Membrane Distillation: Recent Configurations, Membrane Surface Engineering, and Applications
Abstract
:1. Introduction
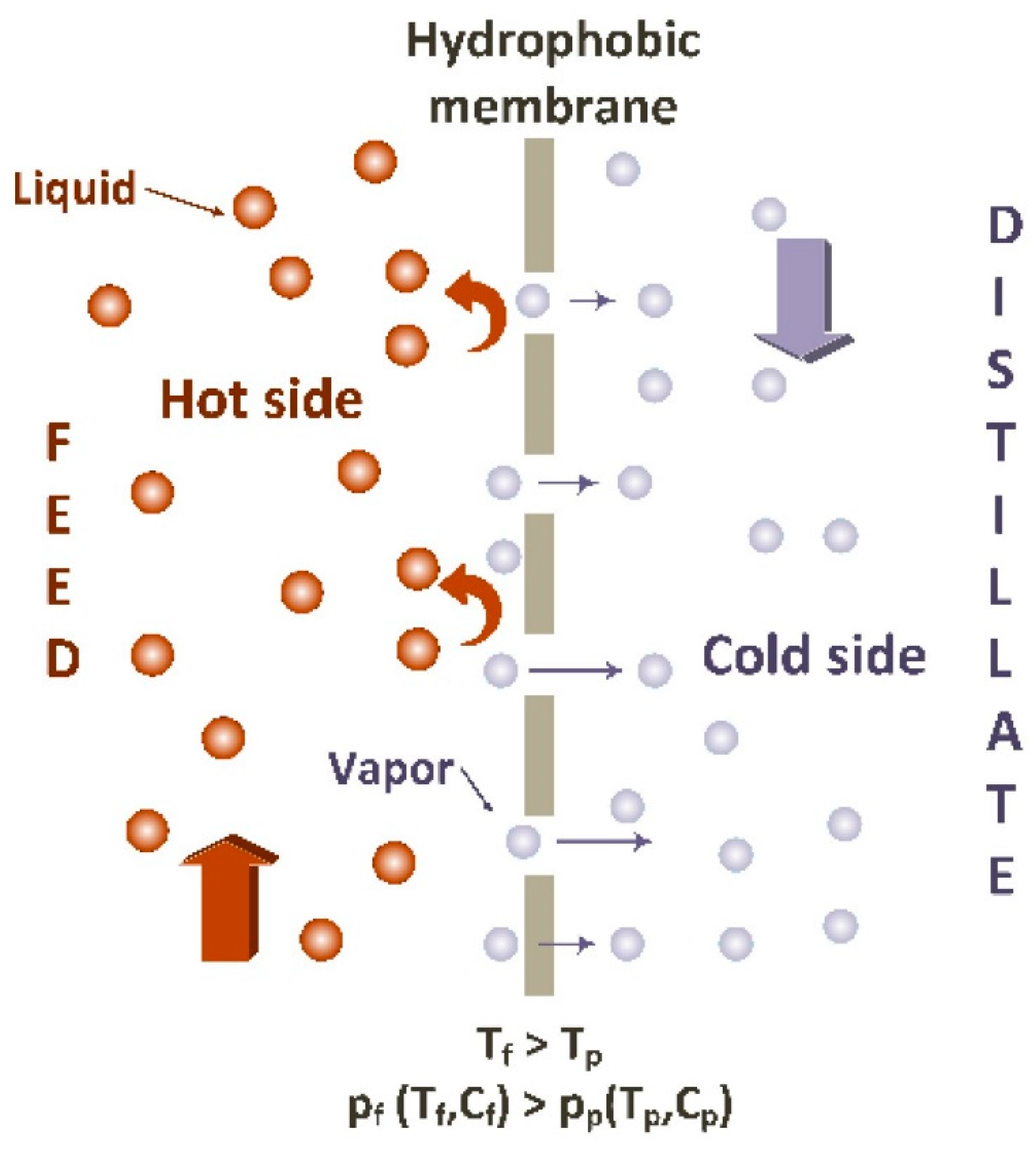
2. Membrane Distillation
3. Conventional Configurations
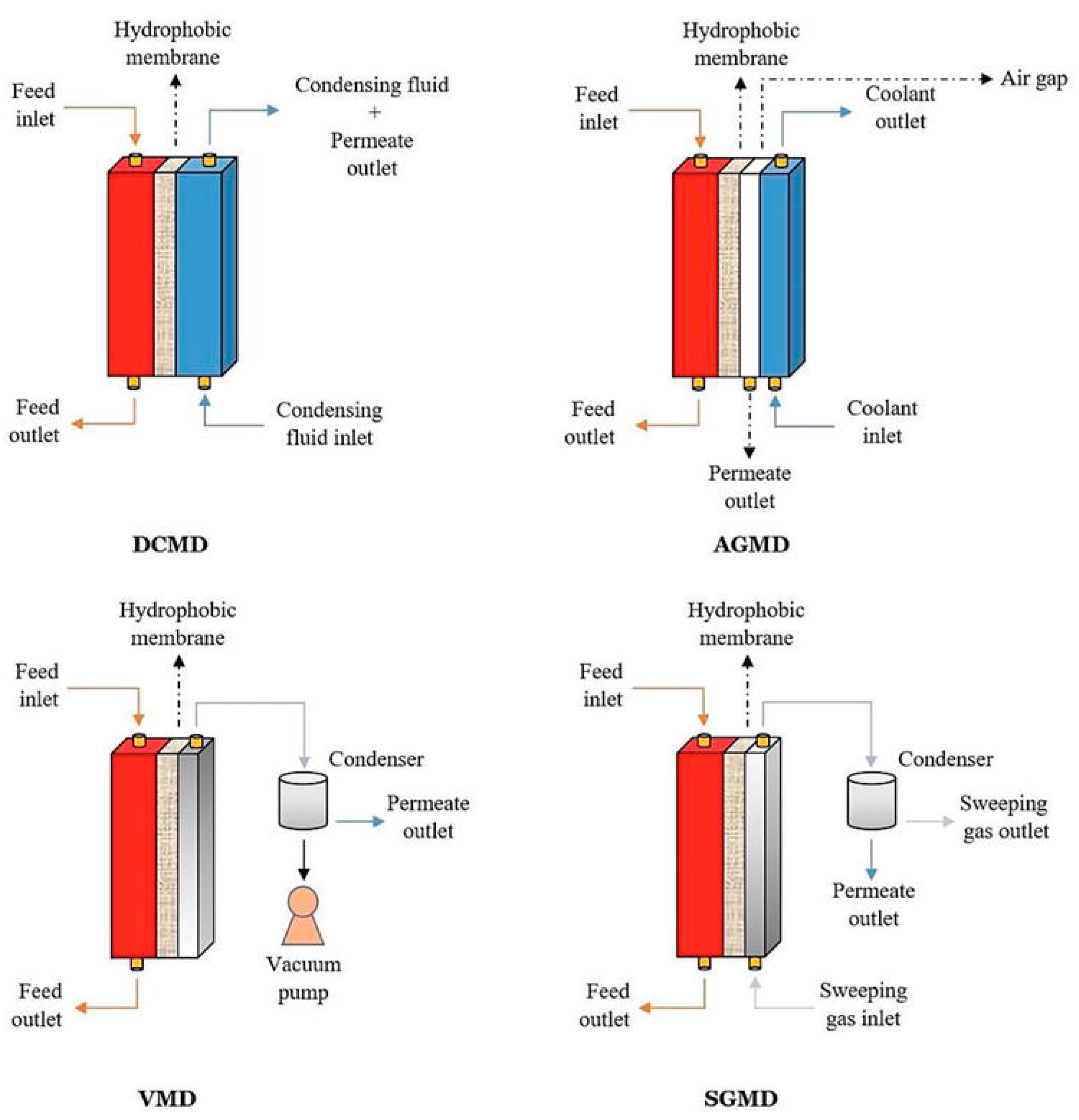
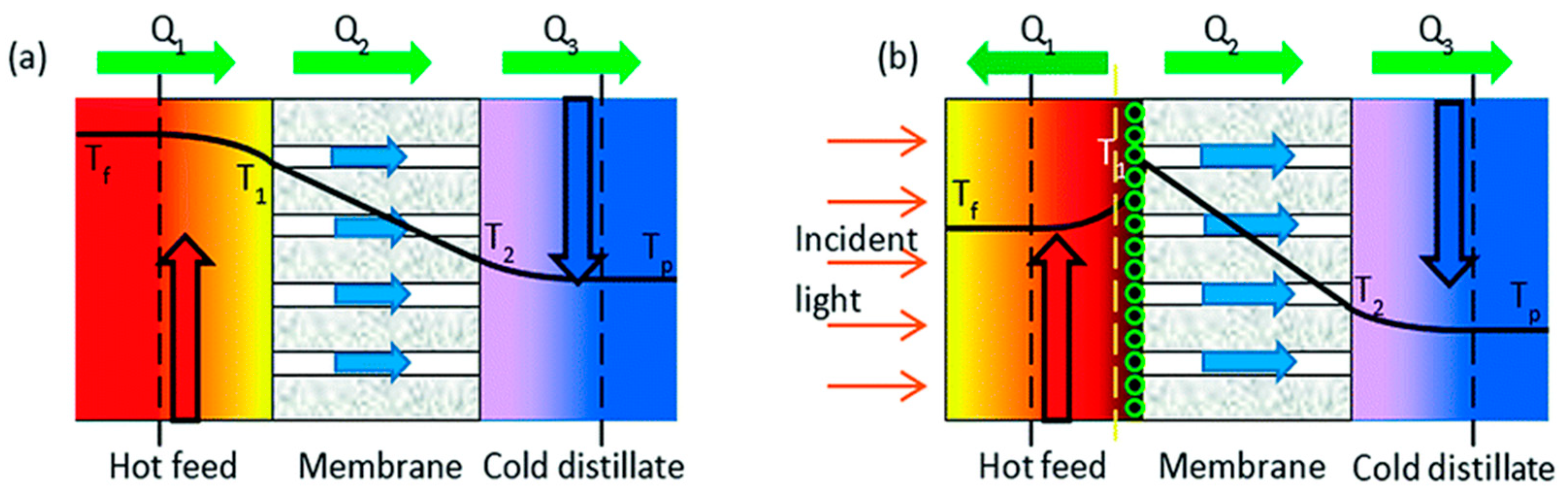
3.1. Direct Contact Membrane Distillation (DCMD)
3.2. Air-Gap Membrane Distillation (AGMD)
3.3. Vacuum Membrane Distillation (VMD)
3.4. Sweeping Gas Membrane Distillation (SGMD)
4. Recent Configurations
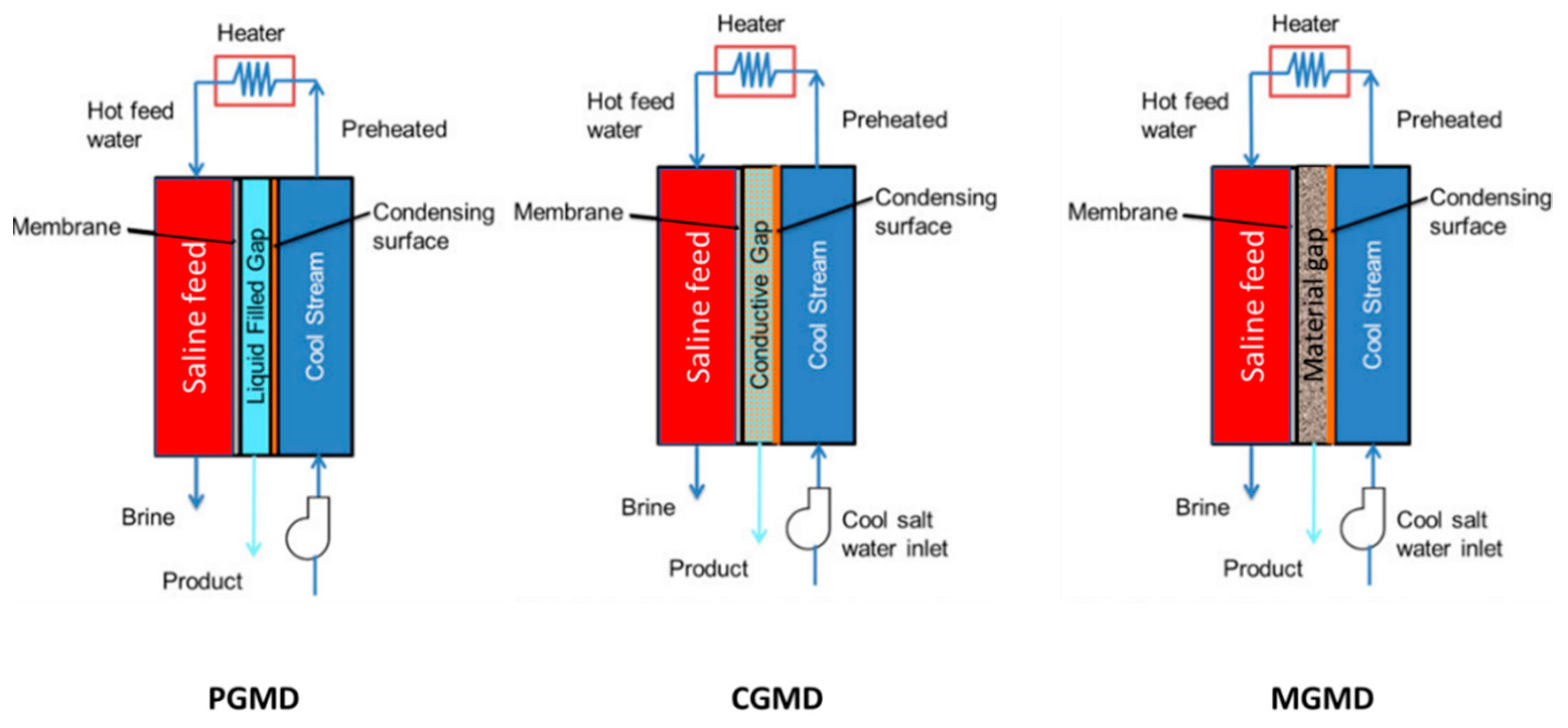
4.1. Permeate Gap Membrane Distillation (PGMD)
4.2. Material Gap Membrane Distillation (MGMD)
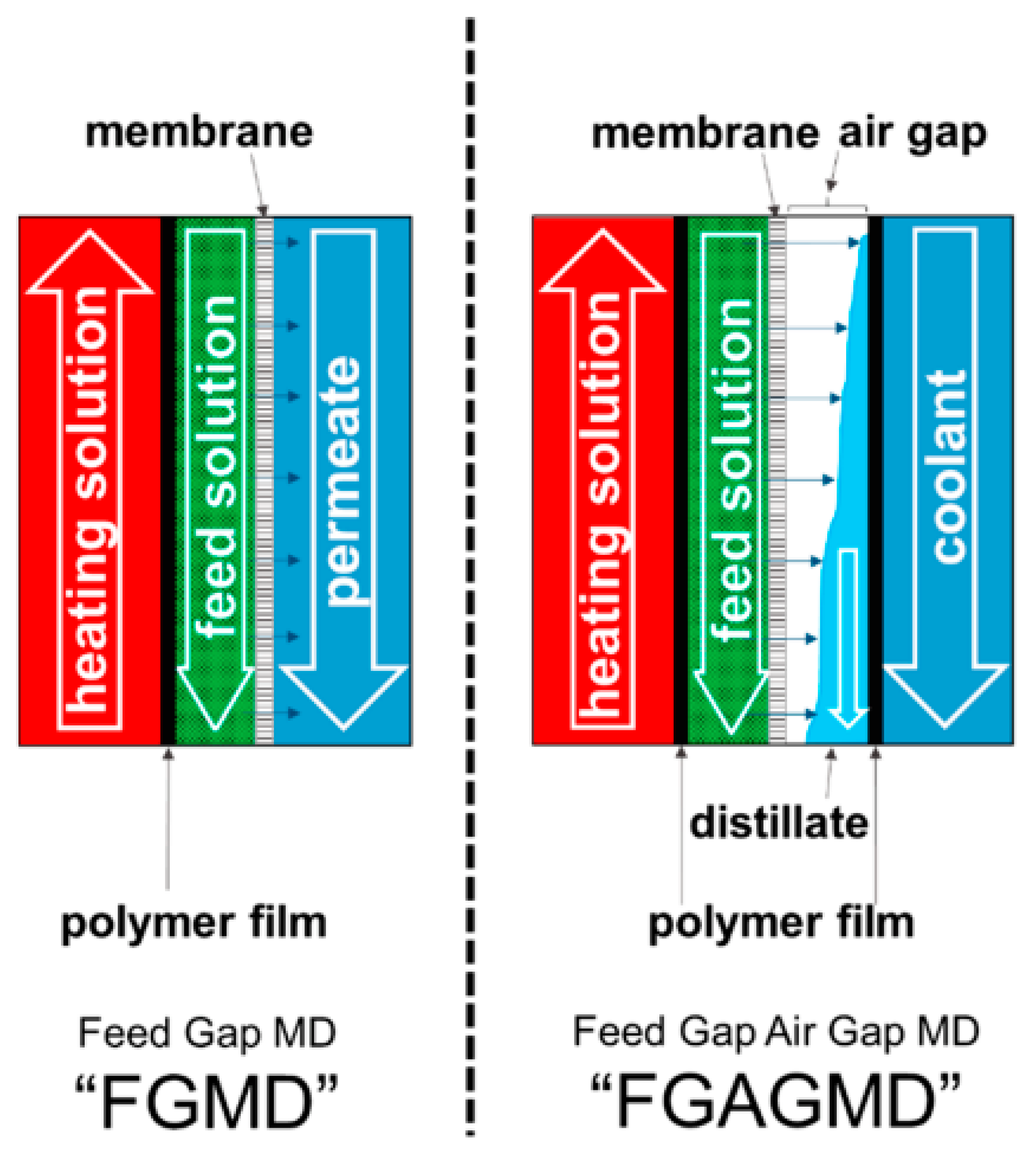
4.3. Feed Gap Membrane Distillation (FGMD)
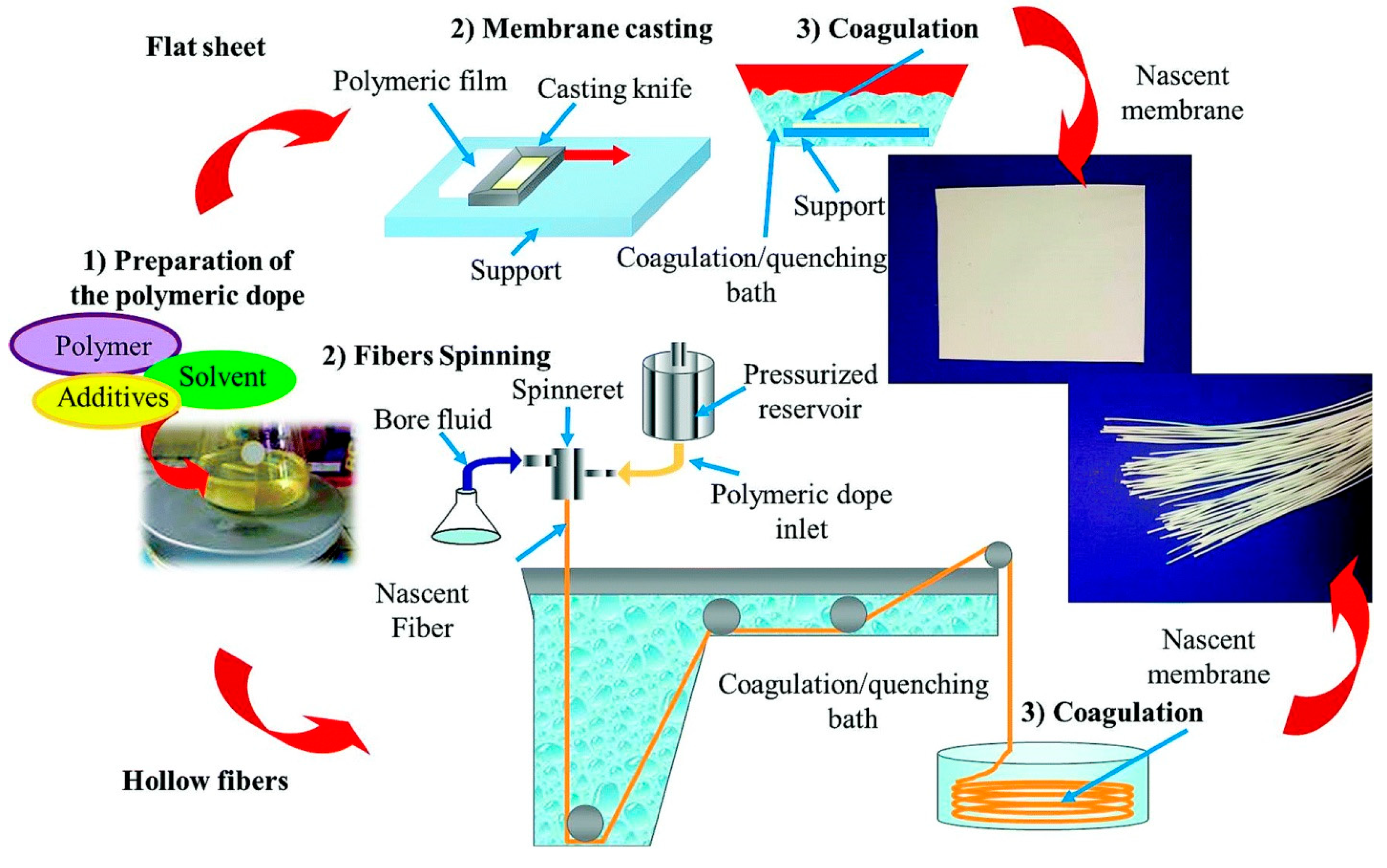
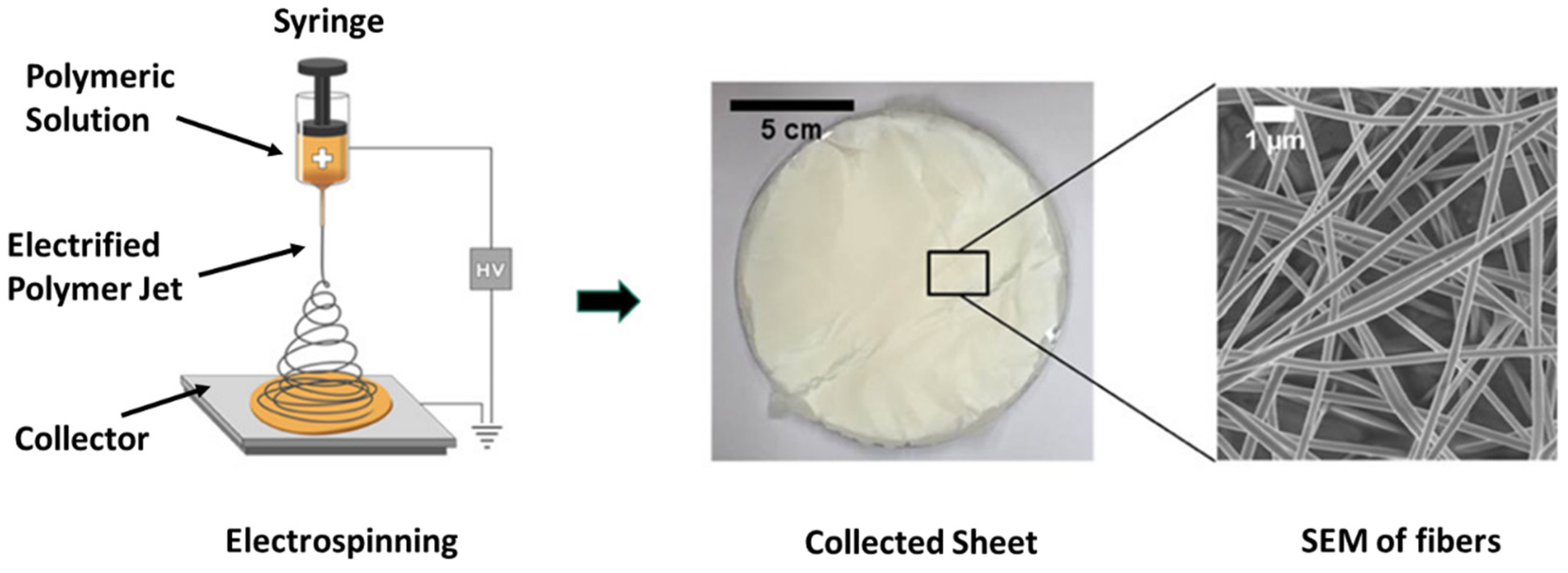
5. Membrane Materials and Fabrication
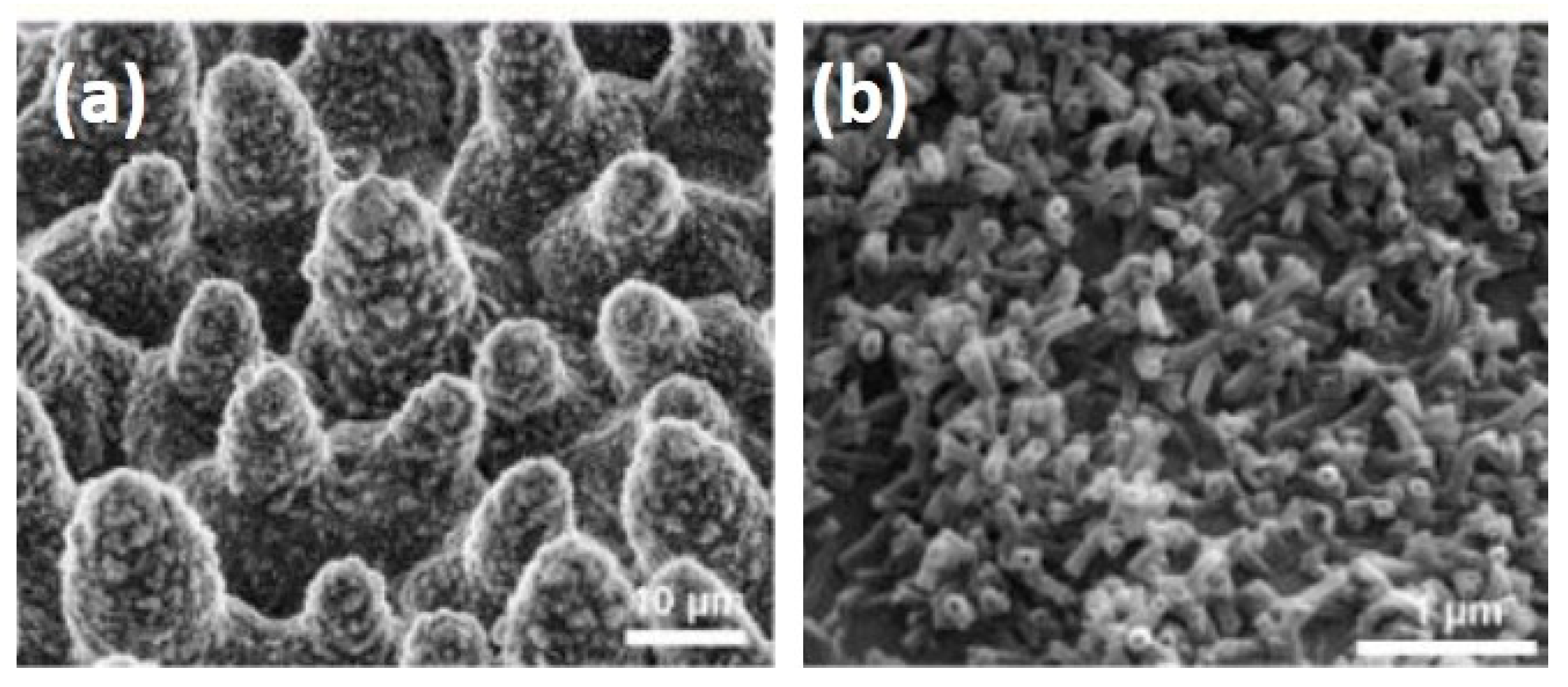
6. Surface Engineering
7. Membrane Fouling
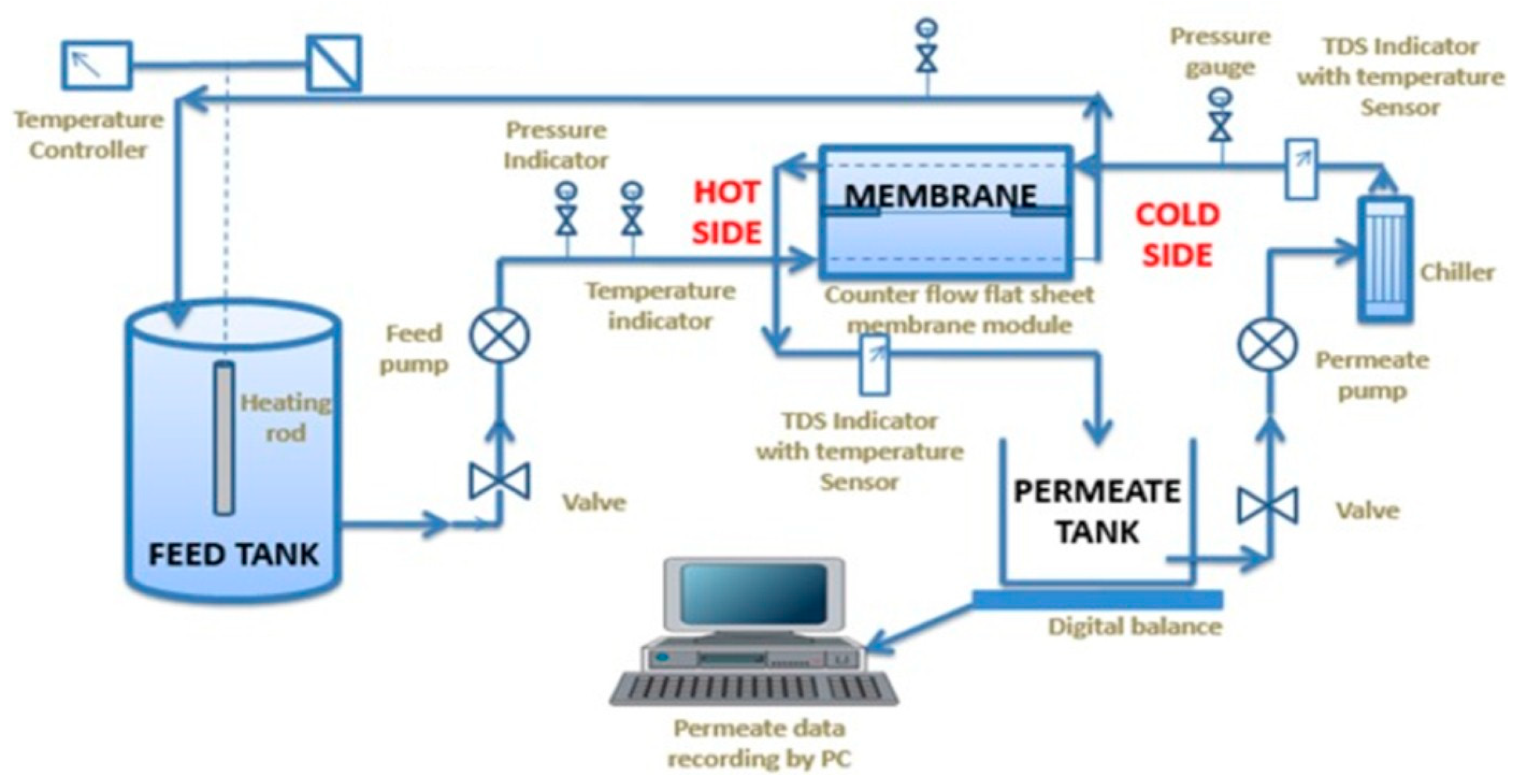
8. Membrane Distillation Experiment
9. Applications
9.1. Using Standalone MD Configurations
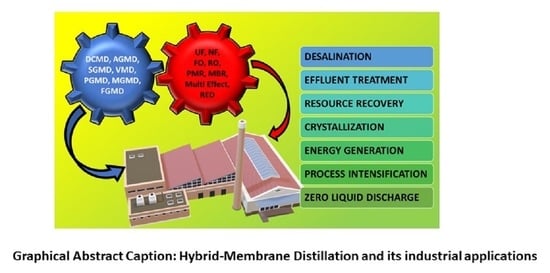
9.2. Using Hybrid Membrane Distillation
10. Membrane Distillation Pilot Plants
11. Conclusions and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization; UNICEF. Progress on Sanitation and Drinking Water—2015 Update and MDG Assessment; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total. Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.J.; Chen, Y.; Chung, T.S. Design of omniphobic interfaces for membrane distillation—A review. Water Res. 2019, 162, 64–77. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Karanasiou, A.; Kostoglou, M.; Karabelas, A. An experimental and theoretical study on separations by vacuum membrane distillation employing hollow-fiber modules. Water 2018, 10, 947. [Google Scholar] [CrossRef] [Green Version]
- Ameen, N.A.M.; Ibrahim, S.S.; Alsalhy, Q.F.; Figoli, A. Highly saline water desalination using direct contact membrane distillation (DCMD): Experimental and simulation study. Water 2020, 12, 1575. [Google Scholar] [CrossRef]
- Gryta, M. Direct contact membrane distillation with crystallization applied to NaCl solutions. Chem. Pap.-Slovak Acad. Sci. 2002, 56, 14–19. [Google Scholar]
- El-Bourawi, M.S.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Qtaishat, M.R.; Banat, F. Desalination by solar powered membrane distillation systems. Desalination 2013, 308, 186–197. [Google Scholar] [CrossRef]
- Kebria, M.R.S.; Rahimpour, A. Membrane Distillation: Basics, Advances, and Applications. Adv. Membr. Technol. 2020, 67. [Google Scholar] [CrossRef] [Green Version]
- Eryildiz, B.; Yuksekdag, A.; Korkut, S.; Zeytuncu, B.; Pasaoglu, M.E.; Koyuncu, I. Effect of operating parameters on removal of boron from wastewater containing high boron concentration by vacuum assisted air gap membrane distillation. J. Water Process. Eng. 2020, 38, 101579. [Google Scholar] [CrossRef]
- Andrés-Mañas, J.A.; Ruiz-Aguirre, A.; Acién, F.G.; Zaragoza, G. Performance increase of membrane distillation pilot scale modules operating in vacuum-enhanced air-gap configuration. Desalination 2020, 475, 114202. [Google Scholar] [CrossRef]
