Application of Response Surface Methodology (RSM) for the Optimization of Chromium(III) Synergistic Extraction by Supported Liquid Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Equipment and Procedure for SLM/DCSLM Transport Experiments
2.3. Determination of the Chromium(III) Ions Concentration
2.4. Experimental Design for RSM
3. Theory
4. Results and Discussion
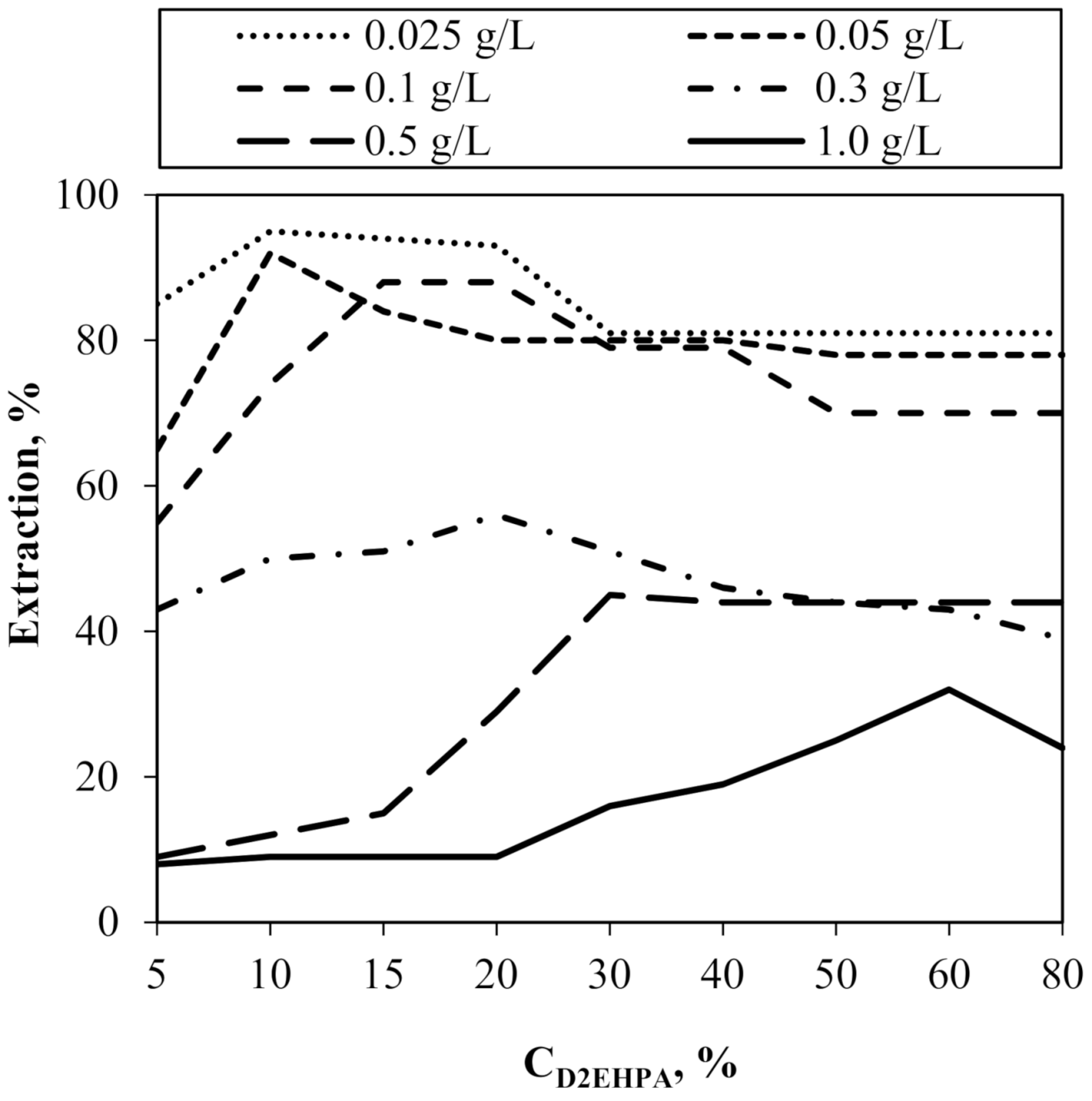
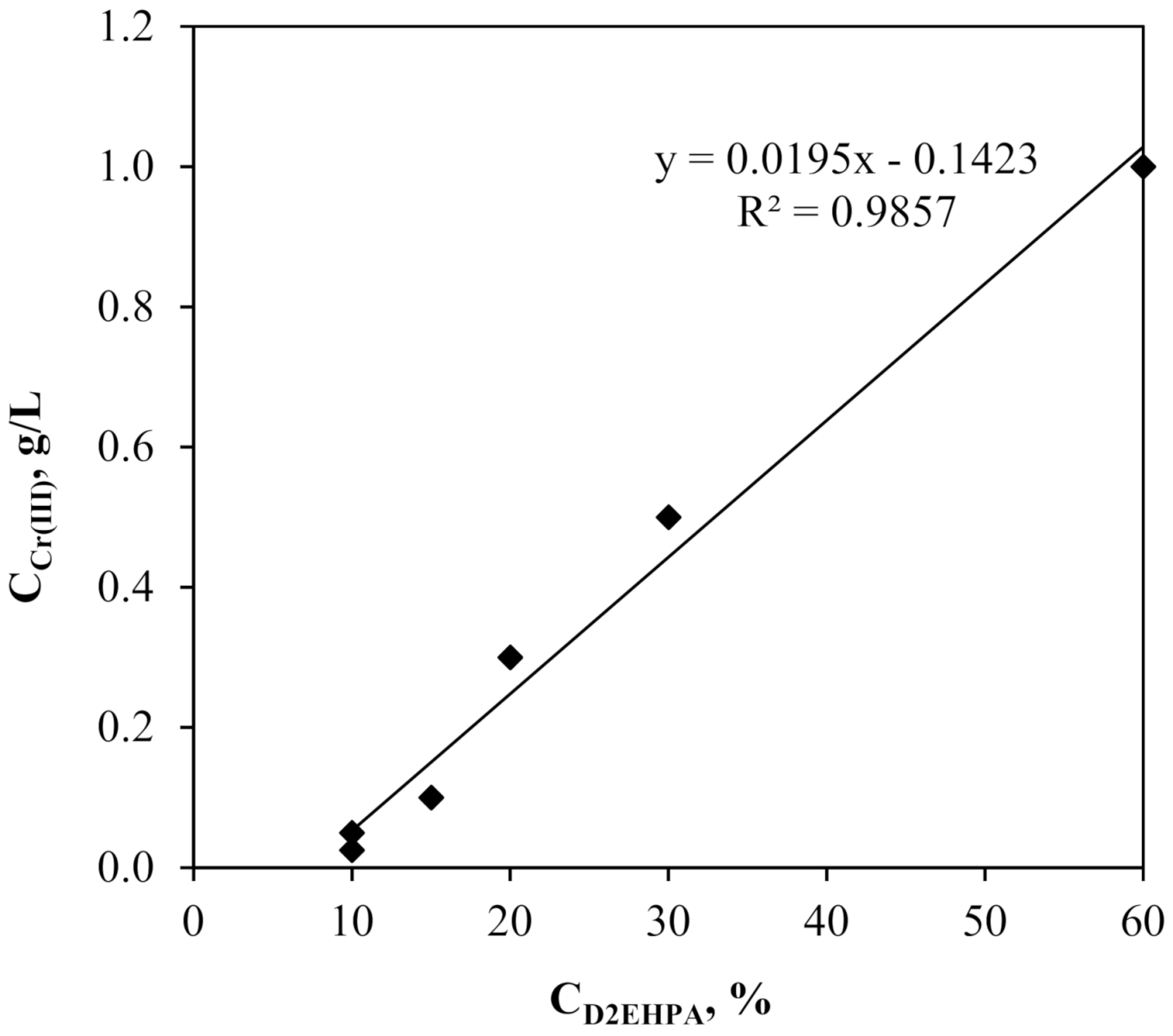
4.1. Subsection Influence of Carrier and Feed Concentration on the Cr(III) Ions Extraction in the SLM Method of “One Experimental Parameter at a Time”
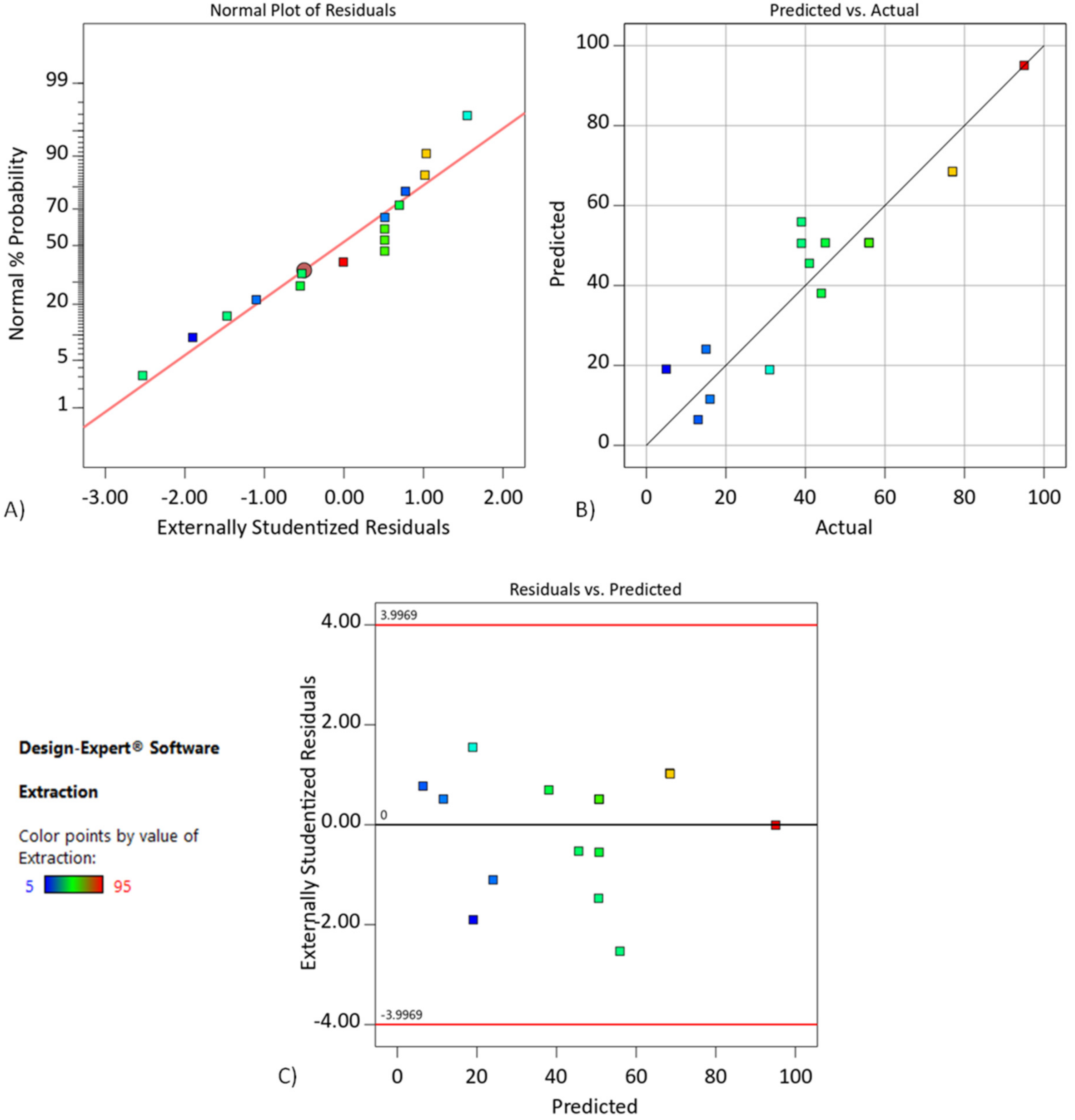
4.2. Effect of the Cyanex272 Addition to the SLM with D2EHPA RSM Modeling and Optimization of Chromium(III) Extraction (Step I)
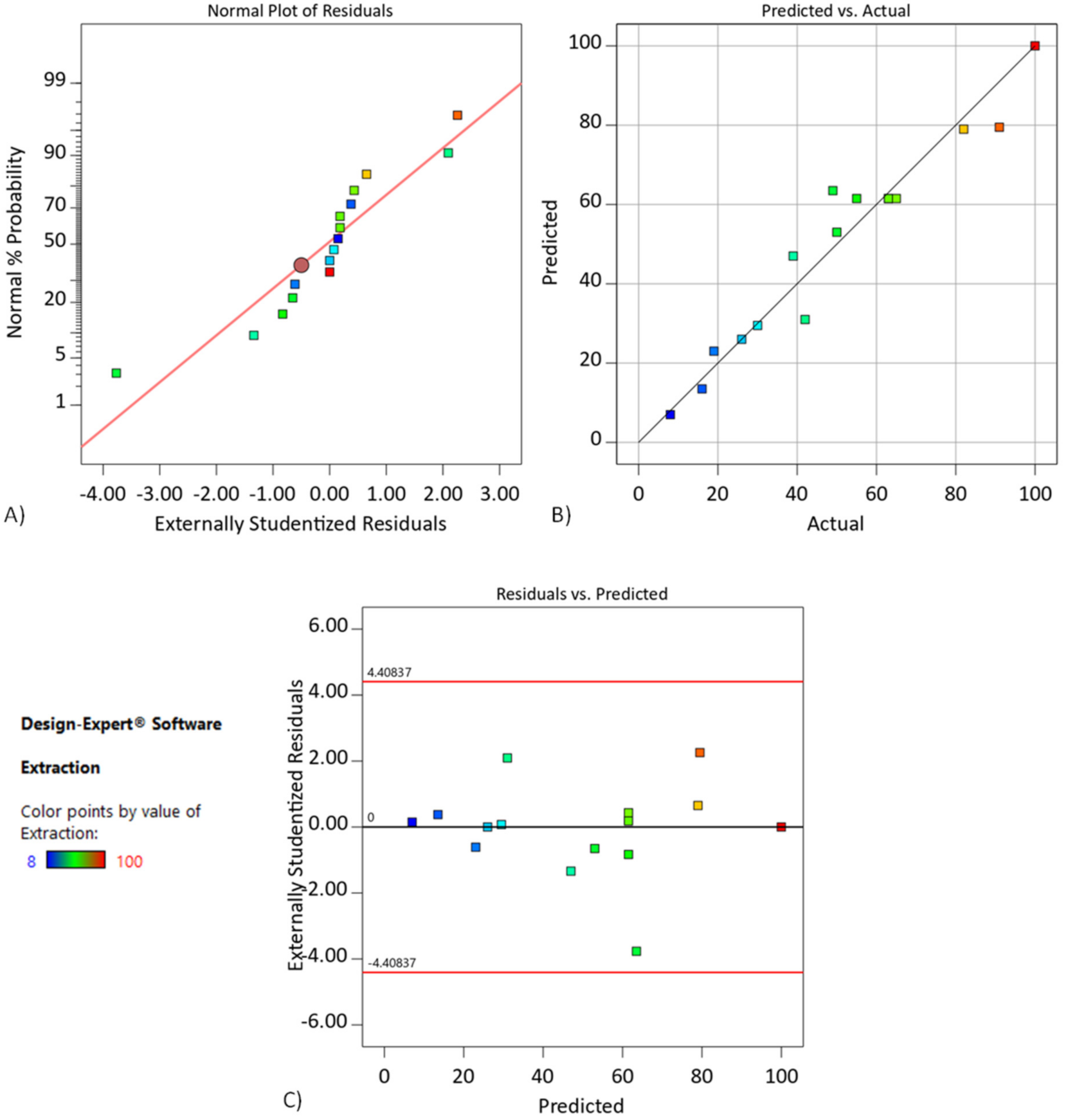
4.3. Effect of the D2EHPA Concentration in DCSLM with Threshold Concentration of Cyanex272 RSM Modeling (Step II)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rajewski, J.; Religa, P.; Gierycz, P. The possibility of using a mathematical model based on consecutive first-order reactions to describe the Cr(III) ions pertraction in DCSLM system. Res. Chem. Intermed. 2017, 43, 5569–5585. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Critical Raw Materials for the EU (2010) Technical Report; European Commission (Enterprise and Industry): Brussels, Belgium, 2010; pp. 22–30. [Google Scholar]
