Mesoporous Membrane Materials Based on Ultra-High-Molecular-Weight Polyethylene: From Synthesis to Applied Aspects
Abstract
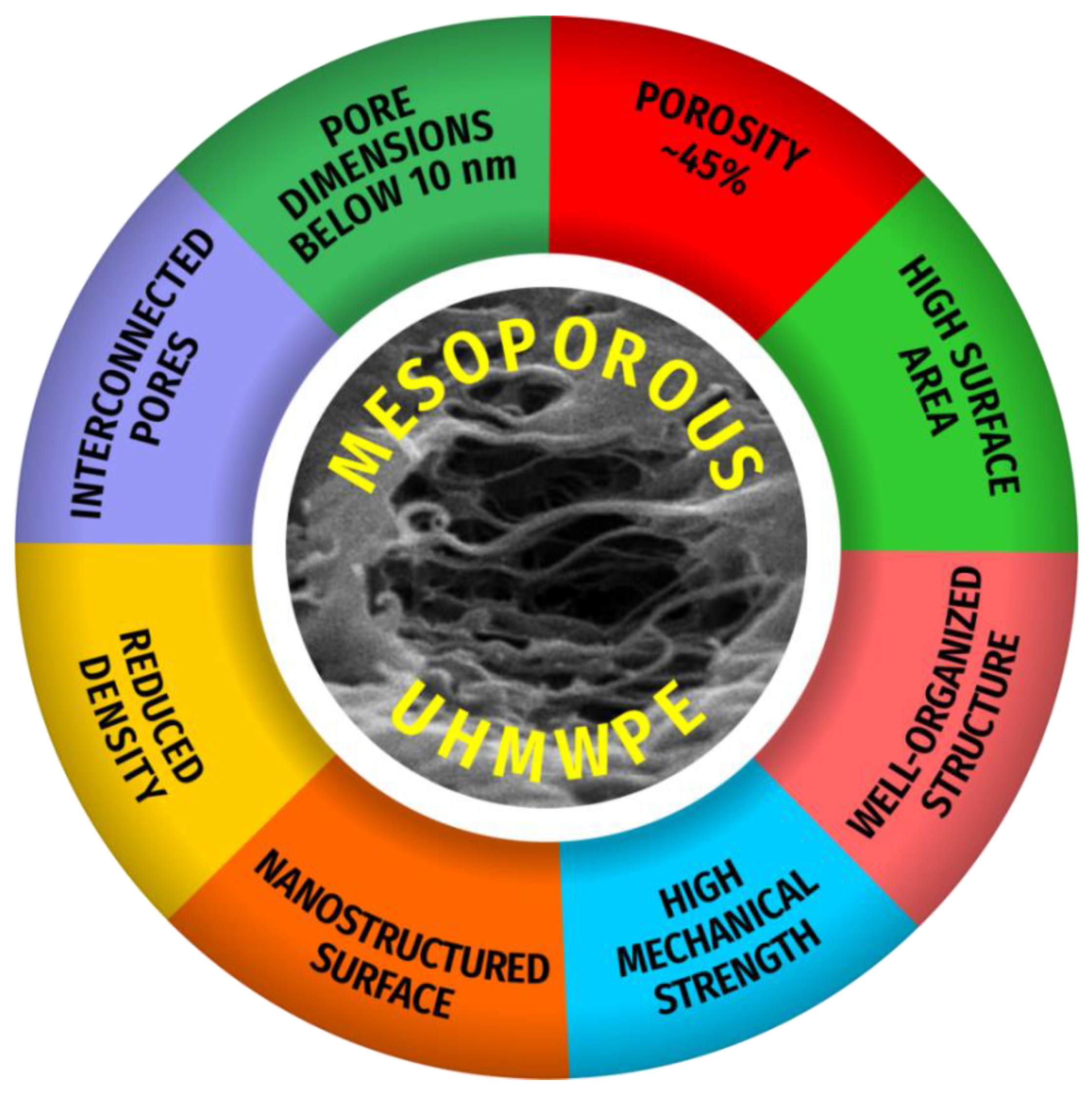
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biphase Oil-in-Water Emulsions
2.3. Preparation of the Test Samples
2.4. Methods
2.4.1. Differential Scanning Calorimetry (DSC)
2.4.2. Wide-Angle X-ray Scattering (WAXS)
2.4.3. Mechanical Tests
2.4.4. Porosity
2.4.5. Liquid Permeability of the Porous UHMWPE Films
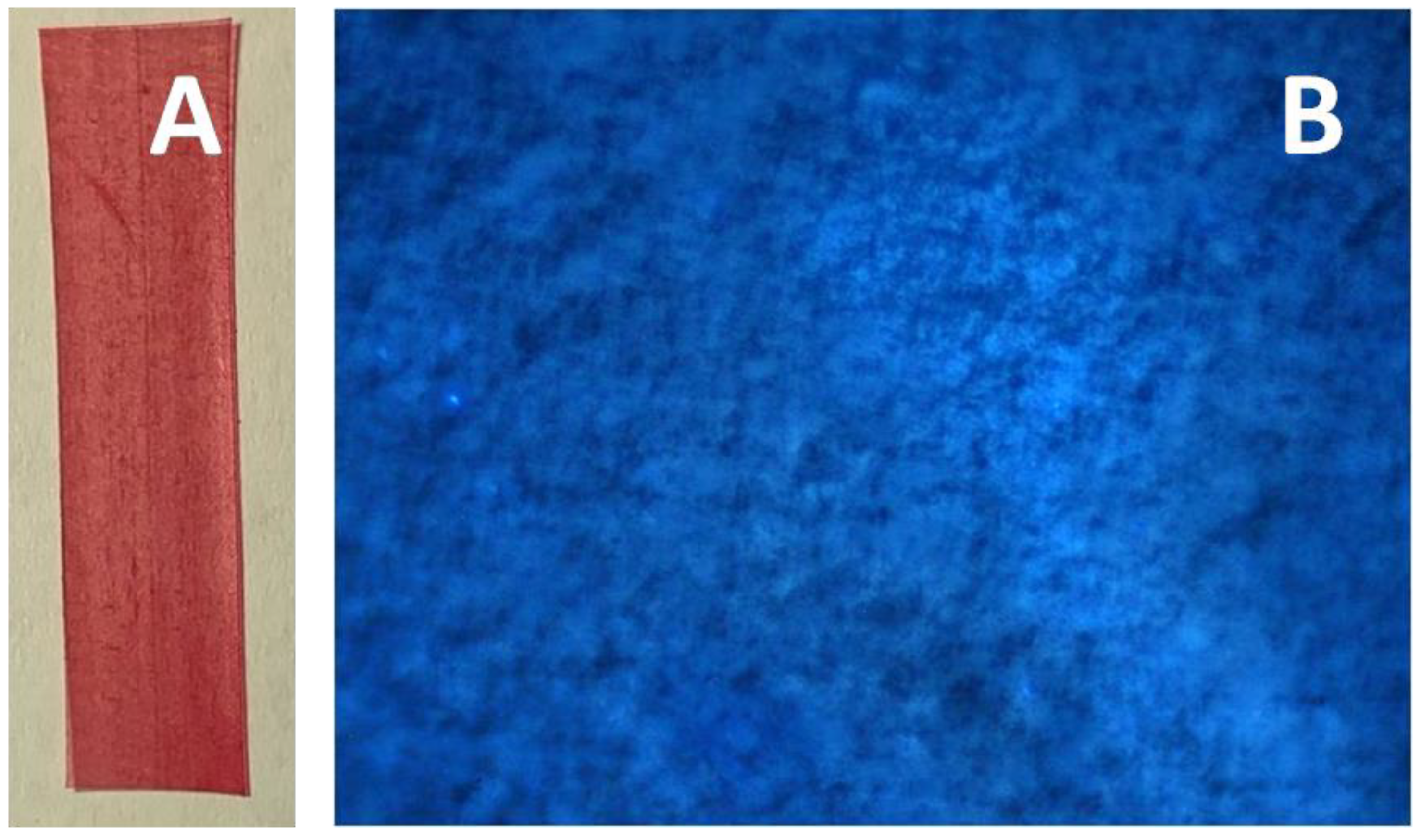
2.4.6. Dye Penetration Tests
2.4.7. Scanning Electron Microscopy (SEM)
2.4.8. Atomic Force Microscopy (AFM)
2.4.9. Permporometry
2.4.10. Confocal Fluorescence Microscopy
2.4.11. Water Vapor Transmission Rate (WVTR) Measurements
2.4.12. Estimation of the Gurley Value
3. Results
3.1. Preparation of Mesoporous Membrane Materials Based on UHMWPE Films via Environmental Crazing
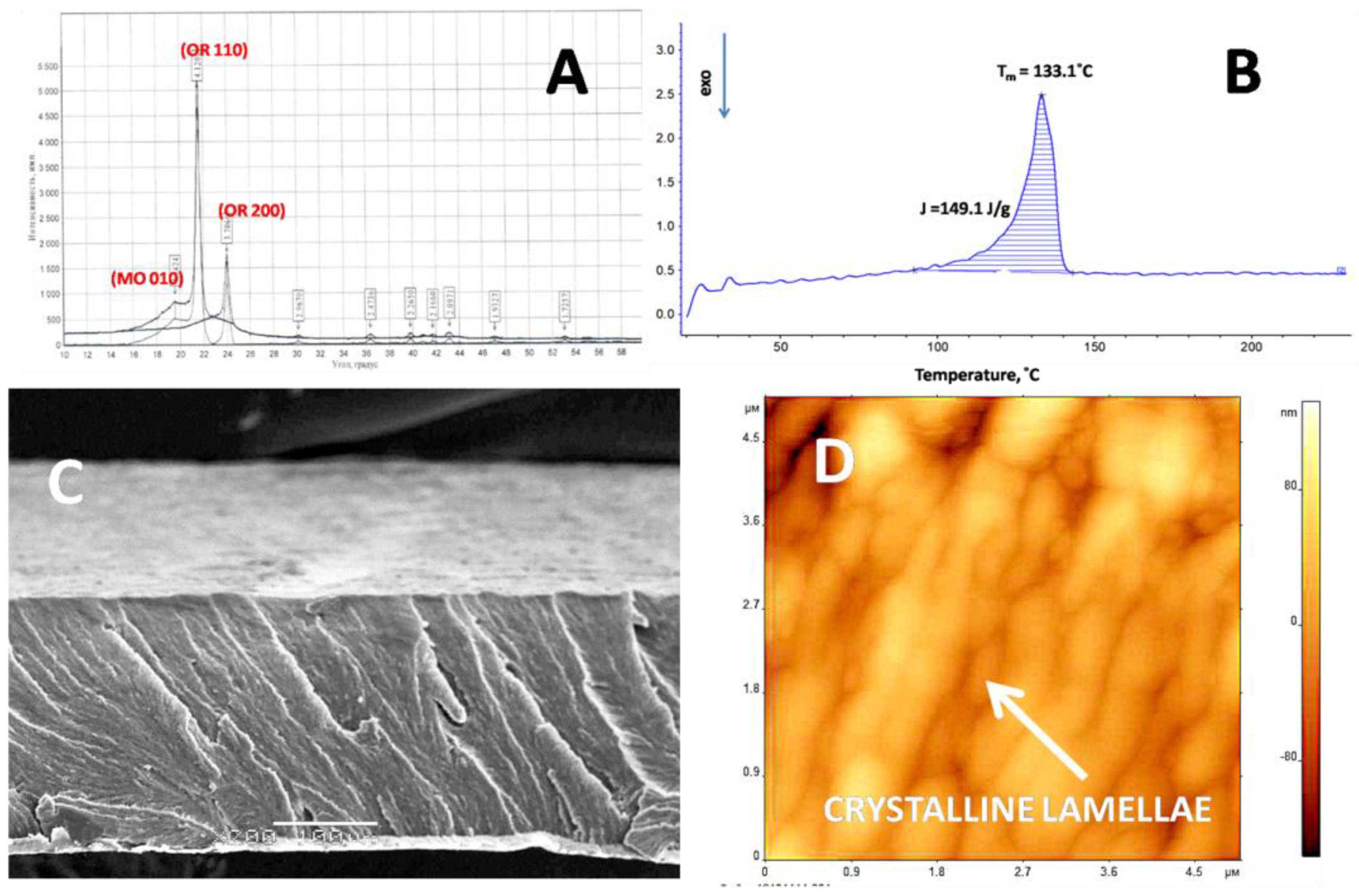
3.1.1. Structure and Morphology of Initial UHMWPE Films
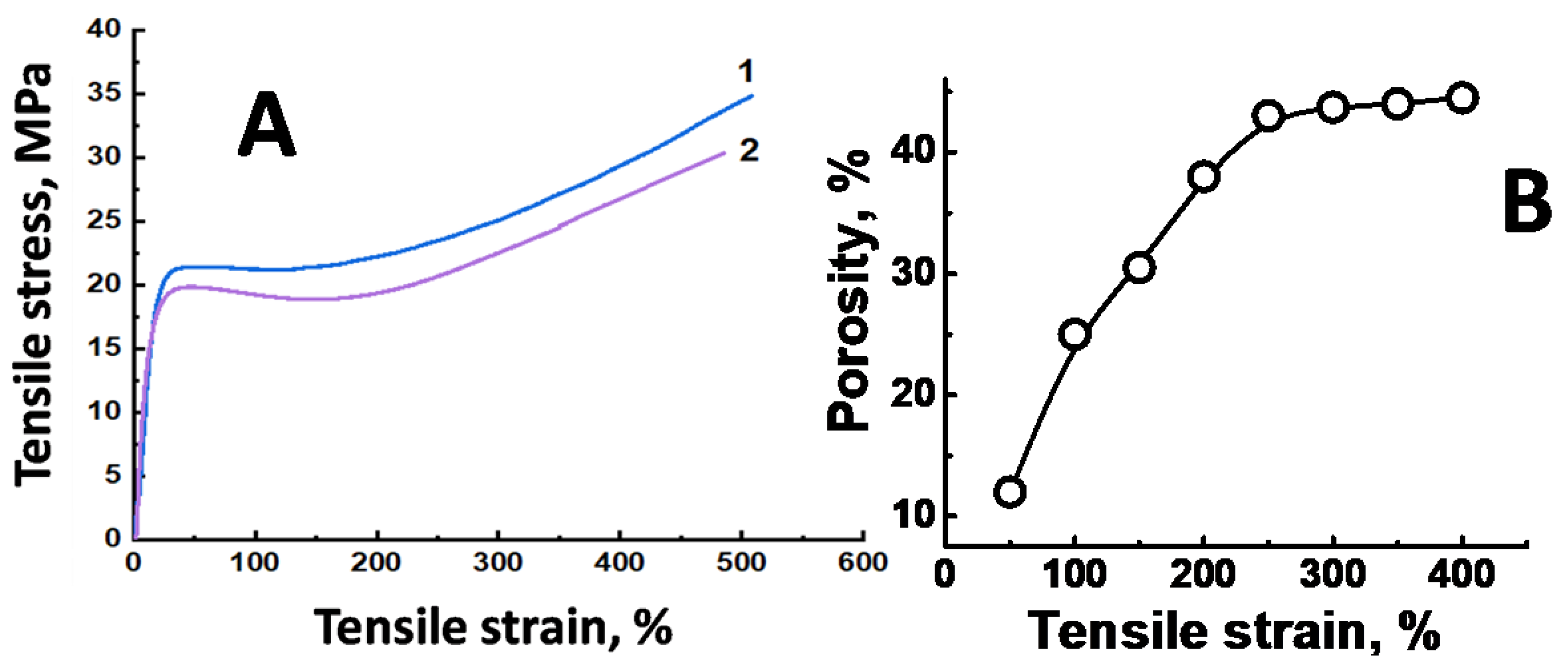
3.1.2. Mechanical Properties of Initial UHMWPE Films
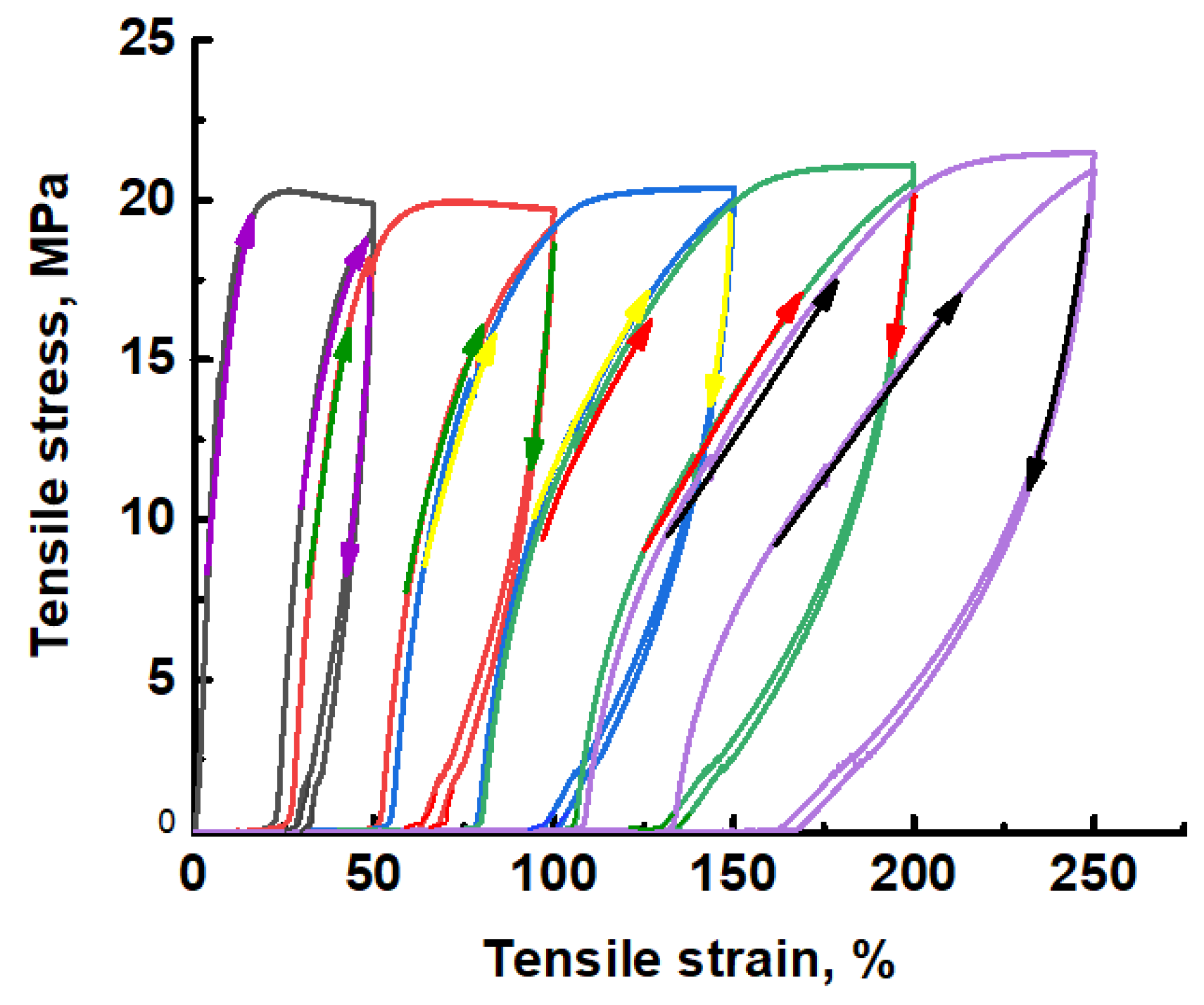
3.1.3. Structural Evolution of Initial UHMWPE Films upon Environmental Crazing
3.1.4. Structural Parameters of Mesoporous UHMWPE Materials
3.1.5. Preparation of Mesoporous Materials with High Shape Stability
3.2. Applied Properties of Mesoporous UHMWPE Membrane Materials
3.2.1. Air Permeability of Mesoporous UHMWPE Materials
3.2.2. Vapor Permeability of Mesoporous UHMWPE Materials
