Fabrication of Polysulfone-Surface Functionalized Mesoporous Silica Nanocomposite Membranes for Removal of Heavy Metal Ions from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
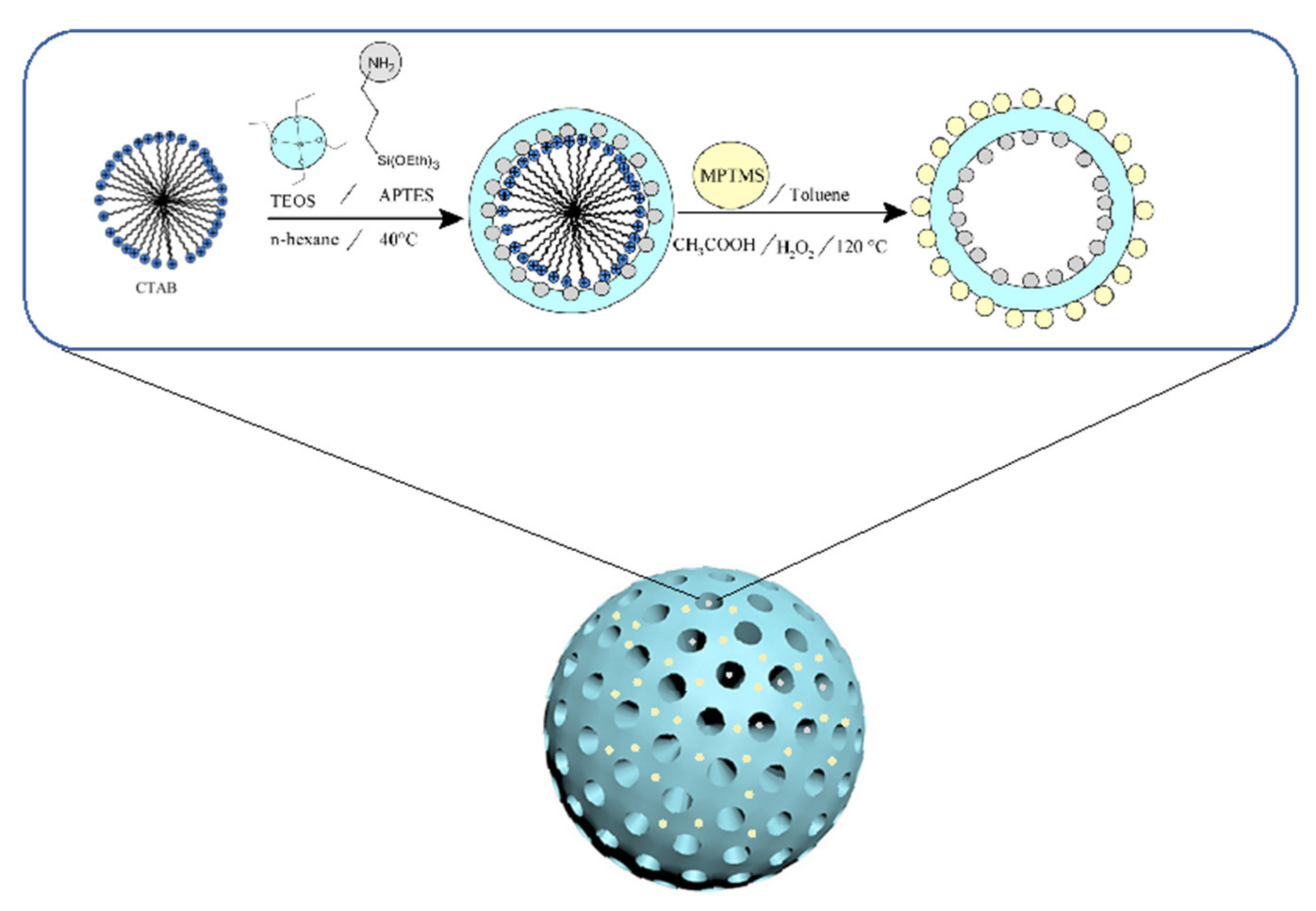
2.2. Surface Functionalization of Mesoporous Silica Nanoparticles
2.3. Membrane Preparation
2.4. Nanoparticles and Membrane Characterization
2.5. Membrane Performance
3. Results and Discussion
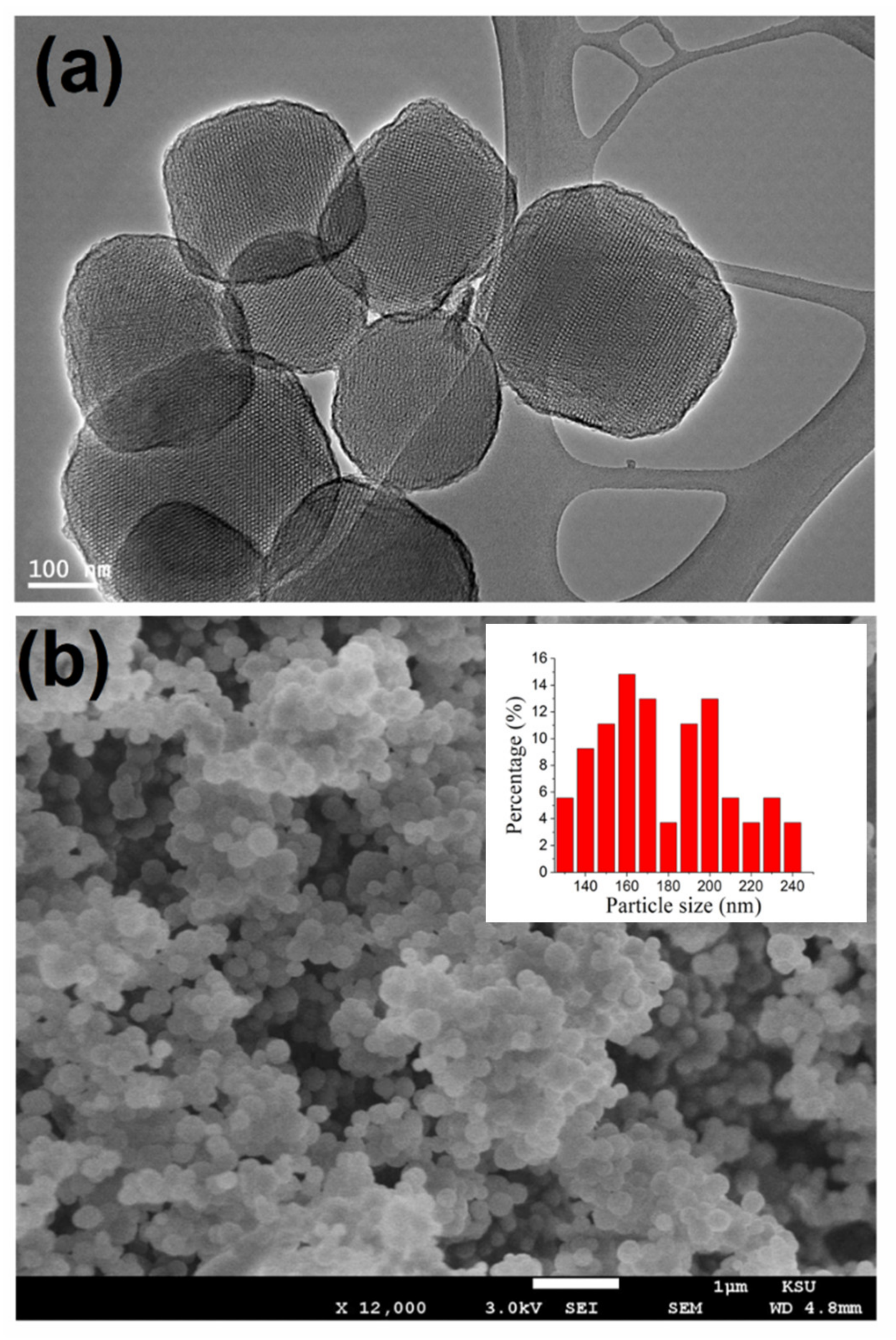
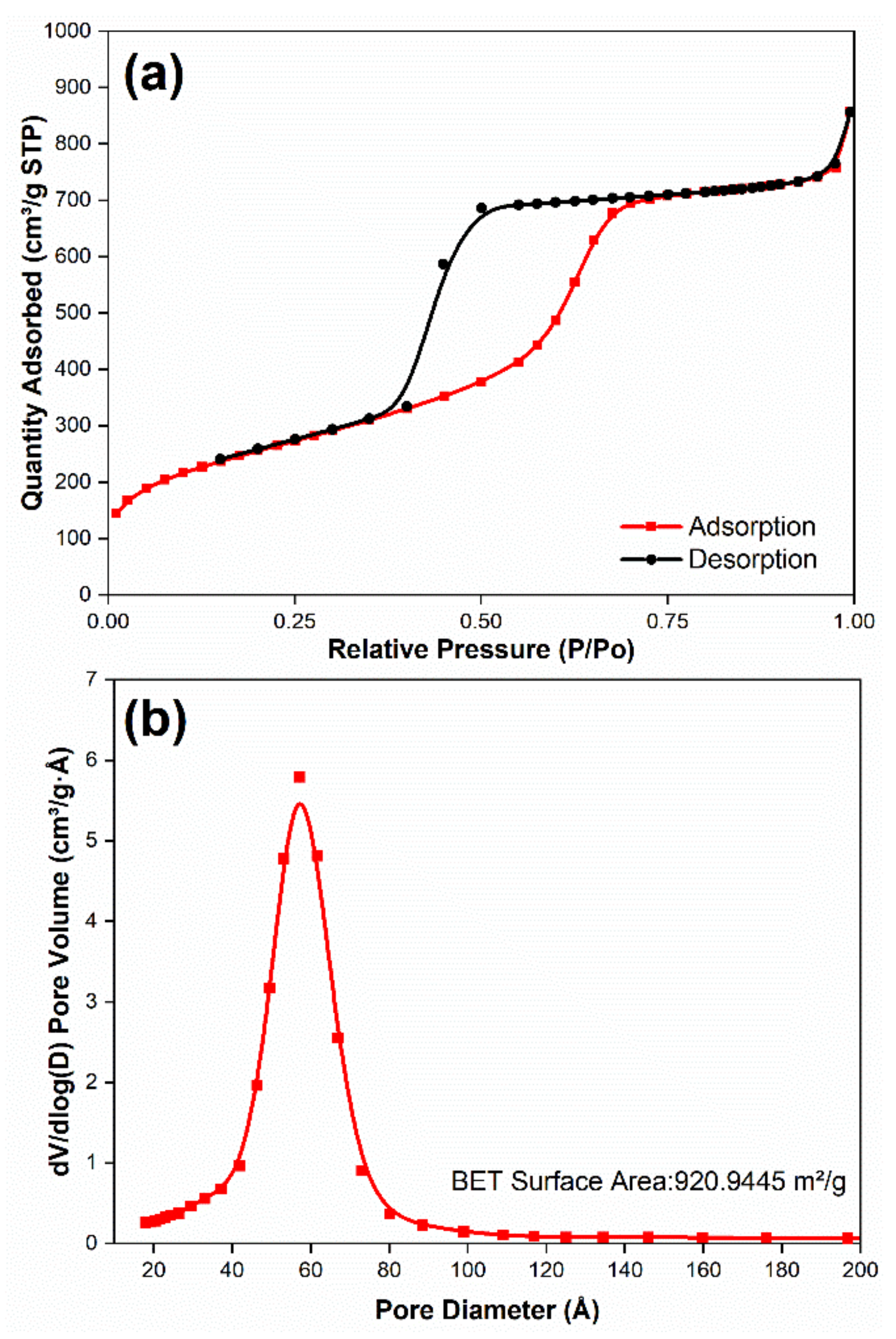
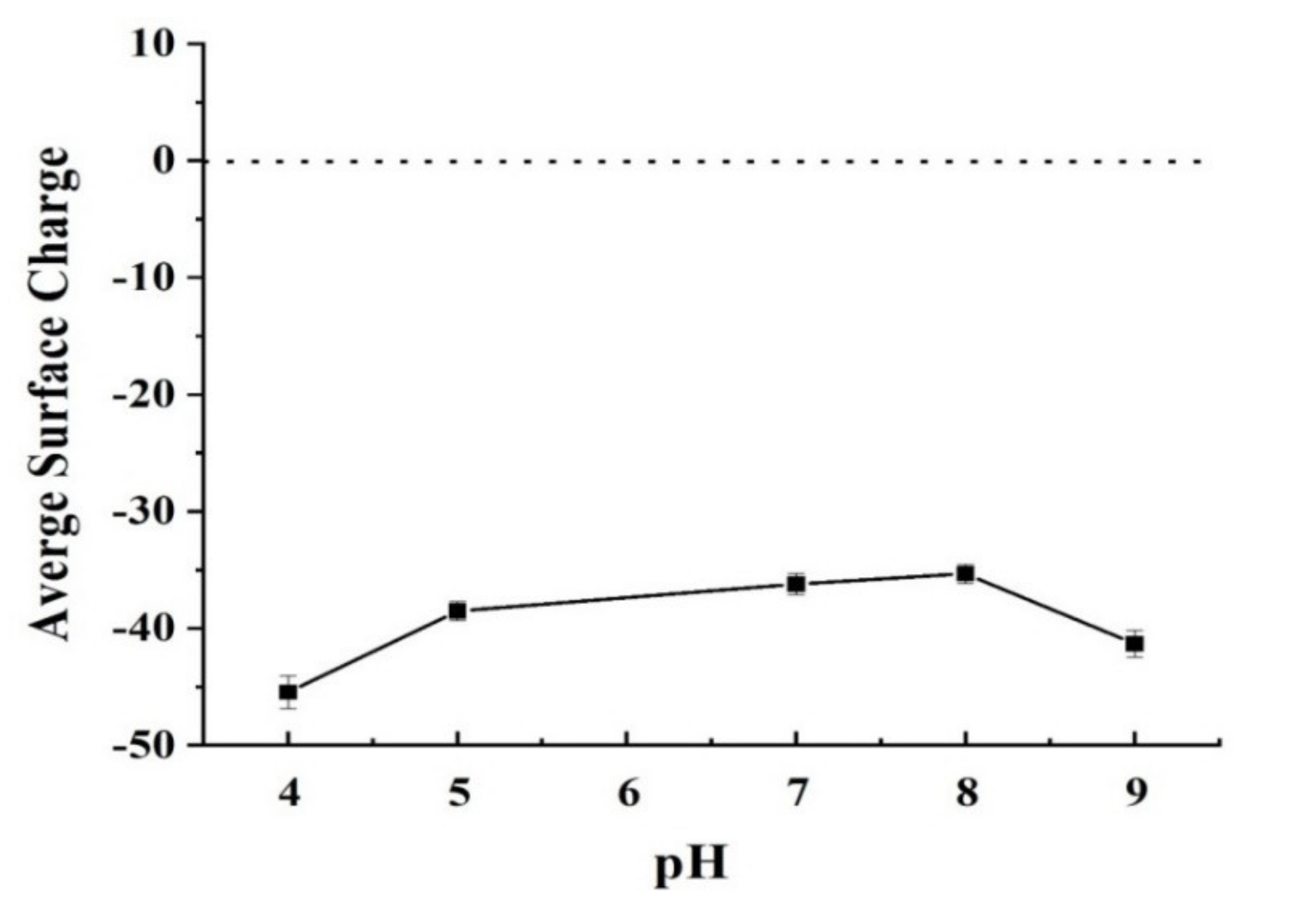
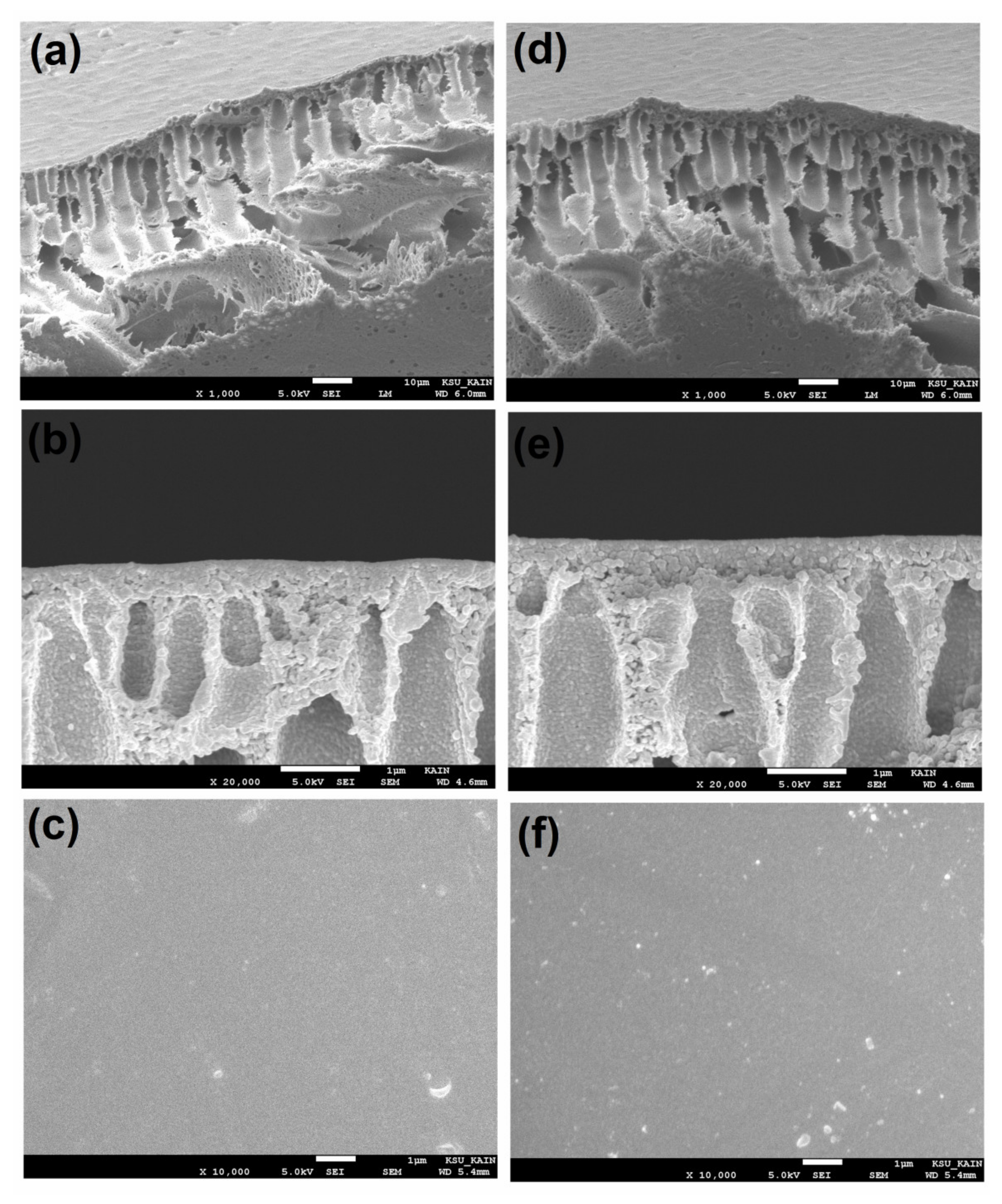
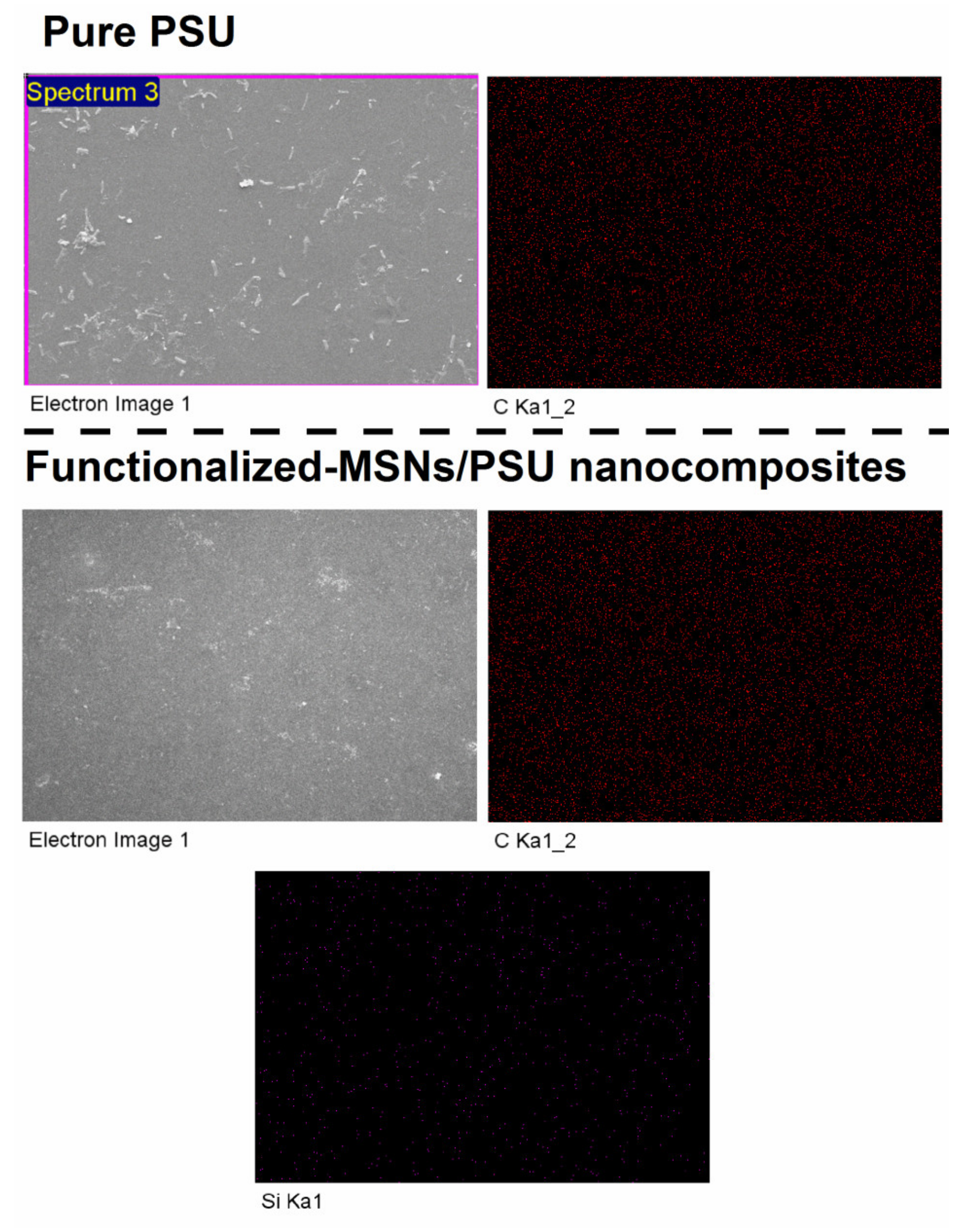
3.1. Analyze the Morphology
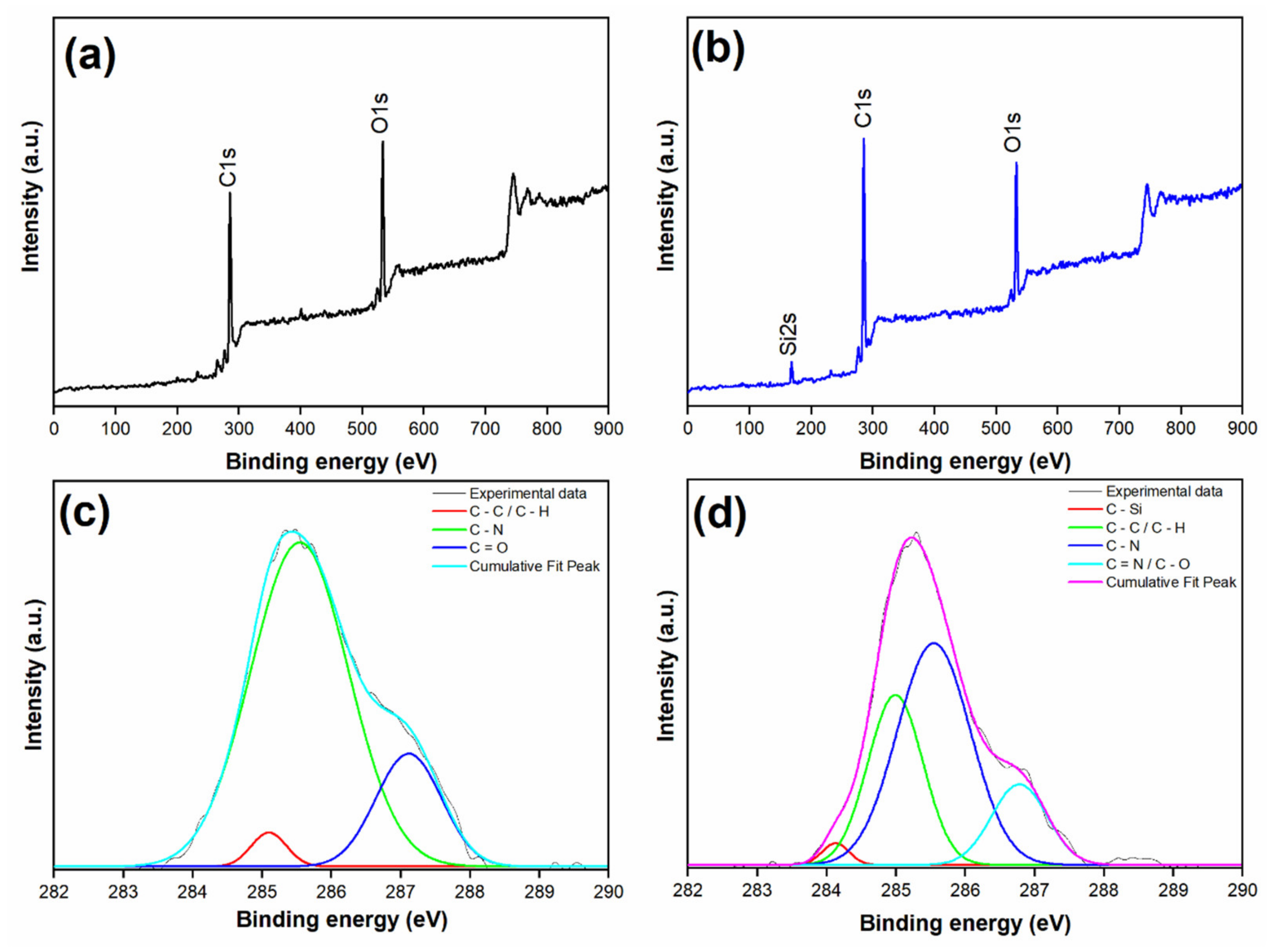
3.2. Spectral Analysis of Membranes
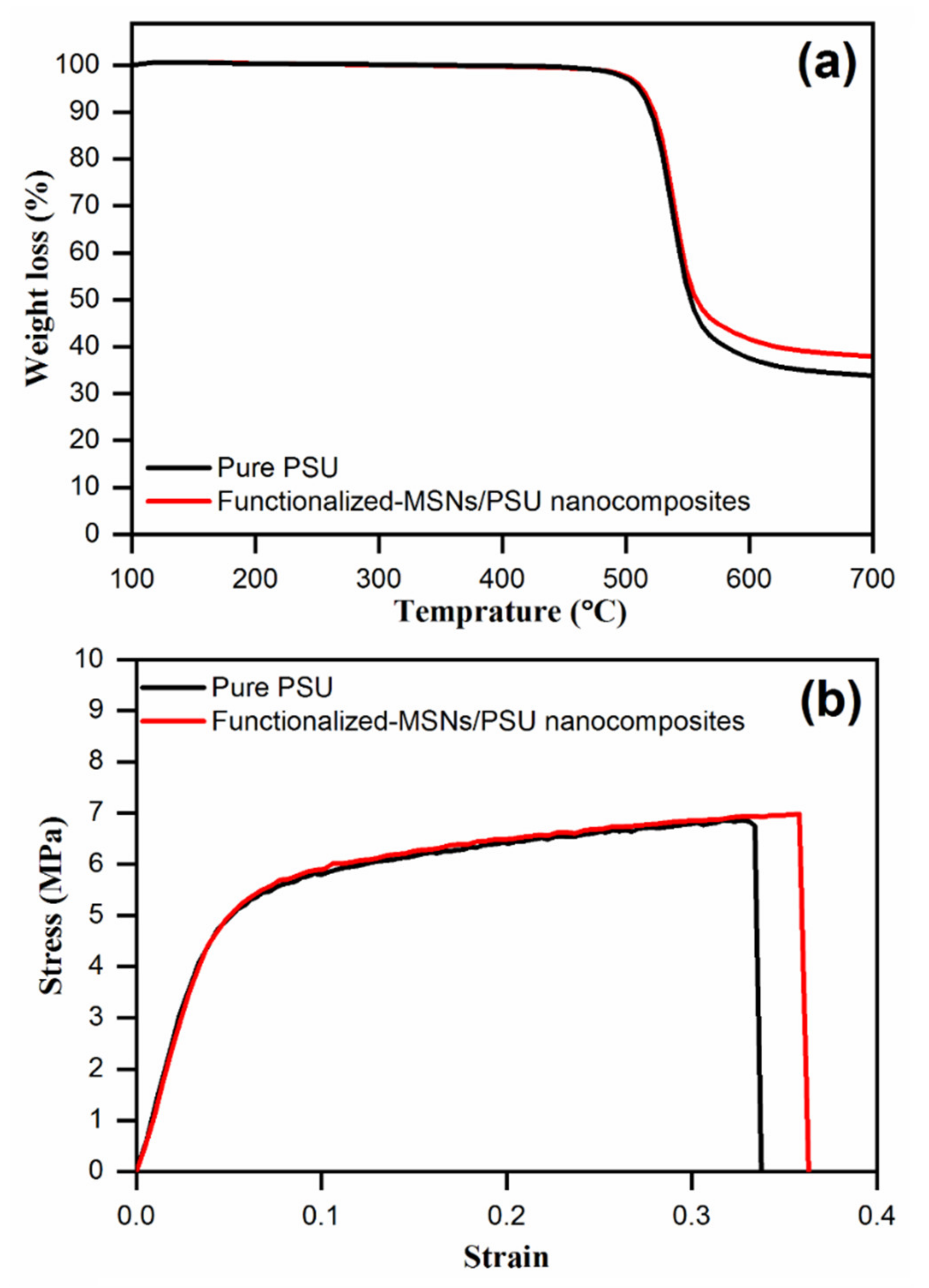
3.3. Thermal Stability of the Membranes
3.4. Mechanical Properties of Membrane
3.5. Equilibrium Water Content and Porosity of Membrane
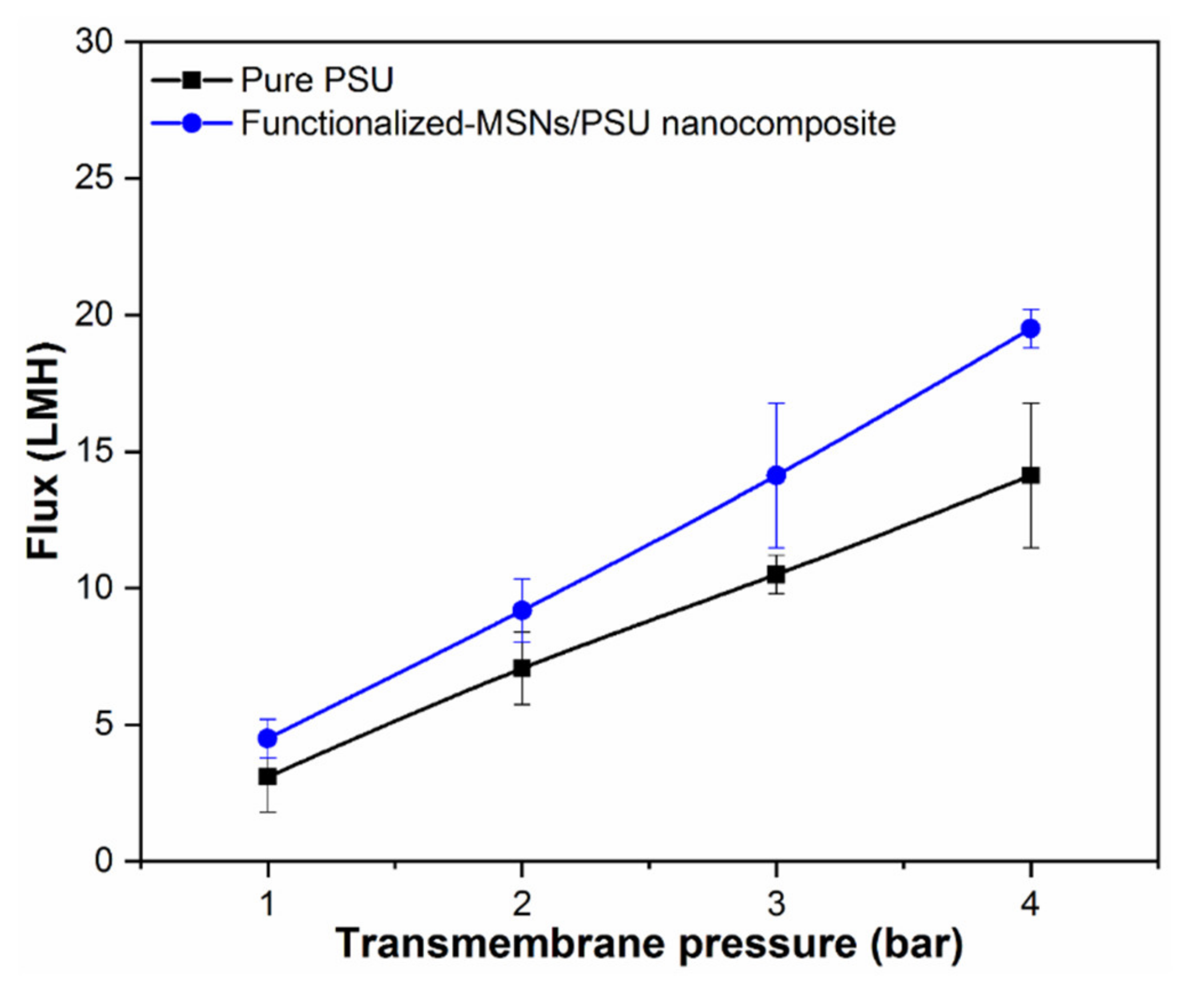
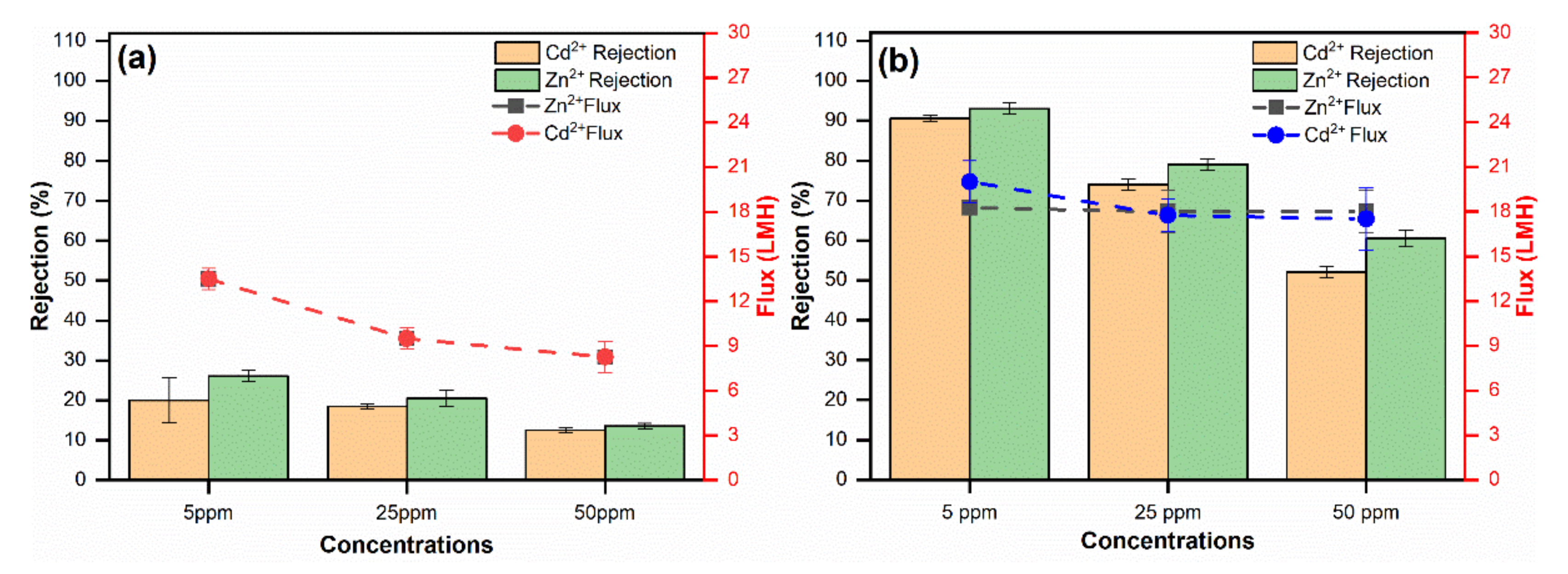
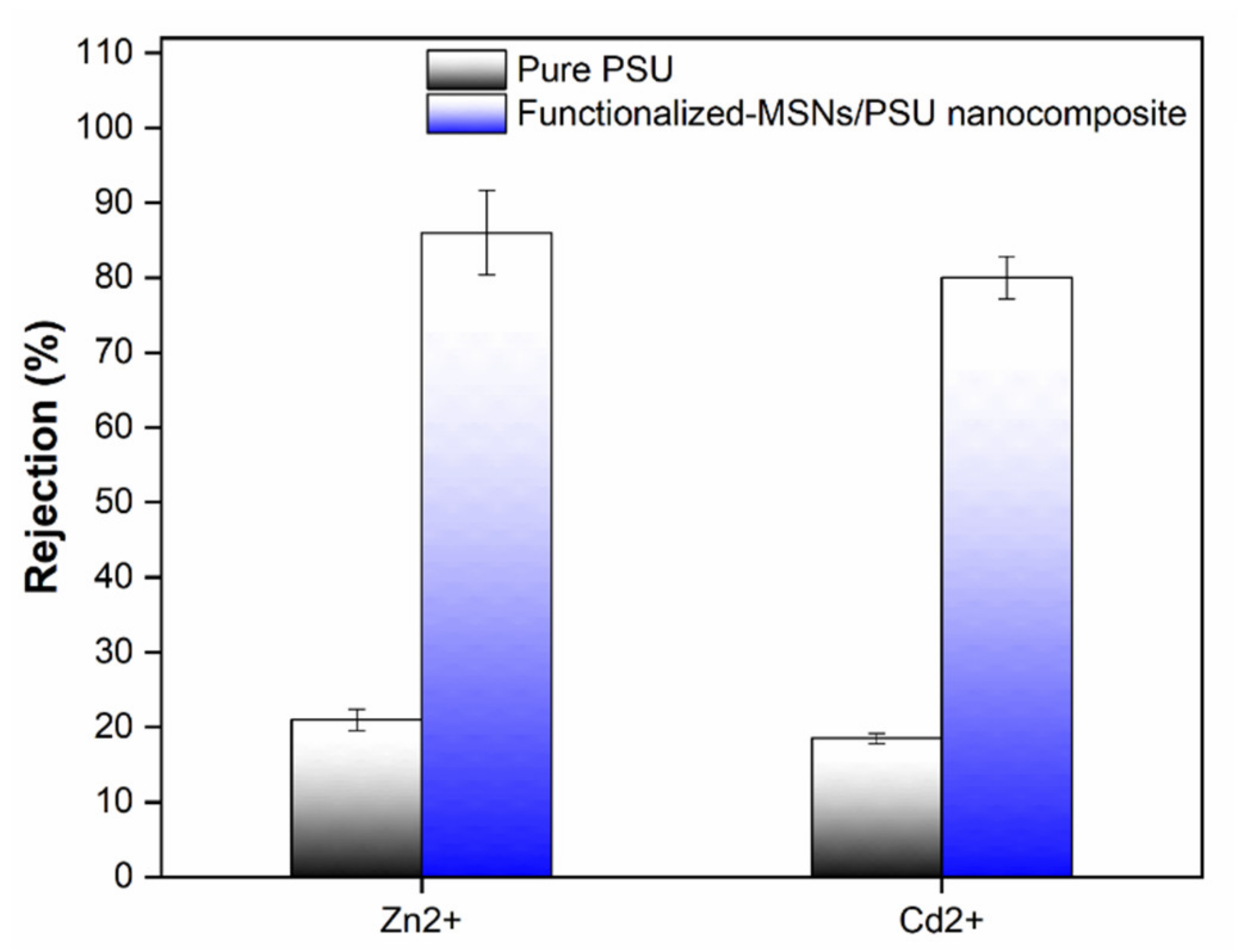
3.6. Membrane Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The Critical Need for Increased Selectivity, Not Increased Water Permeability, for Desalination Membranes. Environ. Sci. Technol. Lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- Abbaszadeh, M.; Krizak, D.; Kundu, S. Layer-by-layer assembly of graphene oxide nanoplatelets embedded desalination membranes with improved chlorine resistance. Desalination 2019, 470, 114116. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- UNESCO. The United Nations World Water Development Report 2019: Leaving No One Behind; UNESCO Publishing: Paris, France, 2019; ISBN 978-92-3-100309-7. [Google Scholar]
- United Nations Children’s Fund (UNICEF); World Health Organization (WHO). Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; United Nations Children’s Fund (UNICEF): New York, NY, USA; World Health Organization (WHO): Geneva, Switzerland, 2017; ISBN 9789241512893. [Google Scholar]
- Baig, N.; Ihsanullah; Sajid, M.; Saleh, T.A. Graphene-based adsorbents for the removal of toxic organic pollutants: A review. J. Environ. Manag. 2019, 244, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Zawierucha, I.; Kozlowski, C.; Malina, G. Removal of toxic metal ions from landfill leachate by complementary sorption and transport across polymer inclusion membranes. Waste Manag. 2013, 33, 2129–2136. [Google Scholar] [CrossRef]
