Two-Dimensional Transition Metal Carbides and Nitrides (MXenes) for Water Purification and Antibacterial Applications
Abstract
:1. Introduction
2. Fabrication of MXene-Based Membranes
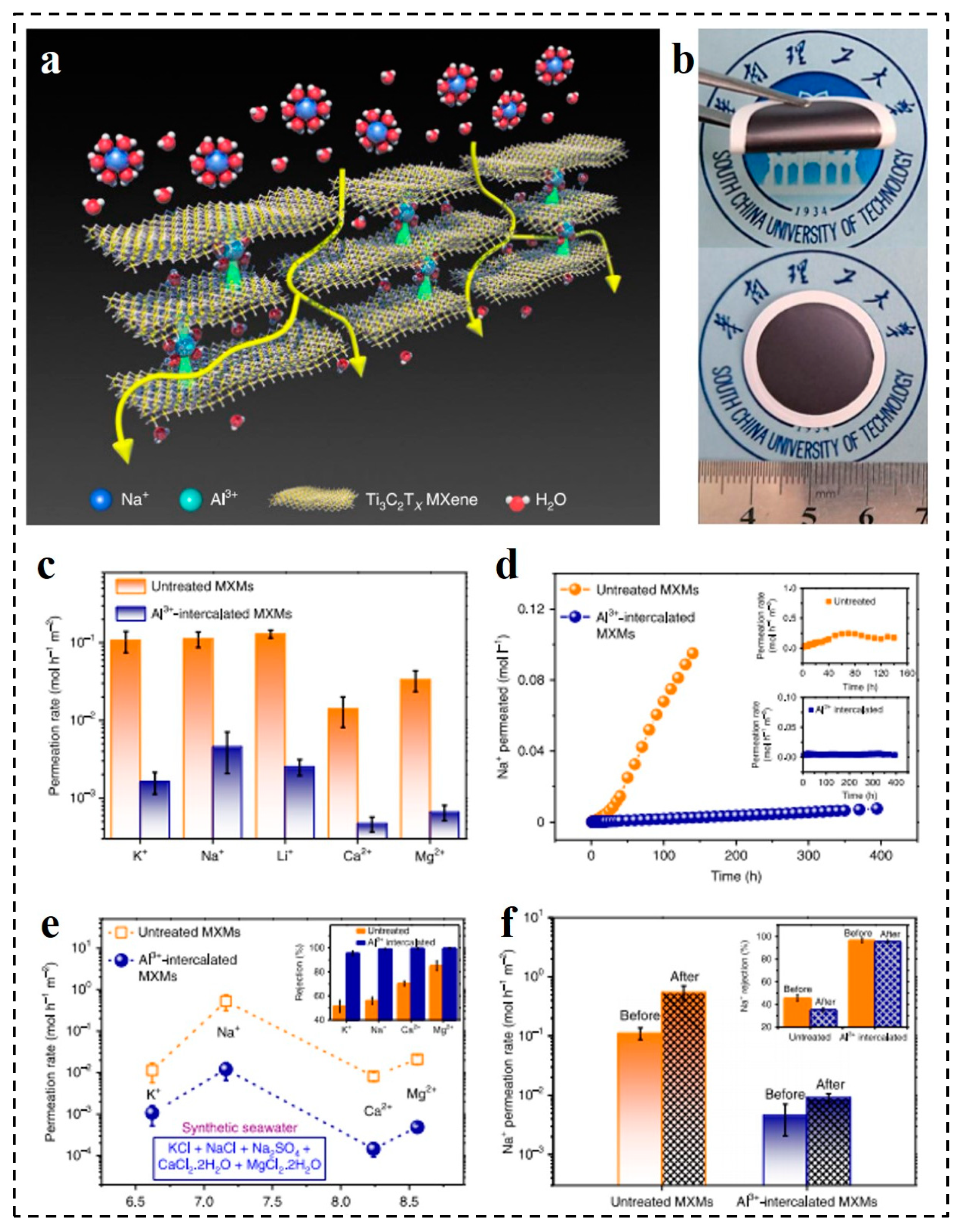
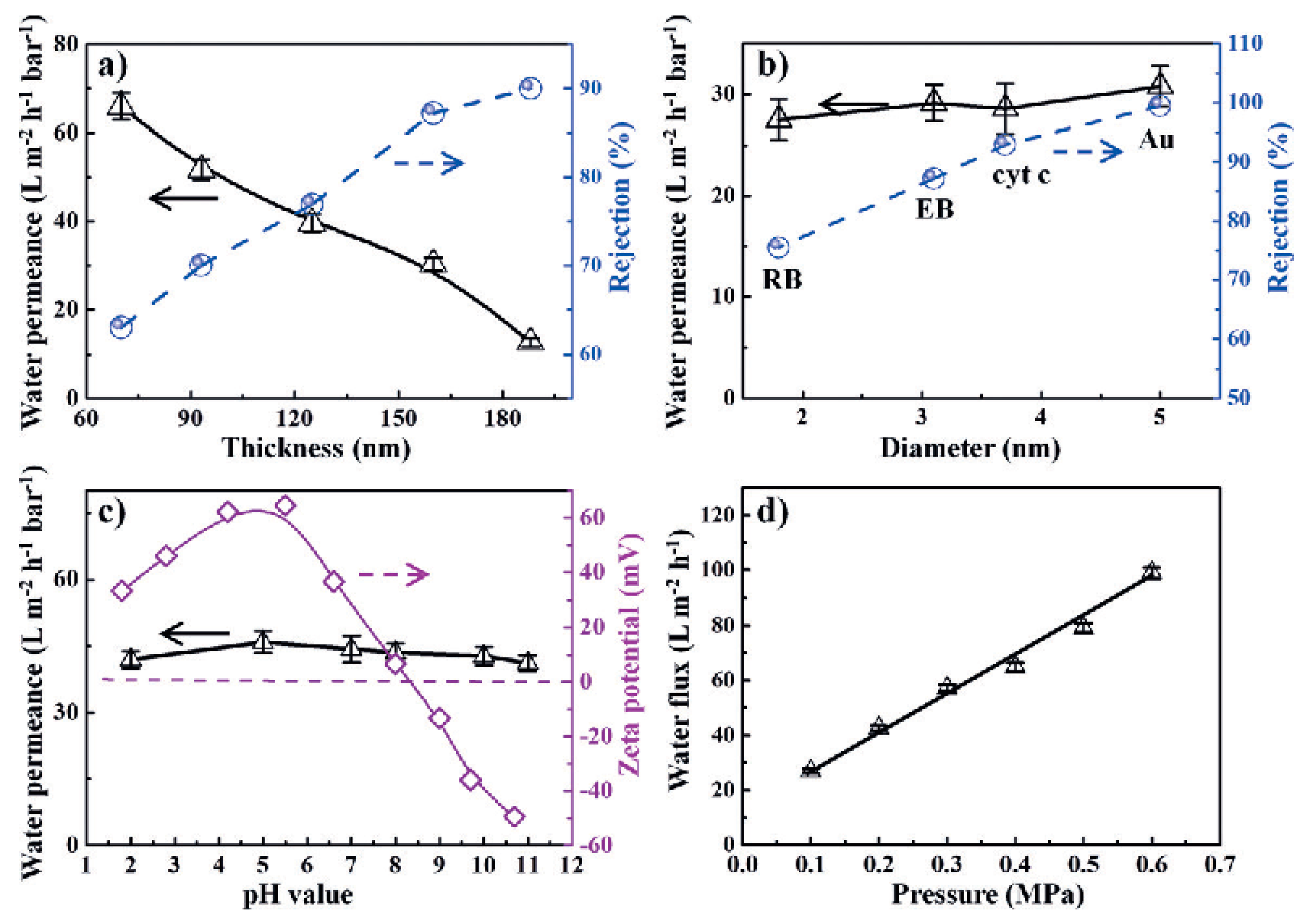
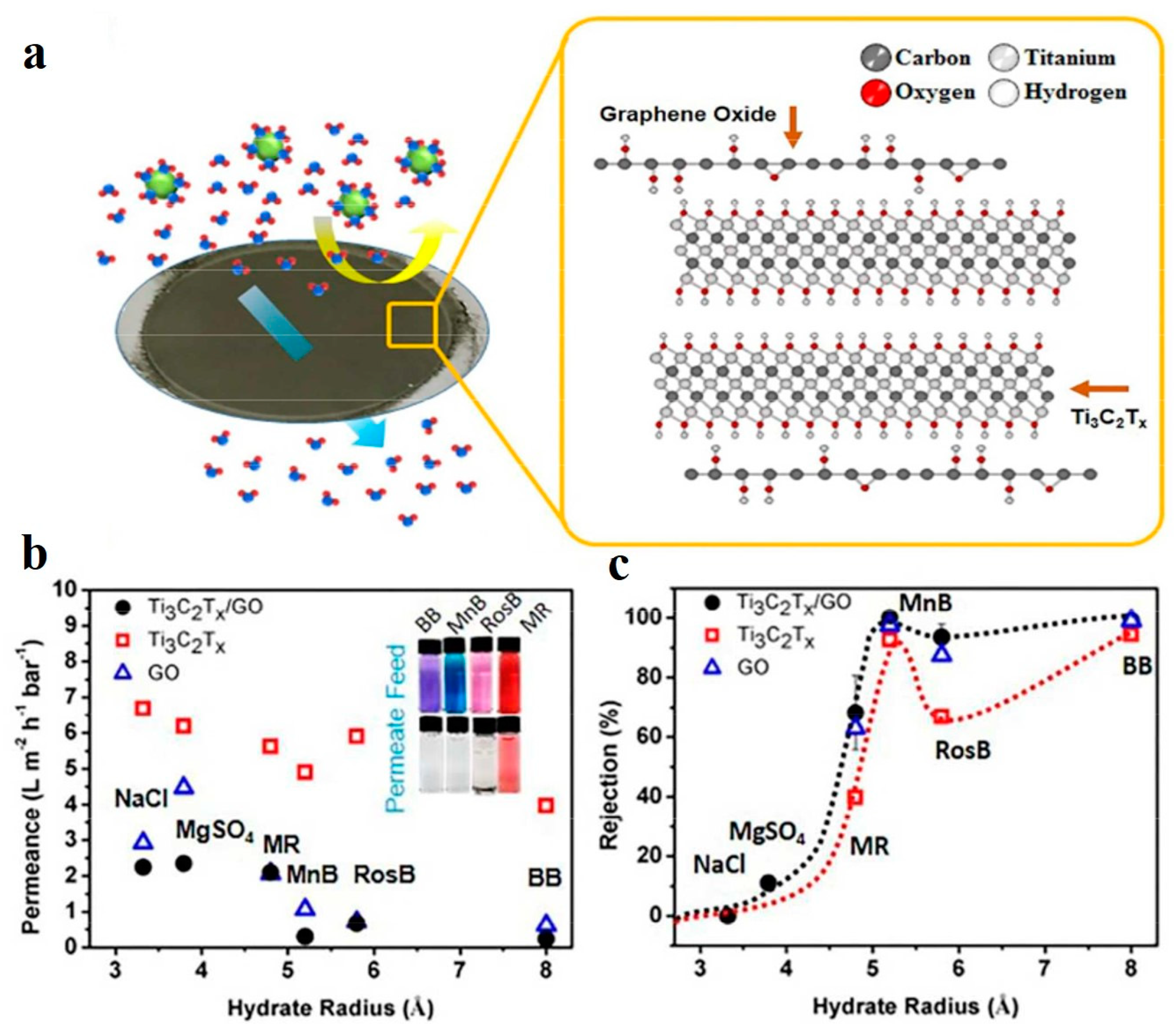
3. Water Purification and Desalination Applications
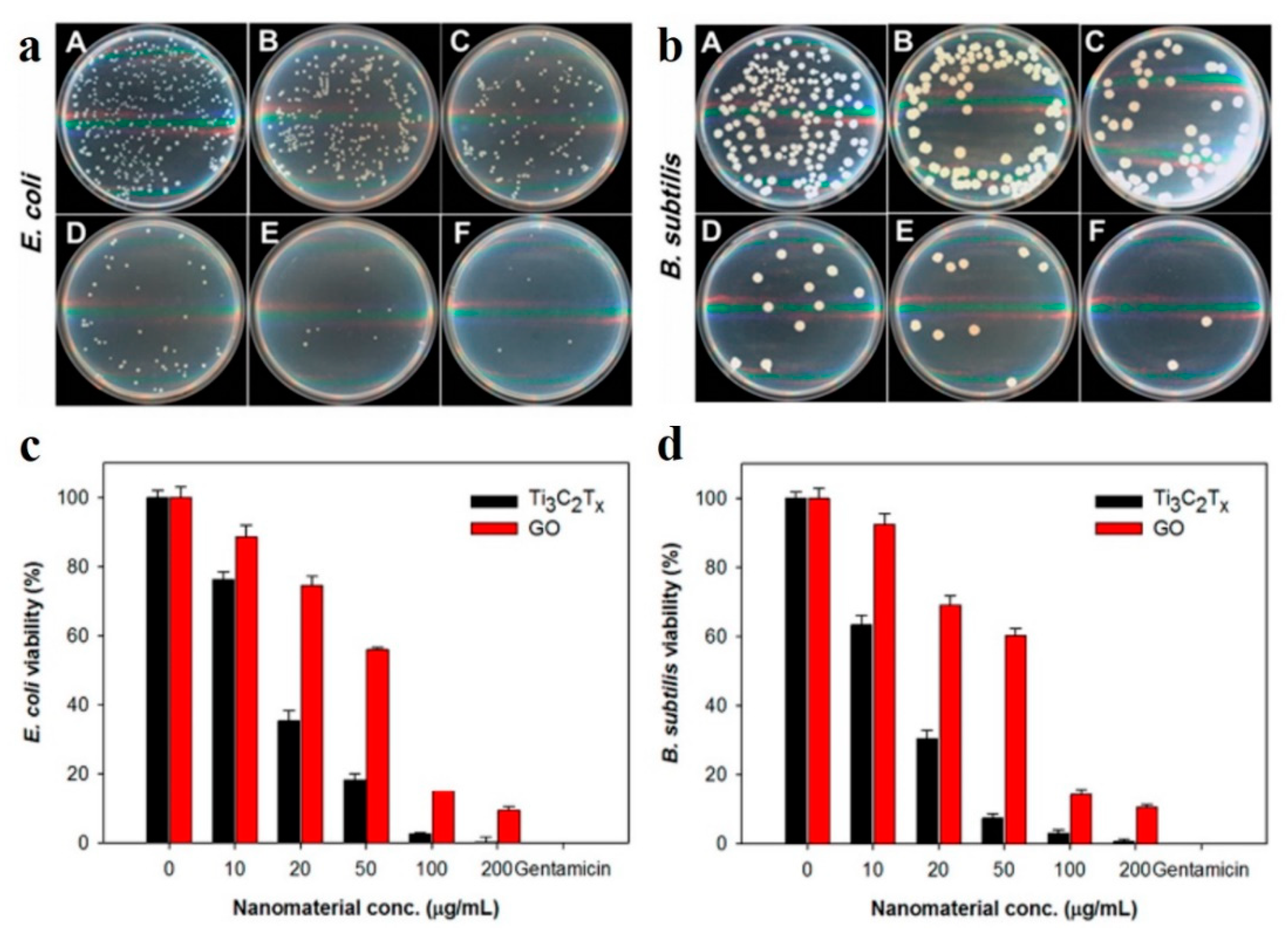
4. Antibacterial Activity of MXene-Based Membranes
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Morris, R.D. Drinking water and cancer. Environ. Health Perspect 1995, 103 (Suppl. S8), 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, M.; Manzoor, S.; Ahmad, H.; Asif, A.; Bangash, T.A.; Latif, A.; Jaleel, S. Purinoceptor expression in hepatocellular virus (HCV)-induced and non-HCV hepatocellular carcinoma: An insight into the proviral role of the P2X4 receptor. Mol. Biol. Rep. 2018, 45, 2625–2630. [Google Scholar] [CrossRef]
- Asif, A.; Khalid, M.; Manzoor, S.; Ahmad, H.; Rehman, A.U. Role of purinergic receptors in hepatobiliary carcinoma in Pakistani population: An approach towards proinflammatory role of P2X4 and P2X7 receptors. Purinergic Signal. 2019, 15, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hrudey, S.; Payment, P.; Huck, P.; Gillham, R.; Hrudey, E. A fatal waterborne disease epidemic in Walkerton, Ontario: Comparison with other waterborne outbreaks in the developed world. Water Sci. Technol. 2003, 47, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Manzoor, S.; Tuz-Zahra, F.; Saalim, M.; Ashraf, M.; Ishtiyaq, J.; Khalid, M. Zika virus: Immune evasion mechanisms, currently available therapeutic regimens, and vaccines. Viral Immunol. 2017, 30, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Tauxe, R.V.; Mintz, E.D.; Quick, R.E. Epidemic cholera in the new world: Translating field epidemiology into new prevention strategies. Emerg. Infect. Dis. 1995, 1, 141. [Google Scholar] [CrossRef]
- Aziz, N.; Hanafiah, M.M. Application of life cycle assessment for desalination: Progress, challenges and future directions. Environ. Pollut. 2021, 268, 115948. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.G. Desalination and sustainability—An appraisal and current perspective. Water Res. 2016, 89, 87–106. [Google Scholar] [CrossRef]
- Pan, S.Y.; Haddad, A.Z.; Kumar, A.; Wang, S.W. Brackish water desalination using reverse osmosis and capacitive deionization at the water-energy nexus. Water Res. 2020, 183, 116064. [Google Scholar] [CrossRef]
- Ahmed, Z.; Rehman, F.; Ali, U.; Ali, A.; Iqbal, M.; Thebo, K.H. Recent Advances in MXene-based Separation Membranes. ChemBioEng Rev. 2021, 8, 110–120. [Google Scholar] [CrossRef]
- Ali, A.; Aamir, M.; Thebo, K.H.; Akhtar, J. Laminar Graphene Oxide Membranes Towards Selective Ionic and Molecular Separations: Challenges and Progress. Chem. Rec. 2020, 20, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Ahmad, K.S.; Rehman, F.; Bhatti, Z.; Thebo, K.H. Two-dimensional graphene oxide based membranes for ionic and molecular separation: Current status and challenges. J. Environ. Chem. Eng. 2021, 9, 105605. [Google Scholar] [CrossRef]
- Wang, E.N.; Karnik, R. Water desalination: Graphene cleans up water. Nat. Nano 2012, 7, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Mehmood, M.; Ahmed, J.; Majeed, A.; Thebo, K.H. CVD grown defect rich-MWCNTs with anchored CoFe alloy nanoparticles for OER activity. Mater. Lett. 2020, 259, 126831. [Google Scholar] [CrossRef]
