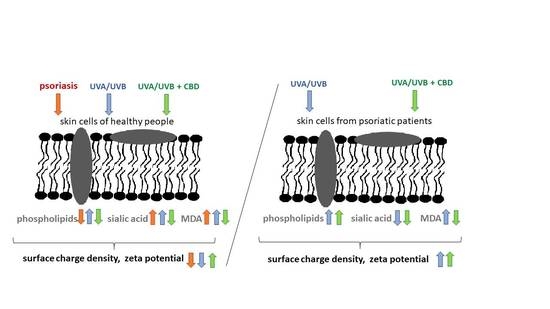
The Differential Effect of Cannabidiol on the Composition and Physicochemical Properties of Keratinocyte and Fibroblast Membranes from Psoriatic Patients and Healthy People
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cell Isolation
2.2. Cell Culture and Treatment
- Control group (Ctr), keratinocytes or fibroblasts from the skin of healthy subjects were cultured in standard medium;
- CBD group (CBD), keratinocytes or fibroblasts from the skin of healthy subjects were cultured for 24 h in medium containing 4 µM CBD;
- UVA group (UVA), keratinocytes or fibroblasts from the skin of healthy subjects were exposed to UVA radiation (30 J/cm2);
- UVB group (UVB), keratinocytes or fibroblasts from the skin of healthy subjects were exposed to UVB radiation (60 mJ/cm2);
- UVA + CBD group (UVA + CBD), keratinocytes or fibroblasts from the skin of healthy subjects were exposed to UVA irradiation (30 J/cm2) and then cultured for 24 h in medium containing 4 µM CBD;
- UVB + CBD group (UVB + CBD), keratinocytes or fibroblasts from the skin of healthy subjects were exposed to UVB irradiation (60 mJ/cm2) and then cultured for 24 h in medium containing 4 µM CBD.
- Psoriasis group (Ps), keratinocytes or fibroblasts from the skin of psoriatic patients were cultured in standard medium;
- Ps + CBD group (Ps + CBD), keratinocytes or fibroblasts from the skin of psoriatic patients were cultured for 24 h in medium containing 4 µM CBD;
- Ps + UVA group (Ps + UVA), keratinocytes or fibroblasts from the skin of psoriatic patients were exposed to UVA radiation (30 J/cm2);
- Ps + UVB group (Ps + UVB), keratinocytes or fibroblasts from the skin of psoriatic patients were exposed to UVB radiation (60 mJ/cm2);
- Ps + UVA + CBD group (Ps + UVA + CBD), keratinocytes or fibroblasts from the skin of psoriatic patients were exposed to UVA radiation (30 J/cm2) and cultured for 24 h in medium containing 4 µM CBD;
- Ps + UVB + CBD group (Ps + UVB + CBD), keratinocytes or fibroblasts from the skin of psoriatic patients were exposed to UVB radiation (60 mJ/cm2) and cultured for 24 h in medium containing 4 µM CBD.
2.3. Isolation and Analysis of Phospholipids
2.4. Determination of the Sialic Acid Level
2.5. Estimation of Lipid Peroxidation
2.6. Electrochemical Methods
2.7. Statistical Analysis
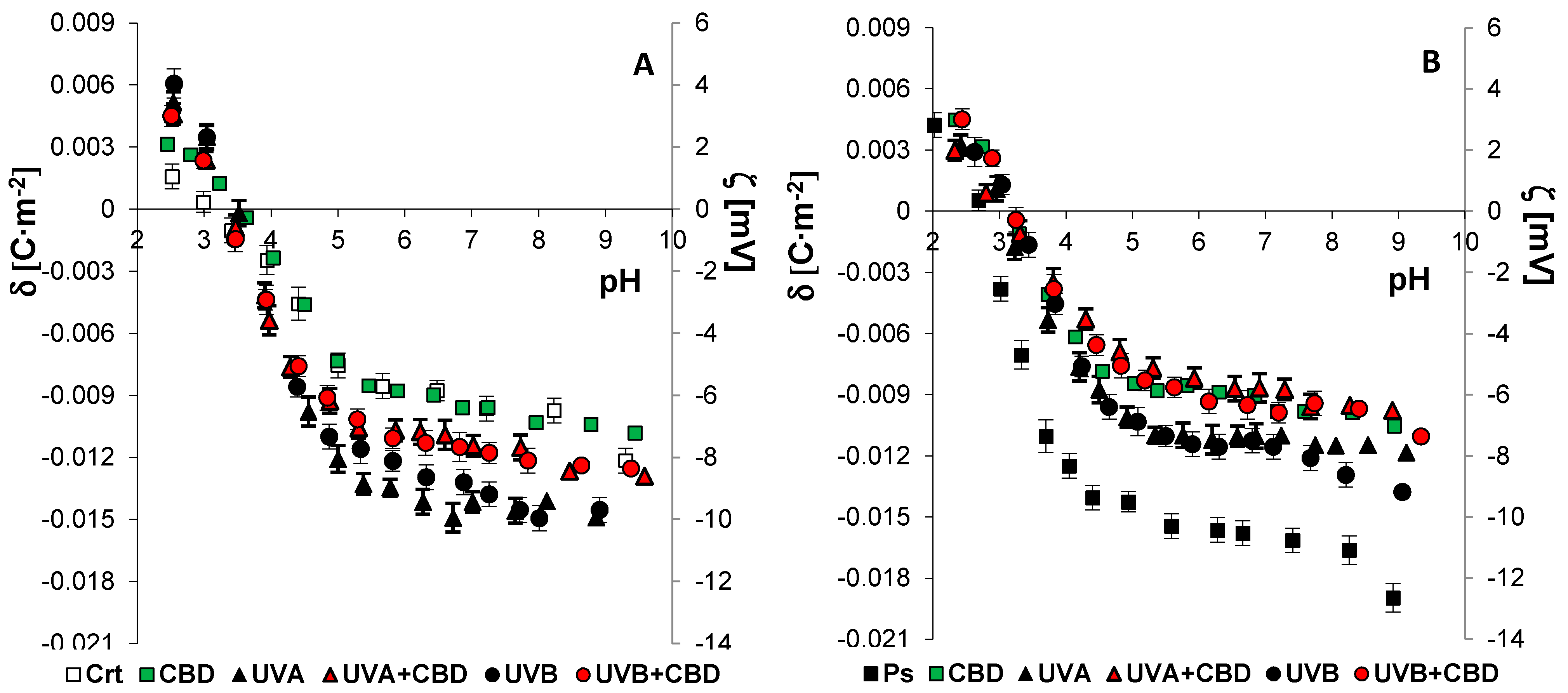