- Muthukrishnan, M.; Guha, B.K. Effect of pH on rejection of hexavalent chromium by nanofiltration. Desalination 2008, 219, 171–178. [Google Scholar] [CrossRef]
- Nasir, A.; Masood, F.; Yasin, T.; Hameed, A. Progress in polymeric nanocomposite membranes for wastewater treatment: Preparation, properties and applications. J. Ind. Eng. Chem. 2019, 79, 29–40. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Chen, S.S.; Hsu, H.T.; Li, C.W. Separation of three divalent cations (Cu2+, Co2+ and Ni2+) by NF membranes from pHs3 to 5. Desalination 2013, 328, 51–57. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Lakhotia, S.R.; Ghosh, A.K.; Bindal, R.C. Removal of arsenic from aqueous media using zeolite/chitosan nanocomposite membrane. Sep. Sci. Technol. 2018, 54, 282–288. [Google Scholar] [CrossRef]
- Abu Tarboush, B.J.; Chouman, A.; Jonderian, A.; Ahmad, M.; Hmadeh, M.; Al-Ghoul, M. Metal–Organic Framework-74 for Ultratrace Arsenic Removal from Water: Experimental and Density Functional Theory Studies. ACS Appl. Nano Mater. 2018, 1, 3283–3292. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Removal of chromium(VI) from aqueous solutions by polymer inclusion membranes. Water Res. 2002, 36, 4870–4876. [Google Scholar] [CrossRef]
- Fatin-Rouge, N.; Dupont, A.; Vidonne, A.; Dejeu, J.; Fievet, P.; Foissy, A. Removal of some divalent cations from water by membrane-filtration assisted with alginate. Water Res. 2006, 40, 1303–1309. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Bordoloi, S.; Nath, S.K.; Gogoi, S.; Dutta, R.K. Arsenic and iron removal from groundwater by oxidation—Coagulation at optimized pH: Laboratory and field studies. J. Hazard. Mater. 2013, 260, 618–626. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tang, Y.P.; Ma, D.; Chung, T.S. UiO-66 incorporated thin-film nanocomposite membranes for efficient selenium and arsenic removal. J. Memb. Sci. 2017, 541, 262–270. [Google Scholar] [CrossRef]
- Masheane, M.L.; Nthunya, L.N.; Malinga, S.P.; Nxumalo, E.N.; Mamba, B.B.; Mhlanga, S.D. Synthesis of Fe-Ag/f-MWCNT/PES nanostructured-hybrid membranes for removal of Cr(VI) from water. Sep. Purif. Technol. 2017, 184, 79–87. [Google Scholar] [CrossRef]