- Francis, L.; Ghaffour, N.; Alsaadi, A.A.; Amy, G.L. A new design for water vapor flux enhancement. J. Membr. Sci. 2013, 448, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, J.; Chung, H.W.; Warsinger, D.M.; AlMarzooqi, F.A.; Arafat, H.A. Energy efficiency of permeate gap and novel conductive gap membrane distillation. J. Membr. Sci. 2016, 502, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Schwantes, R.; Seger, J.; Bauer, L.; Winter, D.; Hogen, T.; Koschikowski, J.; Geißen, S.U. Characterization and assessment of a novel plate and frame MD module for single pass wastewater concentration–FEED gap air gap membrane distillation. Membranes 2019, 9, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Sun, D.; Li, B.; Li, P. Evaluation of commercial PTFE membranes for desalination of brine water through vacuum membrane distillation. Chem. Eng. Process. Process. Intensif. 2016, 110, 52–63. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, H.; Wang, F.; Guo, Y.; Zhang, H.; Chen, J. Preparation and properties of PTFE hollow fiber membranes for desalination through vacuum membrane distillation. J. Membr. Sci. 2013, 446, 145–153. [Google Scholar] [CrossRef]
- Kwong, H.Y.; Wong, M.H.; Wong, Y.W.; Wong, K.H. Superhydrophobicity of polytetrafluoroethylene thin film fabricated by pulsed laser deposition. Appl. Surf. Sci. 2007, 253, 8841–8845. [Google Scholar] [CrossRef]
- Himma, N.F.; Anisah, S.; Prasetya, N.; Wenten, I.G. Advances in preparation, modification, and application of polypropylene membrane. J. Polym. Eng. 2016, 36, 329–362. [Google Scholar] [CrossRef]
- Gryta, M. Influence of Polypropylene Membrane Surface Porosity on the Performance of Membrane Distillation Process. J. Membr. Sci. 2007, 287, 67–78. [Google Scholar] [CrossRef]
- Lin, Y.K.; Chen, G.; Yang, J.; Wang, X.L. Formation of isotactic polypropylene membranes with bicontinuous structure and good strength via thermally induced phase separation method. Desalination 2009, 236, 8–15. [Google Scholar] [CrossRef]
- Othman, N.; Harruddin, N.; Idris, A.; Ooi, Z.Y.; Fatiha, N.; Raja Sulaiman, R.N. Fabrication of polypropylene membrane via thermally induced phase separation as a support matrix of tridodecylamine supported liquid membrane for Red 3BS dye removal. Desalination Water Treat. 2016, 57, 12287–12301. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y. Application and modification of poly (vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2019, 463, 145–165. [Google Scholar] [CrossRef]
- Figoli, A.; Marino, T.; Simone, S.; Nicolo, E.D.; Li, X.M.; He, T.; Tornaghi, S.; Drioli, E. Towards non-toxic solvents for membrane preparation-A review. Green Chem. 2014, 16, 4034–4059. [Google Scholar] [CrossRef]
- Fan, H.; Peng, Y.; Li, Z.; Chen, P.; Jiang, Q.; Wang, S. Preparation and characterization of hydrophobic PVDF membranes by vapor-induced phase separation and application in vacuum membrane distillation. J. Polym. Res. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Bilad, M.R.; Arafat, H.A. Improving Liquid Entry Pressure of Polyvinylidene Fluoride (PVDF) Membranes by Exploiting the Role of Fabrication Parameters in Vapor-Induced Phase Separation VIPS and Non-Solvent-Induced Phase Separation (NIPS) Processes. Appl. Sci. 2017, 7, 181. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.; Fane, A.G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Membr. Sci. 2013, 425, 30–39. [Google Scholar] [CrossRef]
- Josef, E.; Guterman, R. Designing Solutions for Electrospinning of Poly(ionic liquid)s. Macromolecules 2019, 52, 5223–5230. [Google Scholar] [CrossRef]
- Abdallah, H.; Moustafa, A.F.; AlAnezi, A.A.H.; El-Sayed, H.E.M. Performance of a newly developed titanium oxide nanotubes/polyethersulfone blend membrane for water desalination using vacuum membrane distillation. Desalination 2014, 346, 30–36. [Google Scholar] [CrossRef]