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kendall, A.C.; Nicolaou, A. Bioactive lipid mediators in skin inflammation and immunity. Prog. Lipid Res. 2013, 52, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Muallem, S.; Young, W.; Jha, C.A.; Ahuja, M. Lipids at membrane contact sites: Cell signaling andion transport. EMBO Rep. 2017, 18, 1893–1904. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Pędzińska-Betiuk, A.; Figaszewski, Z.A.; Skrzydlewska, E. Effects of hypertension and FAAH inhibitor treatment of rats with primary and secondary hypertension considering the physicochemical properties of erythrocytes. Toxicol. Mech. Method. 2020, 30, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, I.; Gęgotek, A.; Gajko, E.; Skrzydlewska, E.; Figaszewski, Z.A. Effects of rutin on the physicochemical properties of skin fibroblasts membrane disruption following UV radiation. Chem. Biol. Interact. 2018, 282, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol effects on phospholipid metabolism in keratinocytes from patients with psoriasis vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Kemeny, L.; Varga, E.; Novak, Z. Advances in phototherapy for psoriasis and atopic dermatitis. Expet. Rev. Clin. Immunol. 2019, 15, 1205–1214. [Google Scholar] [CrossRef]
- Dang, L.; Wang, Y.; Xue, Y.; He, L.; Li, Y.; Xiong, J. Low-dose UVB irradiation prevents MMP2-induced skin hyperplasia by inhibiting inflammation and ROS. Oncol. Rep. 2015, 34, 1478–1486. [Google Scholar] [CrossRef]
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological alterations of redox signaling and endocannabinoid system in granulocytes and plasma of psoriatic patients. Cells 2018, 7, E159. [Google Scholar] [CrossRef]
- Rácz, E.; Prens, E.P. Phototherapy of Psoriasis, a Chronic Inflammatory Skin Disease. Adv. Exp. Med. Biol. 2017, 996, 287–294. [Google Scholar] [PubMed]
- Lee, C.H.; Wu, S.B.; Hong, C.H.; Yu, H.S.; Wei, Y.H. Molecular mechanism of UV-induced apoptosis and its effects on skin residential cells: The implication in UV-based phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-Inflammatory properties of cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front. Pharmacol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Atalay, S.; Dobrzyńska, I.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol protects keratinocyte cell membranes following exposure to UVB and hydrogen peroxide. Redox Biol. 2020, 36, 101613. [Google Scholar] [CrossRef]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Hasko, G.; Pacher, P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Atalay, S.; Domingues, P.; Skrzydlewska, E. The Differences in the Proteome Profile of cannabidiol-treated skin fibroblasts following UVA or UVB Irradiation in 2D and 3D cell cultures. Cells 2019, 8, 995. