Nanodrug Transmembrane Transport Research Based on Fluorescence Correlation Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
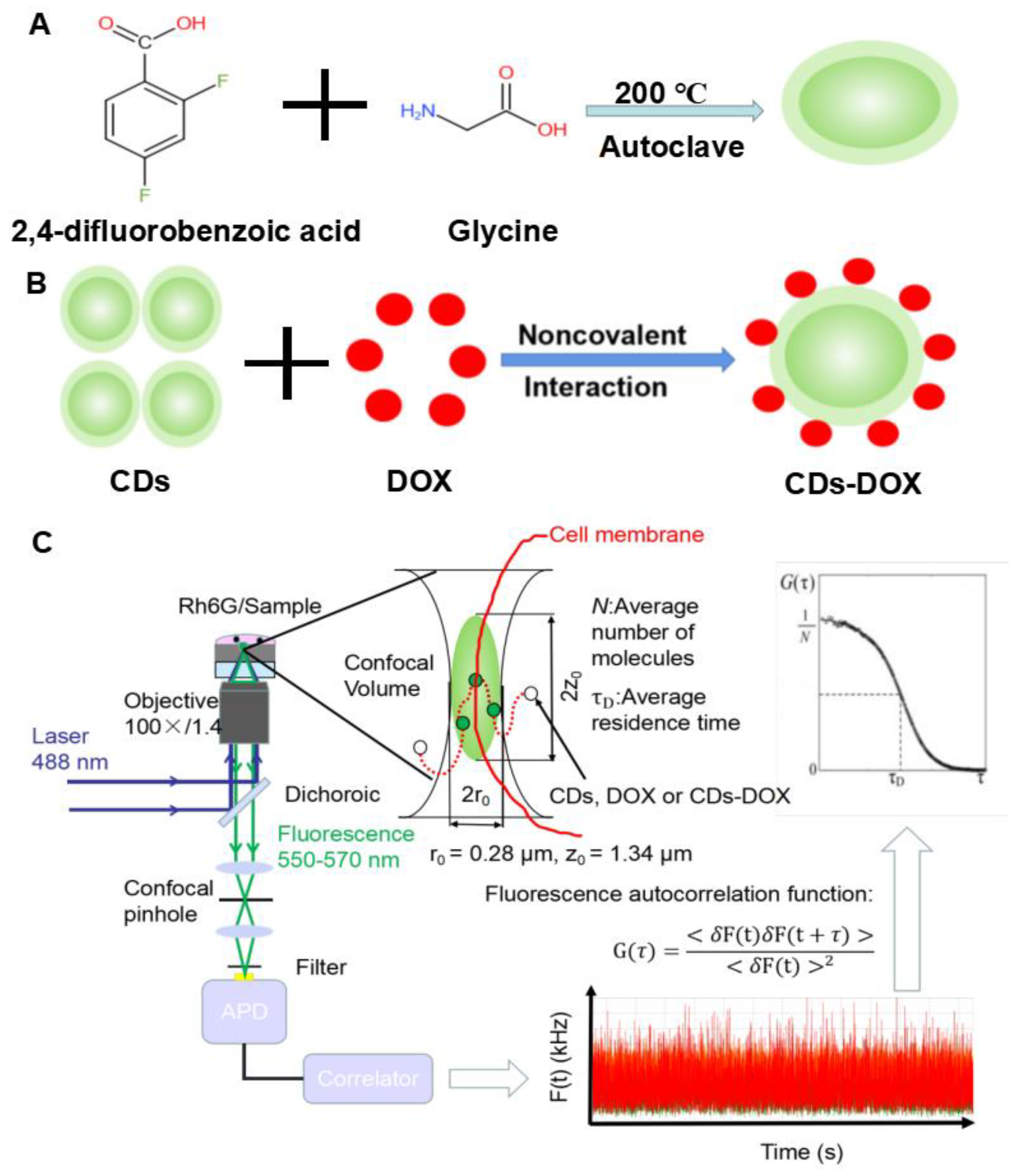
2.2. CDs and CDs-DOX Preparation
2.3. Quantum Yield Measurement
2.4. Cell Imaging
2.5. FCS Setup
2.6. Statistical Analysis
3. Results
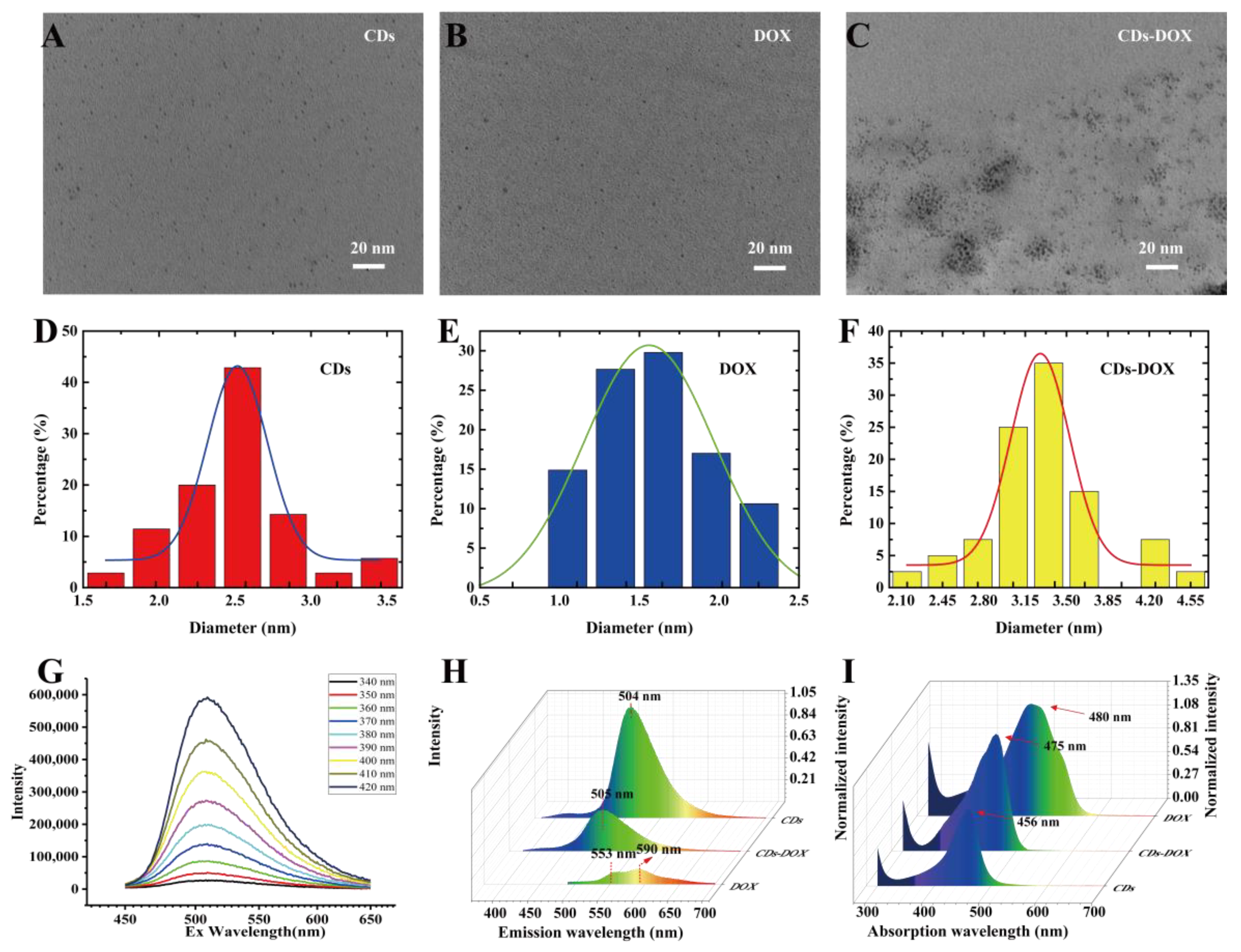
3.1. Synthesis and Characterization of CDs, DOX and CDs-DOX
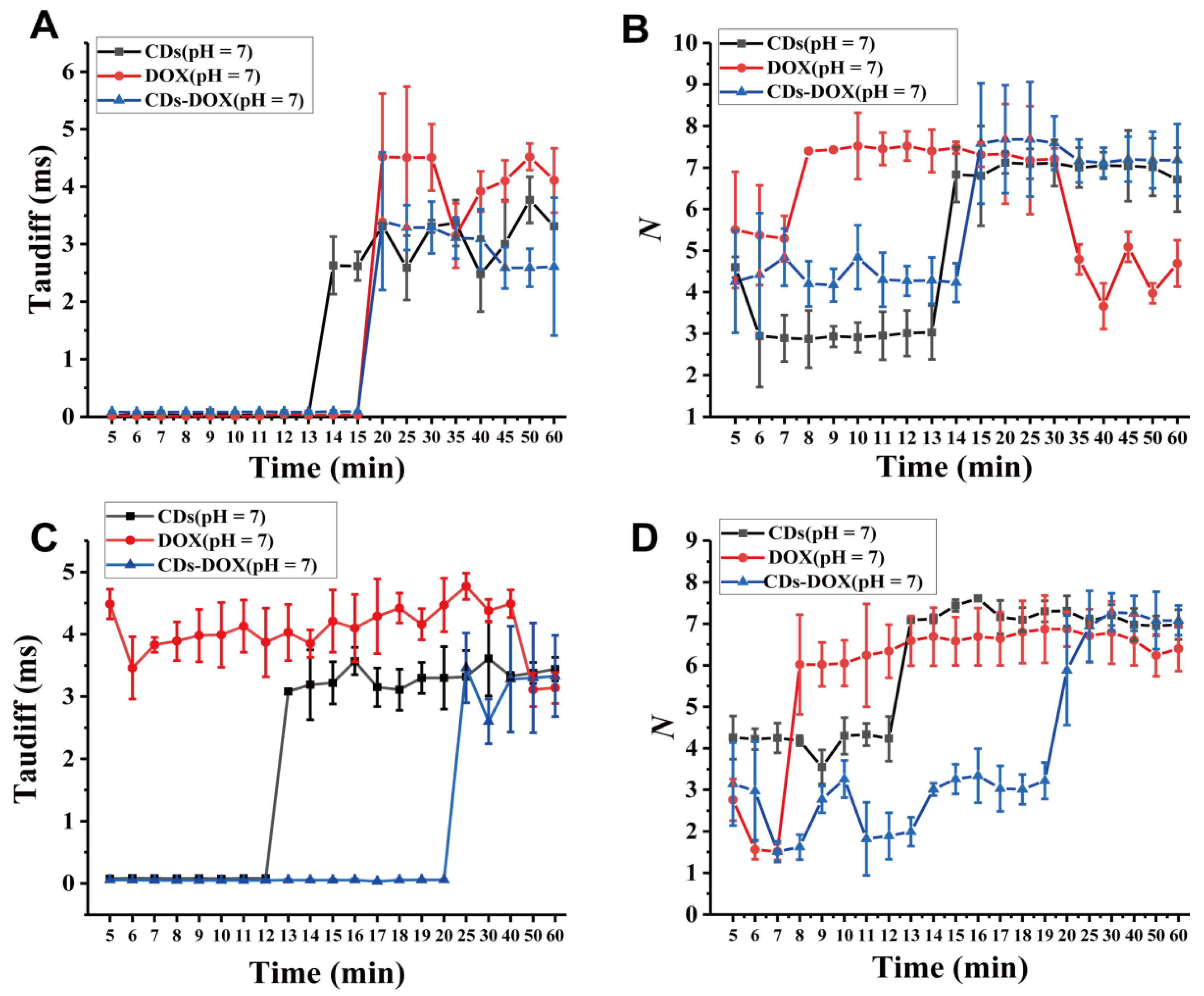
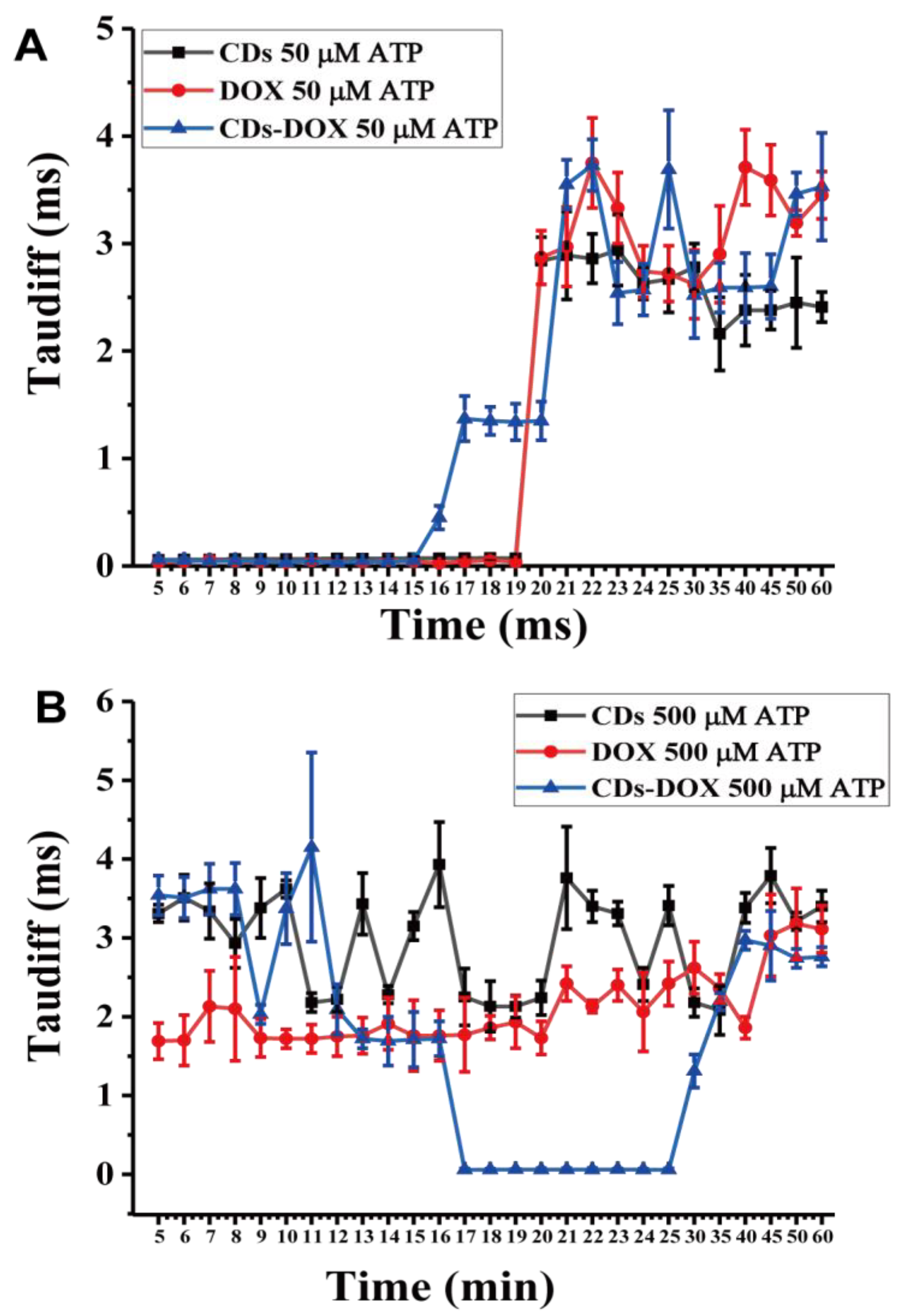
3.2. Investigating Nanodrug Transmembrane Transport
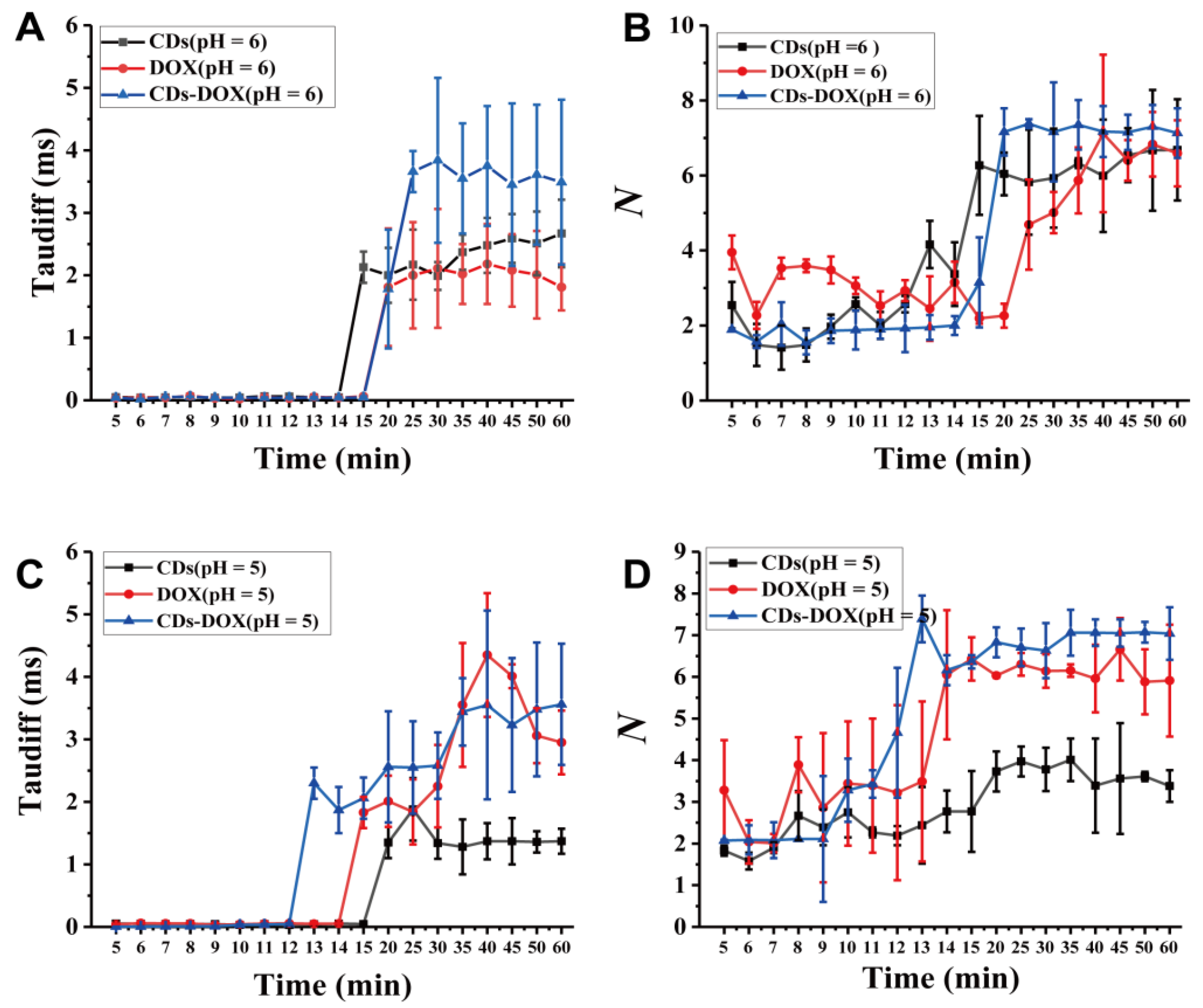
3.3. Investigating Nanodrug Transmembrane Transport under Different Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carvalho, C.; Santos, R.X.; Cardoso, S.; Correia, S.; Oliveira, P.J.; Santos, M.S.; Moreira, P.I. Doxorubicin: The good, the bad and the ugly effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.B.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.P.; Zheng, H.Z. Hollow luminescent carbon dots for drug delivery. Carbon 2013, 59, 192–199. [Google Scholar]
- Wang, B.B.; Wang, S.J.; Wang, Y.F.; Yan, L.; Wu, H.; Ma, X.J.; Tan, M.Q. Highly fluorescent carbon dots for visible sensing of doxorubicin release based on efficient nanosurface energy transfer. Biotechnol. Lett. 2016, 38, 191–201. [Google Scholar] [CrossRef]