- Ali, Z.; Mehmood, M.; Ahmed, J.; Majeed, A.; Thebo, K.H. MWCNTs and carbon onions grown by CVD method on nickel-cobalt alloy nanocomposites prepared via novel alcogel electrolysis technique and its oxygen evolution reaction application. Mater. Res. Express 2019, 6, 105627. [Google Scholar] [CrossRef]
- Fan, H.; Peng, M.; Strauss, I.; Mundstock, A.; Meng, H.; Caro, J. MOF-in-COF molecular sieving membrane for selective hydrogen separation. Nat. Commun. 2021, 12, 38. [Google Scholar] [CrossRef]
- Rehman, F.; Thebo, K.H.; Aamir, M.; Akhtar, J. Chapter 8—Nanomembranes for Water Treatment. In Nanotechnology in the Beverage Industry; Amrane, A., Rajendran, S., Nguyen, T.A., Assadi, A.A., Sharoba, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–240. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L.; Wang, H.; Xu, Z.; Zhao, Y.; Luo, Y.; Nasir, N.; Song, Y.; Wu, H.; Pan, F.; et al. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 2019, 10, 2101. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Chen, L.; Yin, L.; Liu, Z.; Pei, S.; Li, F.; Hou, G.; Chen, S.; Song, L.; Thebo, K.H.; et al. CdPS3 nanosheets-based membrane with high proton conductivity enabled by Cd vacancies. Science 2020, 370, 596–600. [Google Scholar] [CrossRef]
- Thebo, K.H.; Qian, X.; Zhang, Q.; Chen, L.; Cheng, H.M.; Ren, W. Highly stable graphene-oxide-based membranes with superior permeability. Nat. Commun. 2018, 9, 1486. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Qian, X.; Thebo, K.H.; Cheng, H.-M.; Ren, W. Controlling reduction degree of graphene oxide membranes for improved water permeance. Sci. Bull. 2018, 63, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Thebo, K.H.; Qian, X.; Wei, Q.; Zhang, Q.; Cheng, H.-M.; Ren, W. Reduced graphene oxide/metal oxide nanoparticles composite membranes for highly efficient molecular separation. J. Mater. Sci. Technol. 2018, 34, 1481–1486. [Google Scholar] [CrossRef]
- Ali, A.; Pothu, R.; Siyal, S.H.; Phulpoto, S.; Sajjad, M.; Thebo, K.H. Graphene-based membranes for CO2 separation. Mater. Sci. Energy Technol. 2019, 2, 83–88. [Google Scholar] [CrossRef]
- Jaffri, S.B.; Ahmad, K.S.; Thebo, K.H.; Rehman, F. Recent developments in carbon nanotubes-based perovskite solar cells with boosted efficiency and stability. Z. Phys. Chem. 2021. [Google Scholar] [CrossRef]
- Ahmed Janjhi, F.; Chandio, I.; Ali Memon, A.; Ahmed, Z.; Hussain Thebo, K.; Ali Ayaz Pirzado, A.; Ali Hakro, A.; Iqbal, M. Functionalized graphene oxide based membranes for ultrafast molecular separation. Sep. Purif. Technol. 2020, 274, 117969. [Google Scholar] [CrossRef]
- Chandio, I.; Janjhi, F.A.; Memon, A.A.; Memon, S.; Ali, Z.; Thebo, K.H.; Pirzado, A.A.A.; Hakro, A.A.; Khan, W.S. Ultrafast ionic and molecular sieving through graphene oxide based composite membranes. Desalination 2020, 500, 114848. [Google Scholar] [CrossRef]
- Maqbool, I.; Rehman, F.; Soomro, F.; Bhatti, Z.; Ali, U.; Jatoi, A.H.; Lal, B.; Iqbal, M.; Phulpoto, S.; Ali, A.; et al. Graphene-based Materials for Fighting Coronavirus Disease 2019: Challenges and Opportunities. ChemBioEng Rev. 2021, 8, 67–77. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Liu, D.; Yang, C.; Liu, Y.; Ruoff, R.S.; Lei, W. Functionalized boron nitride membranes with ultrafast solvent transport performance for molecular separation. Nat. Commun. 2018, 9, 1902. [Google Scholar] [CrossRef] [PubMed]
