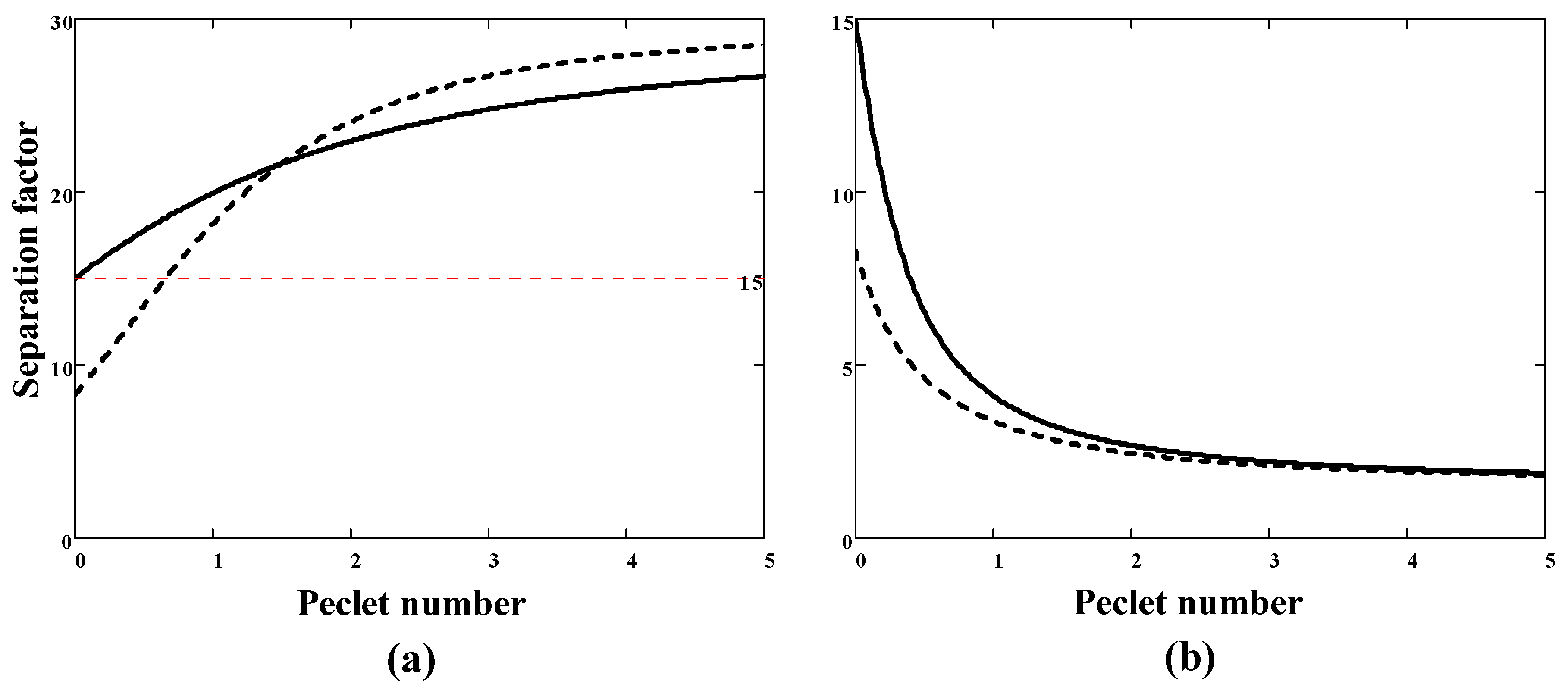4.1. Approximations and Flux Equations
Now, let us return to the differential flux Equations (9) and (10), and consider the permeability coefficients
(Equation (11)). Substituting Formulas (6) into Equation (11) gives the following expressions for the local permeability coefficients:
Since the volume fraction of type
i penetrant
, the above equations become:
where
In the absence of coupling (), the first term in the right-hand side of Equations (22) and (23) vanishes, and the permeability coefficients are reduced to the coefficients , which, as a first approximation, can be assumed to be independent of concentration and identified with the corresponding values for single gases. The relations (22) and (23) play a crucial role in the model development.
