Graphene Oxide Modified Polyamide 66 Ultrafiltration Membranes with Enhanced Anti-Fouling Performance
Abstract
:1. Introduction
2. Experimental
2.1. Materials
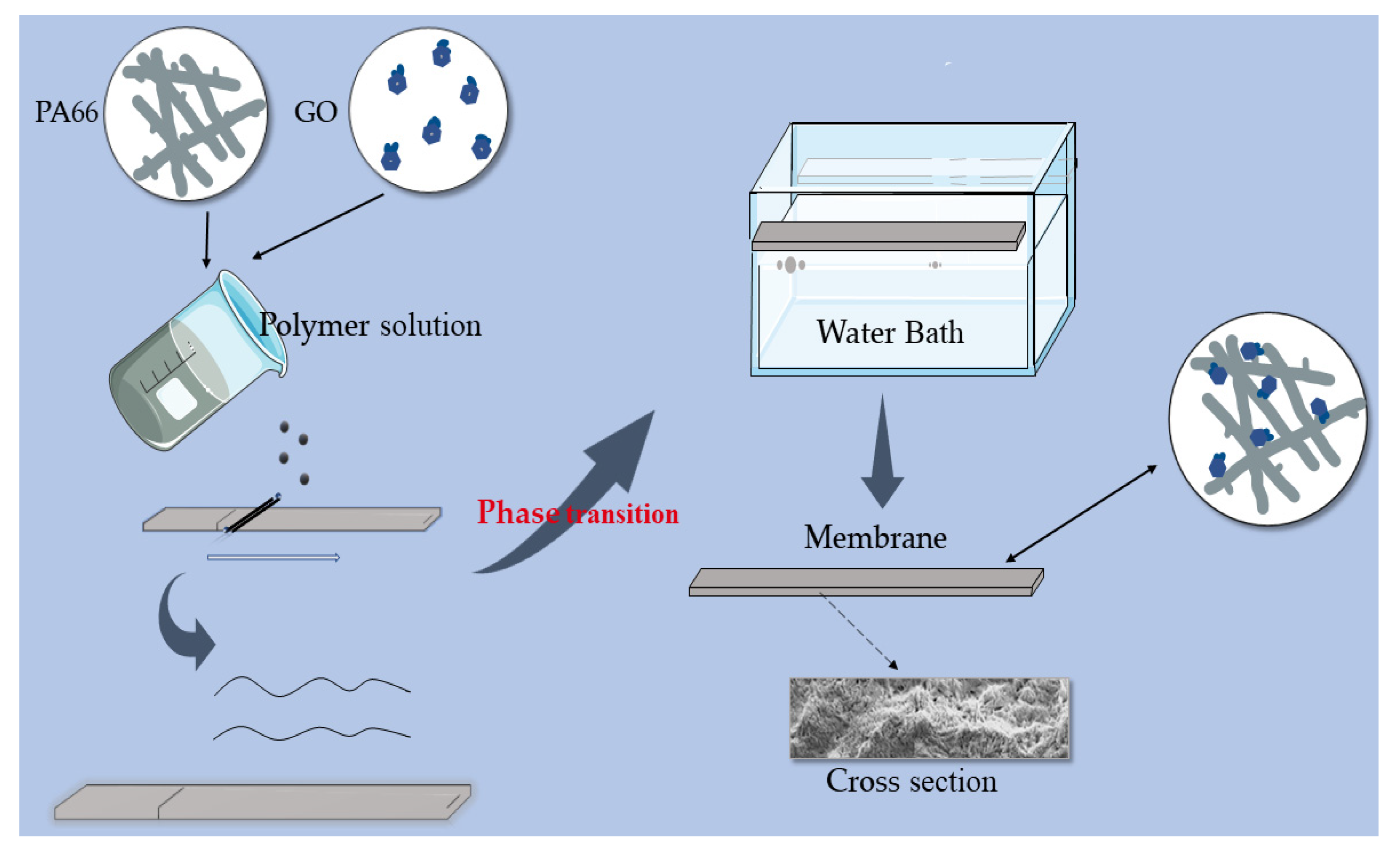
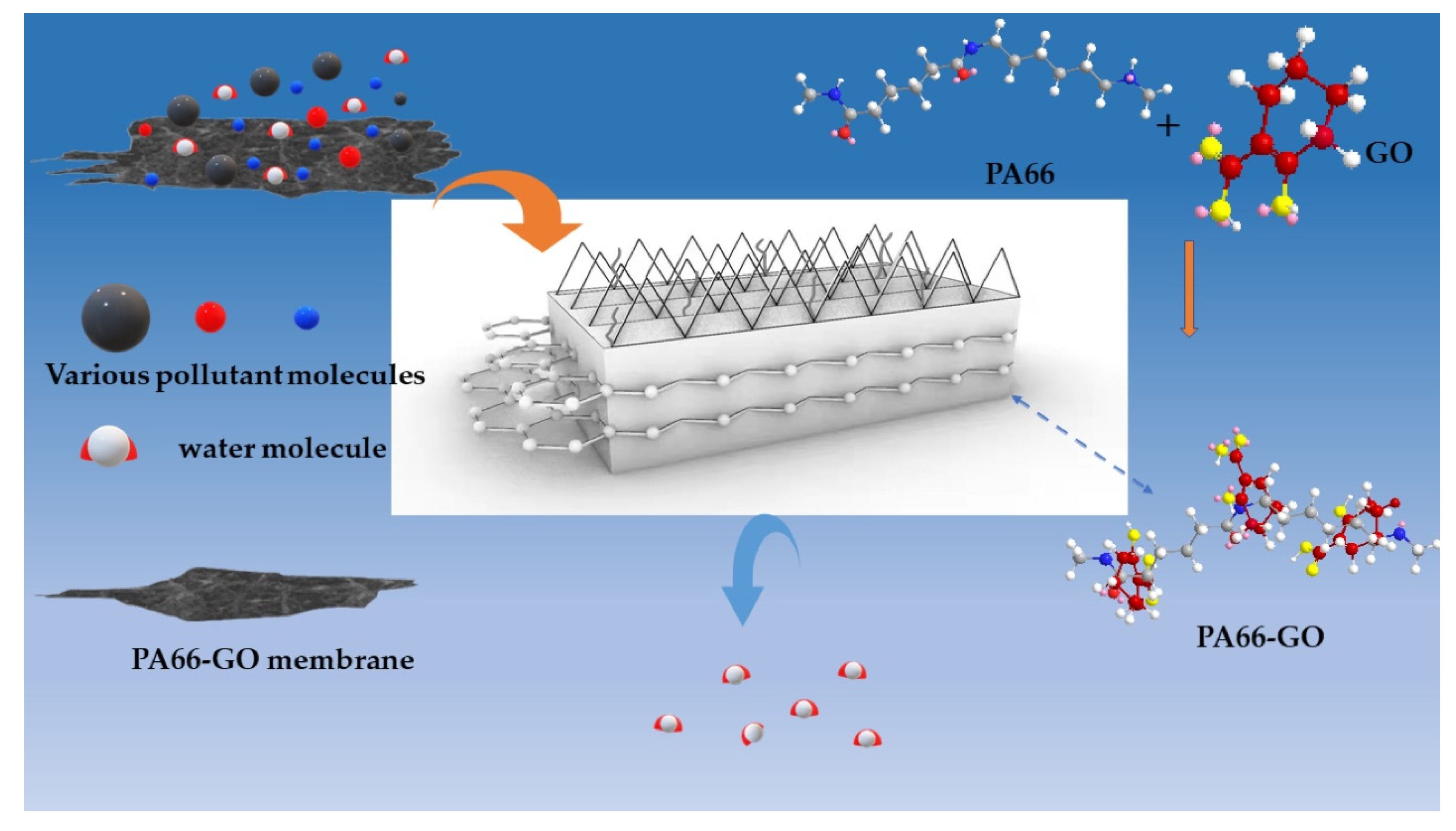
2.2. Preparation of PA66-GO Flat Sheet Membranes
- (1)
- Different amounts of GO (from 0 wt.% to 0.5 wt.%) were added into appropriate amounts of FA and PC mixed solvent. (The ratio of the two solvents was 5/1.) and the solution was treated with ultrasonic for 1 h to disperse GO and minimize agglomeration;
- (2)
- The PA66 solution was prepared in advance. PA66 (22 wt.%) was dissolved in a mixture of the remaining FA and PC. The mixture was blended at 60 °C until a clear homogeneous solution was obtained. The solution was aged for a period of 1 or 2 h in a thermostat held at 45 °C to remove air bubbles. Then the solution was naturally cooled to room temperature;
- (3)
- The GO solution was then added slowly into the PA66 mixture solution under vigorous stirring. The mixed solution of the two was stirred for 5 h to obtain a homogenous solution for casting. The prepared casting solution was sealed and stored for more than 12 h to remove air bubbles. To remove residual air bubbles trapped within the dope solutions, each solution was then subjected to 1 h of ultrasonication, followed by casting on a glass plate, using a self-made casting glass rod. Then, the cast glass plate was left for 3 min at an ambient temperature before being immersed into a water coagulation bath at room temperature for the phase inversion process to take place. Once the membrane was peeled off from the glass plate, it was transferred to another water bath and kept for 24 h to remove residual solvent. For pure PA66 membrane, the same method was applied.