- Chethikkattuveli Salih, A.R.; Hyun, K.; Asif, A.; Soomro, A.M.; Farooqi, H.M.U.; Kim, Y.S.; Kim, K.H.; Lee, J.W.; Huh, D.; Choi, K.H. Extracellular Matrix Optimization for Enhanced Physiological Relevance in Hepatic Tissue-Chips. Polymers 2021, 13, 3016. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Asif, A.; Kim, K.H.; Jabbar, F.; Kim, S.; Choi, K.H. Real-time sensors for live monitoring of disease and drug analysis in microfluidic model of proximal tubule. Microfluid. Nanofluidics 2020, 24, 43. [Google Scholar] [CrossRef]
- Asif, A.; Park, S.H.; Soomro, A.M.; Khalid, M.A.U.; Salih, A.R.C.; Kang, B.; Ahmed, F.; Kim, K.H.; Choi, K.H. Microphysiological system with continuous analysis of albumin for hepatotoxicity modeling and drug screening. J. Ind. Eng. Chem. 2021, 98, 318–326. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Asif, A.; Chethikkattuveli Salih, A.R.; Lee, J.-W.; Hyun, K.-N.; Choi, K.-H. Gravity-Based Flow Efficient Perfusion Culture System for Spheroids Mimicking Liver Inflammation. Biomedicines 2021, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.R.C.; Farooqi, H.M.U.; Kim, Y.S.; Lee, S.H.; Choi, K.H. Impact of serum concentration in cell culture media on tight junction proteins within a multiorgan microphysiological system. Microelectron. Eng. 2020, 232, 111405. [Google Scholar] [CrossRef]
- Waqas, M.; Manzoor Soomro, A.; Ali, S.; Kumar, S.; Chan, S.; Hussain, K.; Hussain Memon, F.; Ahmed Shaikh, S. Multifunctional Cathodic Interlayer with Polysulfide Immobilization Mechanism for High-Performance Li-S Batteries. ChemistrySelect 2020, 5, 12009–12019. [Google Scholar] [CrossRef]
- Soomro, A.M.; Memon, F.H.; Lee, J.-W.; Ahmed, F.; Kim, K.H.; Kim, Y.S.; Choi, K.H. Fully 3D printed multi-material soft bio-inspired frog for underwater synchronous swimming. Int. J. Mech. Sci. 2021, 210, 106725. [Google Scholar] [CrossRef]
- Caro, J. Hierarchy in inorganic membranes. Chem. Soc. Rev. 2016, 45, 3468–3478. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Tang, P.-F.; Liu, C.-J.; Li, R.; Li, X.-D.; Chen, J.; Qiao, Z.-Q.; Zhang, H.-P.; Yang, G.-C. An efficient light-to-heat conversion coupling photothermal effect and exothermic chemical reaction in Au NRs/V2C MXene membranes for high-performance laser ignition. Def. Technol. 2021. Advance online publication. [Google Scholar] [CrossRef]
- Shi, F.; Sun, J.; Wang, J.; Liu, M.; Yan, Z.; Zhu, B.; Li, Y.; Cao, X. MXene versus graphene oxide: Investigation on the effects of 2D nanosheets in mixed matrix membranes for CO2 separation. J. Membr. Sci. 2021, 620, 118850. [Google Scholar] [CrossRef]
- Tong, X.; Liu, S.; Qu, D.; Gao, H.; Yan, L.; Chen, Y.; Crittenden, J. Tannic acid-metal complex modified MXene membrane for contaminants removal from water. J. Membr. Sci. 2021, 622, 119042. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, Y.; Wang, P.; Wang, Y.; Wang, Q.; Yang, Z.; Tang, C.; Xiang, S.; Luo, S.; Huang, S.; et al. Facile synthesis of a sandwiched Ti3C2Tx MXene/nZVI/fungal hypha nanofiber hybrid membrane for enhanced removal of Be(II) from Be(NH2)2 complexing solutions. Chem. Eng. J. 2021, 421, 129682. [Google Scholar] [CrossRef]