Substituting Equations (22) and (23) into Equations (9) and (10), respectively, give
where
is dimensionless membrane thickness, the parameters
are defined in Equation (15). These equations include the ratio of the local solubility coefficients. We make a simplifying assumption
that allows an analytical integration of the above differential equations. The assumption of constant
is fulfilled when the sorption of the mixture is consistent with the Henry model, as well as with the multicomponent Langmuir model. It seems reasonable to equate the ratio
with the ratio of solubility coefficients for pure gases
, i.e.,
Identification (27) means that, within the framework of this work, the deviation of the membrane selectivity from ideal selectivity is due only to frictional interaction of the gas penetrants 1 and 2. Namely, the coupling effect is based on the diffusional coupling only. In practice, there may be noticeable deviations
from the ideal solubility selectivity (see, e.g., [
20,
26]). Given that mixed-gas solubility data are far from always available and their experimental measurement is a time-consuming procedure, the approximation (27) is reasonably adequate for design calculations.
Using Equation (27) and the relation
, Equations (25) and (26) can be rewritten as
where
is the quantity that includes the total molar flux
N of the gas mixture and 1–2 diffusivity
, which is a measure of coupling. In the membrane literature, a dimensionless quantity of this type is often referred to as the “membrane Peclét number”.
Integration of the differential equation system (28) and (29) leads to the result (see
Appendix A for some details):
where
Here r is the pressure ratio ( and denote the feed and permeate pressure, respectively). One can see that besides the partial pressure drop, the flux Equations (31) and (32) include an additional contribution (“coupling pressure”) due to diffusion coupling. Note that this additional contribution has different signs for components 1 and 2, i.e., the coupling results in lower flux (compared to pure gas flux) for one component and higher flux for the other component. This is the apparent difference between the developed model and Kedem’s approach, in which the additional “driven force” (complementary to ) has a positive sign for both components (it is easy to show from the flux equations (10)). In the limit (no coupling), Equations (31) and (32) are reduced in form to Equation (15). However, unlike Equation (15), these equations include permeability coefficients which are identified with corresponding quantities for individual gases.
It follows from Equations (31) and (32) that
Using the equality
, one gets an expression for the total flux
where
In the above equations is the ratio of permeability coefficients for pure gases (the ideal selectivity) and is the ideal diffusivity selectivity. (By the way, note that if the penetrants have the same mobility, i.e., , then, as seen from (35), the total flux coincides with the total flux of the gas mixture without coupling. This fact is a direct consequence of assumption (27)).
Any two of the three Equations (31), (32) and (35) can be used to obtain the equation for calculating the permeate composition. So, dividing Equation (31) by Equation (32) gives
where
. After solving this equation, the SF can be calculated.
4.2. Separation Factor
First, consider the trivial case of no coupling, i.e.,
. In this situation
and Equation (37) reduces to the quadratic equation with the familiar result for the permeate composition [
27]. Using the definition (19), the SF for a binary gas mixture with no coupling is given by
with
. It is evident that
(equal sign for
r = 0).
Next, consider the situation of coupling between component fluxes (
). If the downstream pressure is very small, then Equation (37) reduces formally to the quadratic equation with the following solution for the SF:
It should be noted that this equation is indeed quadratic if one considers the Peclét number
B as the
parameter that does not depend on the permeate composition. In general, this is not the case (the Peclét number is a function of permeate composition, see
Section 4.4 below). Nevertheless, Equation (39) is useful as an approximate starting point for further model development. In the case of nonzero downstream pressure (
), Equation (37) is reduced to a cubic equation for the SF, which is more convenient to solve numerically.