2.3. Characterization
2.3.1. GO Characterization
2.3.2. Characterization of the Prepared Membranes
2.3.3. The Hydrophilicity of Membrane
2.3.4. The Permeability, Anti-Fouling Performance for the Membrane
2.3.5. The Porosity of the Membrane
2.3.6. Mechanical Property of Membrane
3. Results and Discussion
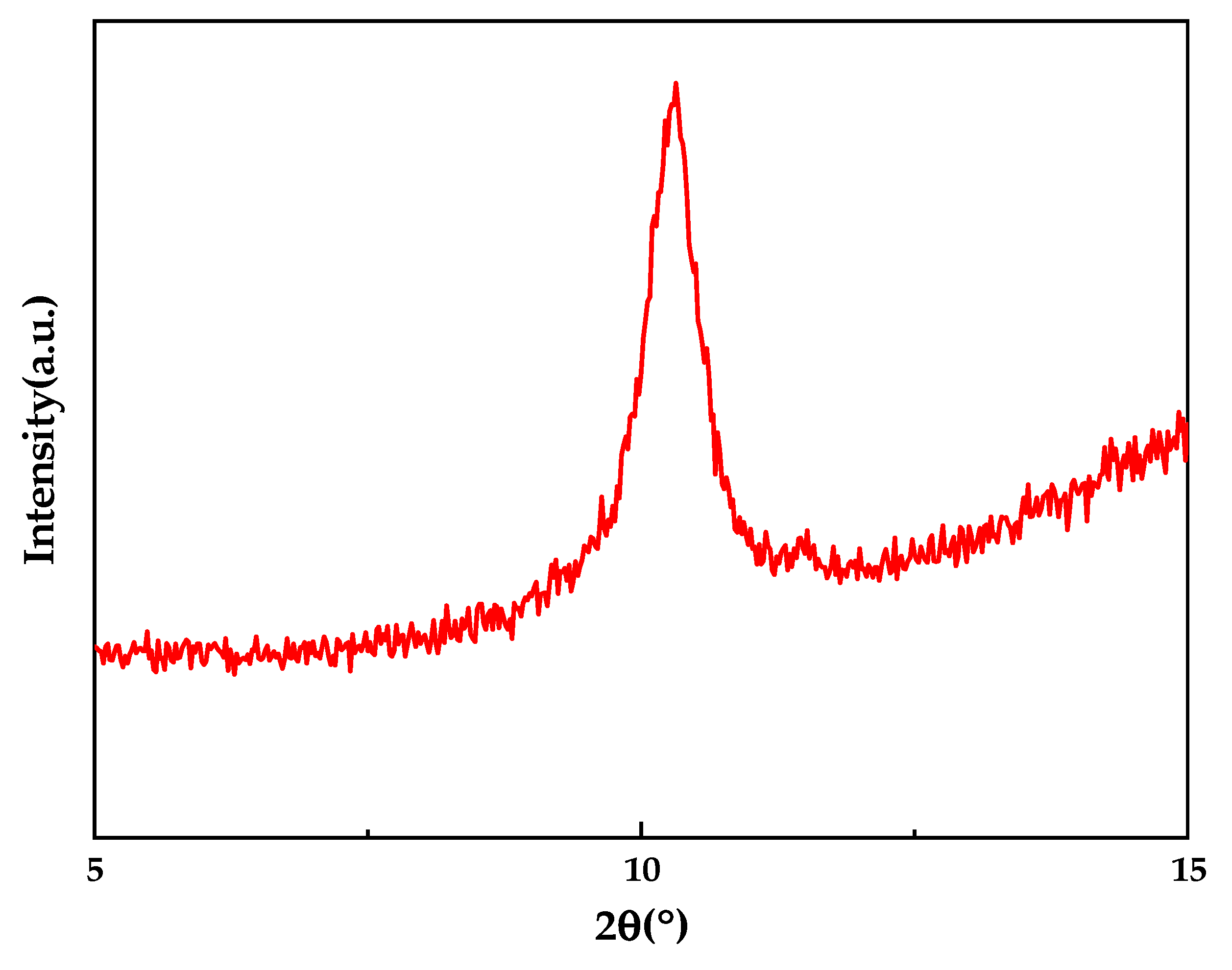
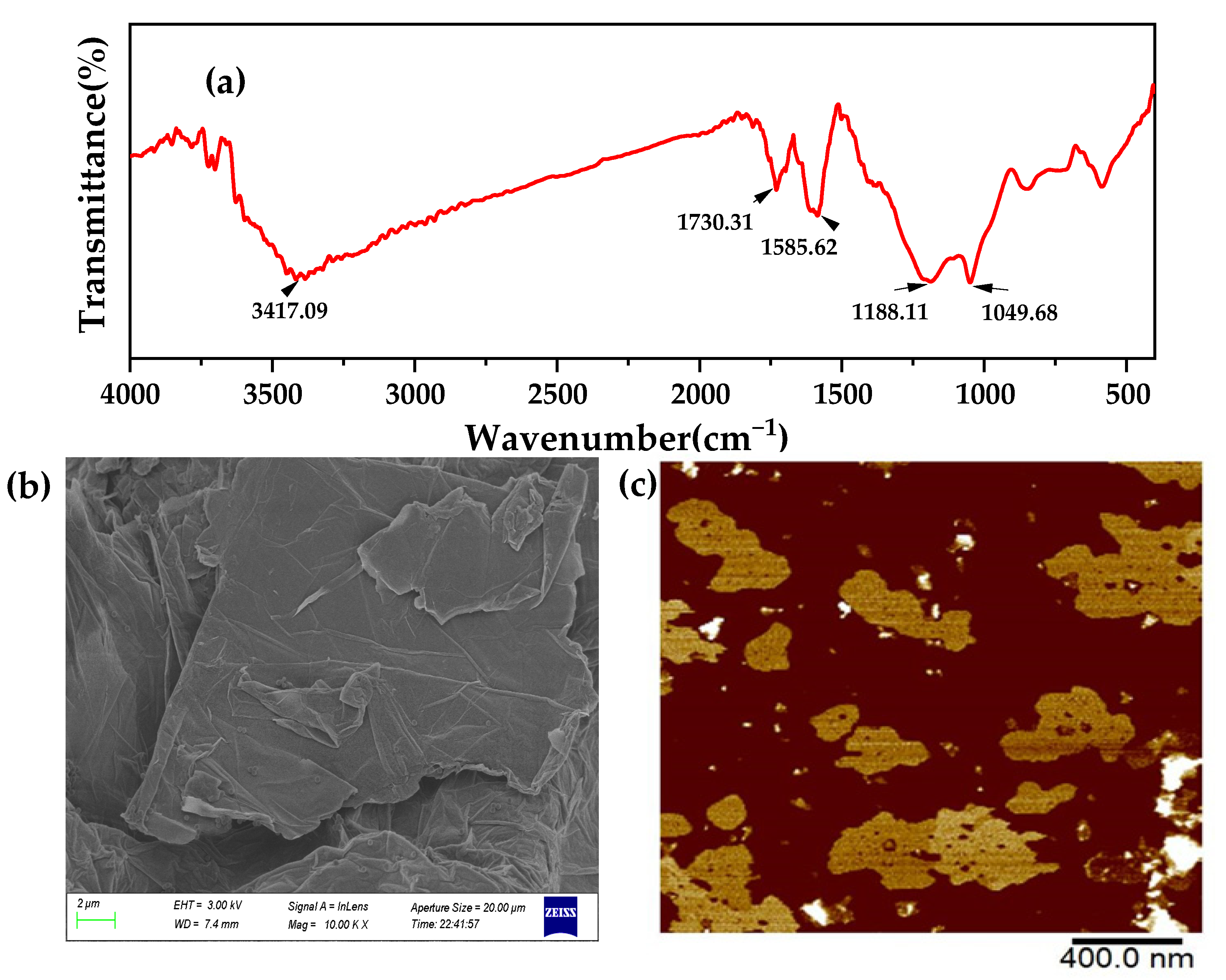
3.1. Characterization of GO
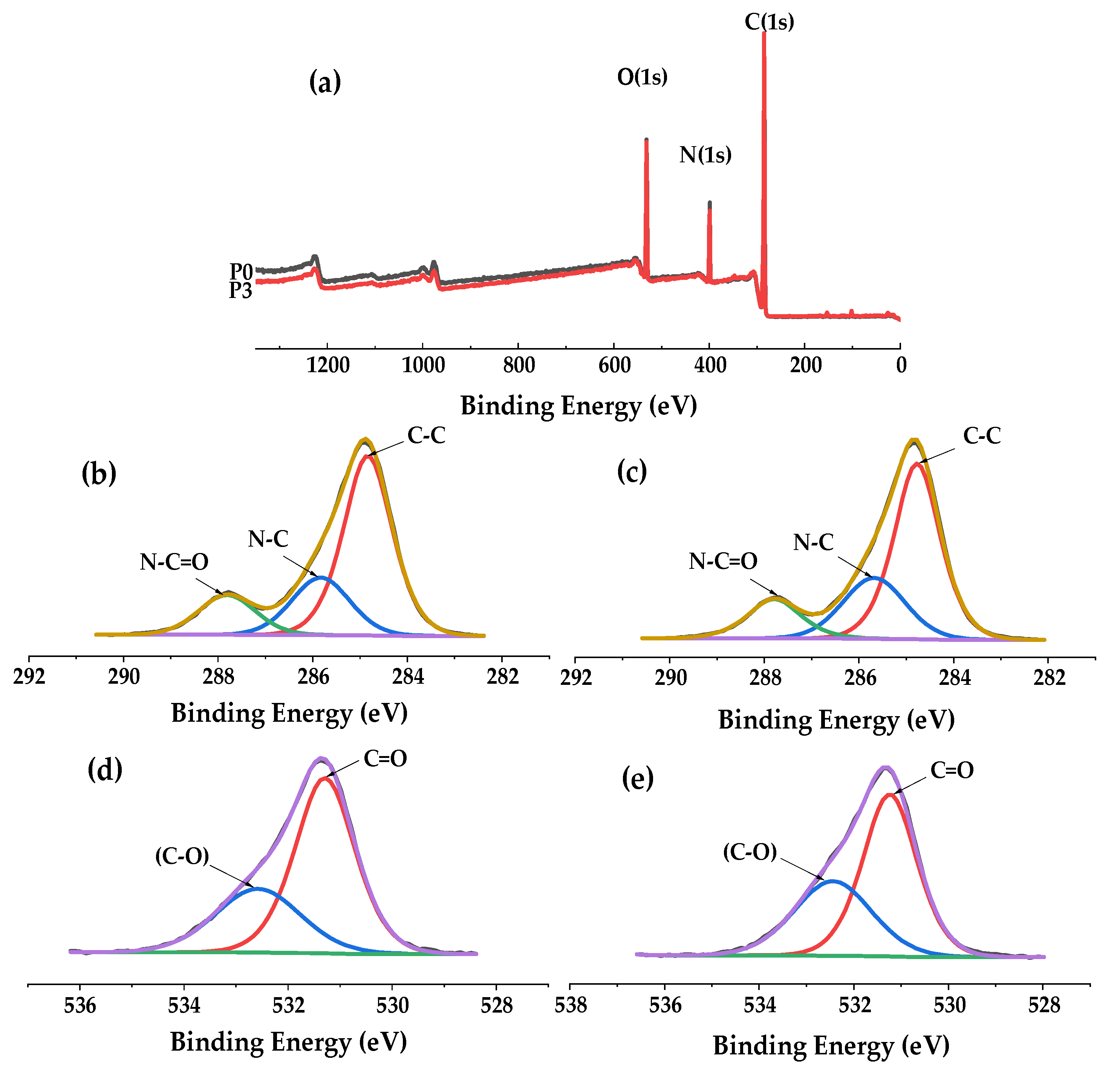
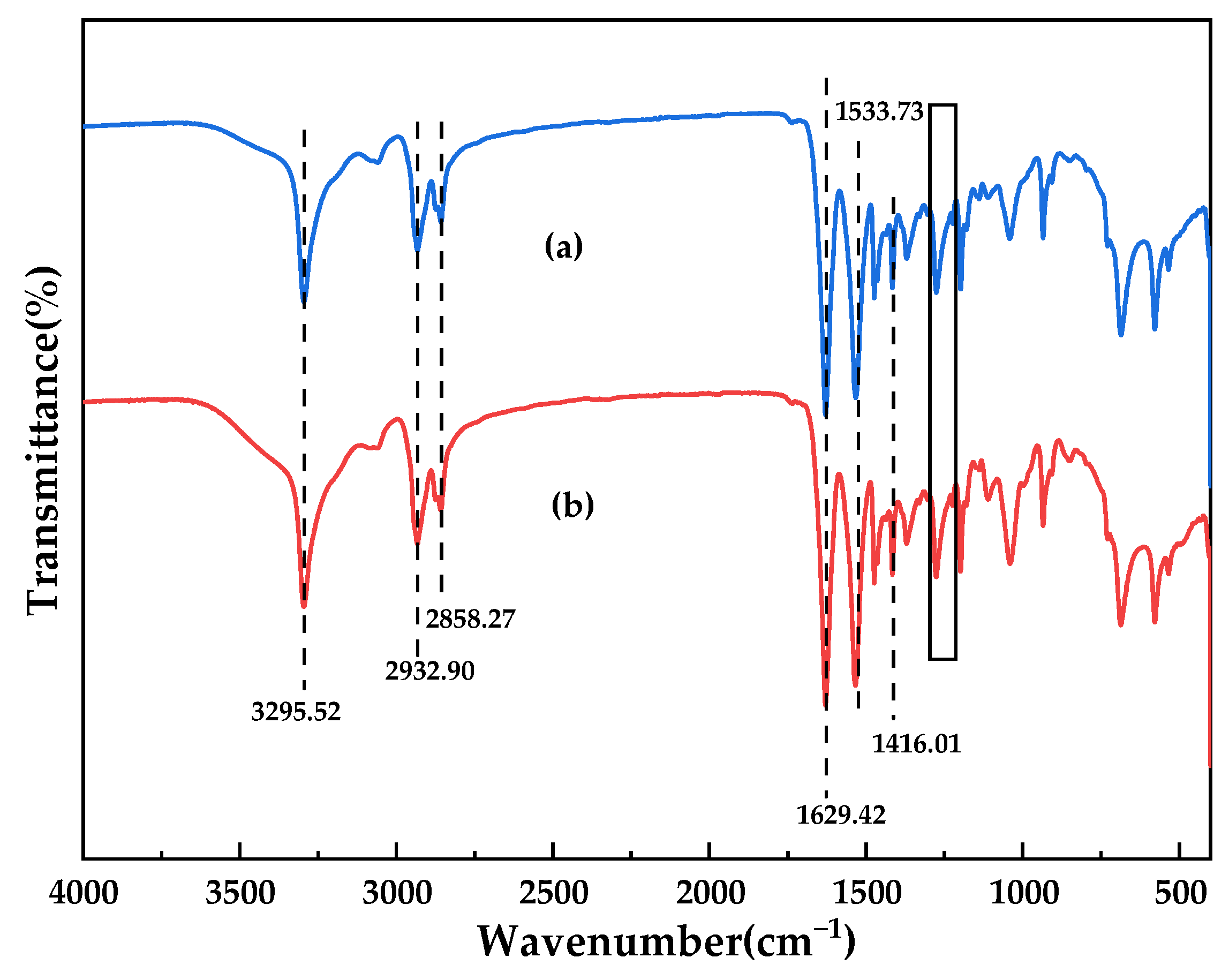
3.2. Chemical Characteristics of the Membranes
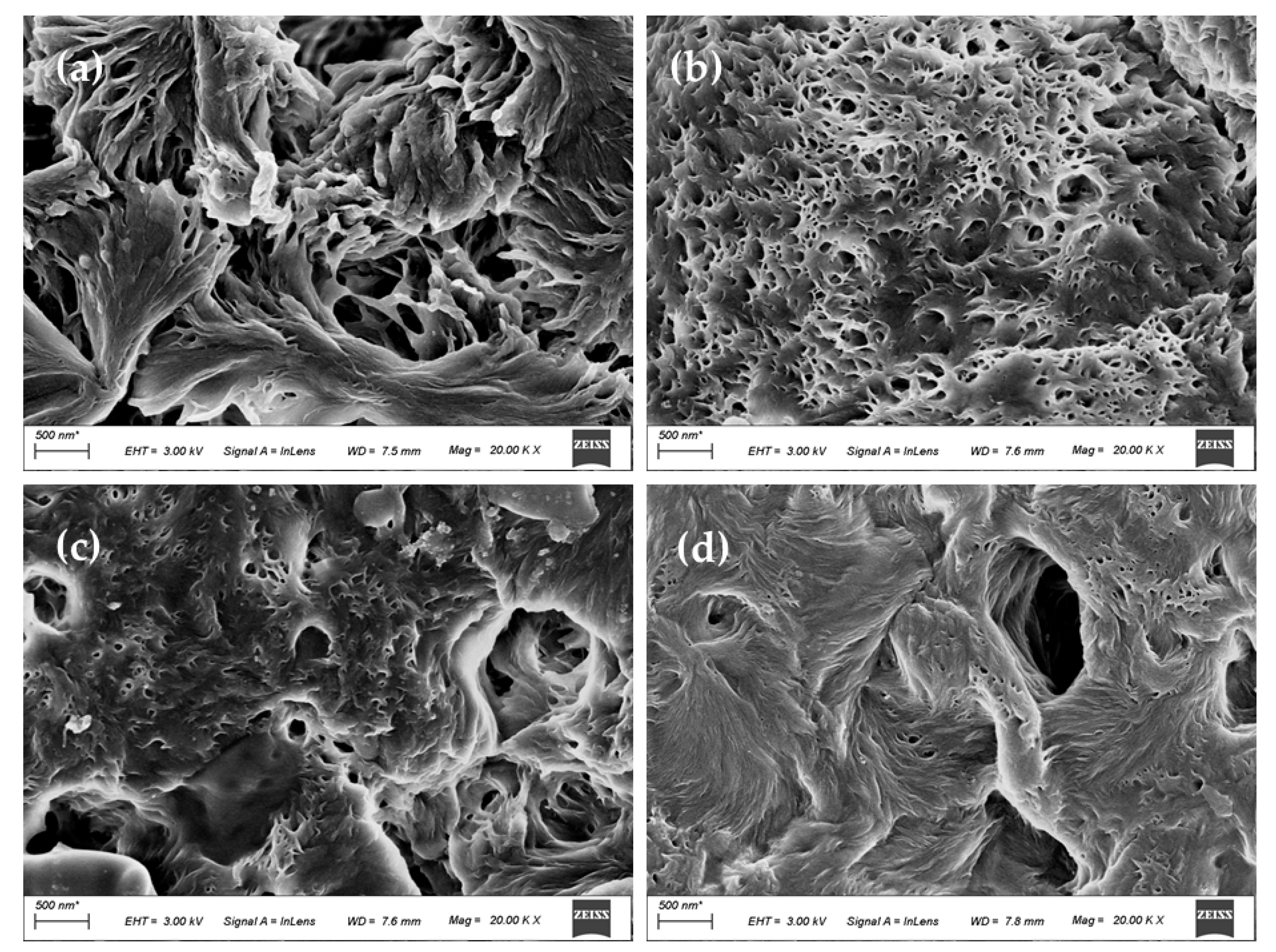
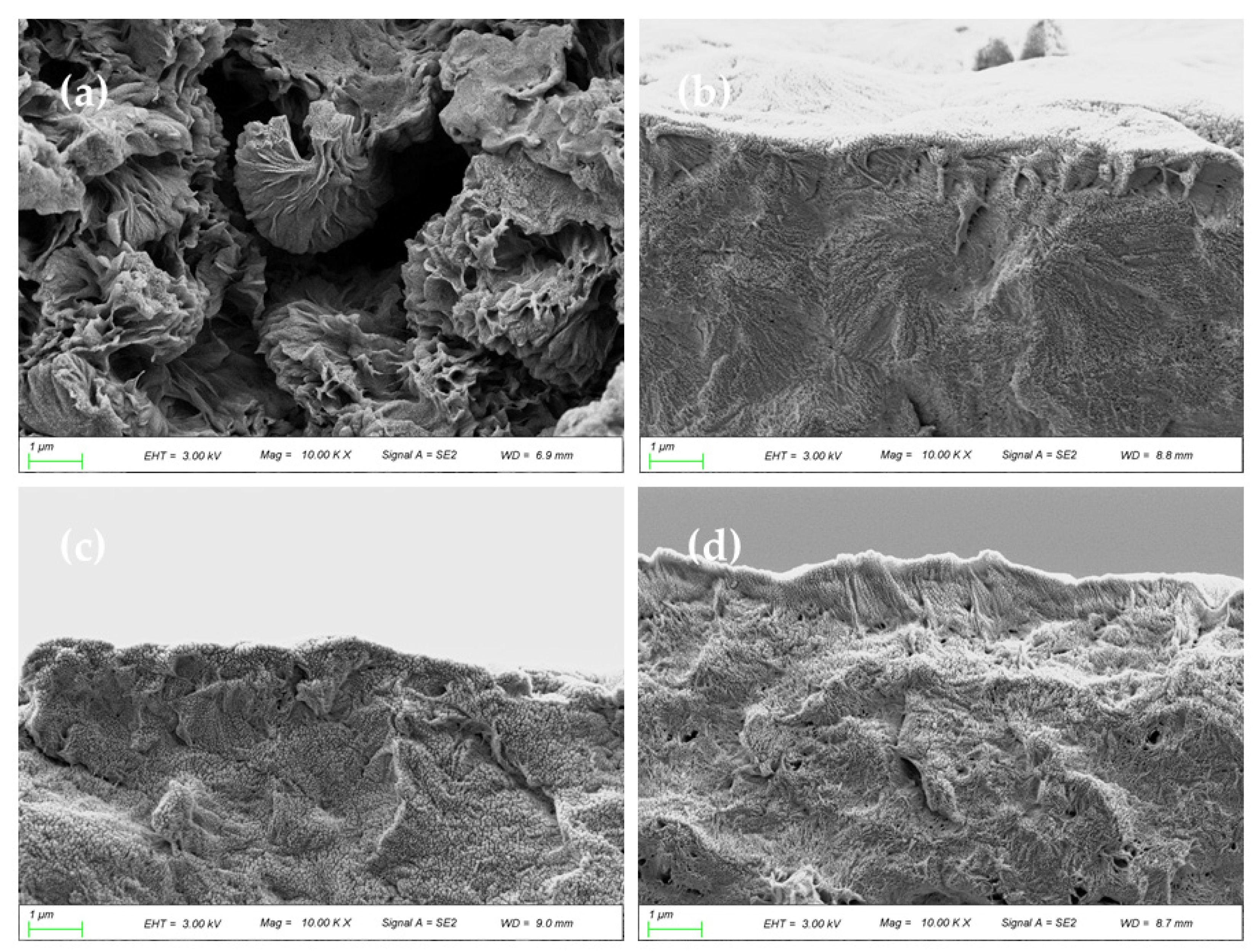
3.3. Morphology of the Membranes
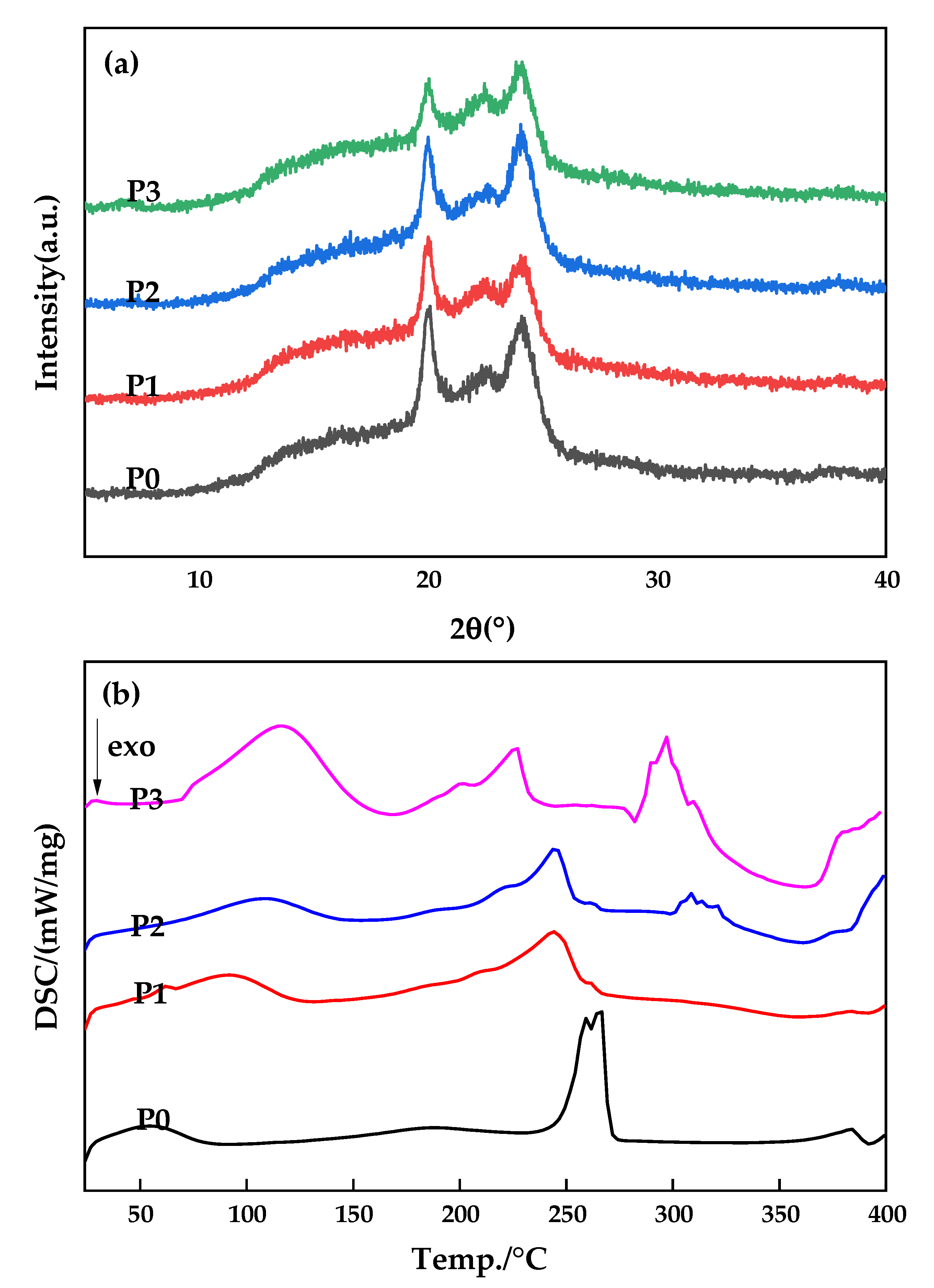
3.4. Crystalline Properties of the Membranes
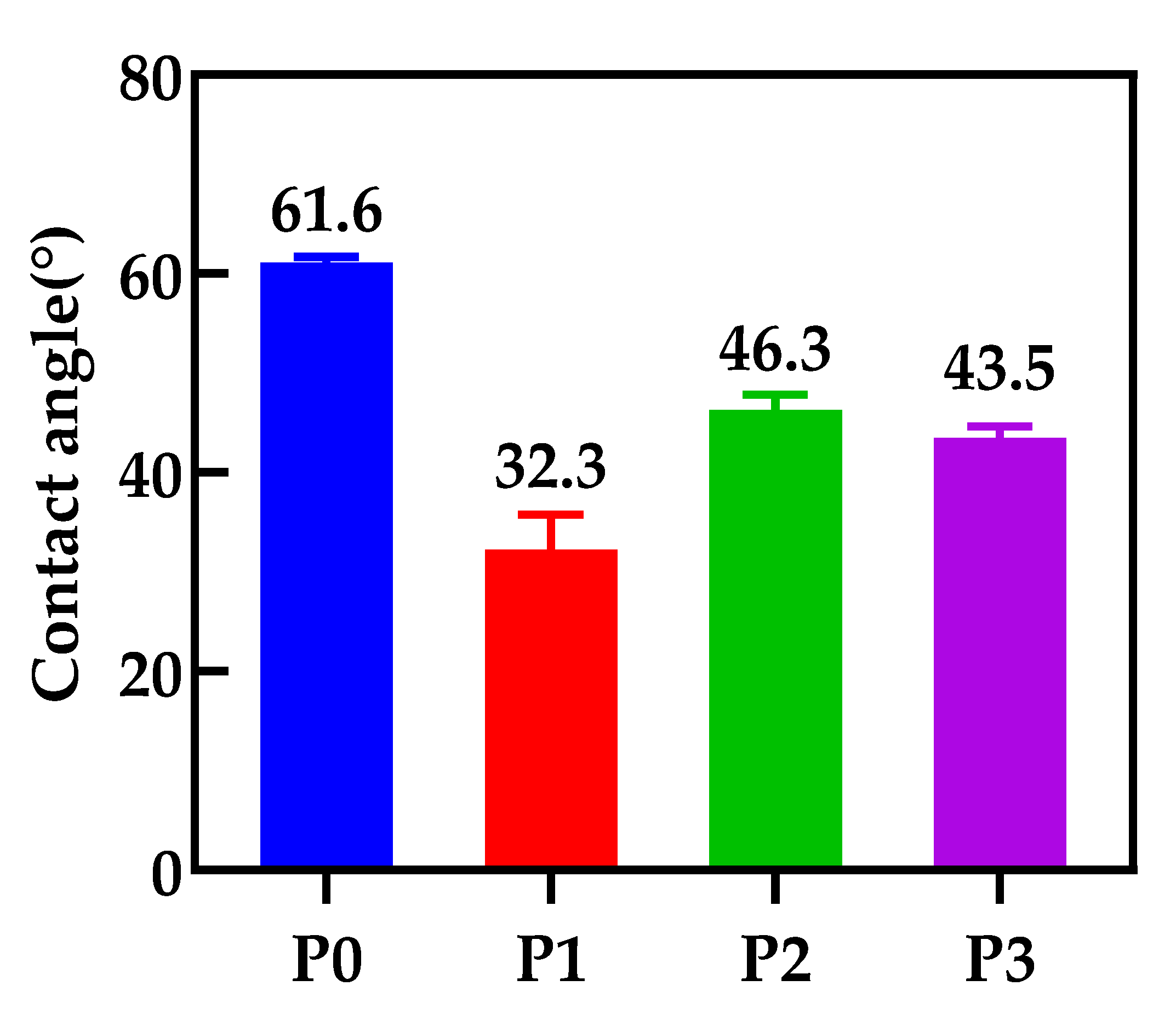
3.5. The Contact Angle of the Membranes
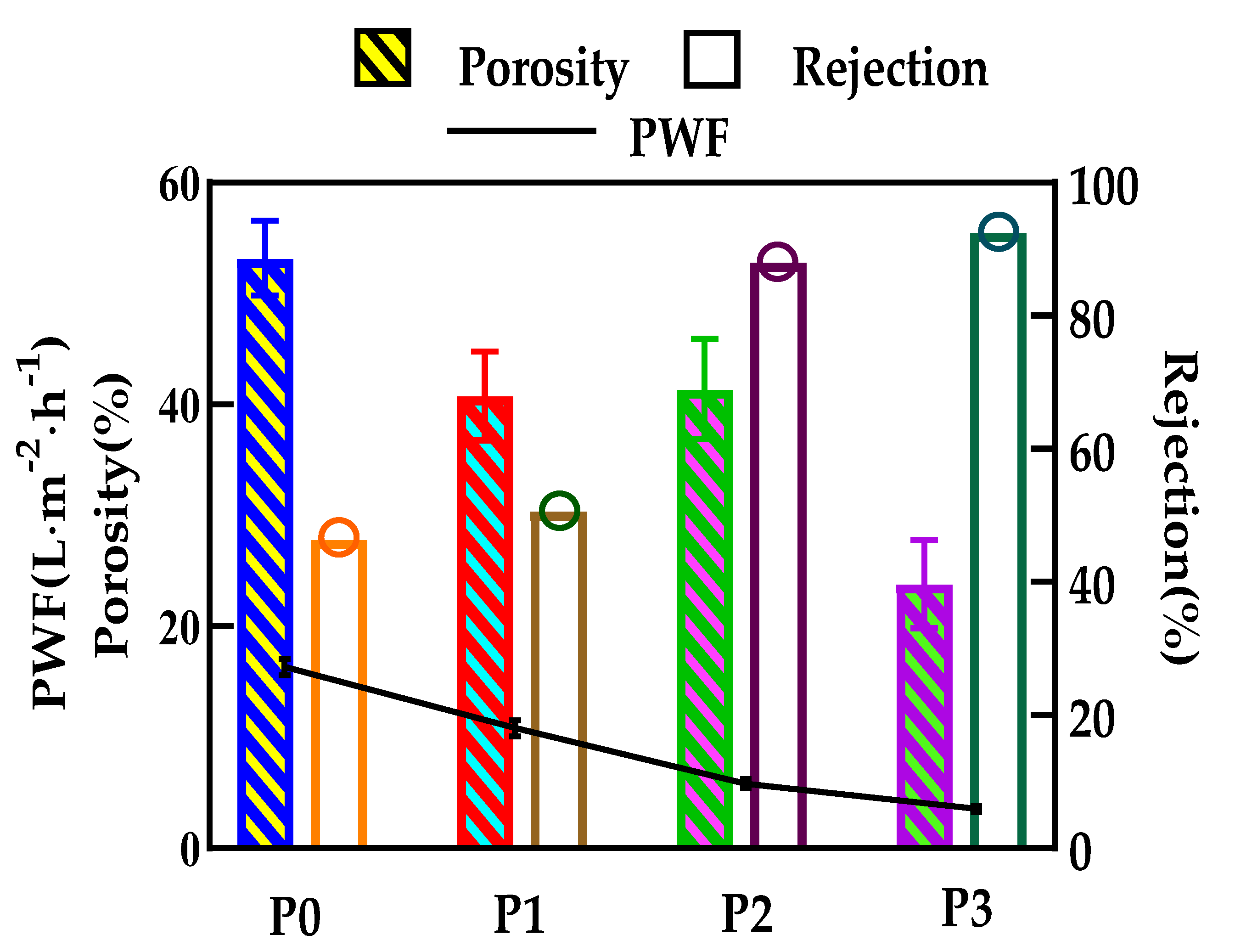
3.6. The Permeability of the Membranes
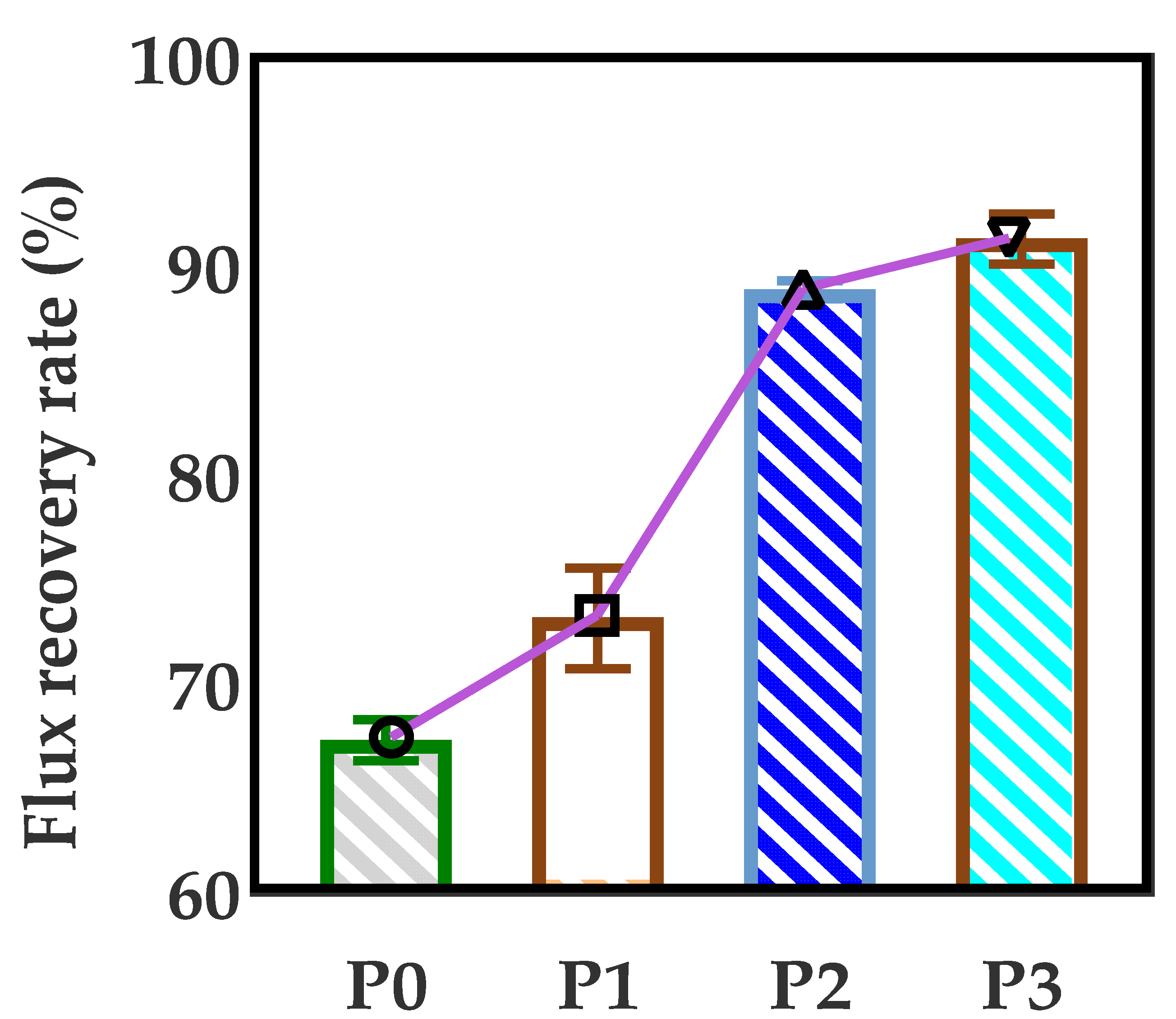
3.7. Anti-Fouling Performance of Developed Membranes
3.8. Mechanical Performance of the Membranes
4. Conclusions
- (1)
- When GO is added to PA66 ultrafiltration membrane, the physicochemical properties of the membrane can be improved from the aspects of microporous structure, hydrophilic properties, surface roughness, and anti-fouling performance.
- (2)
- SEM results show that after the addition of GO, the PA66 membrane becomes denser, and the crystals change from large and sharp axial crystals to small and round spherical crystals in the process of membrane formation. Pure PA66 membrane has larger and more pores than PA66/GO membrane.
- (3)