- Ajibade, T.F.; Tian, H.; Hassan Lasisi, K.; Xue, Q.; Yao, W.; Zhang, K. Multifunctional PAN UF membrane modified with 3D-MXene/O-MWCNT nanostructures for the removal of complex oil and dyes from industrial wastewater. Sep. Purif. Technol. 2021, 275, 119135. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Li, J.; Luo, X.; Xu, D.; Wu, D.; Wang, W.; Cheng, X.; Li, G.; Liang, H. Crumple-textured polyamide membranes via MXene nanosheet-regulated interfacial polymerization for enhanced nanofiltration performance. J. Membr. Sci. 2021, 635, 119536. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, D.; Li, S.; Cui, L.; Zhuang, Y.; Xing, W.; Jing, W. Assembly of multidimensional MXene-carbon nanotube ultrathin membranes with an enhanced anti-swelling property for water purification. J. Membr. Sci. 2021, 623, 119075. [Google Scholar] [CrossRef]
- Zeng, G.; Wei, K.; Zhang, H.; Zhang, J.; Lin, Q.; Cheng, X.; Sengupta, A.; Chiao, Y.-H. Ultra-high oil-water separation membrane based on two-dimensional MXene(Ti3C2Tx) by co-incorporation of halloysite nanotubes and polydopamine. Appl. Clay Sci. 2021, 211, 106177. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Y.; Zeng, G.; Li, X.; Wang, B.; Cheng, X.; Sengupta, A.; Yang, X.; Feng, Z. Bionics inspired modified two-dimensional MXene composite membrane for high-throughput dye separation. J. Environ. Chem. Eng. 2021, 9, 105711. [Google Scholar] [CrossRef]
- Jin, Y.; Fan, Y.; Meng, X.; Li, J.; Li, C.; Sunarso, J.; Yang, N.; Meng, B.; Zhang, W. Modeling of hydrated cations transport through 2D MXene (Ti3C2Tx) membranes for water purification. J. Membr. Sci. 2021, 631, 119346. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Liu, Y.; Xu, Y.; Li, R.; Hong, H.; Shen, L.; Lin, H.; Liao, B.-Q. Enhanced permeability and antifouling performance of polyether sulfone (PES) membrane via elevating magnetic Ni@MXene nanoparticles to upper layer in phase inversion process. J. Membr. Sci. 2021, 623, 119080. [Google Scholar] [CrossRef]
- Li, S.; Geng, X.; Ma, C.; Zhan, X.; Li, J.; Ma, M.; He, J.; Wang, L. Improved performance of three-component structure mixed membrane for pervaporation modified by lignosulfonates@2D-MXene. Sep. Purif. Technol. 2021, 276, 119294. [Google Scholar] [CrossRef]
- Long, Q.; Zhao, S.; Chen, J.; Zhang, Z.; Qi, G.; Liu, Z.-Q. Self-assembly enabled nano-intercalation for stable high-performance MXene membranes. J. Membr. Sci. 2021, 635, 119464. [Google Scholar] [CrossRef]
- Meng, B.; Liu, G.; Mao, Y.; Liang, F.; Liu, G.; Jin, W. Fabrication of surface-charged MXene membrane and its application for water desalination. J. Membr. Sci. 2021, 623, 119076. [Google Scholar] [CrossRef]
- Hu, J.; Zhan, Y.; Zhang, G.; Feng, Q.; Yang, W.; Chiao, Y.-H.; Zhang, S.; Sun, A. Durable and super-hydrophilic/underwater super-oleophobic two-dimensional MXene composite lamellar membrane with photocatalytic self-cleaning property for efficient oil/water separation in harsh environments. J. Membr. Sci. 2021, 637, 119627. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Abuabdou, S.M.A.; Ng, D.-Q.; Bashir, M.J.K. Insight into two-dimensional MXenes for environmental applications: Recent progress, challenges, and prospects. FlatChem 2021, 28, 100256. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Lin, H.; Mo, A. 2D titanium carbide(MXene) nanosheets and 1D hydroxyapatite nanowires into free standing nanocomposite membrane: In vitro and in vivo evaluations for bone regeneration. Mater. Sci. Eng. C 2021, 118, 111367. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Lv, R.; Gao, J.; Wang, A.; Wu, J. Aqueous Stable Ti(3)C(2) MXene Membrane with Fast and Photoswitchable Nanofluidic Transport. ACS Nano 2018, 12, 12464–12471. [Google Scholar] [CrossRef]