- Dżygiel, P.; Wieczorek, P.P. Chapter 3—Supported Liquid Membranes and Their Modifications: Definition, Classification, Theory, Stability, Application and Perspectives. In Liquid Membranes; Elsevier: Amsterdam, The Netherlands, 2010; pp. 73–140. ISBN 9780444532183. [Google Scholar] [CrossRef]
- Rajewski, J.; Religa, P. Synergistic extraction and separation of chromium(III) from acidic solution with a double-carrier supported liquid membrane. J. Mol. Liq. 2016, 218, 309–315. [Google Scholar] [CrossRef]
- Ghorbanpour, P.; Jahanshahi, M. Silver extraction using emulsion liquid membrane system containing D2EHPA-TBP as synergistic carrier: Optimization through response surface methodology. Environ. Technol. 2021, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Thakur, A.; Panesar, P.S. A comparative study on experimental and response surface optimization of lactic acid synergistic extraction using green emulsion liquid membrane. Sep. Purif. Technol. 2019, 211, 54–62. [Google Scholar] [CrossRef]
- Yan, B.; Huang, V.; Chen, K.; Liu, H.; Wang, L. A study of synergetic carrier emulsion liquid membrane for the extraction of amoxicillin from aqueous phase using response surface methodology. J. Ind. Eng. Chem. 2021, 100, 63–74. [Google Scholar] [CrossRef]
- Saw, P.K.; Prajapati, A.K.; Mondal, M.K. The extraction of Cr (VI) from aqueous solution with a mixture of TEA and TOA as synergic extractant by using different diluents. J. Mol. Liq. 2018, 269, 101–109. [Google Scholar] [CrossRef]