3.2.3. Effective Thermal Conductivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jenkins, S.B. Nanoporous Materials: Types, Properties, and Uses; Nova Science Publishers, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Ren, J.; Wang, R. Preparation of Polymeric Membranes. Membrane and Desalination Technologies; Humana Press: Totowa, NJ, USA, 2011; pp. 47–100. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Rodriguez-Perez, M.A. Nanoporous polymeric materials: A new class of materials with enhanced properties. Prog. Mater. Sci. 2016, 78–79, 93–139. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Ali, A.A.; Hameed, A.S. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review. J. Polym. Res. 2018, 25, 75. [Google Scholar] [CrossRef]
- Bettotti, P. (Ed.) Submicron Porous Materials; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.L.; Xu, F.; Li, S.M.; Ma, P.W.; Zhang, X.C.; Liu, Q.H.; Fu, R.W.; Wu, D.C. Porous polymers as multifunctional material platforms toward task-specific applications. Adv. Mater. 2019, 31, 1802922. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Iampol’skii, I.P.; Pinnau, I.; Freeman, B.D. Materials Science of Membranes for Gas and Vapor Separation; Wiley: Chichester, UK; Hoboken, NJ, USA, 2006. [Google Scholar]
- Saleh, T.A.; Gupta, V.K. Nanomaterial and Polymer Membranes: Synthesis, Characterization, and Applications; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Lu, G.Q.; Zhao, X.S. Nanoporous Materials: Science and Engineering; Imperial College Press: London, UK, 2004. [Google