- After loading GO into PA66, the surface of the membrane becomes smooth and the surface morphology changes significantly. However, with further increase of GO, the roughness of the ultrafiltration membrane increases from 60.7 nm to 90.7 nm, which is due to the agglomeration of GO.
- (4)
- The contact angles of all PA66/GO membranes are smaller than the pure PA66 membrane, because of the hydrophilic nature of the oxygen-containing groups on GO. However, when the amount of GO increases, the contact angles increase because the central structure of GO is hydrophobic.
- (5)
- The anti-fouling performance of the membrane was significantly improved after incorporating GO into membranes, which benefited from the optimization effect of GO on the membrane structure, and the flux recovery rate was up to 91.3%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Vatanpour, V.; Faghani, S.; Keyikoglu, R.; Khataee, A. Enhancing the permeability and antifouling properties of cellulose acetate ultrafiltration membrane by incorporation of ZnO@graphitic carbon nitride nanocomposite. Carbohydr. Polym. 2021, 256, 117413. [Google Scholar] [CrossRef] [PubMed]
- Orooji, Y.; Movahedi, A.; Liu, Z.; Asadnia, M.; Ghasali, E.; Ganjkhanlou, Y.; Razmjou, A.; Karimi-Maleh, H.; Kiadeh, N.T.H. Luminescent film: Biofouling investigation of tetraphenylethylene blended polyethersulfone ultrafiltration membrane. Chemosphere 2021, 267, 128871. [Google Scholar] [CrossRef]
- Karimi, A.; Khataee, A.; Ghadimi, A.; Vatanpour, V. Ball-milled Cu2S nanoparticles as an efficient additive for modification of the PVDF ultrafiltration membranes: Application to separation of protein and dyes. J. Environ. Chem. Eng. 2021, 9, 105115. [Google Scholar] [CrossRef]