Equation (39) implies that: (1) the SF is finite over the entire concentration range of the feed mixture (in distinction to expression (20)), in the limit
one has
; (2)
if
, i.e., for (hypothetical) penetrants with the same diffusion mobility the SF is equal to the solubility selectivity; (3)
also if the Peclét number is much greater than one; (4) in the absence of coupling (
) the SF is equal to the ideal selectivity. The last two facts determine the range of SF values:
An important qualitative conclusion follows from (40): the coupling effect is the negative for the diffusivity selectivity membranes (), i.e., real selectivity (mixture selectivity) is less than the ideal one. On the contrary, the coupling effect is positive for the solubility selectivity membranes (), i.e., real selectivity is greater than the ideal one.
4.3. Component Permeabilities
The permeability coefficient of the mixture component
i and mixed-gas selectivity are defined as
From Equation (35) for the total flux, the component fluxes are
With the relations
and
, one gets
As pointed out above in Equation (40), the SF in the limit of large Peclét numbers is equal to the solubility selectivity. Using this result in Equation (43) and for simplicity, setting
r = 0, the
limiting permeability coefficients are
This suggests that
in the case of a strongly coupled system. It can be shown (see
Appendix B) that the expressions (44) are equivalent to the relations previously obtained by Krishna and van Baten [
28] in the model description of gas mixture permeation across micro- and mesoporous membranes.
The implications of the expressions (44) are obvious. If the preferentially permeating component (component 1) of the gas mixture is more mobile, i.e.,
, then its permeability
will be lower compared to the pure gas permeability
; the mixed-gas permeability of component 2 will, on the contrary, be higher than in pure gas conditions. If the preferentially permeating component of the gas mixture is less mobile, i.e.,
, then under conditions of a gas mixture it penetrates through the membrane faster than in a pure state; the second, more diffusion-mobile component, penetrates slower compared to corresponding pure gas. The situation described is reminiscent of Le Chatelier’s principle in chemical thermodynamics. To summarize, one can write
Note that conditions (45) are obtained for the situation of strong coupling (large Peclét numbers) and assuming downstream pressure is very small. Calculations show that inequalities (45) are also valid under less stringent constraints.
Examples of the SF and component permeability calculations are shown in
Figure 1 and
Figure 2, respectively. Consider first the case of a sorption-selective membrane, for which
and
. The SF increases with the degree of coupling (i.e., the Peclét number) (
Figure 1a) and tends to the value of the ideal solubility selectivity. If the pressure ratio
r = 0, then the SF exceeds the value of ideal selectivity
(=15 in this example). At non-zero permeate pressure, the SF also grows monotonically with increasing the Peclét number. In this case, two intervals can be distinguished: at small Peclét numbers (
B less than about 1)
, and at appreciable Peclét numbers (
B more than about 1) the SF even exceeds the corresponding parameter at zero permeate pressure. In the case of a diffusion-selective membrane (
and
), the SF is less than the ideal selectivity and decreases with an increase in the Peclét number (
Figure 1b). In addition, an increase in downstream pressure (from 0 to
) leads to an additional decrease in the SF.
