energy Recovery Devices in Membrane Desalination Processes. In Renewable Energy Powered Desalination Handbook; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 415–444. [Google Scholar]
- Chong, T.H.; Loo, S.L.; Fane, A.G.; Krantz, W.B. Energy-efficient reverse osmosis desalination: Effect of retentate recycle and pump and energy recovery device efficiencies. Desalination 2015, 366, 15–31. [Google Scholar] [CrossRef]
- DIN German Institute for Standardization, DIN 38406-3:2002-03, Deutsche Einheitsverfahren Zur Wasser-, Abwasser- Und Schlammuntersuchung - Kationen (Gruppe E) - Teil 3: Bestimmung von Calcium Und Magnesium, Komplexometrisches Verfahren (E 3); engl.: German standard methods for the examination of water, waste Water and sludge - cations (group E) - Part 3: Determination of calcium and magnesium, complexometric Method (E 3). Berlin: Beuth Verlag GmbH 2002. [CrossRef]
- DIN German Institute for Standardization, DIN 38406-13:1992-07, Deutsche Einheitsverfahren Zur Wasser-, Abwasser- Und Schlammuntersuchung; Kationen (Gruppe E); Bestimmung von Kalium Mittels Atomabsorptionsspektrometrie (AAS) in Der Luft-Acetylen-Flamme (E 13); engl.: German standard methods for the examination of water, waste water and sludge; cations (Group E); Determination of potassium by atomic absorption spectrometry (AAS) using an air-acetylene flame (E 13). Berlin: Beuth Verlag GmbH 1992. [CrossRef]
- DIN German Institute for Standardization, DIN 38406-14:1992-07, Deutsche Einheitsverfahren Zur Wasser-, Abwasser- Und Schlammuntersuchung; Kationen (Gruppe E); Bestimmung von Natrium Mittels Atomabsorptionsspektrometrie (AAS) in Der Luft-Acetylen-Flamme (E 14); engl.: German standard methods for the examination of water, waste water and sludge; cations (Group E); Determination of sodium by atomic absorption spectrometry (ASS) using an air-acetylene flame (E 14). Berlin: Beuth Verlag GmbH 1992. [CrossRef]
- DIN German Institute for Standardization, DIN EN ISO 10304-1:2009-07, Wasserbeschaffenheit - Bestimmung von Gelösten Anionen Mittels Flüssigkeits-Ionenchromatographie - Teil 1: Bestimmung von Bromid, Chlorid, Fluorid, Nitrat, Nitrit, Phosphat Und Sulfat (ISO 10304-1:2007); Deutsche Fassung EN ISO 10304-1:2009; engl.: Water quality - Determination of dissolved anions by liquid chromatography of ions - Part 1: Determination of bromide, chloride, fluoride, nitrate, nitrite, phosphate and sulfate (ISO 10304-1:2007); German Version EN ISO 10304-1:2009. Berlin: Beuth Verlag GmbH 2009. [CrossRef]
- DIN German Institute for Standardization, DIN 38409-7:2005-12, Deutsche Einheitsverfahren Zur Wasser-, Abwasser- Und Schlammuntersuchung - Summarische Wirkungs- Und Stoffkenngrößen (Gruppe H) - Teil 7: Bestimmung Der Säure- Und Basekapazität (H 7); engl.: German standard methods for the examination of water, waste water and sludge - Parameters characterizing effects and substances (Group H) - Part 7: Determination of acid and base-neutralizing capacities (H 7). Berlin: Beuth Verlag GmbH 2005. [CrossRef]
- Van Limpt, B.; Van Der Wal, A. Water and chemical savings in cooling towers by using membrane capacitive deionization. Desalination 2014, 342, 148–155. [Google Scholar] [CrossRef]
- Helmholtz, H. Ueber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern mit Anwendung auf die thierisch-elektrischen Versuche. Ann. Phys. 1853, 165, 211–233. [Google Scholar] [CrossRef]
- Biesheuvel, P.M. Thermodynamic cycle analysis for capacitive deionization. J. Colloid Interface Sci. 2009, 332, 258–264. [Google Scholar] [CrossRef]