- Inger, M.; Dobrzyńska-Inger, A.; Rajewski, J.; Wilk, M. The Use of Response Surface Methodology in Ammonia Oxidation Reaction Study. J. Chem. 2019, 2019, 2641315. [Google Scholar] [CrossRef]
- Inger, M.; Dobrzyńska-Inger, A.; Rajewski, J.; Wilk, M. Optimization of ammonia oxidation using response surface methodology. Catalysts 2019, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 3rd ed.; John Wiley & Sons: London, UK, 2016. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2018, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, K.; Wannachod, T.; Mohde, V.; Pancharoen, U.; Arpornwichanop, A.; Lothongkum, A.W. Mass transfer resistance and response surface methodology for separation of platinum (IV) across hollow fiber supported liquid membrane. J. Eng. Chem. 2016, 42, 23–35. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Huang, J.; Liu, T.; Xue, N.; Shi, Q. Optimization of vanadium (IV) extraction from stone coal leaching solution by emulsion liquid membrane using response surface methodology. Chem. Eng. Res. Des. 2017, 123, 111–119. [Google Scholar] [CrossRef]
- Mesli, M.; Belkhouche, N. Emulsion ionic liquid membrane for recovery process of lead. Comparative study of experimental and response surface design. Chem. Eng. Res. Des. 2018, 129, 160–169. [Google Scholar] [CrossRef]
