Characterization of Extracellular Vesicles Labelled with a Lipophilic Dye Using Fluorescence Nanoparticle Tracking Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
EV-Depletion of the FBS
2.3. EV-Purification from JAr Cell Conditioned Medium
2.4. EV-Purification from Bovine Follicular Fluid
2.5. EV-Purification from Seminal Plasma
2.6. CMG Labeling of EVs
2.6.1. JAr EVs Labeling with CMG Dye
2.6.2. CMG Dye Labeling of Lyophilized HCT116 and Biologically Derived EVs
2.7. NP-40 Detergent Treatment of Neat (Unlabeled) and CMG-Labeled EVs
2.8. Nanoparticle Tracking Analaysis of EVs
2.9. Zeta Potential Measurements
2.10. Statistical Analysis
2.11. Experimental Design
- The fl-NTA brightness threshold affects the detection of fluorescent NPs in a given sample.
- II.
- The CMG dye concentration affects the size distribution, concentration, and ZP values of fl-NPs.
- III.
- Incubation temperature affects the proportion of fl-NPs in a given sample.
- IV.
- The EV purification method affects the proportion of CMG positive fl-NPs present in a given sample.
- V.
- Detergent treatment of EV’s membrane affects the proportion of fl-NPs in a given sample.
- VI.
- The source of material for EV purification affects fl-NPs proportions to the total number of NPs present in a given sample.
3. Results
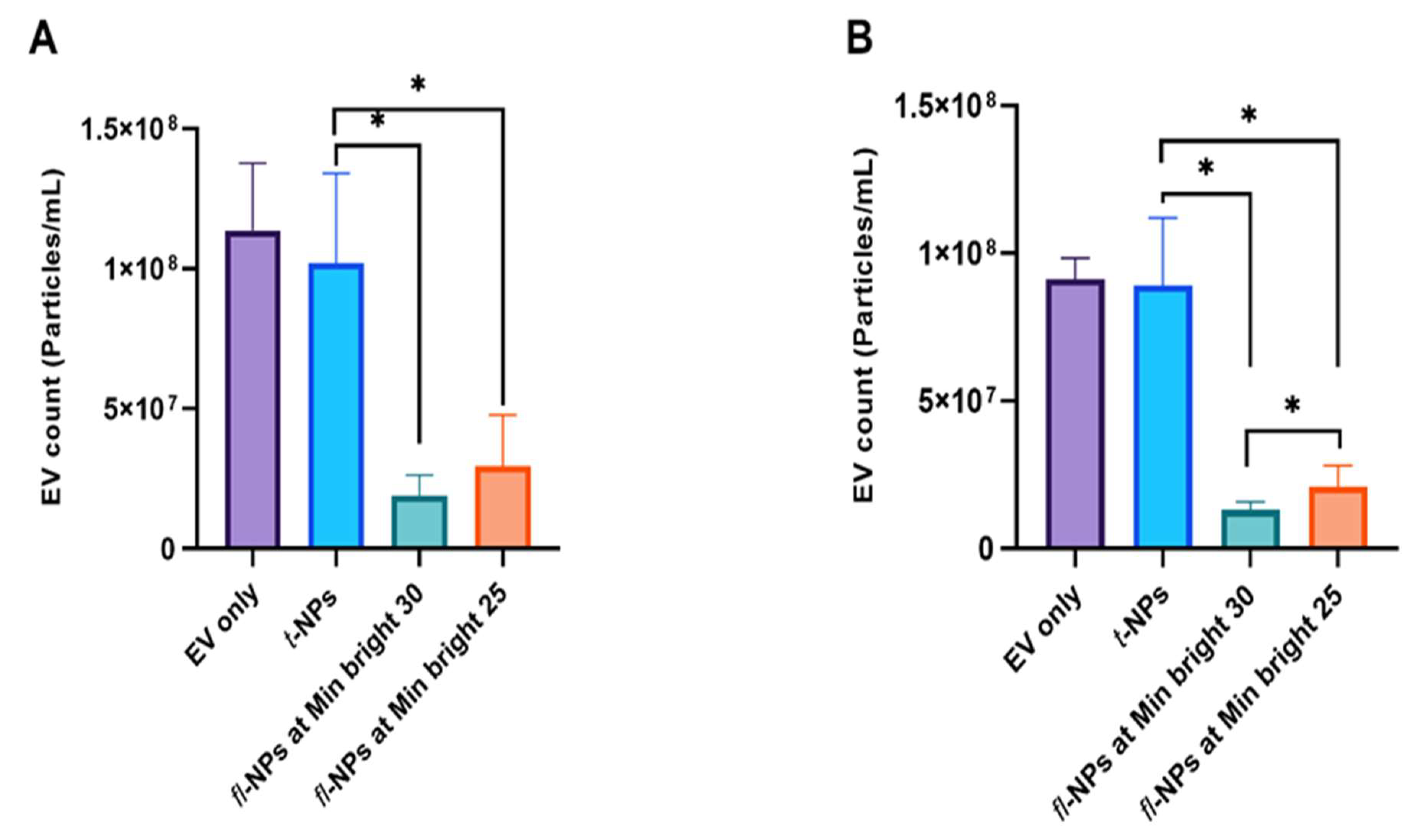
3.1. Effects of Minimum Brightness Threshold Settings on the Detection of Fluorescent Nanoparticles
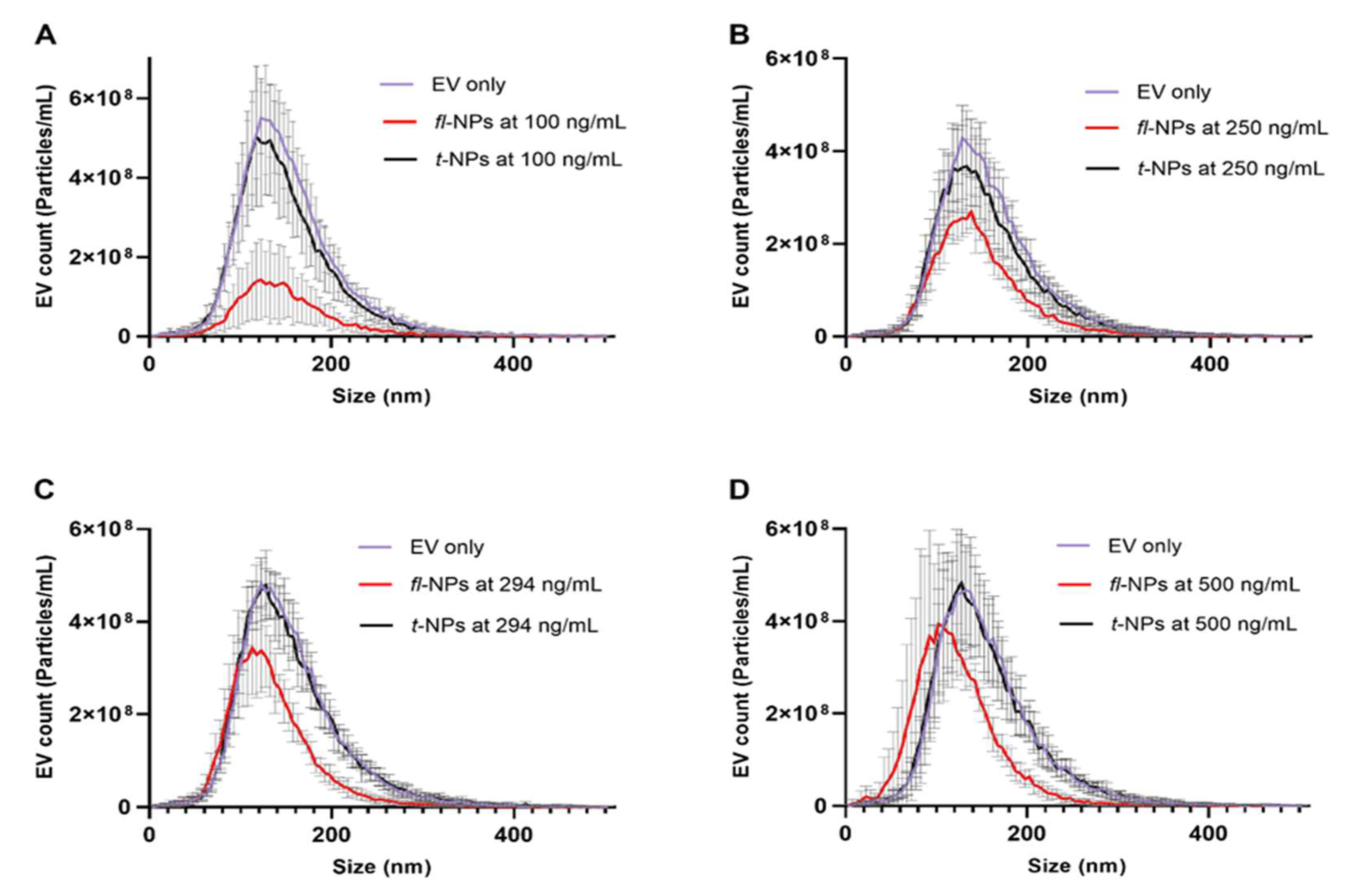
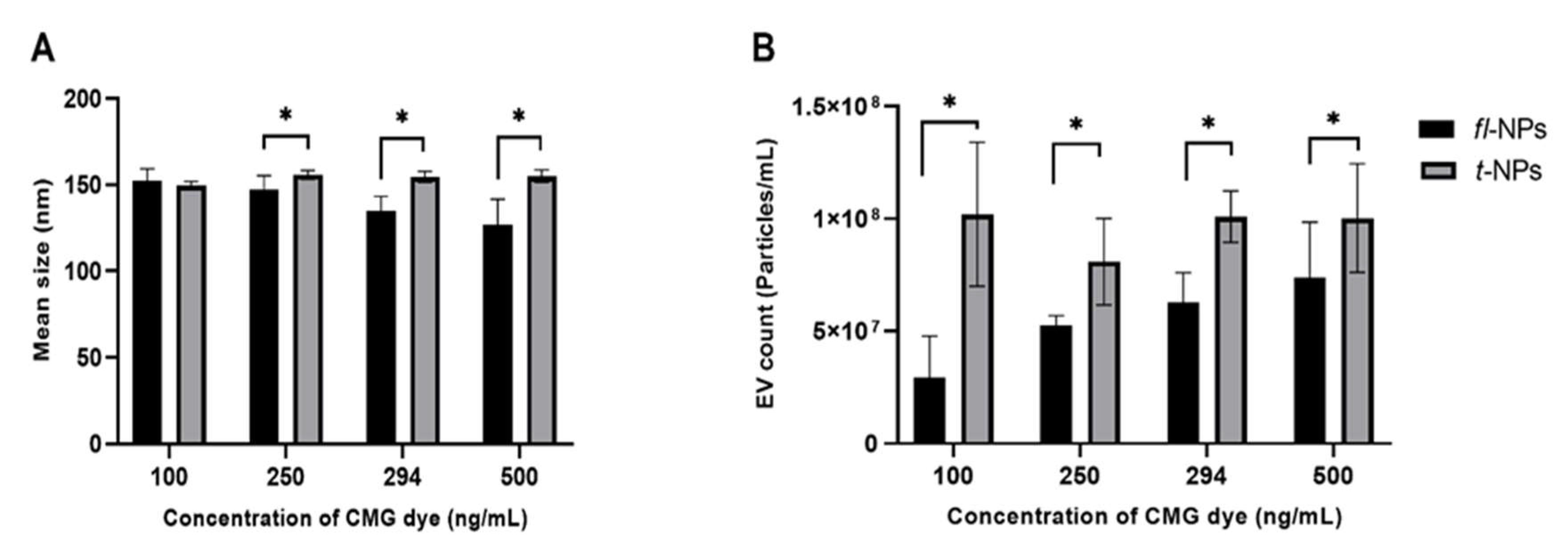
3.2. Effect of CMG Concentration on the Size Distribution, Concentration and ZP Values of fl-NPs of EVs
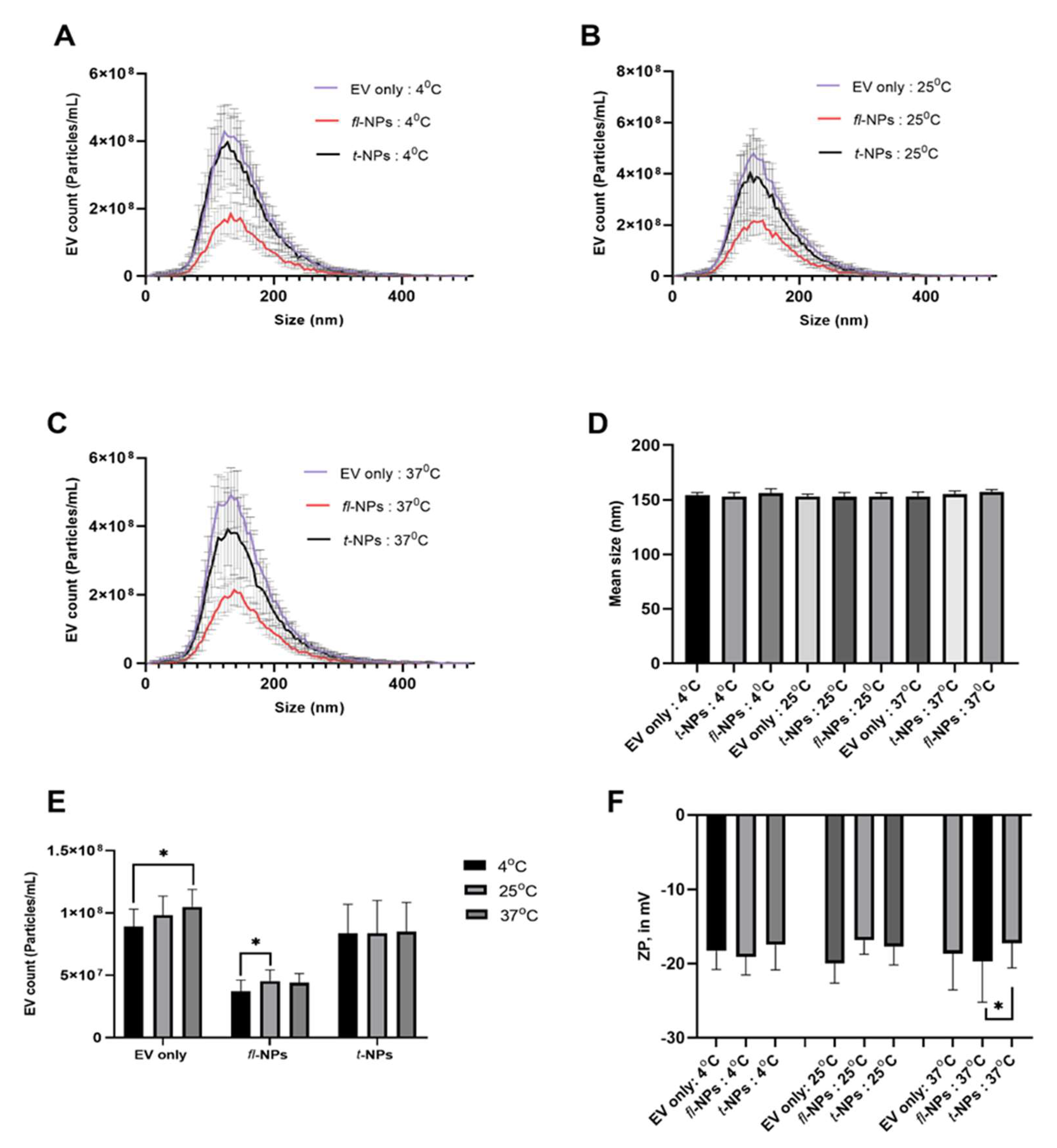
3.3. Effect of Incubation Temperature on the Physical Characteristics of fl-NPs of EVs