- Havanur, S.; Batish, I.; Cheruku, S.P.; Gourishetti, K.; Jagadeeshbabu, P.E.; Kumar, N. Poly(N,N-diethyl acrylamide)/fimctionalized graphene quantum dots hydrogels loaded with doxorubicin as a nano-drug carrier for metastatic lung cancer in mice. Mater. Sci. Eng. 2019, 105, 110094. [Google Scholar] [CrossRef]
- Demirci, S.; McNally, A.B.; Ayyala, R.S.; Lawson, L.B.; Sahiner, N. Synthesis and characterization of nitrogen-doped carbon dots as fluorescent nanoprobes with antimicrobial properties and skin permeabilit. J. Drug Deliv. Sci. Technol. 2020, 59, 101889. [Google Scholar] [CrossRef]
- Biswal, M.R.; Garner, I.; Vichare, R.; Paulson, R.; Appavu, R.; Panguluri, S.K.; Tzekov, R.; Sahiner, N.; Ayyala, R.; Biswal, M.R. Carbon Dots Fabrication: Ocular Imaging and Therapeutic Potential. Front. Bioeng. Biotechnol. 2020, 8, 573470–573484. [Google Scholar]
- Zheng, M.; Liu, S.; Li, J.; Qu, D.; Zhao, H.; Guan, X.; Hu, X.; Xie, Z.; Jing, X.; Sun, Z. Integrating oxaliplatin with highly luminescent carbon dots: An unprecedented theranostic agent for personalized medicine. Adv. Mater. 2014, 26, 3554–3560. [Google Scholar] [CrossRef]
- Shao, D.; Wen, Y.; Xu, S.N.; Xu, J.; Zeng, Q.H.; Shan, C.X. Carbon dots as a trackable drug delivery carrier for localized cancer therapy in vivo. J. Mater. Chem. B 2016, 4, 5119–5126. [Google Scholar]
- Duan, Q.Q.; Ma, Y.; Che, M.X.; Zhang, B.Y.; Zhang, Y.X.; Li, Y.; Zhang, W.D.; Sang, S.B. Fluorescent carbon dots as carriers for intracellular doxorubicin delivery and track. Drug Deliv. Sci. Technol. 2019, 49, 527–533. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, B.; Hao, L.; Liu, N.; Lin, Y.; Guo, W.; Li, X.; Gu, B. Doxorubicin-loaded environmentally friendly carbon dots as a novel, drug delivery system for nucleus targeted cancer therapy. Colloids Surf. B Biointerfaces 2017, 159, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Chen, L.; Wang, J.L.; Du, J.L.; Li, Q.; Gao, Y.D.; Yu, S.P.; Yang, Y.Z. Enhanced-fluorescent imaging and targeted therapy of liver cancer using highly luminescent carbon dots-conjugated foliate. Mater. Sci. Eng. C 2019, 116, 111233–111246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Jiang, J.; Cui, N.; Wang, D. Doxorubicin-Loaded Carbon Dots Lipid-Coated Calcium Phosphate Nanoparticles for Visual Targeted Delivery and Therapy of Tumor. Int. J. Nanomed. 2020, 15, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Fang, T.; Yin, L.; Jiang, J.; He, Y.; Dai, Y.; Wang, D. Multistage delivery of CDs-DOX/ICG-loaded liposome for highly penetration and effective chemo-photothermal combination therapy. Drug Deliv. 2018, 25, 1826–1839. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Wang, Y.Y.; Du, X.F.; Xu, J.J.; Zhao, M.X. Carbon dots-based nanocarrier system with intrinsic tumor targeting ability for cancer treatment. Nano Express 2020, 1, 3–7. [Google Scholar] [CrossRef]
- Douglas, M.; Elliot, E.; Webb, W.W. Thermodynamic Fluctuations in a Reacting System—Measurement by Fluorescence Correlation Spectroscopy. Phys. Rev. Lett. 1972, 29, 705–708. [Google Scholar]