- Han, J.C.; Zhu, Y.K.; Wang, L.F.; Mu, Y.; Feng, G.G.; Liu, K.Q.; Tong, C.H.; Yu, Z.X. Modification of regenerated cellulose ultrafiltration membranes with multi-walled carbon nanotubes for enhanced antifouling ability: Field test and mechanism study. Sci. Total Environ. 2021, 780, 146657. [Google Scholar] [CrossRef]
- Moghimifar, V.; Raisi, A.; Aroujalian, A. Surface modification of polyethersulfone ultrafiltration membranes by corona plasma-assisted coating TiO2 nanoparticles. J. Membr. Sci. 2014, 461, 69–80. [Google Scholar] [CrossRef]
- Kassa, S.T.; Hu, C.C.; Keshebo, D.L.; Belle Marie Ang, M.; Lai, J.Y.; Chu, J.P. Surface modification of high-rejection ultrafiltration membrane with antifouling capability using activated oxygen treatment and metallic glass deposition. Appl. Surf. Sci. 2020, 529, 147131. [Google Scholar] [CrossRef]
- Cheng, K.; Zhang, N.; Yang, N.; Hou, S.; Ma, J.; Zhang, L.; Sun, Y.; Jiang, B. Rapid and robust modification of PVDF ultrafiltration membranes with enhanced permselectivity, antifouling and antibacterial performance. Sep. Purif. Technol. 2021, 262, 118316. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Beltsios, K.; Cheng, L.-P. Formation of bicontinuous, hydrophobic nylon 12 membranes via cold-solvent-induced phase separation for membrane distillation application. J. Appl. Polym. Sci. 2019, 136, 47036. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Shao, F.; Dong, L.; Dong, H.; Zhang, Q.; Zhao, M.; Yu, L.; Pang, B.; Chen, Y. Graphene oxide modified polyamide reverse osmosis membranes with enhanced chlorine resistance. J. Membr. Sci. 2017, 525, 9–17. [Google Scholar] [CrossRef]