- Bordoloi, S.; Nath, S.K.; Dutta, R.K. Iron ion removal from groundwater using banana ash, carbonates and bicarbonates of Na and K, and their mixtures. Desalination 2011, 281, 190–198. [Google Scholar] [CrossRef]
- Pérez-González, A.; Urtiaga, A.M.; Ibáñez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ying, Y.; Peng, X. Graphene oxide nanosheet: An emerging star material for novel separation membranes. J. Mater. Chem. A 2014, 2, 13772–13782. [Google Scholar] [CrossRef]
- Sarango, L.; Paseta, L.; Navarro, M.; Zornoza, B.; Coronas, J. Controlled deposition of MOFs by dip-coating in thin film nanocomposite membranes for organic solvent nanofiltration. J. Ind. Eng. Chem. 2018, 59, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Karkooti, A.; Yazdi, A.Z.; Chen, P.; McGregor, M.; Nazemifard, N.; Sadrzadeh, M. Development of advanced nanocomposite membranes using graphene nanoribbons and nanosheets for water treatment. J. Memb. Sci. 2018, 560, 97–107. [Google Scholar] [CrossRef]
- Al Aani, S.; Gomez, V.; Wright, C.J.; Hilal, N. Fabrication of antibacterial mixed matrix nanocomposite membranes using hybrid nanostructure of silver coated multi-walled carbon nanotubes. Chem. Eng. J. 2017, 326, 721–736. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Yu, C.; Li, Q. Regenerable antimicrobial activity in polyamide thin film nanocomposite membranes. J. Memb. Sci. 2015, 476, 119–127. [Google Scholar] [CrossRef]
- Kong, S.; Lim, M.-y.; Shin, H.; Baik, J.H.; Lee, J.C. High-flux and antifouling polyethersulfone nanocomposite membranes incorporated with zwitterion-functionalized graphene oxide for ultrafiltration applications. J. Ind. Eng. Chem. 2020, 84, 131–140. [Google Scholar] [CrossRef]
- Bikiaris, D. Microstructure and properties of polypropylene/carbon nanotube nanocomposites. Materials 2010, 3, 2884–2946. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, G. Fabrication of high-performance ultrafiltration membranes using zwitterionic carbon nanotubes and polyethersulfone. High Perform. Polym. 2018, 30, 602–611. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, X.; Wei, Y.; Wang, X.; Wang, J.; Zhang, Y.; Gao, C. Enhanced desalination performance of carboxyl functionalized graphene oxide nanofiltration membranes. Desalination 2017, 405, 29–39. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; Arockiasamy Dass, L.; Ali, F.A.A.; Muthumareeswaran, M.R.; Mishra, U.; Ansari, M.A. Removal of heavy metal ions using a carboxylated graphene oxide-incorporated polyphenylsulfone nanofiltration membrane. Environ. Sci. Water Res. Technol. 2018, 4, 438–448. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Alcaina-Miranda, M.-I.; Iborra-Clar, M.-I.; Mendoza-Roca, J.-A.; Pastor-Alcañiz, L. Enhancement in hydrophilicity of different polymer phase-inversion ultrafiltration membranes by introducing PEG/Al2O3 nanoparticles. Sep. Purif. Technol. 2014, 128, 45–57. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Jo, Y.J.; Choi, E.Y.; Choi, N.W.; Kim, C.K. Antibacterial and Hydrophilic Characteristics of Poly(ether sulfone) Composite Membranes Containing Zinc Oxide Nanoparticles Grafted with Hydrophilic Polymers. Ind. Eng. Chem. Res. 2016, 55, 7801–7809. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Zhang, W.H.; Wang, N.; Ji, S.; An, Q.F. Enhanced permeance for PDMS organic solvent nanofiltration membranes using modified mesoporous silica nanoparticles. J. Memb. Sci. 2020, 612, 118257. [Google Scholar] [CrossRef]
- Quaglia, G.; Ambrogi, V.; Pietrella, D.; Nocchetti, M.; Latterini, L. Solid state photoreduction of silver on mesoporous silica to enhance antifungal activity. Nanomaterials 2021, 11, 2340. [Google Scholar] [CrossRef]
- Pérez-Garnes, M.; Morales, V.; Sanz, R.; García-Muñoz, R.A. Cytostatic and cytotoxic effects of hollow-shell mesoporous silica nanoparticles containing magnetic iron oxide. Nanomaterials 2021, 11, 2340. [Google Scholar] [CrossRef]
- Niu, X.; Wang, X.; Niu, B.; Meng, Y.; He, H.; Wang, Y.; Li, G. Costunolide Loaded in pH-Responsive Mesoporous Silica Nanoparticles for Increased Stability and an Enhanced Anti-Fibrotic Effect. Pharmaceuticals 2021, 14, 951. [Google Scholar] [CrossRef] [PubMed]
- Zargar, M.; Hartanto, Y.; Jin, B.; Dai, S. Hollow mesoporous silica nanoparticles: A peculiar structure for thin film nanocomposite membranes. J. Memb. Sci. 2016, 519, 1–10. [Google Scholar] [CrossRef]