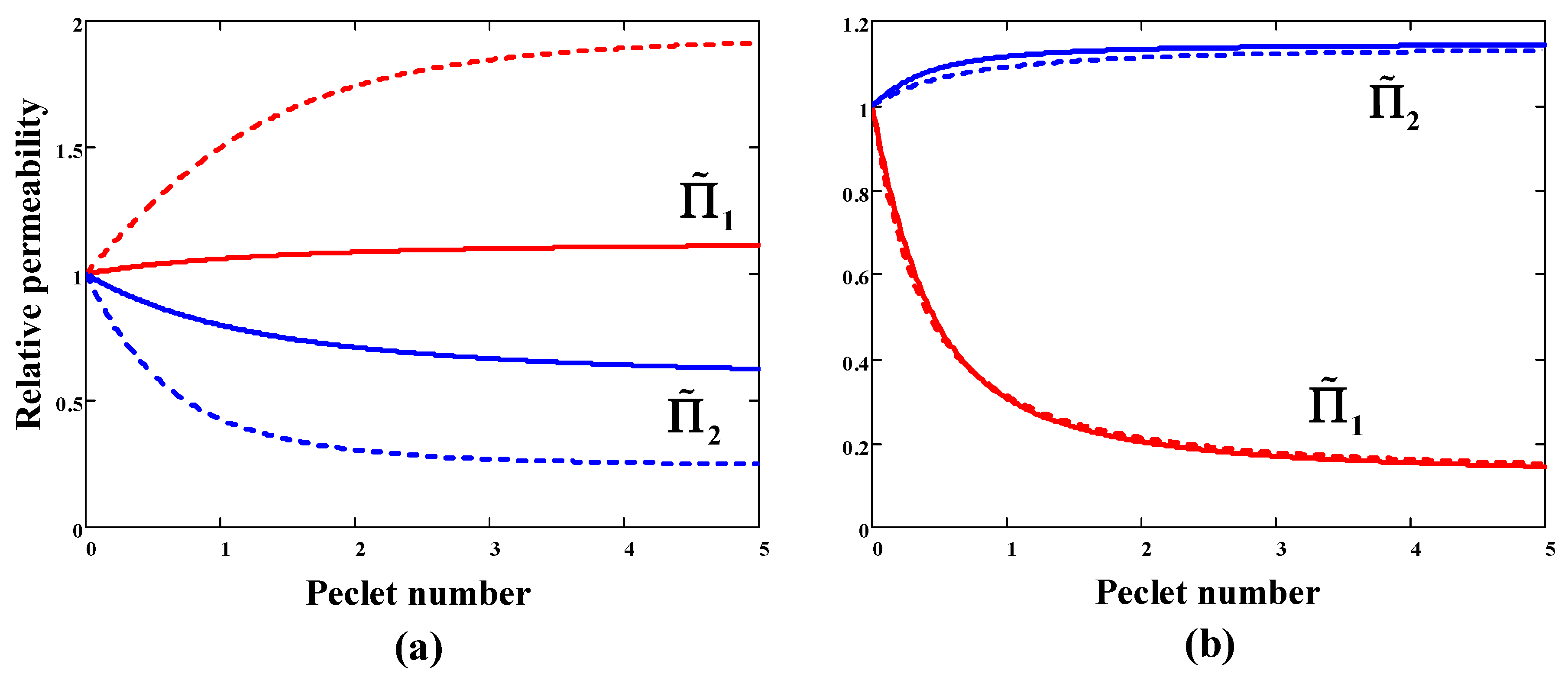
The behavior of the SF will become clearer when considering the dependence of relative permeability coefficients
on Peclét numbers (
Figure 2). For a sorption-selective membrane, component 1 is less mobile than component 2 (
). As evident from
Figure 2a, the mixed-gas permeability of component 1 exceeds pure gas permeability, while the mixed-gas permeability of component 2, on the contrary, is lower than its permeability in pure gas conditions. Namely, slow gas 1 moves faster in the mixture, and fast gas 2, on the contrary, reduces its diffusion mobility. Everything occurs according to the above inequality (45) at
. Calculations have shown (dashed lines in
Figure 2a) that an increase in downstream pressure leads to a larger deviation of the permeability coefficients from the ideal (pure gas) values. In the case of a diffusion-selective membrane, mixed-gas permeabilities behave in the opposite way to the case of a sorption-selective membrane: the permeability of fast component 1 decreases, and the permeability of slow component 2 increases with Peclét’s number (
Figure 2b). At the same time, an increase in downstream pressure has almost no effect on the mixed-gas permeabilities values.