- Ravishankar, H.; Christy, J.; Jegatheesan, V. Graphene Oxide (GO)-Blended Polysulfone (PSf) Ultrafiltration Membranes for Lead Ion Rejection. Membranes 2018, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-H.; Jegal, J.; Kim, W.-N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Arepalli, S.; Nikolaev, P.; Gorelik, O.; Hadjiev, V.G.; Holmes, W.; Files, B.; Yowell, L. Protocol for the characterization of single-wall carbon nanotube material quality. Carbon 2004, 42, 1783–1791. [Google Scholar] [CrossRef]
- Ausman, K.D.; Piner, R.; Lourie, O.; Ruoff, R.S.; Korobov, M. Organic solvent dispersions of single-walled carbon nanotubes: Toward solutions of pristine nanotubes. J. Phys. Chem. B 2000, 104, 8911–8915. [Google Scholar] [CrossRef]
- Xiang, S.; Guo, Z.; Wang, Y.; Liu, H.; Zhang, J.; Cui, Z.; Wang, H.; Li, J. The microstructure regulation, strengthening, toughening and hydrophilicity of polyamide6 in fabricating poly (vinylidene fluoride)-based flat membrane via the thermally induced phase separation technique. Eur. Polym. J. 2020, 126, 109568. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, Z.; Li, T.; Qin, S.; He, B.; Han, N.; Li, J. Fabrication of PVDF-based blend membrane with a thin hydrophilic deposition layer and a network structure supporting layer via the thermally induced phase separation followed by non-solvent induced phase separation process. Appl. Surf. Sci. 2017, 419, 429–438. [Google Scholar] [CrossRef]
- Xu, P.; Hong, J.; Qian, X.; Xu, Z.; Xia, H.; Ni, Q.-Q. “Bridge” graphene oxide modified positive charged nanofiltration thin membrane with high efficiency for Mg2+/Li+ separation. Desalination 2020, 488, 114522. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhang, H.; Zhu, B.; Xu, Y. Antifouling and antimicrobial polymer membranes based on bioinspired polydopamine and strong hydrogen-bonded poly(N-vinyl pyrrolidone). ACS Appl. Mater. Interfaces 2013, 5, 12895–12904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Z.; Mai, W.; Min, C.; Zhou, B.; Shan, M.; Li, Y.; Yang, C.; Wang, Z.; Qian, X. Improved hydrophilicity, permeability, antifouling and mechanical performance of PVDF composite ultrafiltration membranes tailored by oxidized low-dimensional carbon nanomaterials. J. Mater. Chem. A 2013, 1, 3101–3111. [Google Scholar] [CrossRef]
- Islam, M.S.; Yeum, J.H. Electrospun pullulan/poly(vinyl alcohol)/silver hybrid nanofibers: Preparation and property characterization for antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 279–286. [Google Scholar] [CrossRef]
- Linggawati, A.; Mohammad, A.W.; Ghazali, Z. Effect of electron beam irradiation on morphology and sieving characteristics of nylon-66 membranes. Eur. Polym. J. 2009, 45, 2797–2804. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Kim, S.-J.; Lee, K.-H. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. A 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Tesha, J.M.; Dlamini, D.S.; Cui, Z.; Li, J. The effect of Styrene Maleic Anhydride on the microstructure evolution of PSF-based membrane prepared by thermal-induced phase separation method. Mater. Today Commun. 2019, 21, 100687. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Lee, S.Y.; Lee, J.M.; Woo, K.T.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. Poly(vinylidene fluoride) membrane preparation with an environmental diluent via thermally induced phase separation. J. Membr. Sci. 2013, 444, 223–236. [Google Scholar] [CrossRef]