- Abaza, S.F.; Elbialy, N.S.; Mohamed, N. Incorporating silver nanoshell-coated mesoporous silica nanoparticles improves physicochemical and antimicrobial properties of chitosan films. Int. J. Biol. Macromol. 2021, 189, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Abdelsamad, A.M.A.; Khalil, A.S.G.; Ulbricht, M. Influence of controlled functionalization of mesoporous silica nanoparticles as tailored fillers for thin-film nanocomposite membranes on desalination performance. J. Memb. Sci. 2018, 563, 149–161. [Google Scholar] [CrossRef]
- Braun, K.; Pochert, A.; Lindén, M.; Davoudi, M.; Schmidtchen, A.; Nordström, R.; Malmsten, M. Membrane interactions of mesoporous silica nanoparticles as carriers of antimicrobial peptides. J. Colloid Interface Sci. 2016, 475, 161–170. [Google Scholar] [CrossRef]
- Liu, D.; Geng, L.; Fu, Y.; Dai, X.; Lü, C. Novel nanocomposite membranes based on sulfonated mesoporous silica nanoparticles modified sulfonated polyimides for direct methanol fuel cells. J. Memb. Sci. 2011, 366, 251–257. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Y.; Zhang, P.; Zhang, X.; Zhou, Q.; Zhao, J.; Ren, L. Self-enriched mesoporous silica nanoparticle composite membrane with remarkable photodynamic antimicrobial performances. J. Colloid Interface Sci. 2020, 559, 197–205. [Google Scholar] [CrossRef]
- Suwannarat, S.; Amnuaypanich, S.; Chanlek, N.; Amnuaypanich, S. Temperature-enhanced water selectivity in polyvinyl alcohol mixed matrix membranes filled with poly(2-hydroxyethylmethacrylate)-grafted mesoporous silica nanoparticles (PVA/MSNs-g-PHEMA MMMs). Sep. Purif. Technol. 2021, 257, 117875. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; Dass, L.A.; Muthumareeswaran, M.R. Development of a nanocomposite ultrafiltration membrane based on polyphenylsulfone blended with graphene oxide. Sci. Rep. 2017, 7, 41976–41987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.P.; Hou, J.; Chen, F.S.; Meng, X.M. In-situ growth of metal-organic framework film on a polydopamine-modified flexible substrate for antibacterial and forward osmosis membranes. Sep. Purif. Technol. 2020, 236, 116239. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ali, F.A.A.; Alhoshan, M. Efficient soluble anionic dye removal and antimicrobial properties of ZnO embedded-polyphenylsulfone membrane. Water Environ. J. 2021, 35, 670–684. [Google Scholar] [CrossRef]