- Rastegarpanah, A.; Mortaheb, H.R. Surface treatment of polyethersulfone membranes for applying in desalination by direct contact membrane distillation. Desalination 2016, 377, 99–107. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Sirus, Z.; Hossein, A.M.X. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2016, 292, 19–29. [Google Scholar] [CrossRef]
- Guo, F.; Servi, A.; Liu, A.; Gleason, K.K.; Rutledge, G.C. Desalination by membrane distillation using electrospun polyamide fiber membranes with surface fluorination by chemical vapor deposition. ACS Appl. Mater. Interfaces 2015, 7, 8225–8232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Shen, F.; Chen, X.; Luo, J.; Su, Y.; Wu, H.; Wan, Y. A novel plasma-induced surface hydrophobization strategy for membrane distillation: Etching, dipping and grafting. J. Membr. Sci. 2016, 499, 544–554. [Google Scholar] [CrossRef]
- Cao, S.; Wu, X.; Zhu, Y.; Gupta, R.; Tan, A.; Wang, Z.; Jun, Y.S.; Singamaneni, S. Polydopamine/hydroxyapatite nanowire-based bilayered membrane for photothermal-driven membrane distillation. J. Mater. Chem. A 2020, 8, 5147–5156. [Google Scholar] [CrossRef]
- Zuo, J.; Bonyadi, S.; Chung, T.S. Exploring the potential of commercial polyethylene membranes for desalination by membrane distillation. J. Membr. Sci. 2016, 497, 239–247. [Google Scholar] [CrossRef]
- Khayet, M.; Essalhi, M.; Qtaishat, M.R.; Matsuura, T. Robust surface modified polyetherimide hollow fiber membrane for long-term desalination by membrane distillation. Desalination 2019, 466, 107–117. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Gorin, Y.G.; Yeszhanov, A.B.; Kozlovskiy, A.L.; Zdorovets, M.V. Preparation of PET track-etched membranes for membrane distillation by photo-induced graft polymerization. Mater. Chem. Phys. 2018, 205, 55–63. [Google Scholar] [CrossRef]
- Huang, C.Y.; Ko, C.C.; Chen, L.H.; Huang, C.T.; Tung, K.L.; Liao, Y.C. A simple coating method to prepare superhydrophobic layers on ceramic alumina for vacuum membrane distillation. Sep. Purif. Technol. 2018, 198, 79–86. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Rahman, M.A.; Jaafar, J.; Ismail, A.F.; Amin, S.Z.M. Green silica-based ceramic hollow fiber membrane for seawater desalination via direct contact membrane distillation. Sep. Purif. Technol. 2018, 205, 22–31. [Google Scholar] [CrossRef]
- Kujawa, J.; Kujawski, W.; Cerneaux, S.; Li, G.; Al-Gharabli, S. Zirconium dioxide membranes decorated by silanes based-modifiers for membrane distillation. J. Membr. Sci. 2020, 596, 117597. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A. Scaling and fouling in membrane distillation for desalination applications—A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Zhang, H.; Lamb, R.; Lewis, J. Engineering Nanoscale Roughness on Hydrophobic Surface—Preliminary Assessment of Fouling Behaviour. Sci. Technol. Adv. Mater. 2005, 6, 236–239. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Christoph, N.; Wilhelm, B. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.Y.; Lin, H.N.; Tsai, H.A.; Wang, D.M.; Lai, J.Y. Fabrication of a high hydrophobic PVDF membrane via nonsolvent induced phase separation. Desalination 2008, 233, 40–47. [Google Scholar] [CrossRef]
- Teoh, M.M.; Chung, T.S. Membrane distillation with hydrophobic macrovoid-free PVDF–PTFE hollow fiber membranes. Sep. Purif. Technol. 2009, 66, 229–236. [Google Scholar] [CrossRef]
- Sun, D.; Liu, M.Q.; Guo, J.H.; Zhang, J.Y.; Li, B.B.; Li, D.Y. Preparation and characterization of PDMS-PVDF hydrophobic microporous membrane for membrane distillation. Desalination 2015, 370, 63–71. [Google Scholar] [CrossRef]