- Zhang, L.; Han, N.; Tan, L.; Qian, Y.; Cui, Z.; Cai, J. Preparation of hydrolysis of poly(acrylonitrile-co-methyl acrylate) membranes via thermally induced phase separation: Effects of hydrolysis conditions and additives. J. Appl. Polym. Sci. 2018, 135, 46380. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Zhao, W.; Su, Y.; Li, C.; Shi, Q.; Ning, X.; Jiang, Z. Fabrication of antifouling polyethersulfone ultrafiltration membranes using Pluronic F127 as both surface modifier and pore-forming agent. J. Membr. Sci. 2008, 318, 405–412. [Google Scholar] [CrossRef]
- Teng, Z.; Wang, B.; Hu, Y.; Zhang, W.; Wu, Z.; Xu, D. High performance ultrafiltration membrane by coupling magnetic migration and in-situ surface modification. Polym. Test. 2021, 101, 10306. [Google Scholar] [CrossRef]
- Mahdavi, H.; Norouzian, S. Preparation and characterization of modified ultrafiltration nylon 6 membrane modified by poly (acrylamide-co-maleic anhydride). J. Polym. Res. 2018, 25, 222. [Google Scholar] [CrossRef]
- Zhao, J.; Chong, J.Y.; Shi, L.; Wang, R. Explorations of combined nonsolvent and thermally induced phase separation (N-TIPS) method for fabricating novel PVDF hollow fiber membranes using mixed diluents. J. Membr. Sci. 2019, 572, 210–222. [Google Scholar] [CrossRef]



| Code | Membrane | Content (wt.%) | |||
|---|---|---|---|---|---|
| PA66 | GO | FA | PC | ||
| P0 | PA66 | 22 | 0 | 68 | 10 |
| P1 | PA66-GO | 22 | 0.1 | 68 | 9.9 |
| P2 | PA66-GO | 22 | 0.3 | 68 | 9.7 |
| P3 | PA66-GO | 22 | 0.5 | 68 | 9.5 |
| Membrane | C (%) | N (%) | O (%) |
|---|---|---|---|
| PA66 (P0) | 73.7 | 11.6 | 14.7 |
| PA66-GO (P3) | 74.2 | 10.6 | 15.2 |
| Membrane | Conditions | Anti-Fouling Performance (FRR) | Flux (L/(m2·h)) | Reference |
|---|---|---|---|---|
| 0.1 MPa | ||||
| GO+PVP+PVDF | 48.32 cm2(area) | 90.5% | 104.3 | [1] |
| 1 g/L BSA | ||||
| 0.1 MPa | ||||
| DA+DMAPAPS+PVDF | 33.18 cm2 | 96.3% | 364 | [9] |