- Mondal, S.K.; Saha, P. Separation of hexavalent chromium from industrial effluent through liquid membrane using environmentally benign solvent: A study of experimental optimization through response surface methodology. Chem. Eng. Res. Des. 2018, 132, 564–583. [Google Scholar] [CrossRef]
- Ochromowicz, K.; Apostoluk, W. Modelling of carrier mediated transport of Cr(III) in the SLM system with D2EHPA. Sep. Purif. Technol. 2010, 72, 112–117. [Google Scholar] [CrossRef]
- Bouonomenna, M.G.; Oranges, T.; Molinari, R.; Drioli, E. Chromium (III) removal by supported liquid membranes: A comparison among D2EHPA, DNNSA, and a novel extractant as carriers. Water Environ. Res. 2006, 8, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zaghbani, A.; Tayeb, R.; Dhahbi, M. Studies on the transport of chromium(III) through a supported liquid membrane containing D2EHPA as carrier. Desalin. Water Treat. 2009, 12, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Villemin, J.D.; Hlaibi, M.; Lebrun, L. Heavy metal ions extraction using new supported liquid membranes containing ionic liquid as carrier. Sep. Purif. Technol. 2018, 201, 1–9. [Google Scholar] [CrossRef]

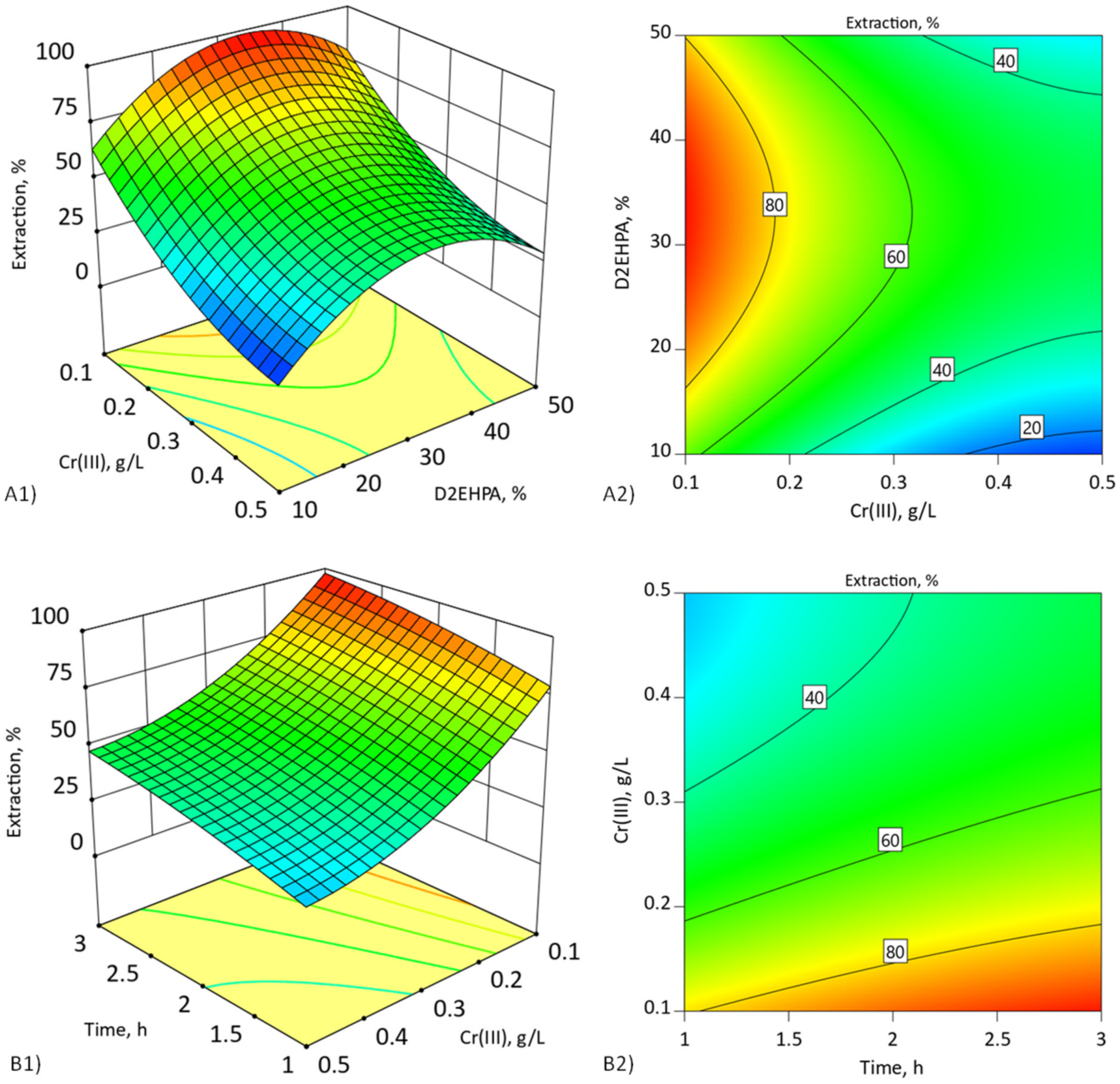
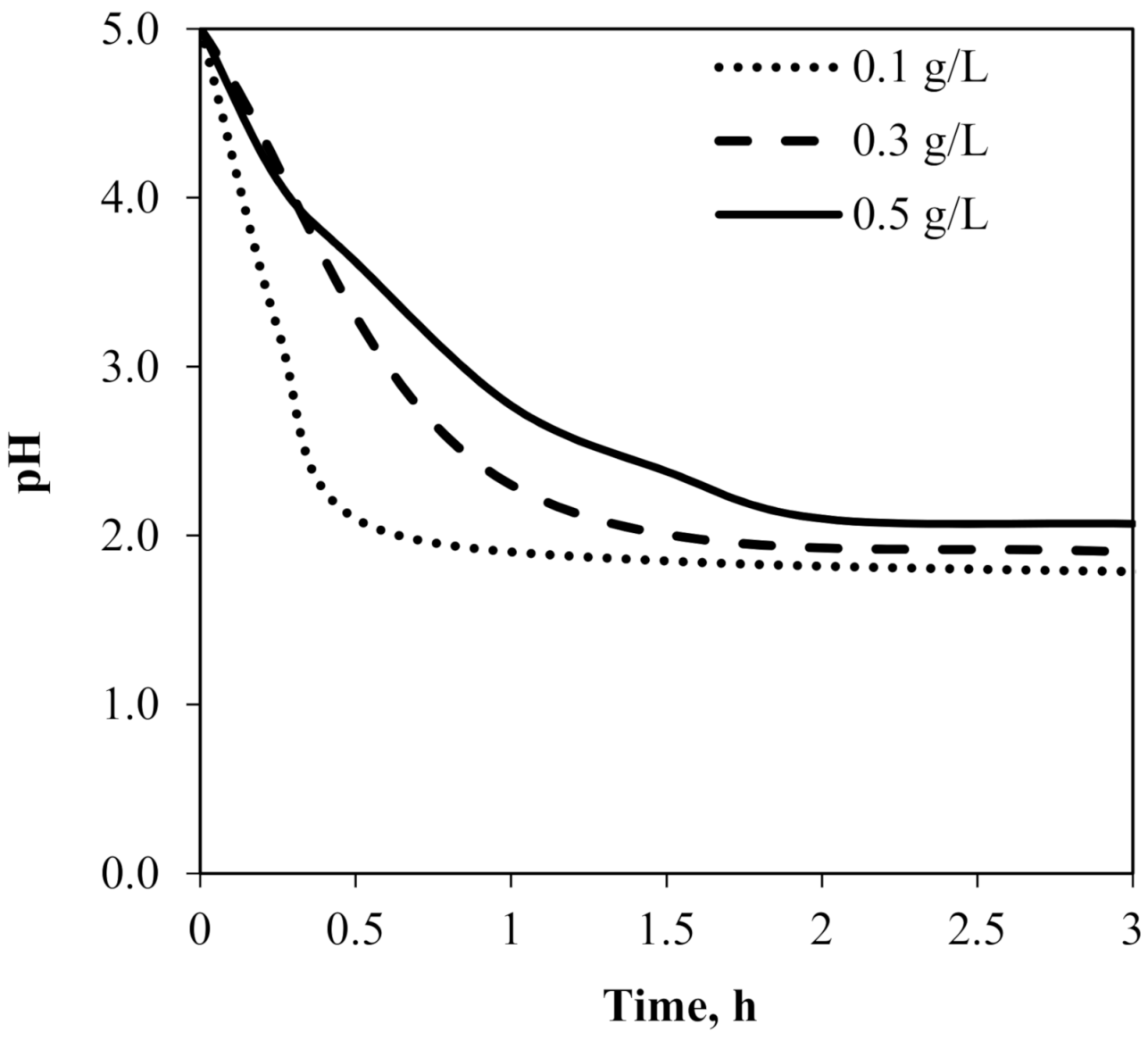
| Varibles | Symbol | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Cyanex272, % v/v | AC | 2 | 5 | 8 |
| Chromium(III), g/L | B | 0.1 | 0.3 | 0.5 |
| Time, h | C | 1 | 2 | 3 |
| Varibles | Symbol | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| D2EHPA, % v/v | AD | 10 | 30 | 50 |