Scholar]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; The “Gold Book”; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Kurtz, S.M. The UHMWPE Handbook Ultra-High Molecular Weight Polyethylene in Total Joint Replacement; Elsevier: San Diego, CA, USA, 2004. [Google Scholar]
- Keller, A.A. Note on single crystals in polymers: Evidence for a folded chain configuration. Philos. Mag. J. Theor. Exp. Appl. Phys. 1957, 2, 1171–1175. [Google Scholar] [CrossRef]
- Lin, L.; Argon, A.S. Structure and plastic deformation of polyethylene. J. Mater. Sci. 1994, 29, 294–323. [Google Scholar] [CrossRef]
- Sobieraj, M.C.; Rimnac, C.M. Ultra high molecular weight polyethylene: Mechanics, morphology, and clinical behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayyoub, T.; Olifirov, L.K.; Chukov, D.I.; Kaloshkin, S.D.; Kolesnikov, E.; Nematulloev, S. The structural and mechanical properties of the UHMWPE films mixed with the PE-wax. Materials 2020, 13, 3422. [Google Scholar] [CrossRef]
- Hussain, M.; Abbas, N.R.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a promising polymer material for biomedical applications: A concise review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Brebbia, C.A. Water Pollution XII; UKWIT Press: Southampton, UK, 2014. [Google Scholar]
- Saleem, J.; Bazargan, A.; Barford, J.; McKay, G. Application of strong porous polymer sheets for superior oil spill recovery. Chem. Eng. Technol. 2015, 38, 482–488. [Google Scholar] [CrossRef]