- Edwie, F.; Teoh, M.M. TS Chung, Effects of additives on dual-layer hydrophobic–hydrophilic PVDF hollow fiber membranes for membrane distillation and continuous performance. Chem. Eng. Sci. 2012, 68, 567–578. [Google Scholar] [CrossRef]
- Khayet, M. Treatment of radioactive wastewater solutions by direct contact membrane distillation using surface modified membranes. Desalination 2013, 321, 60–66. [Google Scholar] [CrossRef]
- Suk, D.E.; Matsuura, T.; Park, H.B.; Lee, Y.M. Development of novel surface modified phase inversion membranes having hydrophobic surface-modifying macromolecule (nSMM) for vacuum membrane distillation. Desalination 2010, 261, 300–312. [Google Scholar] [CrossRef]
- Prince, J.A.; Rana, D.; Singh, G.; Matsuura, T.; Kai, T.J.; Shanmugasundaram, T.S. Effect of hydrophobic surface modifying macromolecules on differently produced PVDF membranes for direct contact membrane distillation. Chem. Eng. J. 2014, 242, 387–396. [Google Scholar] [CrossRef]
- Zheng, Z.; Gu, Z.; Huo, R.; Luo, Z. Superhydrophobic poly (vinylidene fluoride) film fabricated by alkali treatment enhancing chemical bath deposition. Appl. Surf. Sci. 2010, 256, 2061–2065. [Google Scholar] [CrossRef]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Membr. Sci. 2012, 415, 850–863. [Google Scholar] [CrossRef]
- Hou, D.; Wang, J.; Sun, X.; Ji, Z.; Luan, Z. Preparation and properties of PVDF composite hollow fiber membranes for desalination through direct contact membrane distillation. J. Membr. Sci. 2012, 405, 185–200. [Google Scholar] [CrossRef]
- Lee, E.J.; An, A.K.; He, T.; Woo, Y.C.; Shon, H.K. Electrospun nanofiber membranes incorporating fluorosilane-coated TiO2 nanocomposite for direct contact membrane distillation. J. Membr. Sci. 2016, 520, 145–154. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Shim, W.G.; He, T.; Choi, J.S.; Kim, S.H.; Shon, H.K. Superhydrophobic nanofiber membrane containing carbon nanotubes for high-performance direct contact membrane distillation. J. Membr. Sci. 2016, 502, 158–170. [Google Scholar] [CrossRef]
- Kujawski, W.; Kujawa, J.; Wierzbowska, E.; Cerneaux, S.; Bryjak, M.; Kujawski, J. Influence of hydrophobization conditions and ceramic membranes pore size on their properties in vacuum membrane distillation of water–organic solvent mixtures. J. Membr. Sci. 2016, 499, 442–451. [Google Scholar] [CrossRef]
- Liu, L.; Shen, F.; Zhang, B.; Jiang, H.; Li, J.; Luo, J.; Wu, H.; Khan, R.; Wan, Y. Fabrication of PES-Based Membranes with a High and Stable Desalination Performance for Membrane Distillation. RSC Advance 2016, 6, 107840–107850. [Google Scholar] [CrossRef]
- Wang, M.; Liu, G.; Yu, H.; Lee, S.H.; Wang, L.; Zheng, J.; Wang, T.; Yun, Y.; Lee, J.K. ZnO nanorod array modified PVDF membrane with superhydrophobic surface for vacuum membrane distillation application. ACS Appl. Mater. Interfaces 2018, 10, 13452–13461. [Google Scholar] [CrossRef]
- Parani, S.; Oluwafemi, O.S. Fabrication of superhydrophobic polyethersulfone-ZnO rods composite membrane. Mater. Lett. 2020, 281, 128663. [Google Scholar] [CrossRef]