- Fei, M.; Lin, R.; Deng, Y.; Xian, H.; Bian, R.; Zhang, X.; Cheng, J.; Xu, C.; Cai, D. Polybenzimidazole/Mxene composite membranes for intermediate temperature polymer electrolyte membrane fuel cells. Nanotechnology 2018, 29, 035403. [Google Scholar] [CrossRef]
- Liu, G.; Cheng, L.; Chen, G.; Liang, F.; Liu, G.; Jin, W. Pebax-Based Membrane Filled with Two-Dimensional Mxene Nanosheets for Efficient CO(2) Capture. Chem. Asian J. 2020, 15, 2364–2370. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Arabi Shamsabadi, A.; Lin, H.; Luis, P.; Ramakrishna, S.; Aminabhavi, T.M. Sustainable MXenes-based membranes for highly energy-efficient separations. Renew. Sustain. Energy Rev. 2021, 143, 110878. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Jun, B.-M.; Yoon, M.; Taheri-Qazvini, N.; Snyder, S.A.; Jang, M.; Heo, J.; Yoon, Y. Applications of MXene-based membranes in water purification: A review. Chemosphere 2020, 254, 126821. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Z.; Sun, Y.; Long, R.; Shan, M.; Li, X.; Liu, Y.; Liu, J. Review MXenes as a new type of nanomaterial for environmental applications in the photocatalytic degradation of water pollutants. Ceram. Int. 2021, 47, 7321–7343. [Google Scholar] [CrossRef]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Buczek, S.; Gogotsi, Y.; Mahmoud, K.A. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes). Mater. Today 2019, 30, 80–102. [Google Scholar] [CrossRef]
- Hwang, S.K.; Kang, S.-M.; Rethinasabapathy, M.; Roh, C.; Huh, Y.S. MXene: An emerging two-dimensional layered material for removal of radioactive pollutants. Chem. Eng. J. 2020, 397, 125428. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving Through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wei, Y.; Wang, Y.; Chen, H.; Caro, J.; Wang, H. A Two-Dimensional Lamellar Membrane: MXene Nanosheet Stacks. Angew. Chem. Int. Ed. 2017, 56, 1825–1829. [Google Scholar] [CrossRef]
- Kang, K.M.; Kim, D.W.; Ren, C.E.; Cho, K.M.; Kim, S.J.; Choi, J.H.; Nam, Y.T.; Gogotsi, Y.; Jung, H.-T. Selective Molecular Separation on Ti3C2Tx–Graphene Oxide Membranes during Pressure-Driven Filtration: Comparison with Graphene Oxide and MXenes. ACS Appl. Mater. Interfaces 2017, 9, 44687–44694. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Graham, N.; Yu, W.; Sun, K. Two-dimensional MXene incorporated graphene oxide composite membrane with enhanced water purification performance. J. Membr. Sci. 2020, 593, 117431. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Zhu, J.; Tian, M.; Zheng, S.; Wang, F.; Wang, X.; Wang, L. Ion sieving by a two-dimensional Ti3C2Tx alginate lamellar membrane with stable interlayer spacing. Nat. Commun. 2020, 11, 3540. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, R.; Hou, P.-X.; Ma, Y.; Majeed, A.; Wang, X.; Liu, C. MXene-Carbon Nanotube Hybrid Membrane for Robust Recovery of Au from Trace-Level Solution. ACS Appl. Mater. Interfaces 2020, 12, 43032–43041. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; VahidMohammadi, A.; Wang, Z.; Ouyang, L.; Beidaghi, M.; Hamedi, M.M. Layer-by-layer self-assembly of pillared two-dimensional multilayers. Nat. Commun. 2019, 10, 2558. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.; Wyatt, B.C.; Nemani, S.K.; Anasori, B. Double transition-metal MXenes: Atomistic design of two-dimensional carbides and nitrides. MRS Bull. 2020, 45, 850–861. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, X. MXene Nanosheet Templated Nanofiltration Membranes toward Ultrahigh Water Transport. Environ. Sci. Technoogies 2021, 55, 1270–1278. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, e1906697. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Ma, X.; Xie, Y.; Teng, D.; Zhang, S. Preparation of a new 2D MXene/PES composite membrane with excellent hydrophilicity and high flux. RSC Adv. 2017, 7, 56204–56210. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Pu, Y.; Zhao, D. Two-Dimensional Membranes: New Paradigms for High-Performance Separation Membranes. Chem. Asian J. 2020, 15, 2241–2270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Shen, Y.; Mu, P.; Wang, Q.; Li, J. Ultrathin 2D Ti(3)C(2)T(x) MXene membrane for effective separation of oil-in-water emulsions in acidic, alkaline, and salty environment. J. Colloid Interface Sci. 2020, 561, 861–869. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, W.; Xu, H.; Yang, W.; Kong, Q.; Wang, A.; Ding, M.; Shang, J. Fabrication of a Novel Antifouling Polysulfone Membrane with in Situ Embedment of Mxene Nanosheets. Int. J. Environ. Res. Public Health 2019, 16, 4659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zha, X.J.; Zhao, X.; Pu, J.H.; Tang, L.S.; Ke, K.; Bao, R.Y.; Bai, L.; Liu, Z.Y.; Yang, M.B. Flexible Anti-Biofouling MXene/Cellulose Fibrous Membrane for Sustainable Solar-Driven Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 36589–36597. [Google Scholar] [CrossRef]
- Wu, X.; Ding, M.; Xu, H. Scalable Ti(3)C(2)T(x) MXene Interlayered Forward Osmosis Membranes for Enhanced Water Purification and Organic Solvent Recovery. ACS Nano 2020, 14, 9125–9135. [Google Scholar] [CrossRef]
- Kim, S.; Yu, M. Fouling and Retention Mechanisms of Selected Cationic and Anionic Dyes in a Ti(3)C(2)T(x) MXene-Ultrafiltration Hybrid System. ACS Appl. Mater. Interfaces 2020, 12, 16557–16565. [Google Scholar] [CrossRef]
- Hong, S. Two-Dimensional Ti(3)C(2)T(x) MXene Membranes as Nanofluidic Osmotic Power Generators. ACS Nano 2019, 13, 8917–8925. [Google Scholar] [CrossRef]
- Mojtabavi, M.; VahidMohammadi, A. Single-Molecule Sensing Using Nanopores in Two-Dimensional Transition Metal Carbide (MXene) Membranes. ACS Nano 2019, 13, 3042–3053. [Google Scholar] [CrossRef]
- Liu, G.; Shen, J.; Liu, Q.; Liu, G.; Xiong, J.; Yang, J.; Jin, W. Ultrathin two-dimensional MXene membrane for pervaporation desalination. J. Membr. Sci. 2018, 548, 548–558. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasool, K.; Madhavan, V.E.; Aïssa, B.; Gogotsi, Y.; Mahmoud, K.A. Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 2018, 6, 3522–3533. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, Y.; Deng, J.; Ding, L.; Li, Z.-K.; Wang, H. Self-Crosslinked MXene (Ti3C2Tx) Membranes with Good Antiswelling Property for Monovalent Metal Ion Exclusion. ACS Nano 2019, 13, 10535–10544. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, L.; Liu, Y.; Wu, Y.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 2020, 3, 296–302. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wei, Y.; Xue, J.; Chen, H.; Ding, L.; Caro, J.; Wang, H. Water Transport with Ultralow Friction through Partially Exfoliated g-C3N4 Nanosheet Membranes with Self-Supporting Spacers. Angew. Chem. Int. Ed. 2017, 56, 8974–8980. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Qu, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Han, R.; Xie, Y.; Ma, X. Crosslinked P84 copolyimide/MXene mixed matrix membrane with excellent solvent resistance and permselectivity. Chin. J. Chem. Eng. 2019, 27, 877–883. [Google Scholar] [CrossRef]