- Chapman, D.L. LI. A contribution to the theory of electrocapillarity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1913, 25, 475–481. [Google Scholar] [CrossRef]
- Stern, O. Zur Theorie Der Elektrolytischen Doppelschicht. Z. Elektrochem. Angew. Phys. Chemie 1924, 30, 508–516. [Google Scholar] [CrossRef]
- Porada, S.; Bryjak, M.; Van Der Wal, A.; Biesheuvel, P. Effect of electrode thickness variation on operation of capacitive deionization. Electrochim. Acta 2012, 75, 148–156. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Porada, S.; Levi, M.; Bazant, M.Z. Attractive forces in microporous carbon electrodes for capacitive deionization. J. Solid State Electrochem. 2014, 18, 1365–1376. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Zhao, R.; Porada, S.; Van Der Wal, A. Theory of membrane capacitive deionization including the effect of the electrode pore space. J. Colloid Interface Sci. 2011, 360, 239–248. [Google Scholar] [CrossRef]
- Dykstra, J.; Zhao, R.; Biesheuvel, P.M.; Van Der Wal, A. Resistance identification and rational process design in Capacitive Deionization. Water Res. 2016, 88, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Biesheuvel, P.M. Activated Carbon Is an Electron-Conducting Amphoteric Ion Adsorbent. arXiv 2015, 9. arXiv:1509.06354. [Google Scholar]
- Mubita, T.; Dykstra, J.; Biesheuvel, P.; Van Der Wal, A.; Porada, S. Selective adsorption of nitrate over chloride in microporous carbons. Water Res. 2019, 164, 114885. [Google Scholar] [CrossRef]
- Hassanvand, A.; Chen, G.Q.; Webley, P.A.; Kentish, S.E. A comparison of multicomponent electrosorption in capacitive deionization and membrane capacitive deionization. Water Res. 2018, 131, 100–109. [Google Scholar] [CrossRef]
- Tsai, S.-W.; Hackl, L.; Kumar, A.; Hou, C.-H. Exploring the electrosorption selectivity of nitrate over chloride in capacitive deionization (CDI) and membrane capacitive deionization (MCDI). Desalination 2021, 497, 114764. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, K.; Zhang, X.; Zhang, C.; Liang, P. Selective ion separation by capacitive deionization (CDI) based technologies: A state-of-the-art review. Environ. Sci. Water Res. Technol. 2020, 6, 243–257. [Google Scholar] [CrossRef]
- Suss, M.E. Size-Based Ion Selectivity of Micropore Electric Double Layers in Capacitive Deionization Electrodes. J. Electrochem. Soc. 2017, 164, E270–E275. [Google Scholar] [CrossRef]
- Guyes, E.N.; Malka, T.; Suss, M.E. Enhancing the Ion-Size-Based Selectivity of Capacitive Deionization Electrodes. Environ. Sci. Technol. 2019, 53, 8447–8454. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, J.E.; Porada, S.; Van Der Wal, A.; Biesheuvel, P.M. Energy consumption in capacitive deionization—Constant current versus constant voltage operation. Water Res. 2018, 143, 367–375. [Google Scholar] [CrossRef]
- Nicolini, J.V.; Borges, C.P.; Ferraz, H.C. Selective rejection of ions and correlation with surface properties of nanofiltration membranes. Sep. Purif. Technol. 2016, 171, 238–247. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Mohammad, A.; Darwish, N. Nanofiltration of highly concentrated salt solutions up to seawater salinity. Desalination 2005, 184, 315–326. [Google Scholar] [CrossRef]
- Mountadar, S.; Carbonell-Alcaina, C.; Luján-Facundo, M.; Ferrer-Polonio, E.; Soler-Cabezas, J.; Mendoza-Roca, J.; Tahiri, S. Desalination of brackish water and reverse osmotic retentate using nanofiltration membranes: Effects of TMP and feed concentration on the treatment. Desalination Water Treat. 2017, 87, 68–75. [Google Scholar] [CrossRef]