- Tian, M.; Yin, Y.; Yang, C.; Zhao, B.; Song, J.; Liu, J.; Li, X.M.; He, T. CF4 Plasma Modified Highly Interconnective Porous Polysulfone Membranes for Direct Contact Membrane Distillation (DCMD). Desalination 2015, 369, 105–114. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, B.; Li, X.M.; Wang, Z.; He, B.Q.; He, T.; Jiang, B. CF4 plasma surface modification of asymmetric hydrophilic polyethersulfone membranes for direct contact membrane distillation. J. Membr. Sci. 2012, 407, 164–175. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.M.; Gilron, J.; Kong, D.; Yin, Y.; Oren, Y.; Linder, C.; He, T. CF4 plasma-modified superhydrophobic PVDF membranes for direct contact membrane distillation. J. Membr. Sci. 2014, 456, 155–161. [Google Scholar] [CrossRef]
- Pedram, S.; Mortaheb, H.R.; Fakhouri, H.; Arefi-Khonsari, F. Polytetrafluoroethylene Sputtered PES Membranes for Membrane Distillation: Influence of RF Magnetron Sputtering Conditions. Plasma Chem. Plasma Process. 2017, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Woo, Y.C.; Chen, Y.; Tijing, L.D.; Phuntsho, S.; He, T.; Choi, J.S.; Kim, S.H.; Shon, H.K. CF4 plasma-modified omniphobic electrospun nanofiber membrane for produced water brine treatment by membrane distillation. J. Membr. Sci. 2017, 529, 234–242. [Google Scholar] [CrossRef]
- Horseman, T.; Yin, Y.; Christie, K.S.; Wang, Z.; Tong, T.; Lin, S. Wetting, scaling, and fouling in membrane distillation: State-of-the-art insights on fundamental mechanisms and mitigation strategies. ACS EST Eng. 2020, 1, 117–140. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Zhang, Z.; Wadekar, S.S.; Lokare, O.R.; Vidic, R.D. Comparison of calcium scaling in direct contact membrane distillation (DCMD) and nanofiltration (NF). J. Membr. Sci. 2021, 638, 119647. [Google Scholar] [CrossRef]
- Darton, T.; Annunziata, U.; del Vigo Pisano, F.; Gallego, S. Membrane autopsy helps to provide solutions to operational problems. Desalination 2004, 167, 239–245. [Google Scholar] [CrossRef]
- Srisurichan, S.; Jiraratananon, R.; Fane, A.G. Humic acid fouling in the membrane distillation process. Desalination 2005, 174, 63–72. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M.; Grzechulska, J.; Morawski, A.W. Membrane distillation of NaCl solution containing natural organic matter. J. Membr. Sci. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Bogler, A.; Lin, S.; Bar-Zeev, E. Biofouling of membrane distillation, forward osmosis and pressure retarded osmosis: Principles, impacts and future directions. J. Membr. Sci. 2017, 542, 378–398. [Google Scholar] [CrossRef]
- Gryta, M. The assessment of microorganism growth in the membrane distillation system. Desalination 2002, 142, 79–88. [Google Scholar] [CrossRef]
- Krivorot, M.; Kushmaro, A.; Oren, Y.; Gilron, J. Factors affecting biofilm formation and biofouling in membrane distillation of seawater. J. Membr. Sci. 2011, 376, 15–24. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Vrouwenvelder, J.S.; Kruithof, J.C.; Van Loosdrecht, M.C.M. Integrated approach for biofouling control. Water Sci. Technol. 2010, 62, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Nthunya, L.N.; Gutierrez, L.; Nxumalo, E.N.; Verliefde, A.R.; Mhlanga, S.D.; Onyango, M.S. f-MWCNTs/AgNPs-coated superhydrophobic PVDF nanofibre membrane for organic, colloidal, and biofouling mitigation in direct contact membrane distillation. J. Environ. Chem. Eng. 2020, 8, 103654. [Google Scholar] [CrossRef]
- Yun, Y.; Ma, R.; Zhang, W.; Fane, A.G.; Li, J. Direct contact membrane distillation mechanism for high concentration NaCl solutions. Desalination 2006, 188, 251–262. [Google Scholar] [CrossRef]
- Bonyadi, S.; Chung, T.S. Flux enhancement in membrane distillation by fabrication of dual layer hydrophilic–hydrophobic hollow fiber membranes. J. Membr. Sci. 2007, 306, 134–146. [Google Scholar] [CrossRef]