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.N. Effects of cannabidiol interactions withWnt/β-catenin pathway and PPARγon oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Darewicz, B.; Figaszewski, Z.A. Characterization of human bladder cell membrane during cancer transformation. J. Membr. Biol. 2015, 248, 301–307. [Google Scholar] [CrossRef]
- Jourdian, G.W.; Dean, L.; Roseman, S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J. Biol. Chem. 1971, 246, 430–435. [Google Scholar] [CrossRef]
- Luo, X.P.; Yazdanpanah, M.; Bhooi, N.; Lehotay, D.C. Determination of aldehydes and other lipid peroxidation products in biological samples by gas chromatography-mass spectrometry. Anal. Biochem. 1995, 228, 294–298. [Google Scholar] [CrossRef]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Dobrzyńska, I.; Skrzydlewska, E. Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol. 2017, 12, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Weresa, J.; Figaszewski, Z.A.; Skrzydlewska, E. Changes in physicochemical properties of kidney cells membrane as a consequence of hypertension and treatment of hypertensive rats with FAAH inhibitor. Chem. Biol. Interact. 2019, 299, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, I.; Skrzydlewska, E.; Figaszewski, Z.A. Parameters characterizing acid-base equilibria between cell membrane and solution and their application to monitoring the effect of various factors on the membrane. Bioelectrochemistry 2006, 69, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Kadam, D.P.; Suryakar, A.N.; Ankush, R.D.; Kadam, C.Y.; Deshpande, K.H. Role of oxidative stress in various stages of psoriasis. Indian J. Clin. Biochem. 2010, 25, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Ogun, M. Biochemistry of reactive oxygen and nitrogen species. In Basic Principles and Clinical Significance of Oxidative Stress; Gowder, S.J.T., Ed.; IntechOpen: London, UK, 2015; pp. 37–58. [Google Scholar] [CrossRef]
- Dobrzyńska, I.; Szachowicz-Petelska, B.; Wroński, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Changes in the physicochemical properties of blood and skin cell membranes as a result of psoriasis vulgaris and psoriatic arthritis development. Int. J. Mol. Sci. 2020, 21, 9129. [Google Scholar] [CrossRef]