- Salari, M.; Pircheraghi, G. Interdiffusion versus crystallization at semicrystalline interfaces of sintered porous materials. Polymer 2018, 156, 54–65. [Google Scholar] [CrossRef]
- Salimon, A.I.; Statnik, E.S.; Zadorozhnyy, M.Y.; Senatov, F.S.; Zherebtsov, D.D.; Safonov, A.A.; Korsunsky, A.M. Porous open-cell UHMWPE: Experimental study of structure and mechanical properties. Materials 2019, 12, 2195. [Google Scholar] [CrossRef] [Green Version]
- Deplancke, T.; Lame, O.; Rousset, F.; Aguili, I.; Seguela, R.; Vigier, G. Diffusion versus cocrystallization of very long polymer chains at interfaces: Experimental study of sintering of UHMWPE nascent powder. Macromolecules 2013, 47, 197–207. [Google Scholar] [CrossRef]
- Wang, G.; Gao, S.; He, J.; An, Z.; Shao, C.; Ding, Y.; Jin, Z. Study on preparing of ultrahigh-molecular weight polyethylene microporous materials by novel non-dense injection molding method. J. Appl. Polym. Sci. 2021, 138, 51008. [Google Scholar] [CrossRef]
- Shen, L.; Peng, M.; Qiao, F.; Zhang, J.-L. Preparation of microporous ultra high molecular weight polyethylene (UHMWPE) by thermally induced phase separation of a UHMWPE/liquid paraffin mixture. Chin. J. Polym. Sci. 2008, 26, 653–657. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Chen, M.; Li, C.; Wu, H.; Guo, S. Constructing tunable bimodal porous structure in ultrahigh molecular weight polyethylene membranes with enhanced water permeance and retained rejection performance. J. Membr. Sci. 2021, 619, 118778. [Google Scholar] [CrossRef]