- Wonchul, S.; Ge, L.H.; Arpino, G.; Villarreal, S.A.; Hamid, E.; Liu, H.S.; Zhao, W.D.; Wen, P.J.; Chiang, H.C.; Wu, L.G. Visualization of Membrane Pore in Live Cells Reveals a Dynamic-Pore Theory Governing Fusion and Endocytosis. Cell 2018, 4, 934–945. [Google Scholar]
- Malacrida, L.; Hedde, P.N.; Ranjit, S.; Cardarelli, F.; Gratton, E. Visualization of barriers and obstacles to molecular diffusion in live cells by spatial pair-cross-correlation in two dimensions. Biomed. Opt. Express 2017, 9, 303–321. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghomi, M. Green synthesized carbon quantum dots from Prosopis juliflora leaves as a dual off-on fluorescence probe for sensing mercury (II) and chemet drug. Mater. Sci. Eng. C 2019, 98, 887–896. [Google Scholar] [CrossRef]
- Gudimella, K.K.; Appidi, T.; Wu, H.F.; Battula, V.; Jogdand, A.; Rengan, A.K.; Gedda, G. Sand bath assisted green synthesis of carbon dots from citrus fruit peels for free radical scavenging and cell imaging. Colloids Surf. B Biointerfaces 2021, 197, 111362. [Google Scholar] [CrossRef]
- Kong, T.T.; Hao, L.Y.; Wei, Y.Y.; Cai, X.X.; Zhu, B.F. Doxorubicin conjugated carbon dots as a drug delivery system for human breast cancer therapy. Cell Prolif. 2018, 51, e12488. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, S.; Liu, L.; Kong, Y.; Zhang, A.W.; Xu, K.; Han, C.P. The Cost-Effective Preparation of Green Fluorescent Carbon Dots for Bioimaging and Enhanced Intracellular Drug Delivery. Nanoscale Res. Lett. 2020, 15, 55. [Google Scholar] [CrossRef]