- Lubbers, J.; Rodriguez, E.; van Kooyk, Y. Modulation of immune tolerance via Siglec-sialic acid interactions. Front. Immunol. 2018, 9, 2807. [Google Scholar] [CrossRef]
- Ghosh, S. Sialic Acid and Biology of Life: An Introduction. In Sialic Acid and Sialoglycoconjugates in the Biology of Life, Health and Disaease; Ghosh, S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 1–61. [Google Scholar] [CrossRef]
- Łuczaj, W.; Dobrzyńska, I.; Wroński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Cannabidiol-mediated changes to the phospholipid profile of UVB-irradiated keratinocytes from psoriatic patients. Int. J. Mol. Sci. 2020, 21, 6592. [Google Scholar] [CrossRef]
- Kim, W.B.; Jerome, D.; Yeung, J. Diagnosis and management of psoriasis. Can. Fam. Physician 2017, 63, 278–285. [Google Scholar]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Zaręba, I.; Surażyński, A.; Skrzydlewska, E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch. Dermatol. Res. 2017, 309, 285–303. [Google Scholar] [CrossRef]
- Juzeniene, A.; Moan, J. Beneficial effects of UV radiation other than via vitamin D production. Derm. Endocrinol. 2012, 4, 109–117. [Google Scholar] [CrossRef]
- Wäster, P.K.; Ollinger, K.M. Redox-dependent translocation of p53 to mitochondria or nucleus in human melanocytes after UVA- and UVB-induced apoptosis. J. Investig. Dermatol. 2009, 129, 1769–1781. [Google Scholar] [CrossRef]
- Gruber, F. The skin lipidome under environmental stress-technological platforms, molecular pathways and translational opportunities. In Skin Stress Response Pathways; Wondrak, G.T., Ed.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 1–27. [Google Scholar]
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Phenotypic and lipidomic characterization of primary human epidermal keratinocytes exposed to simulated solar UV radiation. J. Dermatol. Sci. 2018, 92, 97–105. [Google Scholar] [CrossRef]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Dargere, D.; Auzeil, N.; Laprevote, O.; Szczur, P. Lipid deregulation in UV irradiated skin cells: Role of 25-hydroxycholesterol in keratinocyte differentiation during photoaging. J. Steroid Biochem. Mol. Biol. 2017, 169, 189–197. [Google Scholar] [CrossRef]
- Zeidan, Y.H.; Wu, B.X.; Jenkins, R.W.; Obeid, L.M.; Hannun, Y.A. A novel role for protein kinase Cδ-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. FASEB J. 2008, 22, 183–193. [Google Scholar] [CrossRef]
- Cha, H.J.; He, C.; Zhao, H.; Dong, Y.; An, I.-S.; Kim, Y.J. Intercellular and intracellular functions of ceramides and their metabolites in skin (Review). Int. J. Mol. Med. 2016, 38, 16–22. [Google Scholar] [CrossRef]