4.4. Explicit Form of the Peclét Number
Above, the SF was estimated as a function of the Peclét number using the ideal selectivity and solubility selectivity as input parameters. The effect of coupling was treated formally, in the sense that the Peclét number was assumed to be a parameter independent of feed and permeate composition. A more rigorous approach, not limited to the description of experimental data, should take this dependence into account and provide an explicit expression for the Peclét number. In addition, it is important to evaluate the range of Peclét numbers characteristic of mixed-gas transport through sorption- or diffusion-selective membranes.
Let us now rewrite Equation (30) for the Peclét number as the following:
where
is the dimensionless total permeate flux. The explicit expression of
follows from Equation (35):
The expression for
B contains the unknown Maxwell–Stefan diffusivity
which is an inverse friction coefficient between penetrants 1 and 2. Given that Maxwell–Stefan diffusivities are not accessible from the experiment, various interpolation relations are used to predict those [
29]. In this paper the simple (geometric mean) approximation is used:
where
is the diffusion coefficient for the pure component
i. Since
then
Note that this expression for 1–2 diffusivity is similar (and even simpler) to the interpolation formula used by Krishna [
14] when modeling water/ethanol pervaporation across a polyimide membrane. Substituting of Equation (48) into Equation (46) gives
where
is the experimental solubility coefficient of component 2 in units cm
3(STP)/(cm
3·atm), the feed pressure expressed in atm, a correction factor
was introduced into the equation to compensate for the approximate nature of equality (48).
Let us estimate the value of
B depending on the composition of the mixture to be separated. For the sake of simplicity, let the pressure ratio
. It follows from Equation (47) that the total dimensionless flux varies from 0 to
when the mixture composition changes from
(the pure component 2) to
(the pure component 1). According to Equation (49), the value of
B increases with the upstream pressure and molar volumes of penetrants. At fixed upstream pressure Equation (49) becomes
For instance, if to take atm, cm3/mol, and , then . Since component 1 is the preferentially permeating component (), the coupling effect is maximum in the limit of infinite dilution of component 2 in the feed mixture. In addition, as can be seen from Equation (50), the value of B decreases with increasing diffusivity selectivity. Therefore, one would expect the coupling effect to be weaker for membranes with diffusion selectivity than for membranes with sorption selectivity.
Diffusivity selectivity glassy polymers (polyimides, microporous polymers), which are used to separate olefins and paraffins C
2+, have almost no sorption selectivity (
), and the ideal selectivity values are
[
8,
30]. If one accepts
and
cm
3(STP)/(cm
3·atm) (as the average solubility of propane in aromatic polyimides) for a rough estimate, then from Equation (50) one obtains that the Peclét numbers vary from 0.2 to 1.9. For solubility selectivity glassy polymers (polyacetylenes, polynorbornenes, PIM-1) used for C
2+/methane separations, the diffusion selectivity is less than 1, and the sorption selectivity is in a wide range from 5 to ~10
3 [
8,
31]. For typical values
,
and
cm
3(STP)/(cm
3·atm) (as the solubility of methane in PTMSP at 25 °C) one gets from Equation (50) the range of Peclét numbers from 0.4 to 3.6. Thus, it can be stated that in the hydrocarbon separation by glassy polymers, the Peclét number reaches values of the order of unity (notice that the above estimates were made at a total feed pressure of 10 atm).
Let us list the basic equations for the mixed-gas selectivity and permeability calculation within the framework of the developed model. Permeate composition is calculated using Equation (37) with the expression (49) for the Peclét number. The relationship of permeate composition with the SF is given by Equation (19). Penetrant fluxes are then calculated using Equations (31) and (32) or, equivalently, via the expressions (42). For negligible pressures of the permeate side, computations are simplified. In this case, the SF can be found by Formula (39). In doing so, the Peclét number can be considered in the first approximation as a model parameter, or it can be estimated using the explicit expression (49). Input parameters for the proposed model are the pure gas permeabilities and solubility coefficients.