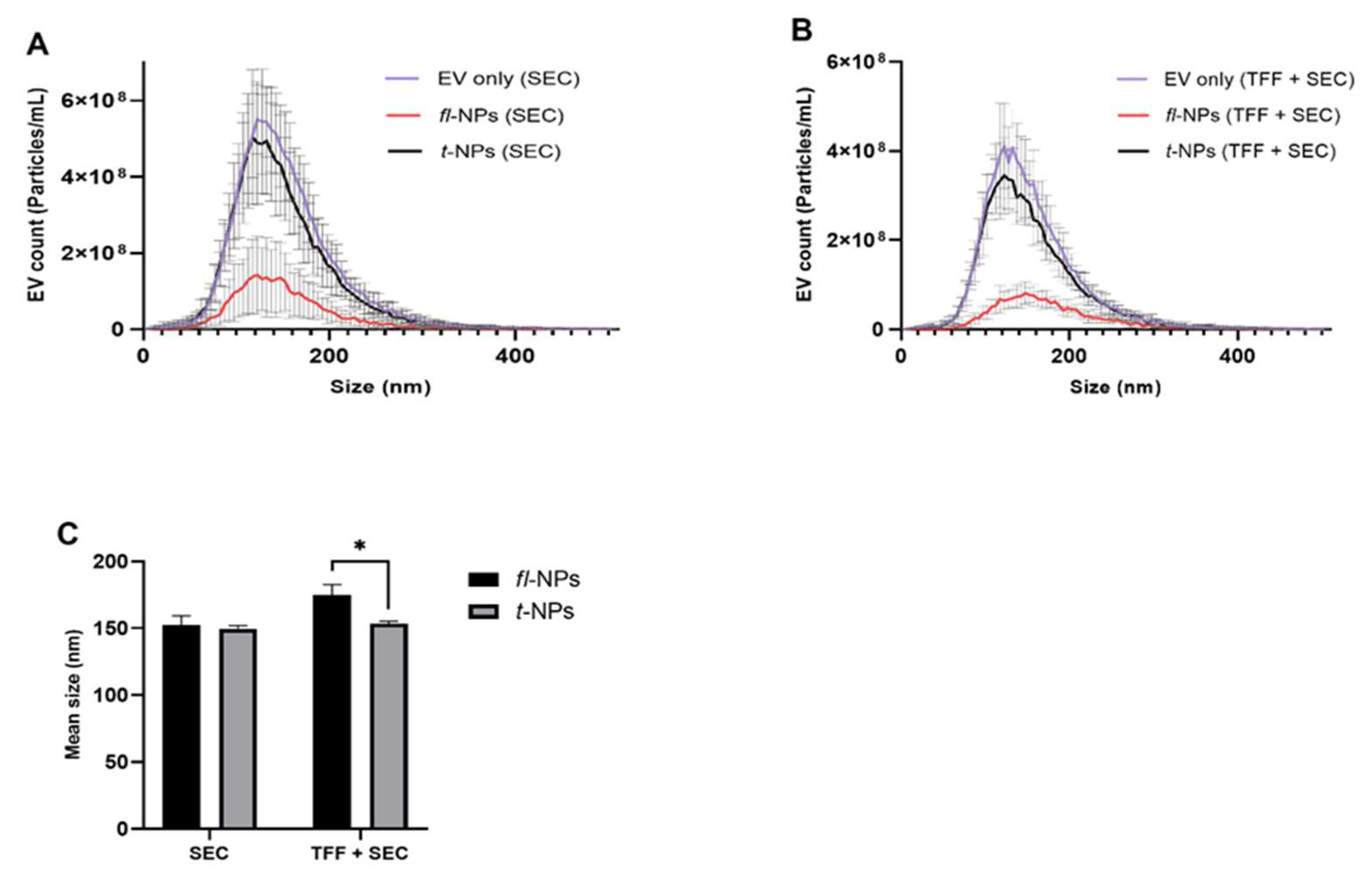
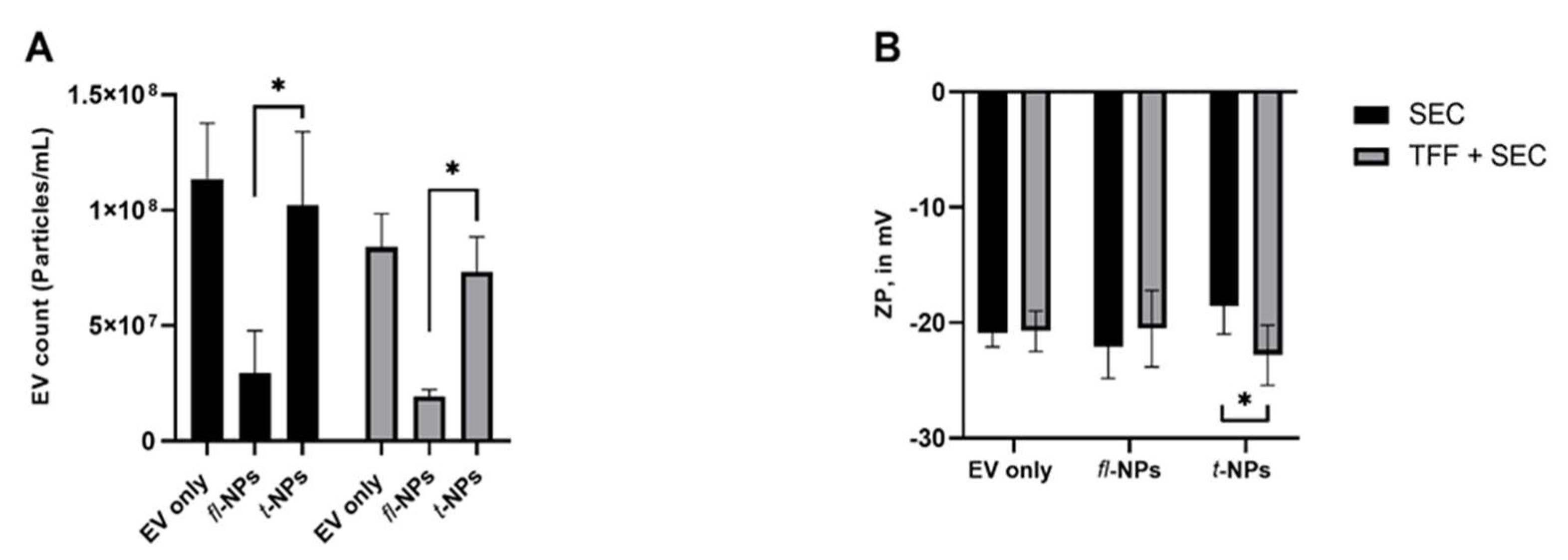
3.4. Effect of EV Purification on the Physical Characteristics of fl-NPs of EVs
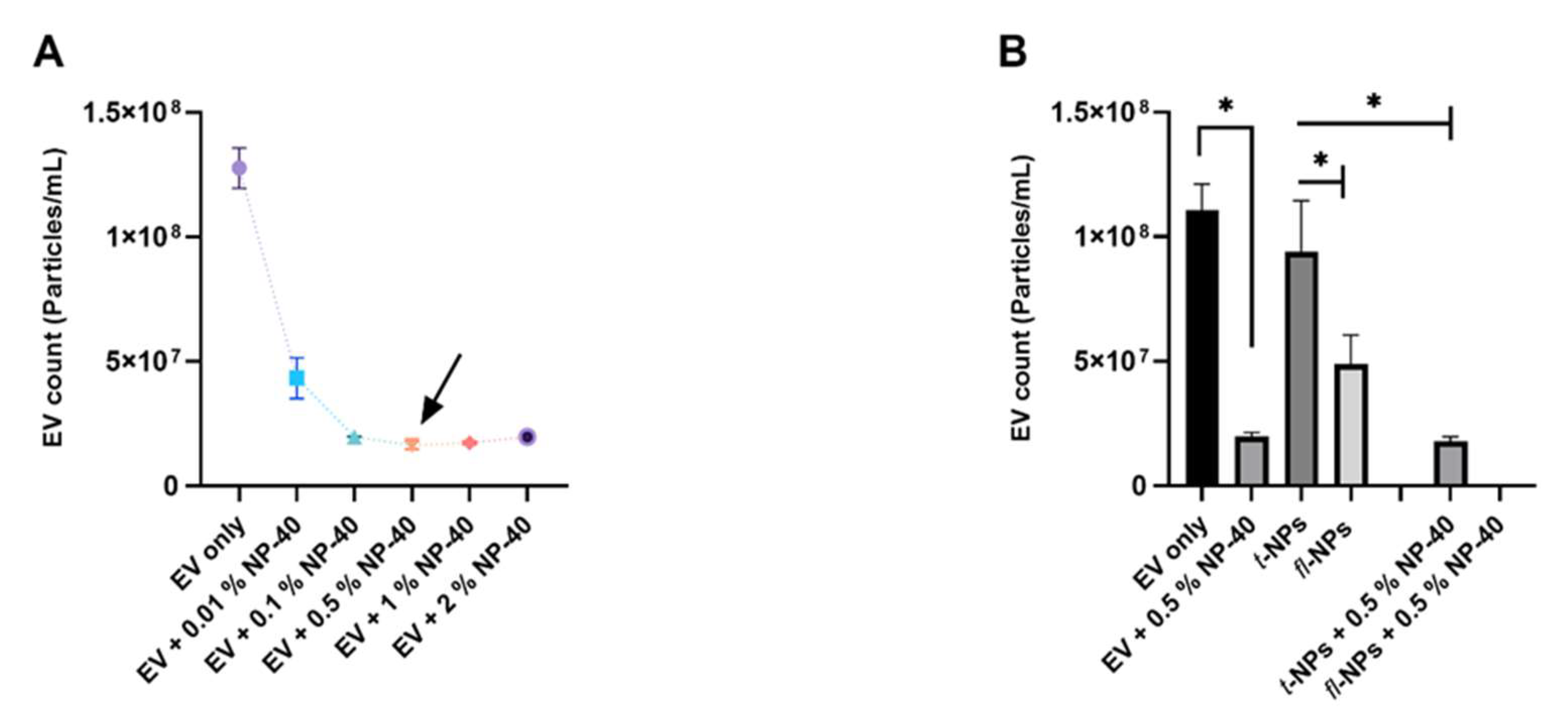
3.5. Effect of Detergent on EV Lipid Bilayer on the Context of Fluorescent EV
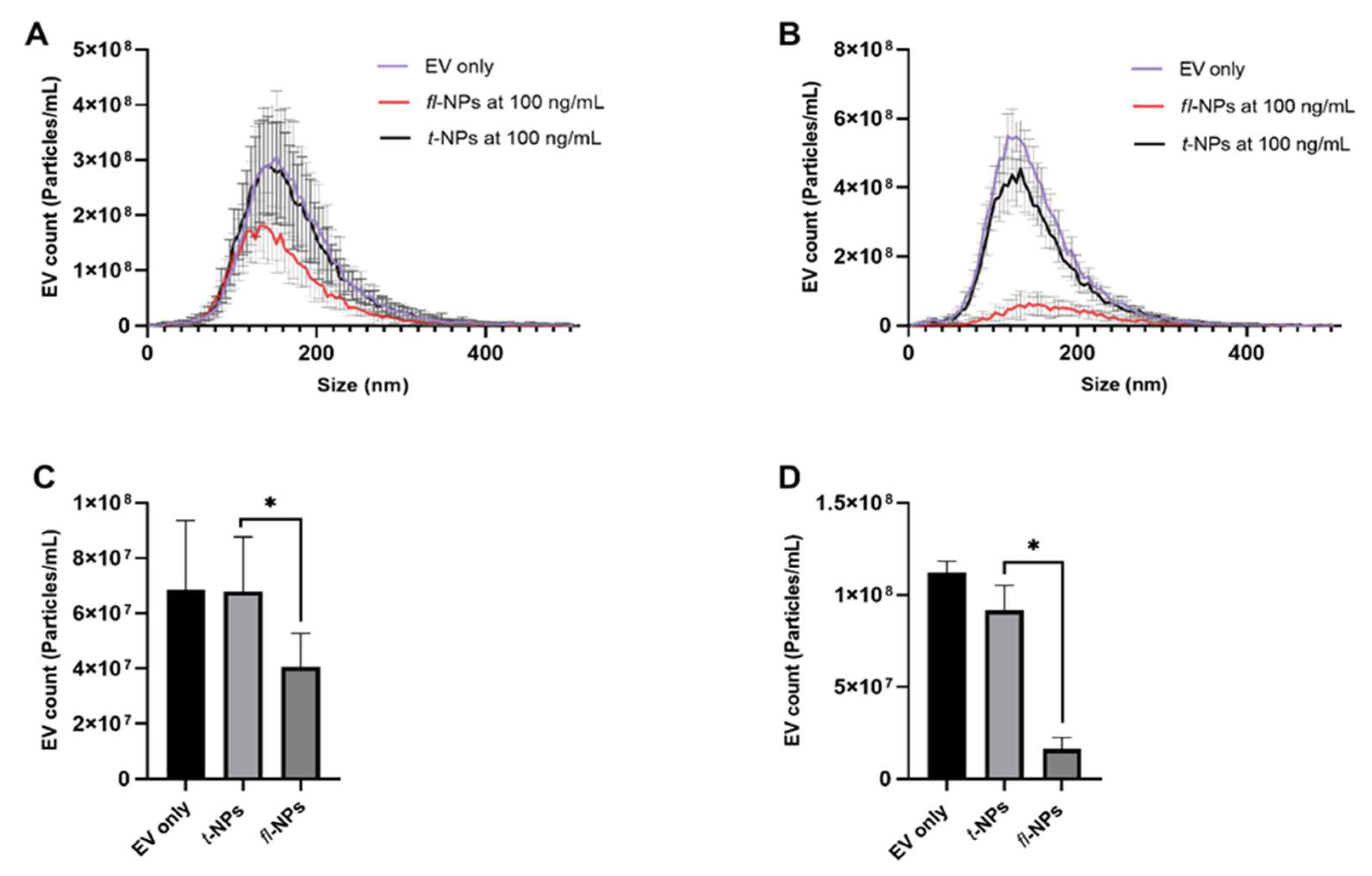
3.6. Effect of Source of EVs on the Fluorescent EV Proportions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Villa, A.; Crescenti, D.; Marzagalli, M.; Kuryk, L.; Limonta, P.; Mazzaferro, V.; Ciana, P. Heterologous and cross-species tropism of can-cer-derived extracellular vesicles. Theranostics 2019, 9, 5681–5693. [Google Scholar] [CrossRef] [PubMed]
- Van der Vlist, E.J.; Nolte-’t Hoen, E.N.M.; Stoorvogel, W.; Arkesteijn, G.J.A.; Wauben, M.H.M. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat. Protoc. 2012, 14, 1311–1326. [Google Scholar] [CrossRef]