- Ramírez de Molina, A.; Gallego-Ortega, D.; Sarmentero-Estrada, J.; Lagares, D.; Gómez del Pulgar, T.; Bandrés, E.; García-Foncillas, J.; Lacal, J.C. Choline kinase as a link connecting phospholipid metabolism and cell cycle regulation: Implications in cancer therapy. Int. J. Biochem. Cell Biol. 2008, 40, 1753–1763. [Google Scholar] [CrossRef]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Ambrożewicz, E.; Skrzydlewska, E. The Proteomic Profile of Keratinocytes and Lymphocytes in Psoriatic Patients. Proteom. Clin. Appl. 2019, 13, E1800119. [Google Scholar] [CrossRef]
- Cannavò, S.P.; Riso, G.; Casciaro, M.; Di Salvo, E.; Gangemi, S. Oxidative stress involvement in psoriasis: A systematic review. Free Radic. Res. 2019, 53, 1–12. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, X.; Geng, R.; Lu, Q.; Rao, R.; Tan, X.; Yang, X.; Liu, W. Increased Level of α2,6-Sialylated Glycans on HaCaT Cells Induced by Titanium Dioxide Nanoparticles under UV Radiation. Nanomaterials 2018, 8, 253. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.Y.; Han, Q.L.; Wang, J.; Li, Y.; Yu, C.H.; Li, Y.R.; Yi, Z.C. Phenolic metabolites of benzene induced caspase-dependent cytotoxicities to K562 cells accompanied with decrease in cell surface sialic acids. Environ. Toxicol. 2014, 29, 1437–1451. [Google Scholar] [CrossRef]
- Suzuki, O.; Abe, M. Galectin-1-mediated cell adhesion, invasion and cell death in human anaplastic large cell lymphoma: Regulatory roles of cell surface glycans. Int. J. Oncol. 2014, 44, 1433–1442. [Google Scholar] [CrossRef][Green Version]
- Weatherhead, S.C.; Farr, P.M.; Jamieson, D.; Hallinan, J.S.; Lloyd, J.J.; Wipat, A.; Reynolds, N.J. Keratinocyte apoptosis in epidermal remodeling and clearance of psoriasis induced by UV radiation. J. Investig. Dermatol. 2011, 131, 1916–1926. [Google Scholar] [CrossRef]
- Meesmann, H.M.; Fehr, E.-M.; Kierschke, S.; Herrmann, M.; Bilyy, R.; Heyder, P.; Blank, N.; Krienke, S.; Lorenz, H.-M.; Schiller, M. Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J. Cell Sci. 2010, 123, 3347–3356. [Google Scholar] [CrossRef]
- Shenoy, C.; Shantaram, M.; Sharanya, K.; Shenoy, M.M. Lipid-bound sialic acid in psoriasis and its correlation with disease severity. Saudi J. Health Sci. 2015, 4, 56–58. [Google Scholar] [CrossRef]