| Chromium(III), g/L | B | 0.1 | 0.3 | 0.5 |
| Time, h | C | 1 | 2 | 3 |
| Initial Cr(III) Concentration, g/L | D2EHPA Threshold Concentration, % | tmax, h | E, % (after 3 h) |
|---|---|---|---|
| 0.025 | 10 | 0.5 | 95 |
| 0.050 | 10 | 0.5 | 92 |
| 0.100 | 15 | 1 | 88 |
| 0.300 | 20 | 3 | 56 |
| 0.500 | 30 | 9 | 45 |
| 1.000 | 60 | 10 | 32 |
| Experiment No. | Concentration of Cyanex272, % | Initial Concentration of the Cr(III), g/L | Time, h | Extraction, % | |
|---|---|---|---|---|---|
| Ac | B | C | Experimental | Predicted | |
| 1 | −1 | 1 | 0 | 13 | 6 |
| 2 | 1 | 0 | 1 | 39 | 51 |
| 3 | 0 | −1 | −1 | 77 | 69 |
| 4 | 0 | 0 | 0 | 56 | 51 |
| 5 | 0 | −1 | 0 | 77 | 68 |
| 6 | 0 | 1 | 0 | 31 | 19 |
| 7 | −1 | 0 | 1 | 44 | 38 |
| 8 | 0 | 1 | −1 | 5 | 19 |
| 9 | 0 | 0 | 0 | 45 | 51 |
| 10 | 1 | 0 | −1 | 15 | 24 |
| 11 | −1 | 0 | −1 | 16 | 12 |
| 12 | −1 | −1 | 0 | 39 | 56 |
| 13 | 0 | 0 | 0 | 56 | 51 |
| 14 | 0 | 1 | 1 | 41 | 46 |
| 15 | 0 | −1 | 1 | 95 | 95 |
| 16 | 0 | 0 | 0 | 56 | 51 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 8610.69 | 9 | 956.74 | 6.13 | 0.0194 | significant |
| A | 312.50 | 1 | 312.50 | 2.00 | 0.2068 | |
| B | 4900.50 | 1 | 4900.50 | 31.41 | 0.0014 | |
| C | 1404.50 | 1 | 1404.50 | 9.00 | 0.0240 | |
| AB | 100.00 | 1 | 100.00 | 0.6409 | 0.4539 | |
| AC | 4.00 | 1 | 4.00 | 0.0256 | 0.8781 | |
| BC | 81.00 | 1 | 81.00 | 0.5191 | 0.4983 | |
| A2 | 1540.56 | 1 | 1540.56 | 9.87 | 0.0200 | |