- Truica-Marasescu, F.; Wertheimer, M.R. Nitrogen-rich plasma-polymer films for biomedical applications. Plasma Process. Polym. 2008, 5, 44–57. [Google Scholar] [CrossRef]
- Pippig, F.; Holländer, A. Fluram labeling of high density NH2 surfaces. Appl. Surf. Sci. 2007, 253, 6817–6823. [Google Scholar] [CrossRef]
- Yang, Y.-n.; Jun, W.; Qing-zhu, Z.; Xue-si, C.; Hui-xuan, Z. The research of rheology and thermodynamics of organic-inorganic hybrid membrane during the membrane formation. J. Memb. Sci. 2008, 311, 200–207. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Li, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Polyethyleneimine, an effective additive for polyethersulfone ultrafiltration membrane with enhanced permeability and selectivity. J. Memb. Sci. 2015, 476, 216–223. [Google Scholar] [CrossRef]
- Li, M.P.; Zhang, X.; Zhang, H.; Liu, W.L.; Huang, Z.H.; Xie, F.; Ma, X.H.; Xu, Z.L. Hydrophilic yolk-shell ZIF-8 modified polyamide thin-film nanocomposite membrane with improved permeability and selectivity. Sep. Purif. Technol. 2020, 247, 116990. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Effect of TiO2 nanoparticles on the performance of polyphenylsulfone biomaterial for orthopaedic implants. J. Mater. Chem. B 2014, 2, 7502–7514. [Google Scholar] [CrossRef] [PubMed]
- Maximous, N.; Nakhla, G.; Wan, W.; Wong, K. Effect of the metal oxide particle distributions on modified PES membranes characteristics and performance. J. Memb. Sci. 2010, 361, 213–222. [Google Scholar] [CrossRef]
- Bakeri, G.; Matsuura, T.; Ismail, A.F. The effect of phase inversion promoters on the structure and performance of polyetherimide hollow fiber membrane using in gas-liquid contacting process. J. Memb. Sci. 2011, 383, 159–169. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Riazi, H.; Almasi, D.; Ismail, A.F. Minimizing structural parameter of thin film composite forward osmosis membranes using polysulfone/halloysite nanotubes as membrane substrates. Desalination 2016, 377, 152–162. [Google Scholar] [CrossRef]