- Rajendinar, K.S.; Ananthanarayanan, R.H.; Satheesh, S.; Rajappa, M. Elevated levels of serum sialic acid and high-sensitivity C-reactive protein: Markers of systemic inflammation in patients with chronic heart failure. Br. J. Biomed. Sci. 2014, 71, 29–32. [Google Scholar]
- Kleiser, S.; Nyström, A. Interplay between cell-surface receptors and extracellular matrix in skin. Biomolecules 2020, 10, 1170. [Google Scholar] [CrossRef]
- Jellusova, J.; Nitschke, L. Regulation of B cell functions by the sialic acid-binding receptors Siglec-G and CD22. Front. Immunol. 2012, 2, 96. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Mihai, G.L.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef] [PubMed]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef] [PubMed]
- Matsura, T. Oxidized phosphatidylserine: Production and bioactivities. Yonago Acta Med. 2014, 57, 119–127. [Google Scholar]
- Chaurio, R.A.; Janko, C.; Muñoz, L.E.; Frey, B.; Herrmann, M.; Gaipl, U.S. Phospholipids: Key players in apoptosis and immune regulation. Molecules 2009, 14, 4892–4914. [Google Scholar] [CrossRef]
- Kagan, V.E.; Bayır, H.; Tyurina, Y.Y.; Bolevich, S.B.; Maguire, J.J.; Fadeel, B.; Balasubramanian, K. Elimination of the unnecessary: Intra- and extracellular signaling by anionic phospholipids. Biochem. Biophys. Res. Commun. 2017, 482, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, I.; Kotynska, J.; Szachowicz-Petelska, B.; Figaszewski, Z.A. Determination of association constants of monovalent ions to sphingomyelin and phosphatidylinositol liposomal membranes by microelectrophoresis. Soft Mater. 2017, 15, 113–120. [Google Scholar] [CrossRef]
- Kotynska, J.; Dobrzyńska, I.; Figaszewski, Z.A. Association of alkali metal cations with phosphatidylcholine liposomal membrane surface. Eur. Biophys. J. 2017, 46, 149–155. [Google Scholar] [CrossRef]
- Montone, C.M.; Cerrato, A.; Botta, B.; Cannazza, G.; Capriotti, A.L.; Cavaliere, C.; Citti, C.; Ghirga, F.; Piovesana, S.; Lagana, A. Improved identification of phytocannabinoids using a dedicated structure-based workflow. Talanta 2020, 219, 121310. [Google Scholar] [CrossRef]
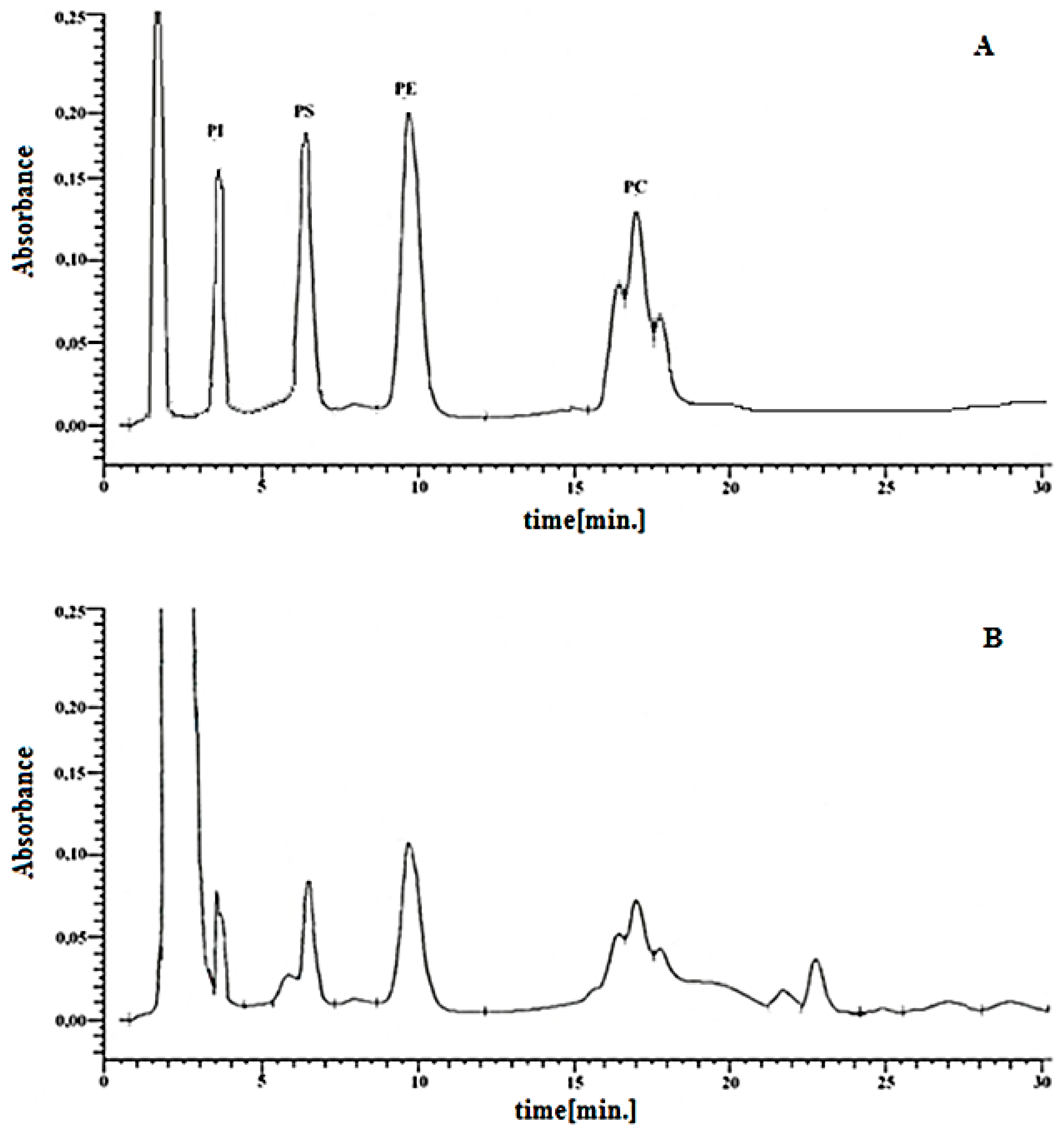

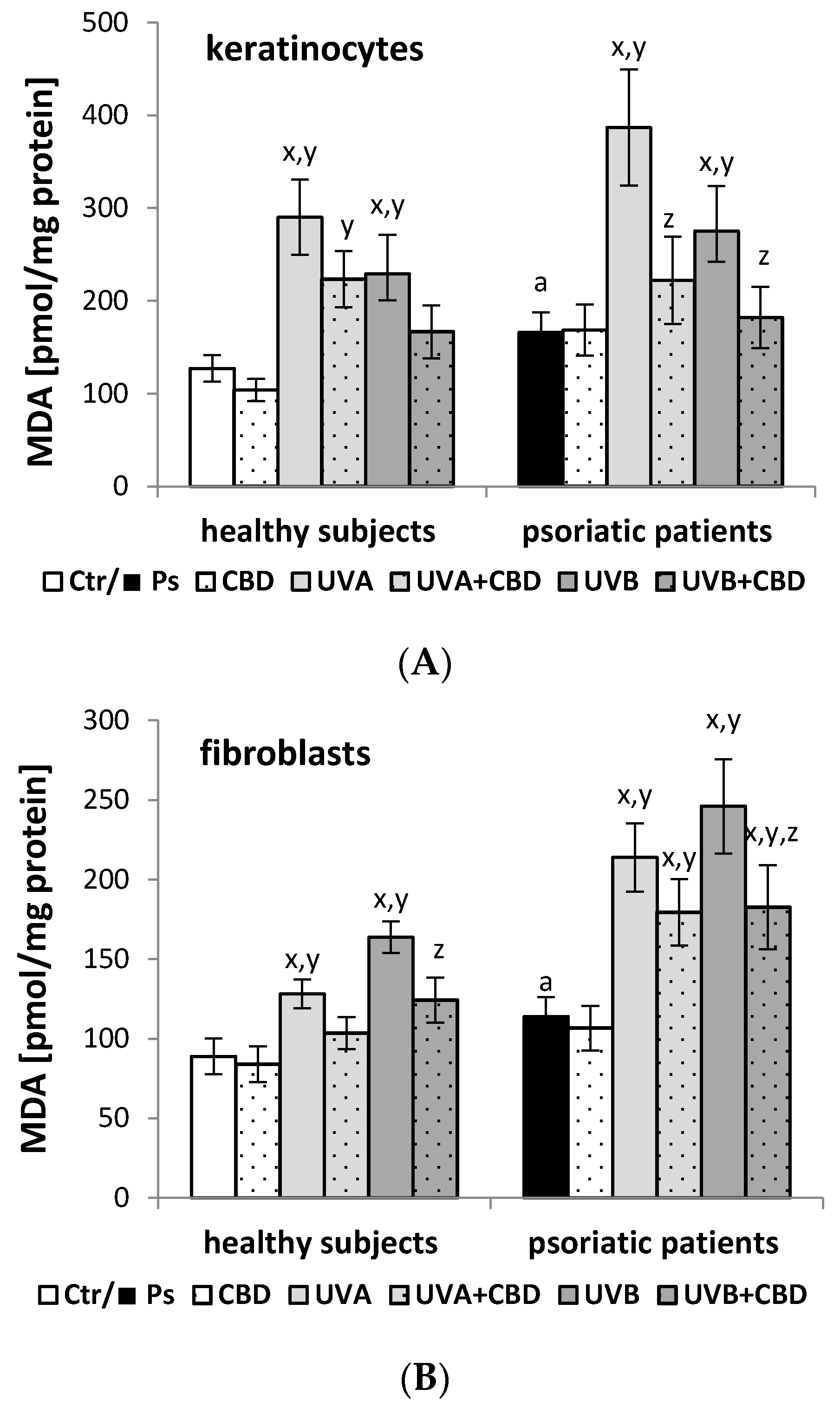
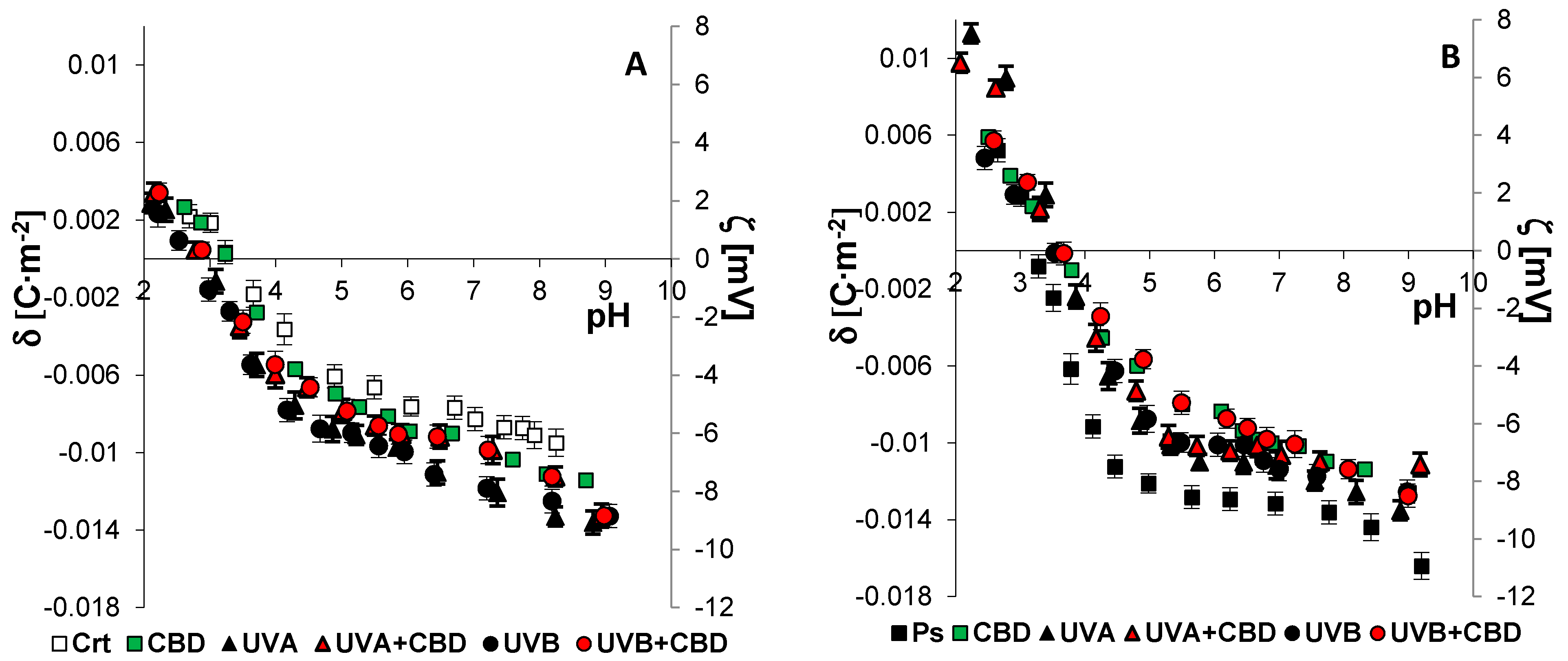
| Groups | PI | PS | PE | PC |
|---|---|---|---|---|
| µg/mg Protein | ||||
| Ctr | 1.14 ± 0.07 | 1.65 ± 0.09 | 3.41 ± 0.32 | 4.01 ± 0.14 |
| CBD | 0.82 ± 0.09 | 1.21 ± 0.07 | 2.81 ± 0.16 | 3.28 ± 0.20 |
| UVA | 1.64 ± 0.10 x,y | 2.12 ± 0.12 x,y | 5.22 ± 0.20 x,y | 5.86 ± 0.32 x,y |
| UVA+ CBD | 1.01 ± 0.07 z | 1.71 ± 0.14 z | 4.00 ± 0.18 y,z | 4.62± 0.20 z |