| 1 g/L BSA | ||||
| 0.2 MPa | ||||
| Cu2S+PVDF | - | 92.4% | 248.25 | [5] |
| 0.5 g/L BSA | ||||
| 0.1 MPa | ||||
| Ar/O2+W50Ni25B25+PSf | - | 91.3% | 321.5 | [8] |
| 0.6–0.8 l/m BSA | ||||
| 0.1 MPa | ||||
| GO-Fe3O4+FAS+PSf | 13.4 cm2 | 98.2% | 323.2 | [31] |
| 1 g/L BSA | ||||
| 0.15 MPa | ||||
| SMA+PSf | - | 91% | 147 | [26] |
| 1 mg/mL BSA | ||||
| 0.4 MPa | ||||
| AM-MA+PA6 | - | 91.1% | 19 | [32] |
| - BSA | ||||
| 0.1 MPa | ||||
| GO+PVDF | - | 85.1% | 163 | [22] |
| 1 g/L BSA | ||||
| 0.1 MPa | ||||
| Pluronic F127+PES | 28.7 cm2 | 94.12% | 140 | [30] |
| 1 g/L BSA | ||||
| 0.15MPa | ||||
| GO+PA66 | 37.4 cm2 | 91.32% | 3.5 | This work |
| 0.1 g/L BSA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Nie, L.; Li, G.; Zhu, Y.; Gao, M.; Wu, R.; Wang, B. Graphene Oxide Modified Polyamide 66 Ultrafiltration Membranes with Enhanced Anti-Fouling Performance. Membranes 2022, 12, 458. https://doi.org/10.3390/membranes12050458
Yan J, Nie L, Li G, Zhu Y, Gao M, Wu R, Wang B. Graphene Oxide Modified Polyamide 66 Ultrafiltration Membranes with Enhanced Anti-Fouling Performance. Membranes. 2022; 12(5):458. https://doi.org/10.3390/membranes12050458
Chicago/Turabian StyleYan, Jiangyi, Lihong Nie, Guiliang Li, Yuanlu Zhu, Ming Gao, Ruili Wu, and Beifu Wang. 2022. "Graphene Oxide Modified Polyamide 66 Ultrafiltration Membranes with Enhanced Anti-Fouling Performance" Membranes 12, no. 5: 458. https://doi.org/10.3390/membranes12050458
APA StyleYan, J., Nie, L., Li, G., Zhu, Y., Gao, M., Wu, R., & Wang, B. (2022). Graphene Oxide Modified Polyamide 66 Ultrafiltration Membranes with Enhanced Anti-Fouling Performance. Membranes, 12(5), 458. https://doi.org/10.3390/membranes12050458