- Fahimirad, S.; Fahimirad, Z.; Sillanpää, M. Efficient removal of water bacteria and viruses using electrospun nanofibers. Sci. Total Environ. 2021, 751, 141673. [Google Scholar] [CrossRef]
- Rasool, K.; Mahmoud, K.A.; Johnson, D.J.; Helal, M.; Berdiyorov, G.R.; Gogotsi, Y. Efficient Antibacterial Membrane based on Two-Dimensional Ti3C2Tx (MXene) Nanosheets. Sci. Rep. 2017, 7, 1598. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Street, R.M.; McDaniel, R.M.; Barsoum, M.W.; Schauer, C.L. Antibacterial properties of electrospun Ti3C2Tz (MXene)/chitosan nanofibers. RSC Adv. 2018, 8, 35386–35394. [Google Scholar] [CrossRef] [Green Version]
- Rozmysłowska-Wojciechowska, A.; Karwowska, E.; Poźniak, S.; Wojciechowski, T.; Chlubny, L.; Olszyna, A.; Ziemkowska, W.; Jastrzębska, A.M. Influence of modification of Ti3C2 MXene with ceramic oxide and noble metal nanoparticles on its antimicrobial properties and ecotoxicity towards selected algae and higher plants. RSC Adv. 2019, 9, 4092–4105. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhu, Y.; Jia, K.; Abraha, B.S.; Li, Y.; Peng, W.; Zhang, F.; Fan, X.; Zhang, L. A near-infrared light-mediated antimicrobial based on Ag/Ti3C2Tx for effective synergetic antibacterial applications. Nanoscale 2020, 12, 19129–19141. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Liu, X.; Li, C.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Zhu, S.; Hu, W.; et al. Interfacial engineering of Bi2S3/Ti3C2Tx MXene based on work function for rapid photo-excited bacteria-killing. Nat. Commun. 2021, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Li, S.; Jing, L.; Chen, P.Y.; Xie, J. Synergistic Antimicrobial Titanium Carbide (MXene) Conjugated with Gold Nanoclusters. Adv. Healthc. Mater. 2020, 9, e2001007. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Karwowska, E.; Wojciechowski, T.; Ziemkowska, W.; Rozmysłowska, A.; Chlubny, L.; Olszyna, A. The Atomic Structure of Ti2C and Ti3C2 MXenes is Responsible for Their Antibacterial Activity Toward E. coli Bacteria. J. Mater. Eng. Perform. 2019, 28, 1272–1277. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.-Z. Development of a MXene-based membrane with excellent anti-fouling for air humidification dehumidification type desalination. J. Membr. Sci. 2022, 641, 119907. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Zhuang, Y.; Liu, G.; Xing, W.; Jing, W. Adjustable interlayer spacing of ultrathin MXene-derived membranes for ion rejection. J. Membr. Sci. 2019, 591, 117350. [Google Scholar] [CrossRef]
- Han, R.; Wu, P. High-performance graphene oxide nanofiltration membrane with continuous nanochannels prepared by the in situ oxidation of MXene. J. Mater. Chem. A 2019, 7, 6475–6481. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Y.; Zhuang, Y.; Jing, W.; Ye, H.; Cui, Z. Assembly of 2D MXene nanosheets and TiO2 nanoparticles for fabricating mesoporous TiO2-MXene membranes. J. Membr. Sci. 2018, 564, 35–43. [Google Scholar] [CrossRef]