| UVB | 1.42 ± 0.10 y | 2.04 ± 0.15 x,y | 5.05 ± 0.19 x,y | 5.52 ± 0.38 x,y |
| UVB + CBD | 1.00 ± 0.07 z | 1.31 ± 0.15 z | 3.67 ± 0.26 z | 4.26± 0.22z |
| Ps | 0.95 ± 0.14 a | 1.37 ± 0.14 a | 2.24 ± 0.19 a | 3.27 ± 0.20 a |
| Ps + CBD | 0.84 ± 0.10 | 1.29 ± 0.10 | 1.85 ± 0.20 | 2.57 ± 0.25 |
| Ps + UVA | 1.02 ± 0.12 | 1.46 ± 0.12 | 3.21 ± 0.26 | 3.83 ± 0.29 y |
| Ps + UVA + CBD | 1.05 ± 0.10 | 1.62 ± 0.13 x | 3.65 ± 0.23 x,y | 4.22 ± 0.21 x,y |
| Ps + UVB | 1.01 ± 0.10 | 1.44 ± 0.13 | 3.12 ± 0.23 | 3.73 ± 0.23 |
| Ps + UVB + CBD | 1.03 ± 0.16 | 1.63 ± 0.14 x | 4.85 ± 0.25 x,y,z | 3.99 ± 0.20 x,y |
| Groups | PI | PS | PE | PC |
|---|---|---|---|---|
| µg/mg Protein | ||||
| Control | 1.69 ± 0.12 | 2.72 ± 0.17 | 5.88 ± 0.25 | 6.99 ± 0.30 |
| CBD | 1.55 ± 0.11 | 2.40 ± 0.10 | 5.02 ± 0.26 | 6.09 ± 0.23 |
| UVA | 3.55 ± 0.31 x,y | 4.52 ± 0.26 x,y | 10.12 ± 0.38 x,y | 12.24 ± 0.81 x,y |
| UVA+ CBD | 2.94 ± 0.20 y | 4.04 ± 0.21 y | 6.24 ± 0.30 z | 7.39 ± 0.30 z |
| UVB | 3.10 ± 0.19 x,y | 4.42 ± 0.22 x,y | 9.05 ± 0.35 x,y | 11.35 ± 0.41 x,y |
| UVB + CBD | 2.51 ± 0.20 | 2.89 ± 0.15 z | 6.01 ± 0.33 z | 7.24± 0.35 z |
| Ps | 1.20 ± 0.10 a | 2.08 ± 0.22 a | 4.60 ± 0.29 a | 5.32 ± 0.26 a |
| Ps + CBD | 0.94 ± 0.10 | 1.66 ± 0.19 | 3.84 ± 0.22 | 4.40 ± 0.25 |
| Ps + UVA | 1.58 ± 0.11 x | 2.26 ± 0.21 | 5.31 ± 0.28 x,y | 6.22 ± 0.33 x,y |
| Ps + UVA + CBD | 1.64 ± 0.12 x,y | 2.52 ± 0.18 x,y | 5.33 ± 0.32 x,y | 6.39 ± 0.36 x,y |
| Ps + UVB | 1.66 ± 0.15 x,y | 2.37 ± 0.21 | 5.25 ± 0.25 y | 6.17 ± 0.33 x,y |
| Ps + UVB +CBD CBD | 1.84 ± 0.18 x,y | 3.66 ± 0.23 x,y | 5.46 ± 0.30 x,y | 6.37 ± 0.28 x,y |
| Cells/Groups | Parameters | |||
|---|---|---|---|---|
| CTA [10−6 mol/m2] | CTB [10−6 mol/m2] | KAH [102 m3/mol] | KBOH [107 m3/mol] | |
| Keratinocytes | ||||
| Ctr | 3.04 ± 0.09 | 0.55 ± 0.05 | 1.85 ± 0.06 | 3.86 ± 0.09 |
| CBD | 3.31 ± 0.11 x | 0.57 ± 0.04 | 1.78 ± 0.05 | 4.09 ± 0.08 |
| UVA | 3.99 ± 0.12 x,y | 0.63 ± 0.05 | 0.88 ± 0.04 x,y | 5.65 ± 0.10 x,y |
| UVA + CBD | 3.65 ± 0.08 x,z | 0.50 ± 0.04 | 1.18 ± 0.05 z | 4.26 ± 0.08 z |
| UVB | 3.96 ± 0.15 x,y | 0.59 ± 0.05 | 0.78 ± 0.04 x,y | 6.50 ± 0.11 x,y |
| UVB + CBD | 3.40 ± 0.07 z | 0.75 ± 0.04 x,y | 1.10 ± 0.06 z | 4.60 ± 0.11 z |
| Ps | 4.89 ± 0.11 a | 1.38 ± 0.06 a | 0.88 ± 0.07 a | 6.16 ± 0.11 a |
| Ps + CBD | 3.42 ± 0.10 x | 0.95 ± 0.05 | 1.68 ± 0.07 x | 3.02 ± 0.08 x |
| Ps + UVA | 3.96 ± 0.12 x,y | 1.69 ± 0.08 x,y | 1.29 ± 0.05 y | 5.15 ± 0.14 y |
| Ps + UVA + CBD | 3.91 ± 0.07 x,y | 1.34 ± 0.06 z | 1.37 ± 0.05 x | 4.99 ± 0.08 x,y |