- Saranya, R.; Kumar, M.; Tamilarasan, R.; Ismail, A.F.; Arthanareeswaran, G. Functionalised activated carbon modified polyphenylsulfone composite membranes for adsorption enhanced phenol filtration. J. Chem. Technol. Biotechnol. 2016, 91, 748–761. [Google Scholar] [CrossRef]
- Chong, J.Y.; Wang, R. From micro to nano: Polyamide thin film on microfiltration ceramic tubular membranes for nanofiltration. J. Memb. Sci. 2019, 587, 117161. [Google Scholar] [CrossRef]
- Miculescu, M.; Thakur, V.K.; Miculescu, F.; Voicu, S.I. Graphene-based polymer nanocomposite membranes: A review. Polym. Adv. Technol. 2016, 27, 844–859. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane Fouling in Osmotically Driven Membrane Processes: A Review. J. Memb. Sci. 2015, 499, 201–233. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Alam, M.; Kaushik, A. Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube/polyphenylsulfone nanocomposite membranes. Mater. Chem. Phys. 2019, 233, 102–112. [Google Scholar] [CrossRef]
- Chandrashekhar Nayak, M.; Isloor, A.M.; Inamuddin; Lakshmi, B.; Marwani, H.M.; Khan, I. Polyphenylsulfone/multiwalled carbon nanotubes mixed ultrafiltration membranes: Fabrication, characterization and removal of heavy metals Pb2+, Hg2+, and Cd2+ from aqueous solutions. Arab. J. Chem. 2019, 13, 4661–4672. [Google Scholar] [CrossRef]
- He, M.; Wang, L.; Lv, Y.; Wang, X.; Zhu, J.; Zhang, Y.; Liu, T. Novel polydopamine/metal organic framework thin film nanocomposite forward osmosis membrane for salt rejection and heavy metal removal. Chem. Eng. J. 2020, 389, 124452. [Google Scholar] [CrossRef]
- Thong, Z.; Han, G.; Cui, Y.; Gao, J.; Chung, T.S.; Chan, S.Y.; Wei, S. Novel nanofiltration membranes consisting of a sulfonated pentablock copolymer rejection layer for heavy metal removal. Environ. Sci. Technol. 2014, 48, 13880–13887. [Google Scholar] [CrossRef]
- Moideen, I.K.; Isloor, A.M.; Qaiser, A.A.; Ismail, A.F.; Abdullah, M.S. Separation of heavy metal and protein from wastewater by sulfonated polyphenylsulfone ultrafiltration membrane process prepared by glycine betaine enriched coagulation bath. Korean J. Chem. Eng. 2018, 35, 1281–1289. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Insight Studies on Metal-Organic Framework Nanofibrous Membrane Adsorption and Activation for Heavy Metal Ions Removal from Aqueous Solution. ACS Appl. Mater. Interfaces 2018, 10, 18619–18629. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.X.; Wu, Y.Z.; Xu, Z.L.; Zhang, H.Z.; Gao, P.; Xu, S.J. Thin-film nanocomposite NF membrane with GO on macroporous hollow fiber ceramic substrate for efficient heavy metals removal. Environ. Res. 2021, 197, 111040. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, S.; Vatanpour, V.; Kariminia, H.-R. Influence of ion interaction on lead removal by a polyamide nanofiltration membrane. Desalination 2015, 362, 84–92. [Google Scholar] [CrossRef]


| Membranes | Water Flux LMH | Rejection (%) | References | |
|---|---|---|---|---|
| Cd2+ | Zn2+ | |||
| PPSU/MWCNTs | 185 | 72 | - | [64] |
| PDA/MOF-TFN | 11 | 82 | - | [65] |
| Matrimid/PEI | 2.9 | 97 | 98 | [66] |
| PSU/sPPSU | 190 | 69 | - | [67] |
| PAN/MOF-808 | 348 | 57 | 64 | [68] |
| eGO3/PA-HFC | 18 | - | 93 | [69] |
| Polyamide NF | 40 | 84 | 87 | [70] |
| Functionalized-MSNs/PSU | 20 | 91 | 94 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, A.A.; Shukla, A.K.; Mrad, M.H.; Alswieleh, A.M.; Alotaibi, K.M. Fabrication of Polysulfone-Surface Functionalized Mesoporous Silica Nanocomposite Membranes for Removal of Heavy Metal Ions from Wastewater. Membranes 2021, 11, 935. https://doi.org/10.3390/membranes11120935
Alotaibi AA, Shukla AK, Mrad MH, Alswieleh AM, Alotaibi KM. Fabrication of Polysulfone-Surface Functionalized Mesoporous Silica Nanocomposite Membranes for Removal of Heavy Metal Ions from Wastewater. Membranes. 2021; 11(12):935. https://doi.org/10.3390/membranes11120935
Chicago/Turabian StyleAlotaibi, Abdullah A., Arun Kumar Shukla, Mohamed Habib Mrad, Abdullah M. Alswieleh, and Khalid M. Alotaibi. 2021. "Fabrication of Polysulfone-Surface Functionalized Mesoporous Silica Nanocomposite Membranes for Removal of Heavy Metal Ions from Wastewater" Membranes 11, no. 12: 935. https://doi.org/10.3390/membranes11120935
APA StyleAlotaibi, A. A., Shukla, A. K., Mrad, M. H., Alswieleh, A. M., & Alotaibi, K. M. (2021). Fabrication of Polysulfone-Surface Functionalized Mesoporous Silica Nanocomposite Membranes for Removal of Heavy Metal Ions from Wastewater. Membranes, 11(12), 935. https://doi.org/10.3390/membranes11120935