- Zheng, Y.W.; Yang, D.; Wu, X.; Yan, H.R.; Zhao, Y.C.; Feng, B.; Weng, J.; Wang, J.X. A facile approach for the synthesis of highly luminescent carbon dots using vitamin-based small organic molecules with benzene ring structure as precursors. RSC Adv. 2015, 5, 90245–90254. [Google Scholar] [CrossRef]
- Lan, M.H.; Di, Y.F.; Zhu, X.Y.; Ng, T.W.; Xia, J.; Liu, W.M.; Meng, X.M.; Wang, P.F.; Lee, C.S.; Zhang, W.J. Carbon dot-based fluorescence turn-on sensor for hydrogen peroxide with a photo-induced electron transfer mechanism. Chem. Commun. 2015, 51, 15574–15577. [Google Scholar] [CrossRef]
- Jing, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.G.; Cai, C.Z.; Lin, H.W. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.O.; Matthew, S. Dual-mode colorimetric and fluorometric probe for ferric ion detection using N-doped carbon dots prepared via hydrothermal synthesis followed by microwave irradiation. Opt. Mater. 2019, 94, 330–336. [Google Scholar]
- Zhou, N.; Zhu, A.S.; Maharjan, B.S.; Hao, A.Z.; Song, A.Y. Elucidating the endocytosis, in-tracellular trafficking, and exocytosis of carbon dots in neural cells. RSC Adv. 2014, 4, 62086–62095. [Google Scholar]