| Ps + UVB | 3.93 ± 0.10 x,y | 0.85 ± 0.07 x | 1.01 ± 0.06 y | 5.04 ± 0.10 y |
| Ps + UVB + CBD | 3.49 ± 0.09 x,z | 0.92 ± 0.06 | 1.38 ± 0.05 x,z | 4.37 ± 0.09 x,z |
| Fibroblasts | ||||
| Ctr | 3.42 ± 0.10 | 0.37 ± 0.04 | 2.88 ± 0.06 | 3.78 ± 0.05 |
| CBD | 3.39 ± 0.08 | 0.56 ± 0.04 x | 3.48 ± 0.05 x | 3.78 ± 0.06 |
| UVA | 4.09 ± 0.11 x,y | 1.58 ± 0.07 x | 1.83 ± 0.04 x,y | 5.25 ± 0.07 x,y |
| UVA+ CBD | 3.64 ± 0.09 z | 1.07 ± 0.04 x,z | 0.98 ± 0.04 x,y,z | 4.29 ± 0.06 z |
| UVB | 3.92 ± 0.11 x,y | 1.84 ± 0.08 x | 0.88 ± 0.04 x,y | 5.67 ± 0.07 x,y |
| UVB + CBD | 3.52 ± 0.10 z | 1.19 ± 0.06 x,z | 1.04 ± 0.04 x,y | 4.52 ± 0.07 z |
| Ps | 5.80 ± 0.09 a | 0.75 ± 0.05 a | 0.58 ± 0.03 a | 7.81 ± 0.12 a |
| Ps + CBD | 3.38 ± 0.07 x | 0.88 ± 0.06 | 0.88 ± 0.05 | 4.19 ± 0.10 x |
| Ps + UVA | 3.93 ± 0.08 x,y | 0.85 ± 0.04 | 0.78 ± 0.04 | 5.24 ± 0.08 x |
| Ps + UVA + CBD | 3.24 ± 0.06 x,z | 0.55 ± 0.04 x,z | 0.98 ± 0.04 x | 4.26 ± 0.08 x,z |
| Ps + UVB | 4.35 ± 0.08 x | 1.06 ± 0.08 x | 1.08 ± 0.06 x | 4.67 ± 0.08 x |
| Ps + UVB + CBD | 3.20 ± 0.07 x,z | 0.56 ± 0.04 y,z | 0.96 ± 0.04 x | 4.28 ± 0.08 x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szachowicz-Petelska, B.; Łuczaj, W.; Wroński, A.; Jastrząb, A.; Dobrzyńska, I. The Differential Effect of Cannabidiol on the Composition and Physicochemical Properties of Keratinocyte and Fibroblast Membranes from Psoriatic Patients and Healthy People. Membranes 2021, 11, 111. https://doi.org/10.3390/membranes11020111
Szachowicz-Petelska B, Łuczaj W, Wroński A, Jastrząb A, Dobrzyńska I. The Differential Effect of Cannabidiol on the Composition and Physicochemical Properties of Keratinocyte and Fibroblast Membranes from Psoriatic Patients and Healthy People. Membranes. 2021; 11(2):111. https://doi.org/10.3390/membranes11020111
Chicago/Turabian StyleSzachowicz-Petelska, Barbara, Wojciech Łuczaj, Adam Wroński, Anna Jastrząb, and Izabela Dobrzyńska. 2021. "The Differential Effect of Cannabidiol on the Composition and Physicochemical Properties of Keratinocyte and Fibroblast Membranes from Psoriatic Patients and Healthy People" Membranes 11, no. 2: 111. https://doi.org/10.3390/membranes11020111
APA StyleSzachowicz-Petelska, B., Łuczaj, W., Wroński, A., Jastrząb, A., & Dobrzyńska, I. (2021). The Differential Effect of Cannabidiol on the Composition and Physicochemical Properties of Keratinocyte and Fibroblast Membranes from Psoriatic Patients and Healthy People. Membranes, 11(2), 111. https://doi.org/10.3390/membranes11020111