- Bilad, M.R.; FAlMarzooqi, F.A.; Arafat, H.A. New Concept for Dual-Layer Hydrophilic/ Hydrophobic Composite Membrane for Membrane Distillation. J. Membr. Sep. Technol. 2015, 4, 122–133. [Google Scholar] [CrossRef]
- Schofield, R.W.; Fane, A.G.; Fell, C.J.D.; Macoun, R. Factors affecting flux in membrane distillation. Desalination 1990, 77, 279–294. [Google Scholar] [CrossRef]
- Manzoor, K.; Khan, S.J.; Jamal, Y.; Shahzad, M.A. Heat extraction and brine management from salinity gradient solar pond and membrane distillation. Chem. Eng. Res. Des. 2017, 118, 226–237. [Google Scholar] [CrossRef]
- Boubakri, A.; Hafiane, A.; Bouguecha, S.A.T. Direct contact membrane distillation: Capability to desalt raw water. Arab. J. Chem. 2017, 10, 3475–3481. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Song, K.G.; Kim, J.; Shin, J.; Maeng, S.K.; Park, J. Feasibility of membrane distillation process for potable water reuse: A barrier for dissolved organic matters and pharmaceuticals. J. Hazard. Mater. 2021, 409, 124499. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zodrow, K.R.; Szemraj, P.B.; Li, Q. Photothermal nanocomposite membranes for direct solar membrane distillation. J. Mater. Chem. 2017, 5, 23712–23719. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A low cost hydrophobic kaolin hollow fiber membrane (h-KHFM) for arsenic removal from aqueous solution via direct contact membrane distillation. Sep. Purif. Technol. 2019, 214, 31–39. [Google Scholar] [CrossRef]
- Sivakumar, M.; Ramezanianpour, M.; O’Halloran, G. Mine water treatment using a vacuum membrane distillation system. APCBEE Procedia 2013, 5, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Aloulou, H.; Aloulou, W.; Daramola, M.O.; Amar, R.B. Silane-grafted sand membrane for the treatment of oily wastewater via air gap membrane distillation: Study of the efficiency in comparison with microfiltration and ultrafiltration ceramic membranes. Mater. Chem. Phys. 2021, 261, 124186. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Aboosadi, Z.A.; Mansourizadeh, A.; Honarvar, B. Surface modified porous polyetherimide hollow fiber membrane for sweeping gas membrane distillation of dyeing wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125439. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G.; Harasimowicz, M.; Chmielewski, A.G. Membrane processes in nuclear technology-application for liquid radioactive waste treatment. Sep. Purif. Technol. 2001, 22, 617–625. [Google Scholar] [CrossRef]
- Zakrzewska-Trznadel, G.; Harasimowicz, M.; Chmielewski, A.G. Concentration of radioactive components in liquid low-level radioactive waste by membrane distillation. J. Membr. Sci. 1999, 163, 257–264. [Google Scholar] [CrossRef]
- Wen, X.; Li, F.; Zhao, X. Removal of nuclides and boron from highly saline radioactive wastewater by direct contact membrane distillation. Desalination 2016, 394, 101–107. [Google Scholar] [CrossRef]
- Nie, X.; Hu, X.; Liu, C.; Xia, X.; Dong, F. Decontamination of uranium contained low-level radioactive wastewater from UO2 fuel element industry with vacuum membrane distillation. Desalination 2021, 516, 115226. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hakami, M.; Zacharof, M.P.; Abu Homod, H.; Alsadun, A. Mercury, arsenic and lead removal by air gap membrane distillation: Experimental study. Water 2020, 12, 1574. [Google Scholar] [CrossRef]