- Tian, Y.; Manfei, G.; Gong, M.; Su, G.; Zhu, S.; Zhang, W.; Wang, S.; Li, Z.; Chen, C.; Li, L.; et al. Protein Profiling and Sizing of Extracellular Vesicles from Colorectal Cancer Patients via Flow Cytometry. ACS Nano 2018, 12, 671–680. [Google Scholar] [CrossRef]
- Caponnetto, F.; Manini, I.; Skrap, M.; Palmai-Pallag, T.; Di Loreto, C.; Beltrami, A.P.; Cesselli, D.; Ferrari, E. Size-dependent cellular uptake of exosomes. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1011–1020. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Song, Z.; Lu, S.; Yu, Y.; Tian, J.; Li, X.; Guan, F. ExoTracker: A low-pH-activatable fluorescent probe for labeling exosomes and monitoring endocytosis and trafficking. Chem. Commun. 2020, 56, 14869–14872. [Google Scholar] [CrossRef]
- Marcu, I.C.; Eberhard, N.; Yerly, A.; Balmer, V.; Hemphill, A.; Mogel, H.; Gaschen, V.; Stoffel, M.H.; Bluteau, J. Isolation of Human Small Extracellular Vesicles and Tracking of Their Uptake by Retinal Pigment Epithelial Cells In Vitro. Int. J. Mol. Sci. 2020, 21, 3799. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell. Biochem. 2010, 111, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.; Melo, S.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nat. Cell Biol. 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Yang, Y.; Oh, S.J.; Hong, Y.; Seo, M.; Jang, M. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Control. Release 2017, 266, 8–16. [Google Scholar] [CrossRef]
- Meckes, D.G.; Shair, K.H.Y.; Marquitz, A.R.; Kung, C.-P.; Edwards, R.H.; Raab-Traub, N. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. USA 2010, 107, 20370–20375. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.W.; Sceneay, J.; Lima, L.G.; Wong, C.S.; Becker, M.; Krumeich, S.; Lobb, R.; Castillo, V.; Ni Wong, K.; Ellis, S.; et al. The Biodistribution and Immune Suppressive Effects of Breast Cancer–Derived Exosomes. Cancer Res. 2016, 76, 6816–6827. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-J.; Wu, J.-Y.; Wang, J.-M.; Hu, X.-B.; Cai, J.-X.; Xiang, D.-X. Gemcitabine loaded autologous exosomes for effective and safe chem-otherapy of pancreatic cancer. Acta Biomaterialia 2020, 101, 519–530. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Zhang, H.; Xu, P.; Liu, J.; Ma, J.; Lv, M.; Li, D.; Katirai, F.; Shen, G.-X.; et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 2012, 3, 1282. [Google Scholar] [CrossRef] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P.; Wilson, J.; Hunt, T. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21054 (accessed on 26 February 2021).