- Yang, L.; Wang, Z.; Wang, J.; Jiang, W.H.; Jiang, X.W.; Bai, Z.S.; He, Y.P.; Jiang, J.Q.; Wang, D.K.; Yang, L. Doxorubicin conjugated function-alizable carbon dots for nucleus targeted delivery and enhanced therapeutic efficacy. Nanoscale 2016, 8, 6801–6809. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, H.; Li, W.K.; Hua, W.; Yong, Y. Highly-expressed P2X7 receptor promotes growth and metastasis of human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3β/β-catenin and mTOR/HIF1α/VEGF signaling. Int. J. Cancer 2019, 145, 1068–1082. [Google Scholar] [CrossRef]
- Norris, A.; Grant, B.D. Endosomal microdomains: Formation and function—ScienceDirect. Curr. Opin. Cell Biol. 2020, 65, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Weeratunga, S.; Paul, B.; Collins, B.M. Recognising the signals for endosomal trafficking. Curr. Opin. Cell Biol. 2020, 65, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.M.; Ma, Y.Q. Theoretical and Computational Investigations of Nanoparticle–Biomembrane Interactions in Cellular Delivery. Small 2015, 11, 1055–1071. [Google Scholar] [CrossRef]
- Li, H.; Ye, S.; Guo, J.Q.; Wang, H.B.; Yan, W.; Song, J.; Qu, J.L. Biocompatible carbon dots with low-saturation-intensity and high-photobleaching-resistance for STED nanoscopy imaging of the nucleolus and tunneling nanotubes in living cells. Nano Res. 2019, 12, 3075–3084. [Google Scholar] [CrossRef]
- Rehor, A.; Tirelli, N.; Hubbell, J.A. Drug Loading, Release and Bioavailability, 1st ed.; ETH Zurich: Zurich, Switzerland, 2005; pp. 71–92. [Google Scholar]