- Koeman-Stein, N.E.; Creusen, R.J.M.; Zijlstra, M.; Groot, C.K.; Van den Broek, W.B.P. Membrane distillation of industrial cooling tower blowdown water. Water Resour. Ind. 2016, 14, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Macedonio, F.; Ali, A.; Poerio, T.; El-Sayed, E.; Drioli, E.; Abdel-Jawad, M. Direct contact membrane distillation for treatment of oilfield produced water. Sep. Purif. Technol. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Lau, W.J.; Ismail, A.F.; Veerasamy, D. Membrane distillation technology for treatment of wastewater from rubber industry in Malaysia. Procedia CIRP 2015, 26, 792–796. [Google Scholar] [CrossRef] [Green Version]
- Mokhtar, N.M.; Lau, W.J.; Ismail, A.F. Dye wastewater treatment by direct contact membrane distillation using polyvinylidene fluoride hollow fiber membranes. J. Polym. Eng. 2015, 35, 471–479. [Google Scholar] [CrossRef]
- Hausmann, A.; P Sanciolo, P.; Vasiljevic, T.; Kulozik, U.; Duke, M. Performance assessment of membrane distillation for skim milk and whey processing. J. Dairy Sci. 2014, 97, 56–71. [Google Scholar] [CrossRef] [Green Version]
- Mozia, S.; Tomaszewska, M.; Morawski, A.W. Photocatalytic membrane reactor (PMR) coupling photocatalysis and membrane distillation—Effectiveness of removal of three azo dyes from water. Catal. Today 2007, 129, 3–8. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Fane, A.G.; Pasquier, A.C.S.; Bing, W.; Wong, F.S. Experimental study and design of a submerged membrane distillation bioreactor. Chem. Eng. Technol. 2009, 32, 38–44. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Conidi, C.; Cassano, A.; Aljlil, S.; Alharbi, O.A.; Drioli, E. Direct contact membrane distillation for the concentration of clarified orange juice. J. Food Eng. 2016, 187, 37–43. [Google Scholar] [CrossRef]
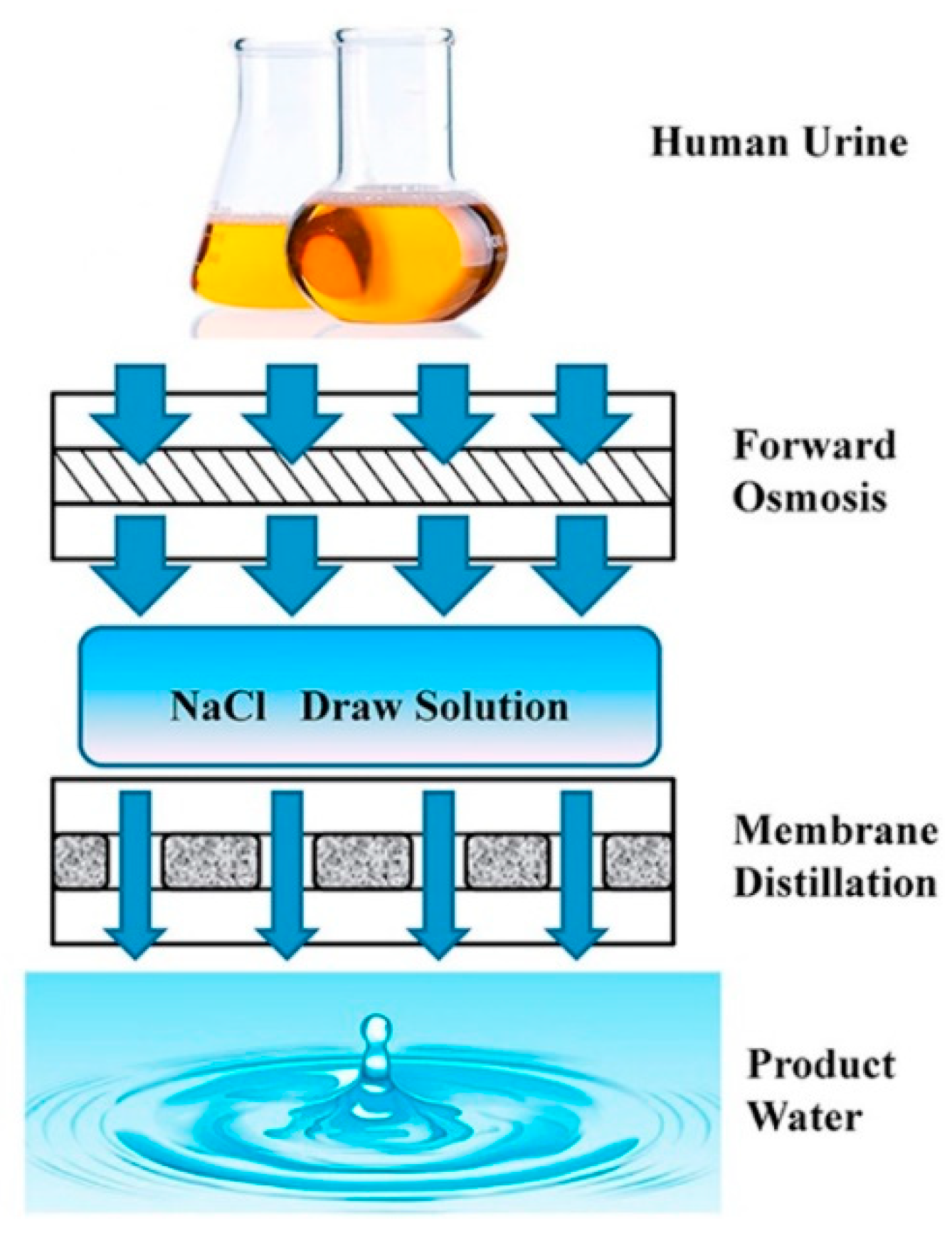
- Liu, Q.; Liu, C.; Zhao, L.; Ma, W.; Liu, H.; Ma, J. Integrated forward osmosis-membrane distillation process for human urine treatment. Water Res. 2016, 91, 45–54. [Google Scholar] [CrossRef]
- Bindels, M.; Carvalho, J.; Gonzalez, C.B.; Brand, N.; Nelemans, B. Techno-economic assessment of seawater reverse osmosis (SWRO) brine treatment with air gap membrane distillation (AGMD). Desalination 2020, 489, 114532. [Google Scholar] [CrossRef]
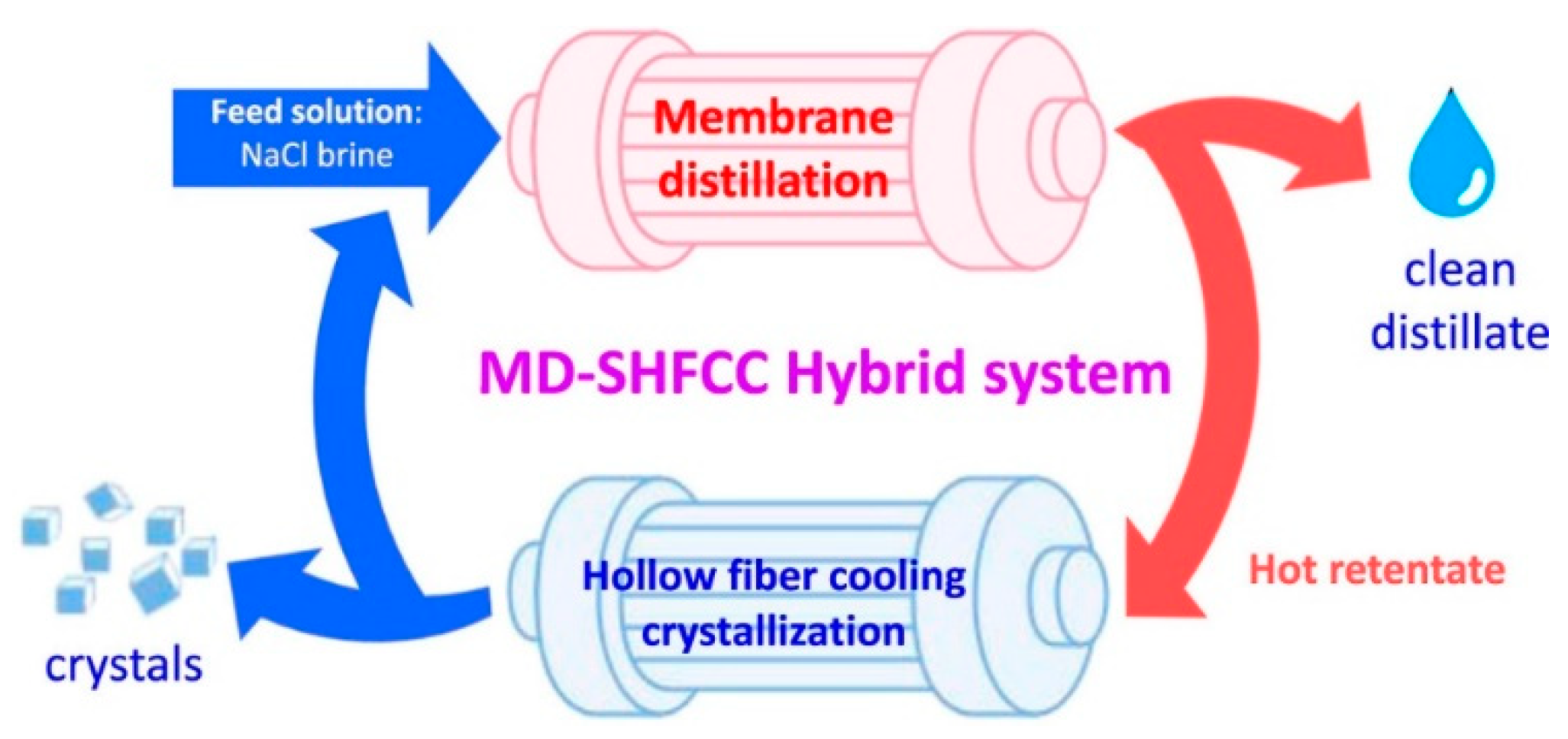
- Luo, L.; Zhao, J.; Chung, T.S. Integration of membrane distillation (MD) and solid hollow fiber cooling crystallization (SHFCC) systems for simultaneous production of water and salt crystals. J. Membr. Sci. 2018, 564, 905–915. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Nghiem, L.D.; Lee, S.; Vigneswaran, S. Membrane distillation crystallization for brine mining and zero liquid discharge: Opportunities, challenges, and recent progress. Environ. Sci. Water Res. Technol. 2019, 5, 1202–1221. [Google Scholar] [CrossRef]
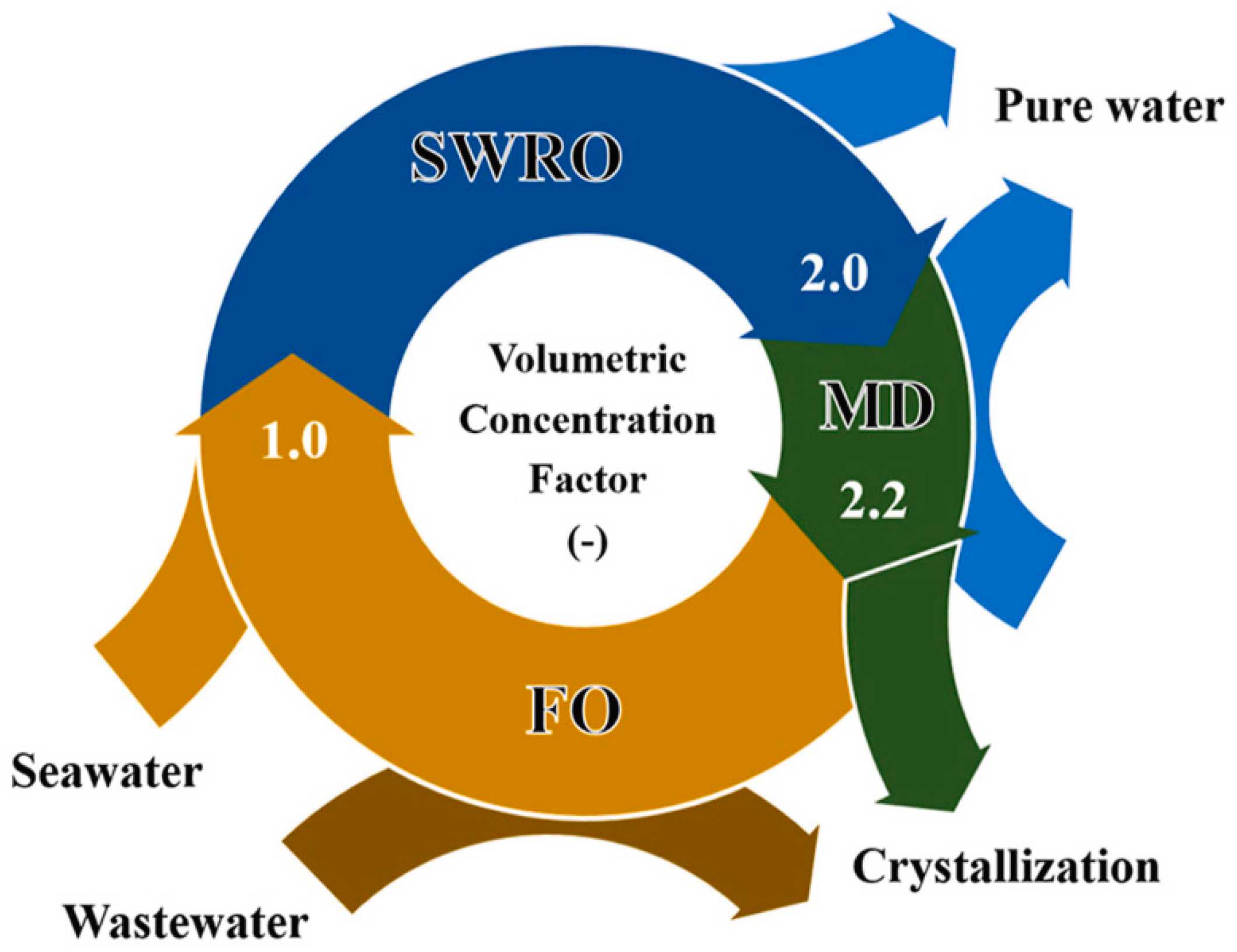
- Son, H.S.; Soukane, S.; Lee, J.; Kim, Y.; Kim, Y.-D.; Ghaffour, N. Towards sustainable circular brine reclamation using seawater reverse osmosis, membrane distillation and forward osmosis hybrids: An experimental investigation. J. Environ. Manag. 2021, 293, 112836. [Google Scholar] [CrossRef]
- Al-Shammiri, M.; Safar, M. Multi-effect distillation plants: State of the art. Desalination 1999, 126, 45–59. [Google Scholar] [CrossRef]
- Engelien, H.K.; Skogestad, S. Multi-effect distillation applied to an industrial case study. Chem. Eng. Process. Process Intensif. 2005, 44, 819–826. [Google Scholar] [CrossRef]
- Skuse, C.; Gallego-Schmid, A.; Azapagic, A.; Gorgojo, P. Can emerging membrane-based desalination technologies replace reverse osmosis? Desalination 2020, 500, 114844. [Google Scholar] [CrossRef]