- Wei, S.; Xie, Y.; Xing, Y.; Wang, L.; Ye, H.; Xiong, X.; Wang, S.; Han, K. Two-dimensional graphene Oxide/MXene composite lamellar membranes for efficient solvent permeation and molecular separation. J. Membr. Sci. 2019, 582, 414–422. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, K. MXene nanocomposite nanofiltration membrane for low carbon and long-lasting desalination. J. Membr. Sci. 2021, 640, 119808. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Wang, H.; Han, L.; Tanis-Kanbur, M.B.; Pranav, M.V.; Chew, J.W. Photothermal-enhanced and fouling-resistant membrane for solar-assisted membrane distillation. J. Membr. Sci. 2018, 565, 254–265. [Google Scholar] [CrossRef]

| Type of Membrane | Fabrication Method | Feed Solution/Concentration | Rejection (%) | Permeability (Lm−2 h−1 bar−1) | Ref. |
|---|---|---|---|---|---|
| Ti3C2Tx | Vacuum filtration | RB | 85 | 1084 | [64] |
| EB | 90 | ||||
| CC (Each 10–20 mg/L) | 97 | ||||
| Ti3C2Tx | Vacuum filtration | NaCl (10,000 mg/L) | 56–64 | 10 | [100] |
| BSA (2000 mg/L) | |||||
| Ti3C2Tx | Vacuum filtration | CR | 92 | 405 | [74] |
| GN | 80 | ||||
| MgCl2 | 2.3 | ||||
| Na2SO4 | 13.2 | ||||
| NaCl (Each 100–1000 mg/L) | 13.8% | ||||
| Ti3C2Tx | Vacuum filtration | E. coli | >99 | 37.4 | [91] |
| B. subtilis | >99 | ||||
| Ti3C2Tx | Vacuum filtration | Na2SO4 | 50–99 | 5–15.25 | [101] |
| Mg2SO4 | |||||
| MgCl2 | |||||
| NaCl | |||||
| VOSO4 | |||||
| Ti3C2Tx-Ag | Vacuum-assisted filtration | RB | 79.9 | ~420 | [84] |
| MG | 92.3 | ||||
| BSA (50–100 mg/L) | >99% | ||||
| Ti3C2Tx-GO | Vacuum filtration | BB | 95.4 | ~25 L | [65] |
| Rose Bengal | 94.6 | ||||
| MLB | 40 | ||||
| MLR | 5 | ||||
| MgSO4 | <1 | ||||
| NaCl (Each 10 mg/L) | |||||
| Ti3C2Tx-GO | Vacuum filtration | RB | >97 (dyes) | 89.6 | [102] |
| MB | |||||
| CV | |||||
| NR (Each 10 mg/L) | |||||
| Na2SO4 | 61 | ||||
| NaCl (Each 5 mM) | 23 | ||||
| Ti3C2Tx-GO | Vacuum filtration | Chrysoidine G | >99% (dyes) | 71.9 | [80] |
| MLB | |||||
| NR | |||||
| CV | |||||
| BB | |||||
| HA | |||||
| BSA | |||||
| Na2SO4 | 61 | ||||
| NaCl (Each 10 mg/L) | 23 | ||||
| Ti3C2Tx-GO | Vacuum filtration | MO | >95 | ~8.5–11 | [101] |
| MLB | |||||
| Acid yellow 14 | |||||
| IC | |||||
| Eosin (Each 10 mg/L) | |||||
| Ti3C2Tx-TiO2 | Spin coating | Dextran (3000 mg/L) | >95 | ~90 | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahar, I.; Memon, F.H.; Lee, J.-W.; Kim, K.H.; Ahmed, R.; Soomro, F.; Rehman, F.; Memon, A.A.; Thebo, K.H.; Choi, K.H. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes) for Water Purification and Antibacterial Applications. Membranes 2021, 11, 869. https://doi.org/10.3390/membranes11110869
Mahar I, Memon FH, Lee J-W, Kim KH, Ahmed R, Soomro F, Rehman F, Memon AA, Thebo KH, Choi KH. Two-Dimensional Transition Metal Carbides and Nitrides (MXenes) for Water Purification and Antibacterial Applications. Membranes. 2021; 11(11):869. https://doi.org/10.3390/membranes11110869
Chicago/Turabian StyleMahar, Inamullah, Fida Hussain Memon, Jae-Wook Lee, Kyung Hwan Kim, Rafique Ahmed, Faheeda Soomro, Faisal Rehman, Ayaz Ali Memon, Khalid Hussain Thebo, and Kyung Hyun Choi. 2021. "Two-Dimensional Transition Metal Carbides and Nitrides (MXenes) for Water Purification and Antibacterial Applications" Membranes 11, no. 11: 869. https://doi.org/10.3390/membranes11110869
APA StyleMahar, I., Memon, F. H., Lee, J.-W., Kim, K. H., Ahmed, R., Soomro, F., Rehman, F., Memon, A. A., Thebo, K. H., & Choi, K. H. (2021). Two-Dimensional Transition Metal Carbides and Nitrides (MXenes) for Water Purification and Antibacterial Applications. Membranes, 11(11), 869. https://doi.org/10.3390/membranes11110869