| B2 | 162.56 | 1 | 162.56 | 1.04 | 0.3468 | |
| C2 | 105.06 | 1 | 105.06 | 0.6733 | 0.4433 | |
| Residual | 936.25 | 6 | 156.04 | |||
| Lack of fit | 845.50 | 3 | 281.83 | 9.32 | 0.0497 | significant |
| Pure error | 90.75 | 3 | 30.25 | |||
| Cor total | 9546.94 | 15 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 8320.63 | 5 | 1664.13 | 13.57 | 0.0003 | significant |
| A | 312.50 | 1 | 312.50 | 2.55 | 0.1415 | |
| B | 4900.50 | 1 | 4900.50 | 39.96 | <0.0001 | |
| C | 1404.50 | 1 | 1404.50 | 11.45 | 0.0070 | |
| A2 | 1540.56 | 1 | 1540.56 | 12.56 | 0.0053 | |
| B2 | 162.56 | 1 | 162.56 | 1.33 | 0.2764 | |
| Residual | 1226.31 | 10 | 122.63 | |||
| Lack of fit | 1135.56 | 7 | 162.22 | 5.36 | 0.0977 | not significant |
| Pure error | 90.75 | 3 | 30.25 | |||
| Cor total | 9546.94 | 15 |
| Initial Cr(III) Concentration, g/L | D2EHPA Threshold Concentration, % | Cyanex272 Threshold Concentration, % | tmax, h | E, % |
|---|---|---|---|---|
| 0.100 | 15 | 5 | 0.3 | 96 |
| 0.300 | 20 | 1 | 65 | |
| 0.500 | 30 | 3 | 59 |
| Experiment No. | Concentration of D2EHPA, % | Initial Concentration of the Cr, g/L | Time, h | Extraction, % | |
|---|---|---|---|---|---|
| Ad | B | C | Experimental | Predicted | |
| 1 | −1 | 1 | 1 | 16 | 14 |
| 2 | 0 | 0 | 0 | 63 | 62 |
| 3 | −1 | 0 | 1 | 42 | 31 |
| 4 | 1 | 1 | 0 | 30 | 30 |
| 5 | −1 | 0 | −1 | 8 | 7 |
| 6 | 0 | 1 | −1 | 26 | 26 |
| 7 | 0 | 1 | 1 | 50 | 53 |