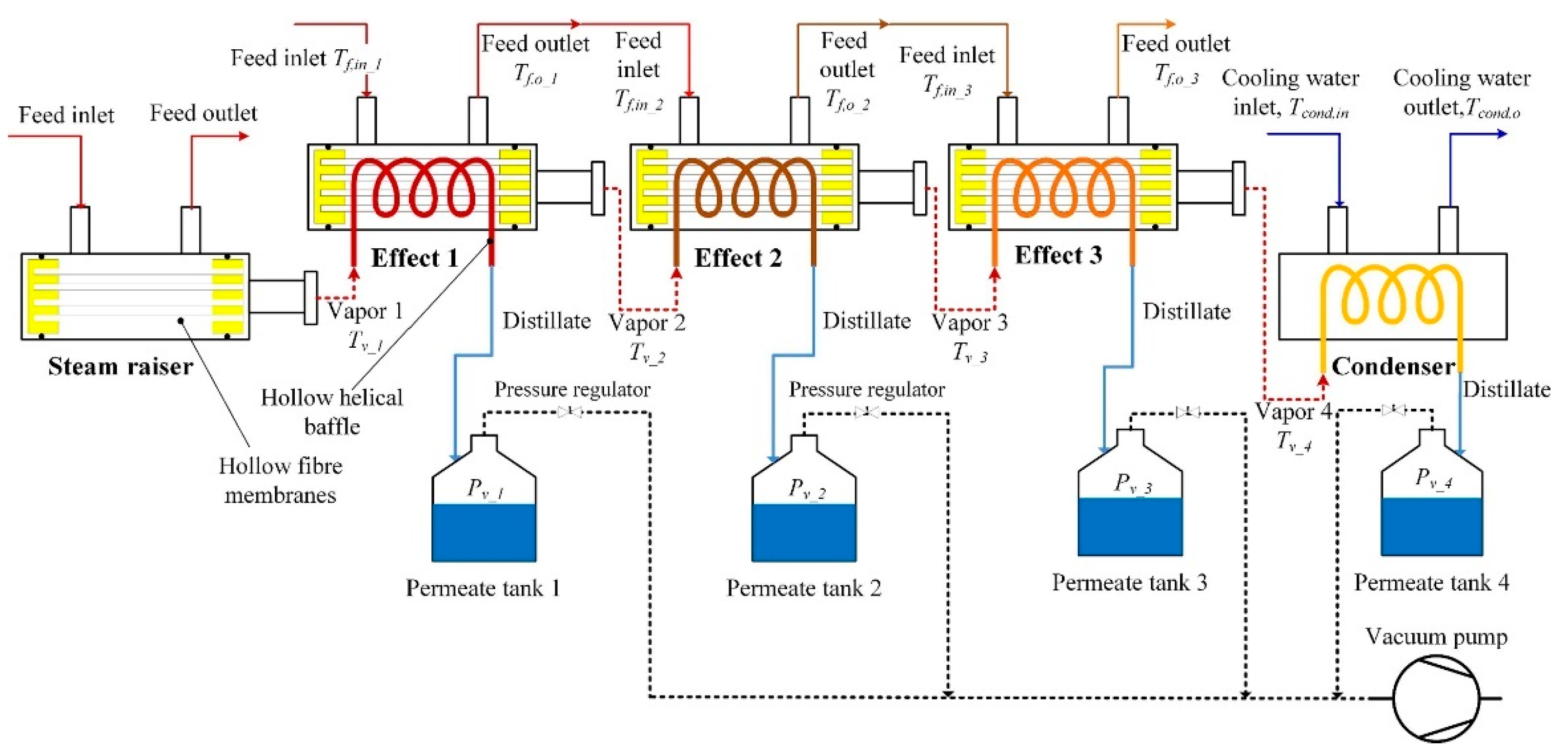
- Li, Q.; Omar, A.; Cha-Umpong, W.; Liu, Q.; Li, X.; Wen, J.; Wang, Y.; Razmjou, A.; Guan, J.; Taylor, R.A. The potential of hollow fiber vacuum multi-effect membrane distillation for brine treatment. Appl. Energy 2020, 276, 115437. [Google Scholar] [CrossRef]
- Turek, M.; Bandura, B. Renewable energy by reverse electrodialysis. Desalination 2007, 205, 67–74. [Google Scholar] [CrossRef]
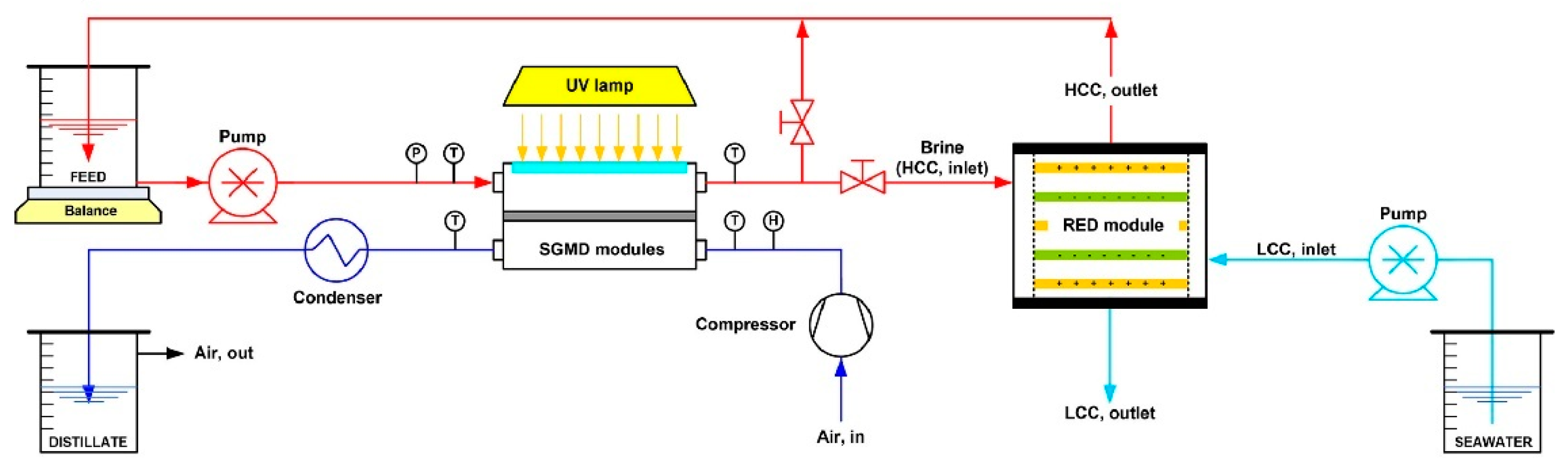
- Avci, A.H.; Santoro, S.; Politano, A.; Propato, M.; Micieli, M.; Aquino, M.; Wenjuan, Z.; Curcio, E. Photothermal Sweeping Gas Membrane Distillation and Reverse Electrodialysis for light-to-heat-to-power conversion. Chem. Eng. Process.-Process Intensif. 2021, 164, 108382. [Google Scholar] [CrossRef]
- Tufa, R.A.; Noviello, Y.; Di Profio, G.; Macedonio, F.; Ali, A.; Drioli, E.; Fontananova, E.; Bouzek, K.; Curcio, E. Integrated membrane distillation-reverse electrodialysis system for energy-efficient seawater desalination. Appl. Energy 2019, 253, 113551. [Google Scholar] [CrossRef]
- Hussain, A.; Janson, A.; Matar, J.M.; Adham, S. Membrane distillation: Recent technological developments and advancements in membrane materials. Emergent Mater. 2021, 1–21. [Google Scholar] [CrossRef]
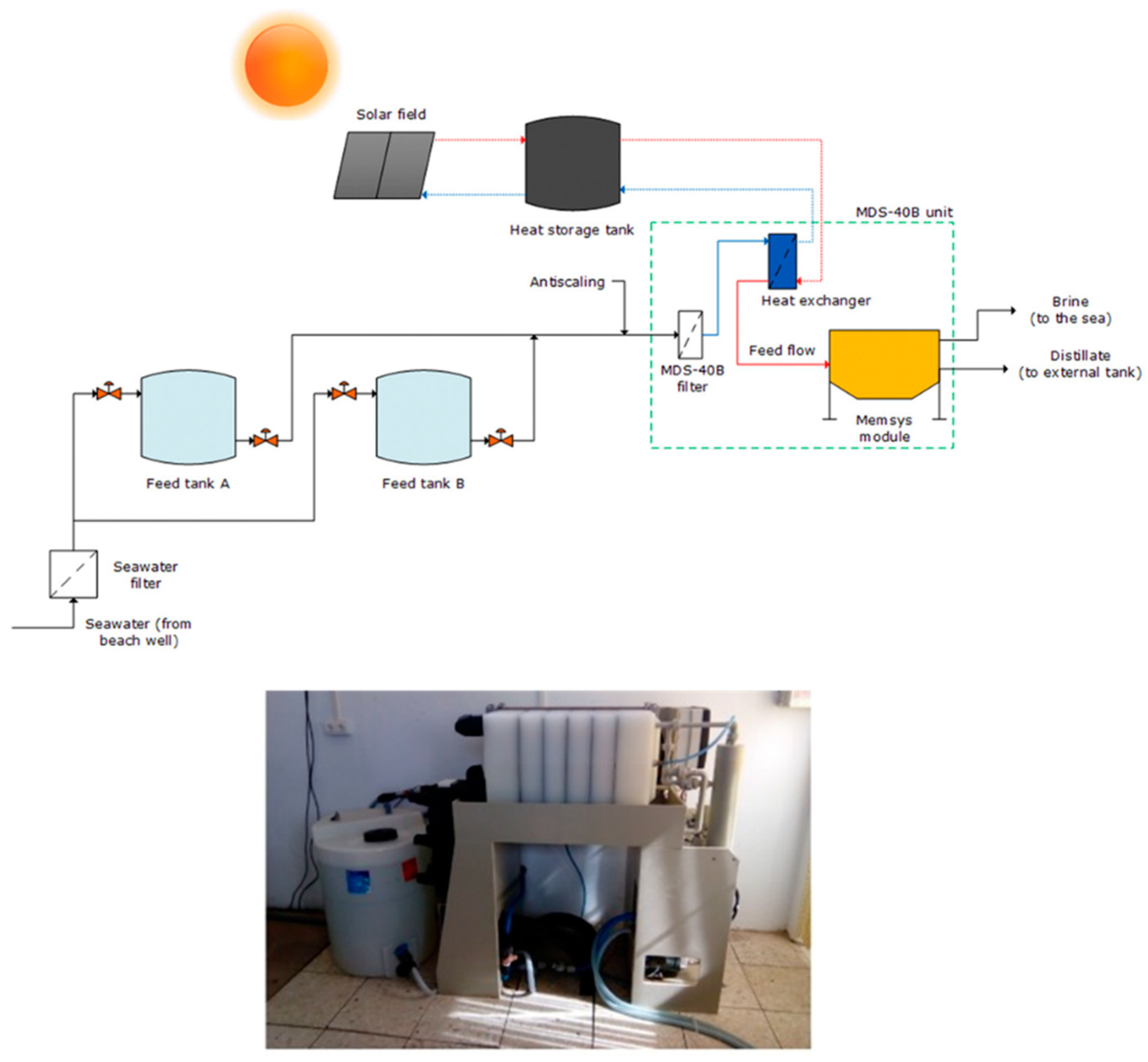
- Andrés-Mañas, J.A.; Ruiz-Aguirre, A.; Acién, F.G.; Zaragoza, G. Assessment of a pilot system for seawater desalination based on vacuum multi-effect membrane distillation with enhanced heat recovery. Desalination 2018, 443, 110–121. [Google Scholar] [CrossRef]
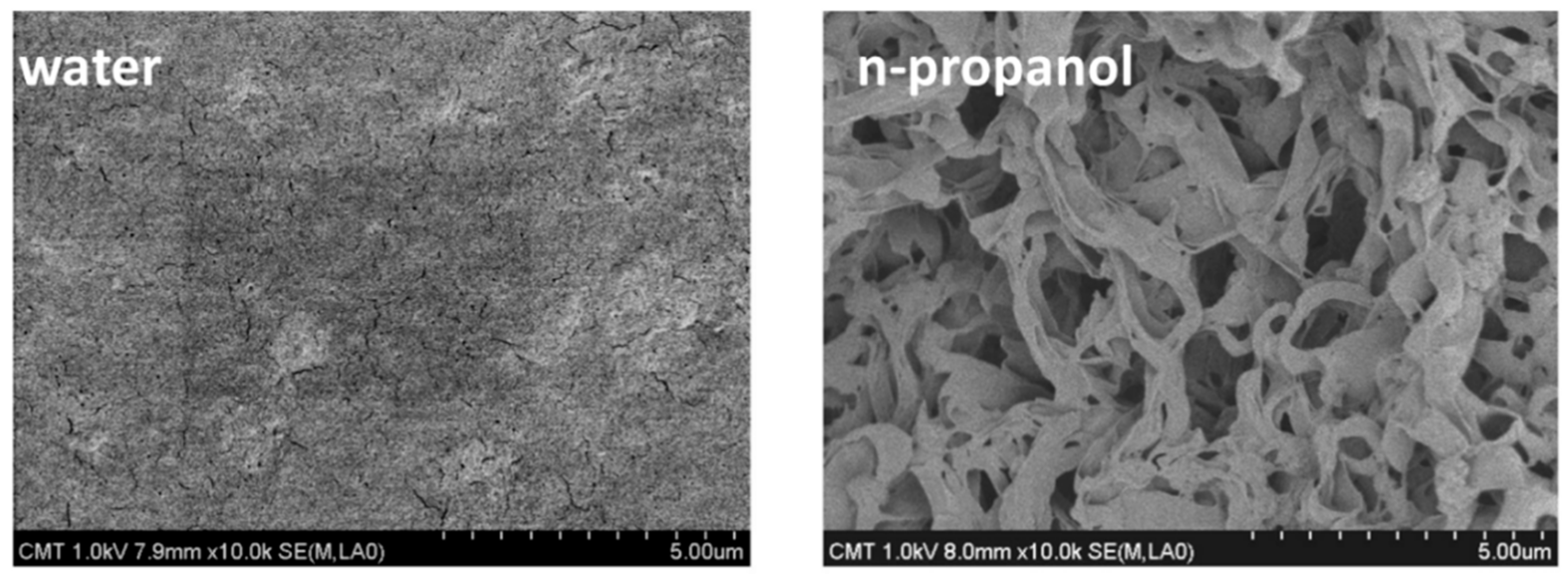
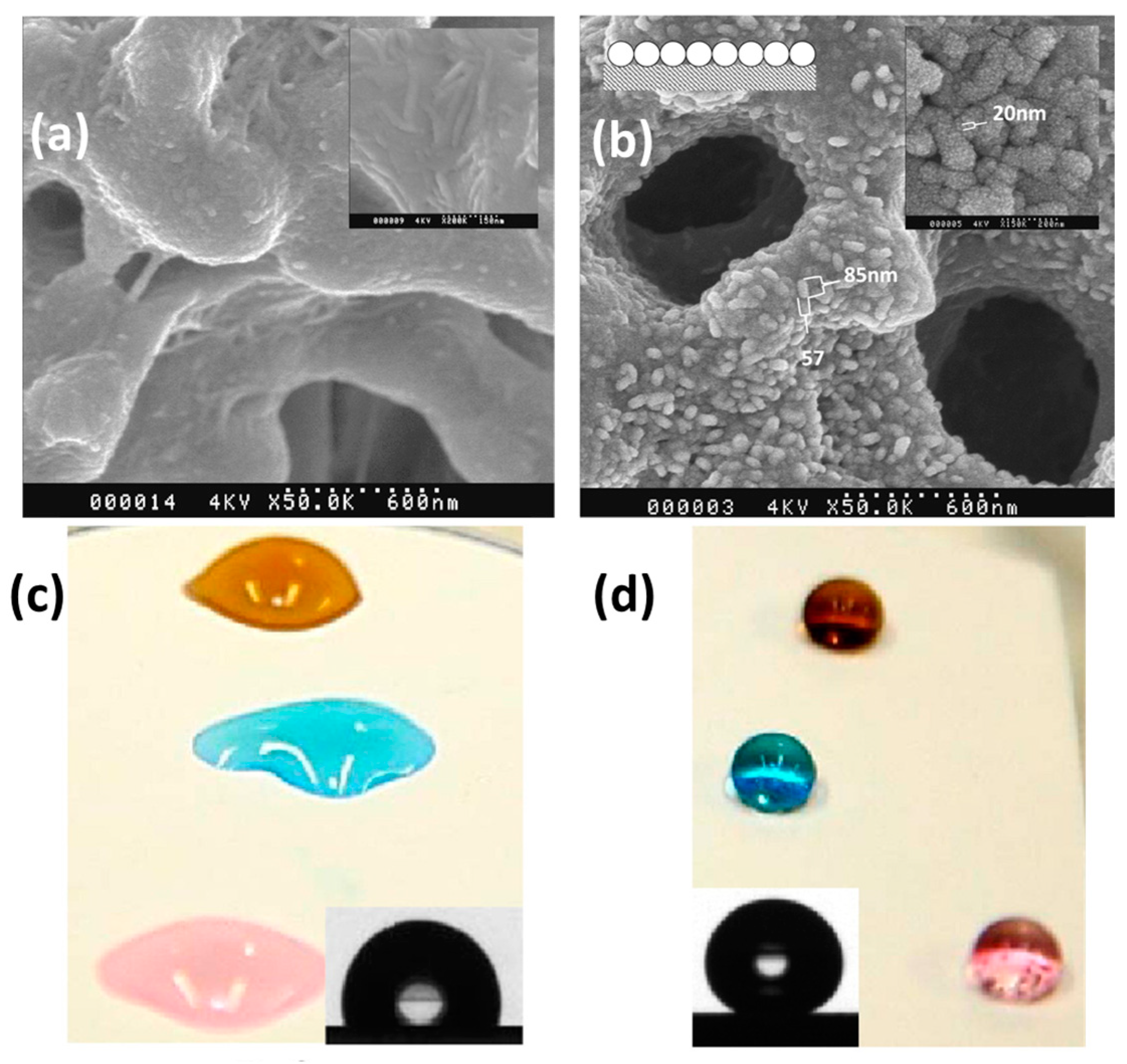
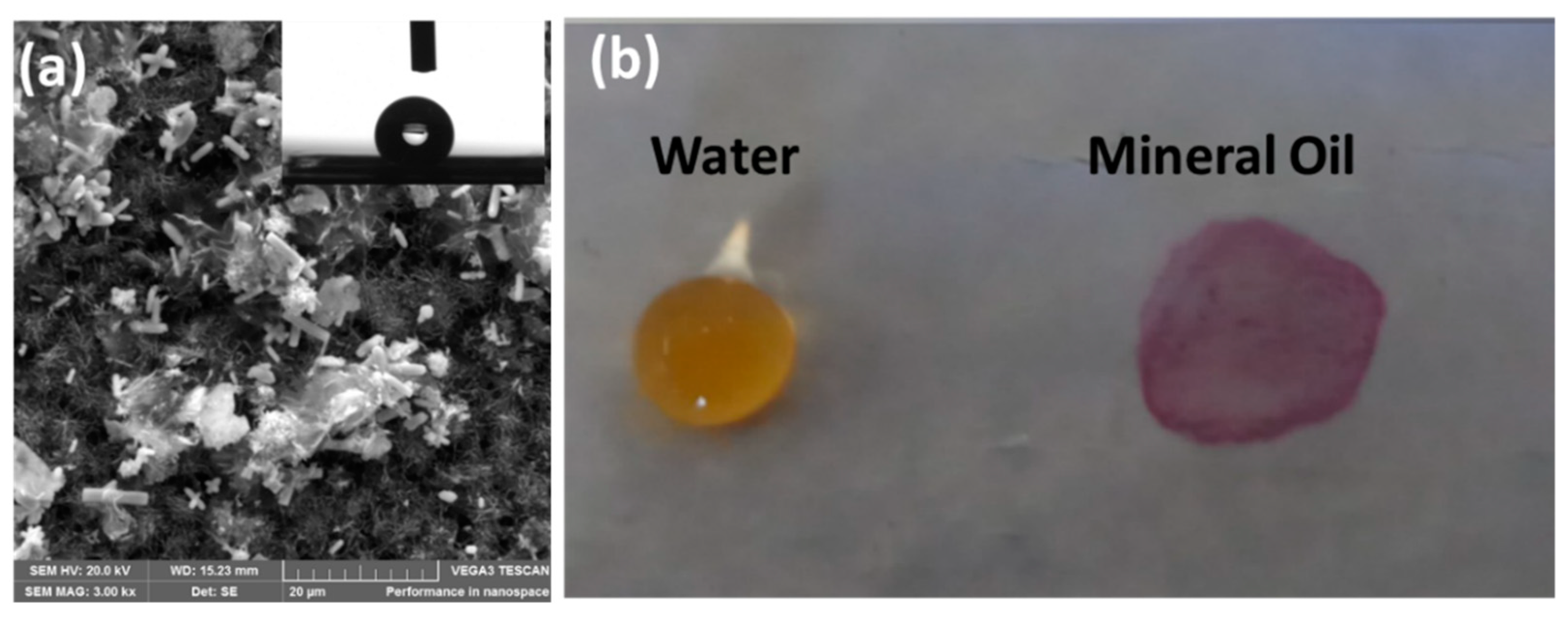
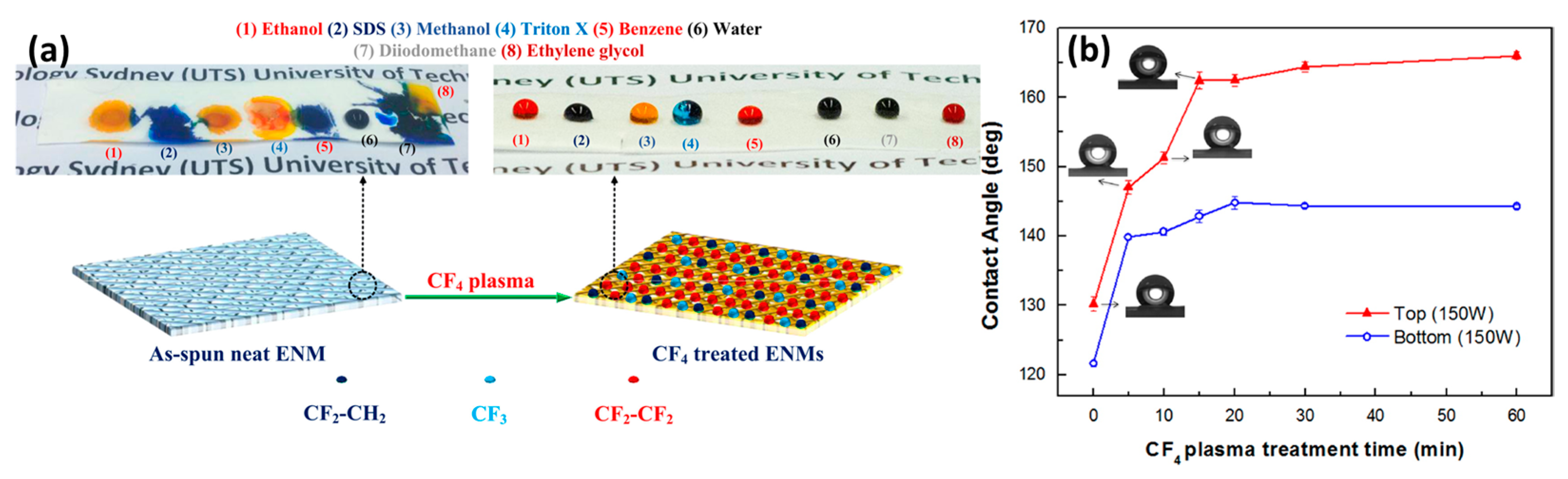
| Configuration | Advantages | Disadvantages | Applications |
|---|---|---|---|
| DCMD | Simple design and operation, high flux | High conductive heat loss, low non-volatile rejection | Desalination of sea water, brackish water, removal of various contaminants |
| VMD | Negligible heat loss, high flux | Risk of membrane wetting due to high pressure difference | Concentration of fruit juice, inorganic acids, recovery of volatile organic compounds, concentration of RO brine |
| SGMD | Low conductive heat loss, high flux | Requires large condenser, expensive | Concentration of fruit juice, removal of non-volatile organics, ethanol processing |
| AGMD | High non-volatile rejection, high purity Low membrane wetting, low heat loss | High mass transfer resistance, low permeate flux | Wastewater produced in water treatment, separation/removal of inorganic acids and organic compounds |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parani, S.; Oluwafemi, O.S. Membrane Distillation: Recent Configurations, Membrane Surface Engineering, and Applications. Membranes 2021, 11, 934. https://doi.org/10.3390/membranes11120934
Parani S, Oluwafemi OS. Membrane Distillation: Recent Configurations, Membrane Surface Engineering, and Applications. Membranes. 2021; 11(12):934. https://doi.org/10.3390/membranes11120934
Chicago/Turabian StyleParani, Sundararajan, and Oluwatobi Samuel Oluwafemi. 2021. "Membrane Distillation: Recent Configurations, Membrane Surface Engineering, and Applications" Membranes 11, no. 12: 934. https://doi.org/10.3390/membranes11120934
APA StyleParani, S., & Oluwafemi, O. S. (2021). Membrane Distillation: Recent Configurations, Membrane Surface Engineering, and Applications. Membranes, 11(12), 934. https://doi.org/10.3390/membranes11120934