- Altaee, A.; Sharif, A.O. Alternative design to dual stage NF seawater desalination using high rejection brackish water membranes. Desalination 2011, 273, 391–397. [Google Scholar] [CrossRef]
- Okamoto, Y.; Lienhard, J.H. How RO membrane permeability and other performance factors affect process cost and energy use: A review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Hofs, B.; Schurer, R.; Harmsen, D.; Ceccarelli, C.; Beerendonk, E.; Cornelissen, E. Characterization and performance of a commercial thin film nanocomposite seawater reverse osmosis membrane and comparison with a thin film composite. J. Membr. Sci. 2013, 446, 68–78. [Google Scholar] [CrossRef]
- Długołęcki, P.; Van Der Wal, A. Energy Recovery in Membrane Capacitive Deionization. Environ. Sci. Technol. 2013, 47, 4904–4910. [Google Scholar] [CrossRef]
- Chen, L.; Yin, X.; Zhu, L.; Qiu, Y. Energy recovery and electrode regeneration under different charge/discharge conditions in membrane capacitive deionization. Desalination 2018, 439, 93–101. [Google Scholar] [CrossRef]
- Mossad, M.; Zou, L. Study of fouling and scaling in capacitive deionisation by using dissolved organic and inorganic salts. J. Hazard. Mater. 2013, 387–393. [Google Scholar] [CrossRef]
- Oest, J.; Elbracht, J.; Schloemer, S.; Illing, C.; Blumenberg, M. Methan im Grundwasser Niedersachsens; GeoBerichte 35; Landesamt für Bergbau, Energie und Geologie: Hannover, Germany, 2019; pp. 6–43. ISSN 1864–7529. [Google Scholar]
- Luo, J. Effects of PH and Salt on Nanofiltration—A Critical Review. J. Membr. Sci. 2013, 11, 18–28. [Google Scholar] [CrossRef]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. Can batch or semi-batch processes save energy in reverse-osmosis desalination? Desalination 2017, 402, 109–122. [Google Scholar] [CrossRef]
- Shrivastava, A.; Rosenberg, S.; Peery, M. Energy efficiency breakdown of reverse osmosis and its implications on future innovation roadmap for desalination. Desalination 2015, 368, 181–192. [Google Scholar] [CrossRef]
- Tan, C.; He, C.; Fletcher, J.; Waite, T.D. Energy recovery in pilot scale membrane CDI treatment of brackish waters. Water Res. 2020, 168, 115146. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Cath, T.Y.; Robertson, A.P.; Reinhard, M.; Leckie, J.O.; Drewes, J.E. Critical Review of Desalination Concentrate Management, Treatment and Beneficial Use. Environ. Eng. Sci. 2013, 30, 502–514. [Google Scholar] [CrossRef]
- Hemmatifar, A.; Ramachandran, A.; Liu, K.; Oyarzun, D.I.; Bazant, M.Z.; Santiago, J.G. Thermodynamics of Ion Separation by Electrosorption. Environ. Sci. Technol. 2018, 52, 10196–10204. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dykstra, J.E.; Lin, S. Energy Efficiency of Capacitive Deionization. Environ. Sci. Technol. 2019, 53, 3366–3378. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, H.-J.; Moon, S.-H. Electrodialysis Desalination. In Desalination; Wiley: Hoboken, NJ, USA, 2019; pp. 287–326. [Google Scholar]
- Mossad, M.; Zou, L. A study of the capacitive deionisation performance under various operational conditions. J. Hazard. Mater. 2012, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Yoon, N.; Park, S.; Son, M.; Lee, J.; Park, J.; Cho, K.H. Optimization of a nanofiltration and membrane capacitive deionization (NF-MCDI) hybrid system: Experimental and modeling studies. Desalination 2020, 493, 114658. [Google Scholar] [CrossRef]
- Choi, J.; Oh, Y.; Chae, S.; Hong, S. Membrane capacitive deionization-reverse electrodialysis hybrid system for improving energy efficiency of reverse osmosis seawater desalination. Desalination 2019, 462, 19–28. [Google Scholar] [CrossRef]


| Classification of Water Salinity | ||