- Quan, J.; Wang, H.; Yu, J.; Wang, Y.; Zhu, J.; Hu, Z. UHMWPE/nanoparticle composite membrane for personal radiation shielding. Compos. Sci. Technol. 2021, 201, 108500. [Google Scholar] [CrossRef]
- Zalepugin, D.Y.; Maksimkin, A.V.; Tilkunova, N.A.; Chernyshova, I.V.; Senatov, F.S.; Vlasov, M.I. Preparation of porous ultrahigh-molecular-weight polyethylene using subcritical water. Russ. J. Phys. Chem. B. 2015, 9, 1157–1161. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Maksimkin, A.V.; Sipyagina, N.A.; Malkova, A.N.; Kolesnikov, E.A.; Zadorozhnyy, M.Y.; Strau, E.A. Ultra-high molecular weight polyethylene with hybrid porous structure. Polymer 2020, 202, 122744. [Google Scholar] [CrossRef]
- Volynskii, A.L.; Bakeev, N.F. Solvent Crazing of Polymers; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1995. [Google Scholar]
- Volynskii, A.L.; Bakeev, N.F. Surface Phenomena in the Structural and Mechanical Behaviour of Solid Polymers; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Arzhakova, O.V.; Prishchepa, D.V.; Dolgova, A.A.; Volynskii, A.L. The effect of preliminary orientation on environmental crazing of high-density polyethylene films. Polymer 2019, 170, 179–189. [Google Scholar] [CrossRef]
- Arzhakova, O.V.; Dolgova, A.A.; Rukhlya, E.G.; Volynskii, A.L. Environmental crazing and properties of mesoporous and nanocomposite materials based on poly(tetrafluoroethylene) films. Polymer 2019, 161, 151–161. [Google Scholar] [CrossRef]
- Arzhakova, O.V.; Dolgova, A.A.; Yarysheva, A.Y.; Nikishin, I.I.; Volynskii, A.L. Mechanoresponsive hard elastic materials based on semicrystalline polymers: From preparation to applied properties. ACS Appl. Polym. Mater. 2020, 2, 2338–2349. [Google Scholar] [CrossRef]