| 8 | 1 | −1 | 0 | 91 | 80 |
| 9 | 1 | 0 | −1 | 19 | 23 |
| 10 | 1 | 0 | 1 | 39 | 47 |
| 11 | 0 | 0 | 0 | 65 | 62 |
| 12 | 0 | −1 | −1 | 82 | 79 |
| 13 | −1 | −1 | 0 | 49 | 64 |
| 14 | 0 | 0 | 0 | 63 | 62 |
| 15 | 0 | −1 | 1 | 100 | 100 |
| 16 | 0 | 0 | 0 | 55 | 62 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 10,207.75 | 7 | 1458.25 | 18.58 | 0.0002 | significant |
| A | 512.00 | 1 | 512.00 | 6.52 | 0.0340 | |
| B | 3536.33 | 1 | 3536.33 | 45.05 | 0.0002 | |
| C | 1093.50 | 1 | 1093.50 | 13.93 | 0.0058 | |
| BC | 9.00 | 1 | 9.00 | 0.1146 | 0.7436 | |
| A2 | 2756.25 | 1 | 2756.25 | 35.11 | 0.0004 | |
| B2 | 506.25 | 1 | 506.25 | 6.45 | 0.0347 | |
| C2 | 272.25 | 1 | 272.25 | 3.47 | 0.0996 | |
| Residual | 628.00 | 8 | 78.50 | |||
| Lack of fit | 569.00 | 5 | 113.80 | 5.79 | 0.0896 | not significant |
| Pure error | 59.00 | 3 | 19.67 | |||
| Cor total | 10,835.75 | 15 |
| Initial Cr(III) Concentration, g/L | Cyanex272 Threshold Concentration, % | D2EHPA Threshold Concentration, % | E, % | |
|---|---|---|---|---|
| DCSLM | SLM/ (D2EHPA Threshold Concentration, %) | |||
| 0.100 | 5 | 30 | 99 (after 2 h) | 88/(15) |
| 0.300 | 30 | 73 | 56/(20) | |
| 0.500 | 40 | 65 | 45/(30) | |
| No. of Experiments | Cr, g/L | t, h | Response: E, % | Stdandard Deviation | SE Mean | 95% CI Low for Mean | 95% CI High for Mean | 95% TI Low for 99% Pop | 95% TI High for 99% Pop | |
|---|---|---|---|---|---|---|---|---|---|---|