- Sengupta, S.; Rothenberg, K.E.; Li, H.; Hoffman, B.D.; Bursac, N. Altering integrin engagement regulates membrane localization of K ir 2.1 channels. J. Cell Sci. 2019, 132, jcs225383. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, F.; Pereno, V.; George, J.H.; Thompson, M.S.; Ye, H. Membrane Mechanical Properties Regulate the Effect of Strain on Spontaneous Electrophysiology in Human iPSC-Derived Neurons. Neuroscience 2019, 404, 165–174. [Google Scholar] [CrossRef]
- Vogt, E.-J.; Tokuhiro, K.; Guo, M.; Dale, R.; Yang, G.; Shin, S.-W.; Movilla, M.J.; Shroff, H.; Dean, J. Anchoring cortical granules in the cortex ensures trafficking to the plasma membrane for post-fertilization exocytosis. Nat. Commun. 2019, 10, 1–13. (accessed on 26 February 2021). [Google Scholar]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular ves-icles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Bachurski, D.; Schuldner, M.; Nguyen, P.-H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Schauss, A.C.; Hansen, H.P.; Hallek, M.; Von Strandmann, E.P. Extracellular vesicle measurements with na-noparticle tracking analysis—An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J. Extracell. Vesicles 2019, 8, 1596016. [Google Scholar] [CrossRef]
- Dissanayake, K.; Nõmm, M.; Lättekivi, F.; Ressaissi, Y.; Godakumara, K.; Lavrits, A.; Midekessa, G.; Viil, J.; Bæk, R.; Jørgensen, M.M.; et al. Individually cultured bovine embryos produce extracellular vesicles that have the potential to be used as non-invasive embryo quality markers. Theriogenology 2020, 149, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Giebel, B.; Helmbrecht, C. Methods to Analyze EVs. Methods Mol. Biol. 2017, 1545, 1–20. [Google Scholar]
- Dissanayake, K.; Midekessa, G.; Lättekivi, F.; Fazeli, A. Measurement of the Size and Concentration and Zeta Potential of Extracellular Vesicles Using Nanoparticle Tracking Analyzer. In Next Generation Culture Platforms for Reliable In Vitro Models. Methods in Molecular Biology, 1st ed.; Brevini, T.A., Fazeli, A., Turksen, K., Eds.; Humana: New York, NY, USA, 2021; Volume 2273, pp. 207–218. [Google Scholar]
- Carnell-Morris, P.; Tannetta, D.; Siupa, A.; Hole, P.; Dragovic, R. Analysis of Extracellular Vesicles Using Fluorescence Nanoparticle Tracking Analysis. In Membrane Trafficking; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1660, pp. 153–173. [Google Scholar]
- CellMaskTM Green Plasma Membrane Stain. Available online: https://www.thermofisher.com/order/catalog/product/C37608 (accessed on 25 May 2021).