|---|---|---|
| mg/L | mg/L | |
| Freshwater | 0–500 | <100 |
| Slightly saline groundwater | 500–1500 | 100–250 |
| Moderately saline groundwater | 1500–7000 | 250–500 |
| Highly saline groundwater | 7000–35,000 | 500–10,000 |
| Seawater | >35,000 | >10,000 |
| Seawater | Dalian, China | Salalah, Oman | Nile Delta, Egypt | Global | |||||
|---|---|---|---|---|---|---|---|---|---|
| Freshwater | Slightly SGW | Freshwater | Moderately SGW | Freshwater | Highly SGW | Irrigation | Drinking Water | ||
| Ref. | [45,46] | [47] w. n. 3 | [47] w. n. 13 | [48] w. n. 29 | [49] w. n. 4 | [50] w. n. 10 | [50] w. n. 10 | [13] | [43,44] |
| 412 | 65 SAR | 172 | 102 SAR | 223 | 40 SAR | 546 | n.s. SAR | n.s. | |
| 10,782 | 19 SAR | 81 | 38 SAR | 340 | 20 SAR | 4658 | 69 SAR | 200 | |
| 1284 | 12 SAR | 32 | 16 SAR | 107 | 14 SAR | 1130 | n.s. SAR | n.s. | |
| 399 | 3 | 2 | 1 | 7 | 9 | 100 | n.s. | n.s. | |
| 19,353 | 34 | 273 | 90 | 702 | 30 | 10,645 | 106 | 250 | |
| 113 | 126 | 118 | 234 | 242 | 160 | 154 | 92 | n.s. | |
| 2712 | 66 | 97 | 27 | 176 | 19 | 1057 | 200 | 250 | |
| n.s. | 47 | 230 | 24 | 416 | n.s. | n.s. | 130 1 | 50 | |
| TDS | 35,055 | 372 | 1004 | 532 | 2213 | 292 | 18,289 | 450 | 600 |
| Conductivity | n.s. | 560 | 1740 | 794 | 4510 | 440 | 32,000 | 700 | n.s. |
| pH | 8 | 7.8 | 7.6 | n.s. | 7.1 | 7.8 | 7.2 | 6.5–8.5 | |
| Temperature 2 | n.s. | 13.1 | 13.6 | 29.5 2 | 29.5 2 | 22.0 2 | 22.0 2 | n.s. | n.s. |
| Membrane | |||||||
|---|---|---|---|---|---|---|---|
| Experiments | Simulation | Literature | |||||
| Slightly SGW | Moderately SGW | Highly SGW | Slightly SGW | Moderately SGW | Highly SGW | ||
| DOW FILMTEC™ NF90-400/34i | 7.37 | 5.18 | - | 9.43 | 9.55 | - | 4.0–10.2 [88,89,90] |
| LEWABRANE® RO B085 HF 4040 | 3.45 | 3.09 | 0.91 | 3.43 | 3.47 | 2.29 | 2.0–5.3 1 [61,91,92] |
| DOW FILMTEC™ SW30XLE-400i | 1.03 | 1.04 | 0.51 | 2.17 | 2.07 | 1.87 | 0.9–3.0 1 [6,91,93] |
| Figure | Recovered Energy during Discharging | Energy Recovery | ||||
|---|---|---|---|---|---|---|
| A | % | kWh/m3 | kWh/m3 | % | % | |
| Slightly SGW | 10 | 76.11 | 0.15 | 0.01 | 9.57 | 37.10 |
| 14 | 67.48 | 0.20 | 0.03 | 14.12 | 54.41 | |
| 18 | 61.46 | 0.27 | 0.05 | 18.01 | 68.09 | |
| 22 | 54.29 | 0.39 | 0.08 | 20.89 | 87.18 | |
| 26 | 31.45 | 0.62 | 0.15 | 23.98 | 93.49 | |
| Moderately SGW | 20 | 66.34 | 0.29 | 0.06 | 20.95 | 25.18 |
| 40 | 48.87 | 0.68 | 0.24 | 36.04 | 64.64 | |
| 45 | 39.10 | 0.94 | 0.34 | 36.59 | 80.27 | |
| 50 | 35.42 | 1.12 | 0.35 | 31.29 | 90.46 | |
| 55 | 24.22 | 2.44 | 0.69 | 28.05 | 96.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosentreter, H.; Walther, M.; Lerch, A. Partial Desalination of Saline Groundwater: Comparison of Nanofiltration, Reverse Osmosis and Membrane Capacitive Deionisation. Membranes 2021, 11, 126. https://doi.org/10.3390/membranes11020126
Rosentreter H, Walther M, Lerch A. Partial Desalination of Saline Groundwater: Comparison of Nanofiltration, Reverse Osmosis and Membrane Capacitive Deionisation. Membranes. 2021; 11(2):126. https://doi.org/10.3390/membranes11020126
Chicago/Turabian StyleRosentreter, Hanna, Marc Walther, and André Lerch. 2021. "Partial Desalination of Saline Groundwater: Comparison of Nanofiltration, Reverse Osmosis and Membrane Capacitive Deionisation" Membranes 11, no. 2: 126. https://doi.org/10.3390/membranes11020126
APA StyleRosentreter, H., Walther, M., & Lerch, A. (2021). Partial Desalination of Saline Groundwater: Comparison of Nanofiltration, Reverse Osmosis and Membrane Capacitive Deionisation. Membranes, 11(2), 126. https://doi.org/10.3390/membranes11020126