| Predicted Mean | Experimant Results | |||||||||
| 1 | 0.3 | 1.0 | 30.68 | 37 | 8,86 | 5.31 | 18.44 | 42.94 | −18.24 | 79.61 |
| 2 | 0.3 | 2.0 | 42.88 | 54 | 8.86 | 4.14 | 33.32 | 52.43 | −3.88 | 89.63 |
| 3 | 0.3 | 3.0 | 50.94 | 65 | 8.86 | 4.29 | 41.04 | 60.83 | 3.916 | 97.96 |
| 4 | 0.3 | 1.5 | 74.67 | 73 | 8.86 | 6.32 | 60.10 | 89.25 | 23.87 | 125.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajewski, J.; Dobrzyńska-Inger, A. Application of Response Surface Methodology (RSM) for the Optimization of Chromium(III) Synergistic Extraction by Supported Liquid Membrane. Membranes 2021, 11, 854. https://doi.org/10.3390/membranes11110854
Rajewski J, Dobrzyńska-Inger A. Application of Response Surface Methodology (RSM) for the Optimization of Chromium(III) Synergistic Extraction by Supported Liquid Membrane. Membranes. 2021; 11(11):854. https://doi.org/10.3390/membranes11110854
Chicago/Turabian StyleRajewski, Jakub, and Agnieszka Dobrzyńska-Inger. 2021. "Application of Response Surface Methodology (RSM) for the Optimization of Chromium(III) Synergistic Extraction by Supported Liquid Membrane" Membranes 11, no. 11: 854. https://doi.org/10.3390/membranes11110854
APA StyleRajewski, J., & Dobrzyńska-Inger, A. (2021). Application of Response Surface Methodology (RSM) for the Optimization of Chromium(III) Synergistic Extraction by Supported Liquid Membrane. Membranes, 11(11), 854. https://doi.org/10.3390/membranes11110854