- Es-Haghi, M.; Godakumara, K.; Häling, A.; Lättekivi, F.; Lavrits, A.; Viil, J.; Andronowska, A.; Nafee, T.; James, V.; Jaakma, U.; et al. Specific trophoblast transcripts transferred by ex-tracellular vesicles affect gene expression in endometrial epithelial cells and may have a role in embryo-maternal crosstalk. Cell Commun. Signal. 2019, 17, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EV-TRACK Consortium; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Kornilov, R.; Puhka, M.; Mannerström, B.; Hiidenmaa, H.; Peltoniemi, H.; Siljander, P.; Seppänen-Kaijansinkko, R.; Kaur, S. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 2018, 7, 1422674. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Viil, J.; Lättekivi, F.; Ord, J.; Reshi, Q.; Jääger, K.; Velthut-Meikas, A.; Andronowska, A.; Jaakma, Ü.; Salumets, A.; et al. Bovine Follicular Fluid and Extracellular Vesicles Derived from Follicular Fluid Alter the Bovine Oviductal Epithelial Cells Transcriptome. Int. J. Mol. Sci. 2020, 21, 5365. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2, 19671. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Seino, R.; Umezaki, K.; Shimoda, A.; Ezoe, T.; Ishiyama, M.; Akiyoshi, K. New Lipophilic Fluorescent Dyes for Labeling Extracellular Vesicles: Characterization and Monitoring of Cellular Uptake of Exosomes. Bioconjugate Chem. 2021, 32, 680–684. (accessed on 21 May 2021). [Google Scholar] [CrossRef]
- Dehghani, M.; Gulvin, S.M.; Flax, J.; Gaborski, T.R. Systematic Evaluation of PKH Labelling on Extracellular Vesicle Size by Na-noparticle Tracking Analysis. Sci. Rep. 2020, 10, 9533. [Google Scholar] [CrossRef]
- Auría-Soro, C.; Nesma, T.; Juanes-Velasco, P.; Landeira-Viñuela, A.; Fidalgo-Gomez, H.; Acebes-Fernandez, V.; Gongora, R.; Parra, M.J.A.; Manzano-Roman, R.; Fuentes, M. Interactions of Nanoparticles and Biosystems: Microenvironment of Nanoparticles and Biomolecules in Nanomedicine. Nanomaterials 2019, 9, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, E.C. Use of Fluorescent Probes: Their Effect on Cell Biology and Limitations. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Tertel, T.; Bremer, M.; Maire, C.; Lamszus, K.; Peine, S.; Jawad, R.; Andaloussi, S.E.; Giebel, B.; Ricklefs, F.L.; Görgens, A. High-Resolution Imaging Flow Cytometry Reveals Impact of Incubation Temperature on Labeling of Extracellular Vesicles with Antibodies. Cytom. Part A 2020, 97, 602–609. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Sódar, B.; Németh, A.; Szabó-Taylor, K.; Pálóczi, K.; Vukman, K.V.; Tamasi, V.; Balogh, A.; Kittel, A.; Pallinger, E.; et al. Differential detergent sensitivity of extra-cellular vesicle subpopulations. Organ. Biomol. Chem. 2015, 13, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Musante, L.; Saraswat, M.; Duriez, E.; Byrne, B.; Ravida, A.; Domon, B.; Holthofer, H. Biochemical and Physical Characterisation of Urinary Nanovesicles following CHAPS Treatment. PLoS ONE 2012, 7, e37279. [Google Scholar] [CrossRef] [Green Version]
- György, B.; Módos, K.; Pállinger, É.; Pálóczi, K.; Pásztói, M.; Misják, P.; Deli, M.; Sipos, Á.; Szalai, A.; Voszka, I.; et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 2011, 117, e39–e48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annes, K.; Müller, D.B.; Vilela, J.A.P.; Valente, R.S.; Caetano, D.P.; Cibin, F.W.S.; Milazzotto, M.; Mesquita, F.S.; Belaz, K.R.A.; Eberlin, M.N.; et al. Influence of follicle size on bovine oocyte lipid composition, follicular metabolic and stress markers, embryo development and blastocyst lipid content. Reprod. Fertil. Dev. 2019, 31, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Komarek, R.; Pickett, B.; Lanz, R.; Jensen, R. Lipid Composition of Bovine Spermatozoa and Seminal Plasma. J. Dairy Sci. 1964, 47, 531–534. [Google Scholar] [CrossRef]
- Am-In, N.; Kirkwood, R.; Techakumphu, M.; Tantasuparuk, W. Lipid profiles of sperm and seminal plasma from boars having normal or low sperm motility. Theriogenology 2011, 75, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Vignon, F.; Koll-back, M.H.; Clavert, A.; Cranz, C. Lipid Composition of Human Seminal Plasma. Arch. Androl. 1989, 22, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Pužar Dominkuš, P.; Stenovec, M.; Sitar, S.; Lasič, E.; Zorec, R.; Plemenitaš, A.; Žagar, E.; Kreft, M.; Lenassi, M. PKH26 labeling of extracellular vesicles: Characterization and cellular internalization of contaminating PKH26 nanoparticles. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 1350–1361. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Midekessa, G.; Godakumara, K.; Dissanayake, K.; Hasan, M.M.; Reshi, Q.U.A.; Rinken, T.; Fazeli, A. Characterization of Extracellular Vesicles Labelled with a Lipophilic Dye Using Fluorescence Nanoparticle Tracking Analysis. Membranes 2021, 11, 779. https://doi.org/10.3390/membranes11100779
Midekessa G, Godakumara K, Dissanayake K, Hasan MM, Reshi QUA, Rinken T, Fazeli A. Characterization of Extracellular Vesicles Labelled with a Lipophilic Dye Using Fluorescence Nanoparticle Tracking Analysis. Membranes. 2021; 11(10):779. https://doi.org/10.3390/membranes11100779
Chicago/Turabian StyleMidekessa, Getnet, Kasun Godakumara, Keerthie Dissanayake, Mohammad Mehedi Hasan, Qurat Ul Ain Reshi, Toonika Rinken, and Alireza Fazeli. 2021. "Characterization of Extracellular Vesicles Labelled with a Lipophilic Dye Using Fluorescence Nanoparticle Tracking Analysis" Membranes 11, no. 10: 779. https://doi.org/10.3390/membranes11100779
APA StyleMidekessa, G., Godakumara, K., Dissanayake, K., Hasan, M. M., Reshi, Q. U. A., Rinken, T., & Fazeli, A. (2021). Characterization of Extracellular Vesicles Labelled with a Lipophilic Dye Using Fluorescence Nanoparticle Tracking Analysis. Membranes, 11(10), 779. https://doi.org/10.3390/membranes11100779