- Mey-Merom, A.; Katz, M. Measurement of the active pore size distribution of microporous membranes. A new approach. J. Membr. Sci. 1986, 27, 119. [Google Scholar] [CrossRef]
- Cuperus, F.P.; Bargeman, D.; Smolders, C.A. Permporometry: The determination of the size distribution of active pores in UF membranes. J. Membr. Sci. 1992, 71, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Yarysheva, A.Y.; Rukhlya, E.G.; Grokhovskaya, T.E.; Dolgova, A.A.; Arzhakova, O.V. Breathable polymeric materials based on high-density polyethylene prepared by environmental crazing. J. Appl. Polym. Sci. 2019, 137, 48567. [Google Scholar] [CrossRef]
- Hantel, M.M.; Armstrong, M.J.; DaRosa, F.; l’Abee, R. Characterization of tortuosity in polyetherimide membranes based on Gurley and electrochemical impedance spectroscopy. J. Electrochem. Soc. 2016, 164, A334–A339. [Google Scholar] [CrossRef]
- Pawlak, A.; Galeski, A. Cavitation during tensile deformation of polypropylene. Macromolecules 2008, 41, 2839–2851. [Google Scholar] [CrossRef]
- Dudnik, A.O.; Trofimchuk, E.S.; Efimov, A.V.; Nikonorova, N.I.; Rukhlya, E.G.; Nikitin, L.N.; Yaminsky, I.V.; Volynskii, A.L. Evolution of the nanoporous structure of high-density polyethylene during drawing in supercritical carbon dioxide. Macromolecules 2018, 51, 1129–1140. [Google Scholar] [CrossRef]