- Verma, A.; Stallacci, F. Effect of Surface Properties on Nanoparticle-cell Interactions. Small 2009, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Humez, S.; Monet, M.; Coppenolle, F.V.; Delcourt, P.; Prevarskaya, N. The role of intracellular pH in cell growth arrest induced by ATP. Am. J. Physiol. Cell Physiol. 2004, 287, 1733–1746. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imamura, H.; Nhat, K.P.H.; Togawa, H.; Saito, K.; Lino, R.; Kato-Yamada, Y.; Nagai, T.; Noji, H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc. Natl. Acad. Sci. USA 2009, 106, 15652–15656. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, S.; Zhang, Y.; Xu, J. pH-Responsive polymer based on dynamic imine bonds as a drug delivery material with pseudo target release behavior. Polym. Chem. 2018, 9, 878–884. [Google Scholar] [CrossRef]
- Qi, B.; Yu, T.; Wang, C.; Wang, T.; Yao, J.; Zhang, X.; Deng, P.; Xia, Y.; Junger, W.G.; Sun, D. Shock wave-induced ATP release from osteosarcoma U2OS cells promotes cellular uptake and cytotoxicity of methotrexate. J. Exp. Clin. Cancer Res. 2016, 35, 161. [Google Scholar] [CrossRef]
- Ben-Dov, N.; Korenstein, R. Proton-induced endocytosis is dependent on cell membrane fluidity, lipid-phase order and the membrane resting potential. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2672–2681. [Google Scholar] [CrossRef]
- Trebinska, S.A.; Swiech, O.; Opuchlik, L.J.; Grzybowska, E.A.; Bilewicz, R. Impact of Medium pH on DOX Toxicity toward HeLa and A498 Cell Lines. ACS Omega 2020, 5, 7979–7986. [Google Scholar] [CrossRef] [PubMed]