- Arzhakova, O.V.; Kopnov, A.Y.; Nazarov, A.I.; Dolgova, A.A.; Volynskii, A.L. “Green” environmental crazing of polymers in oil-in-water emulsions with high water content. Polymer 2019, 186, 122020. [Google Scholar] [CrossRef]
- Deblieck, R.A.C.; van Beek, D.J.M.; Remerie, K.; Ward, I.M. Failure mechanisms in polyolefines: The role of crazing, shear yielding and the entanglement network. Polymer 2011, 52, 2979–2990. [Google Scholar] [CrossRef] [Green Version]
- Saintyves, B.; Dauchot, O.; Bouchaud, E. Bulk elastic fingering instability in Hele-Shaw cells. Phys. Rev. Lett. 2013, 111, 047801. [Google Scholar] [CrossRef] [Green Version]
- Sprague, B.S. Relationship of structure and morphology to properties of “hard” elastic fibers and films. J. Macromol. Sci. Part B 1973, 8, 157–187. [Google Scholar] [CrossRef]
- Lutzweiler, G.; NdreuHalili, A.; Engin Vrana, N. The overview of porous, bioactive scaffolds as instructive biomaterials for tissue regeneration and their clinical translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, M.-Y.; Lin, C.-E.; Zhu, B.-K. Progress in polymeric separators for lithium-ion batteries. RSC Adv. 2015, 5, 89848–89860. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of lithium-ion battery separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Bjorn, W.-J.; Douglas, M.; Orawan, W.-J. Breathable Electrode and Method for Use in Water Splitting; Monash: Melbourne, Australia, 2018. [Google Scholar]
- Cao, L.; Fu, Q.; Si, Y.; Ding, B.; Yu, J. Porous materials for sound absorption. Comp. Comm. 2018, 10, 25–35. [Google Scholar] [CrossRef]
- Wan, C.; Cao, T.; Jing, J.; Lv, F.; Zhang, W.; Li, L. Modification of UHMWPE porous fibers by acrylic acid and its adsorption kinetics for Cu2+removal. Polym. Bull. 2017, 74, 3855–3870. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arzhakova, O.V.; Nazarov, A.I.; Solovei, A.R.; Dolgova, A.A.; Kopnov, A.Y.; Chaplygin, D.K.; Tyubaeva, P.M.; Yarysheva, A.Y. Mesoporous Membrane Materials Based on Ultra-High-Molecular-Weight Polyethylene: From Synthesis to Applied Aspects. Membranes 2021, 11, 834. https://doi.org/10.3390/membranes11110834
Arzhakova OV, Nazarov AI, Solovei AR, Dolgova AA, Kopnov AY, Chaplygin DK, Tyubaeva PM, Yarysheva AY. Mesoporous Membrane Materials Based on Ultra-High-Molecular-Weight Polyethylene: From Synthesis to Applied Aspects. Membranes. 2021; 11(11):834. https://doi.org/10.3390/membranes11110834
Chicago/Turabian StyleArzhakova, Olga V., Andrei I. Nazarov, Arina R. Solovei, Alla A. Dolgova, Aleksandr Yu. Kopnov, Denis K. Chaplygin, Polina M. Tyubaeva, and Alena Yu. Yarysheva. 2021. "Mesoporous Membrane Materials Based on Ultra-High-Molecular-Weight Polyethylene: From Synthesis to Applied Aspects" Membranes 11, no. 11: 834. https://doi.org/10.3390/membranes11110834
APA StyleArzhakova, O. V., Nazarov, A. I., Solovei, A. R., Dolgova, A. A., Kopnov, A. Y., Chaplygin, D. K., Tyubaeva, P. M., & Yarysheva, A. Y. (2021). Mesoporous Membrane Materials Based on Ultra-High-Molecular-Weight Polyethylene: From Synthesis to Applied Aspects. Membranes, 11(11), 834. https://doi.org/10.3390/membranes11110834