| Time (min) | 5 min | 6 min | 7 min | 8 min | |
|---|---|---|---|---|---|
| Drug Diffusion Coefficient (×10−6 cm2 s−1) | CDs | 5.91 ± 1.29 (*) | 6.412 ± 1.25 (*) | 7.32 ± 0.81 (**) | 6.95 ± 1.89 (*) |
| DOX | 7.90 ± 1.15 (*) | 5.5 ± 0.563 (***) | 5.94 ± 0.32 (***) | 5.94 ± 1.98 (*) | |
| CDs-DOX | 69.58 ± 9.48 (**) | 65.41 ± 10.9 (**) | 46.72 ± 10.5 (*) | 32.7 ± 10.9 (*) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Liu, Y.; Zhang, J.; Wang, L.; Guo, Y.; Zhu, Y.; Yang, Z.; Yan, W.; Qu, J. Nanodrug Transmembrane Transport Research Based on Fluorescence Correlation Spectroscopy. Membranes 2021, 11, 891. https://doi.org/10.3390/membranes11110891
Gao X, Liu Y, Zhang J, Wang L, Guo Y, Zhu Y, Yang Z, Yan W, Qu J. Nanodrug Transmembrane Transport Research Based on Fluorescence Correlation Spectroscopy. Membranes. 2021; 11(11):891. https://doi.org/10.3390/membranes11110891
Chicago/Turabian StyleGao, Xinwei, Yanfeng Liu, Jia Zhang, Luwei Wang, Yong Guo, Yinru Zhu, Zhigang Yang, Wei Yan, and Junle Qu. 2021. "Nanodrug Transmembrane Transport Research Based on Fluorescence Correlation Spectroscopy" Membranes 11, no. 11: 891. https://doi.org/10.3390/membranes11110891
APA StyleGao, X., Liu, Y., Zhang, J., Wang, L., Guo, Y., Zhu, Y., Yang, Z., Yan, W., & Qu, J. (2021). Nanodrug Transmembrane Transport Research Based on Fluorescence Correlation Spectroscopy. Membranes, 11(11), 891. https://doi.org/10.3390/membranes11110891