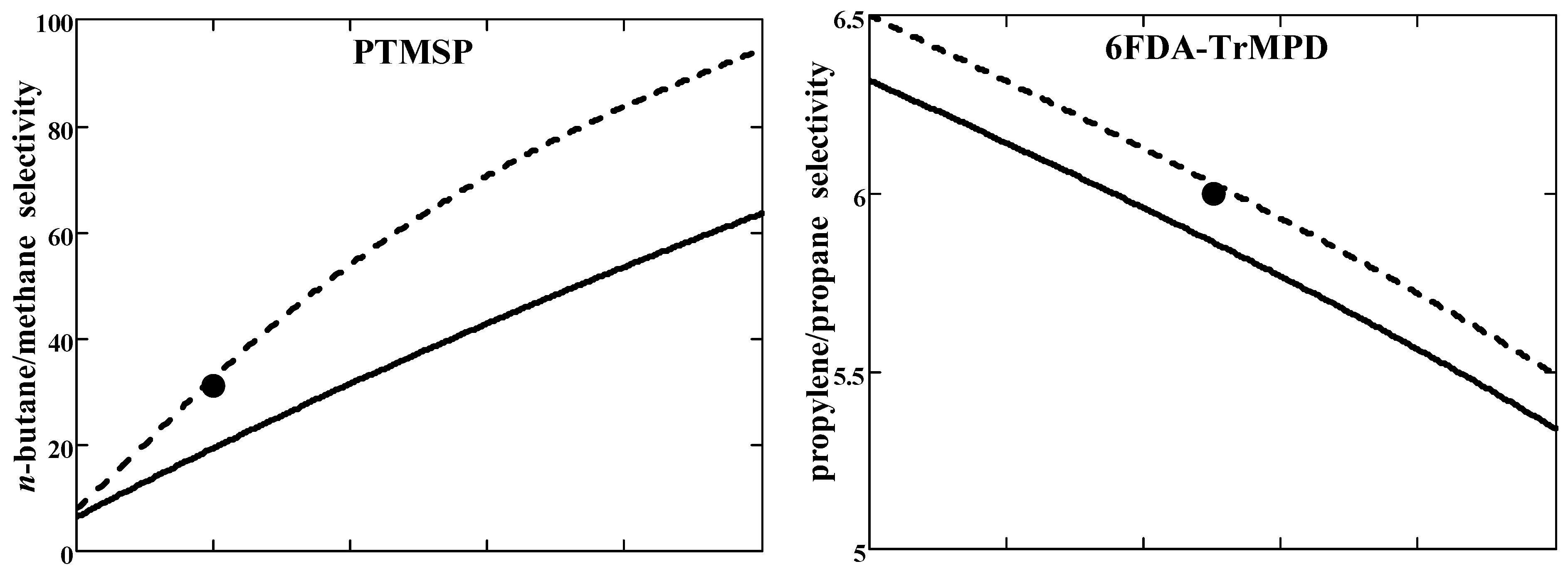
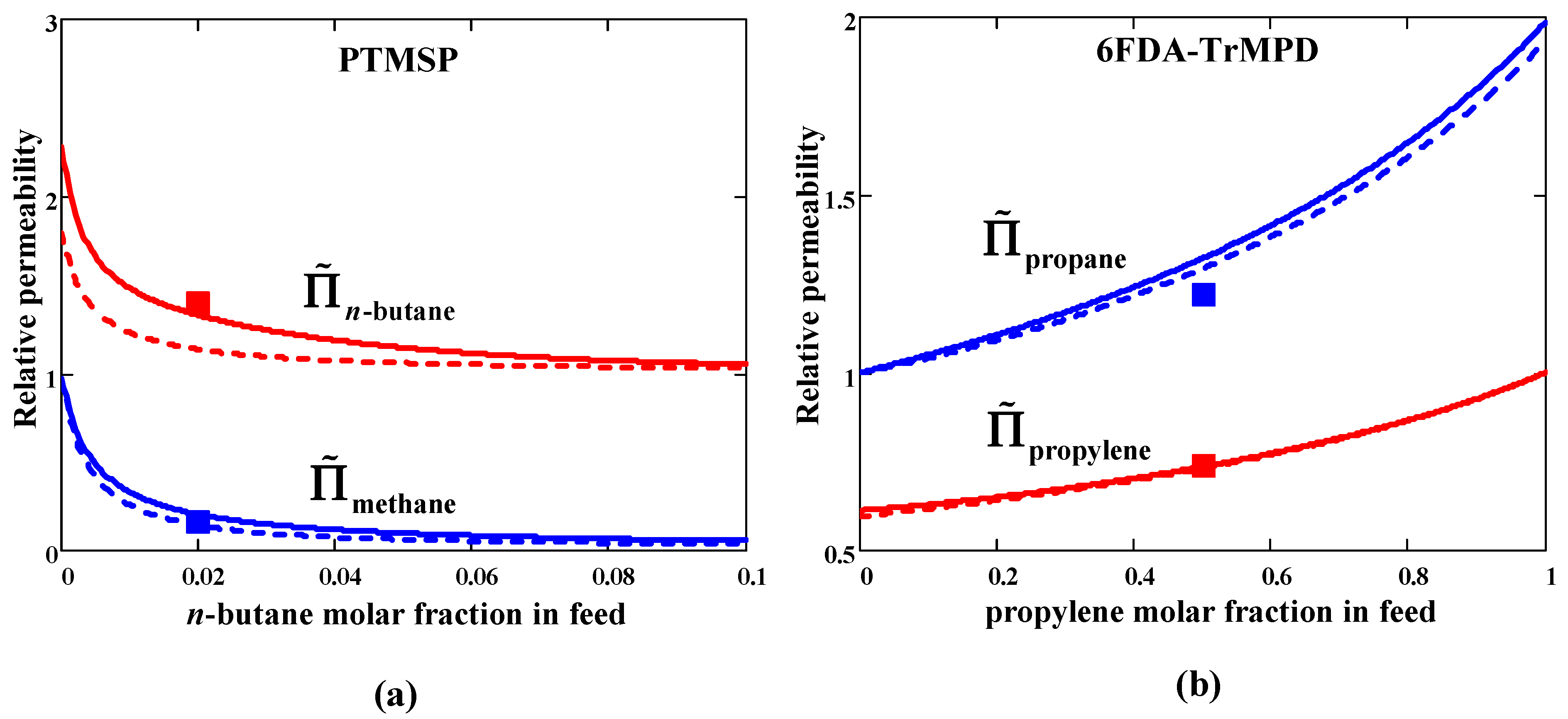
4.5. Comparison of the Model with Experimental Data
The results of the model calculations were validated against the experimental data for two examples of hydrocarbon separation by the glassy polymers. The first example is
n-butane/methane separation through high free volume disubstituted polyacetylene—poly(1-trimethylsilyl-1-propyne) (PTMSP). The second example is propylene/propane separation through polyimide 6FDA-TrMPD. These examples illustrate the transport properties of sorption- and diffusion-selective membranes, respectively. Pure gas values of selectivity and solubility coefficients were used as input parameters for calculations (
Table 1). Results of the model calculations and their comparison with mixed-gas experimental data are presented in
Figure 3.
In the case of PTMSP, the
n-C
4H
10/CH
4 selectivity is noticeably greater than the ideal value (=3.5) and grows with
n-butane concentration in feed (
Figure 3a (top)). The component permeabilities (in units of pure gas values), on the contrary, decrease with the increasing concentration of
n-butane (
Figure 3a (bottom)). Light gas CH
4 permeability in mixed-gas conditions decreases as compared to the pure gas value (
), whereas the situation is the opposite for heavier
n-C
4H
10 (
). This behavior is consistent with the second inequality (45).
In the case of polyimide 6FDA-TrMPD, the mixed-gas C
3H
6/C
3H
8 selectivity is almost half that of pure gas selectivity and decreases with increasing concentration of preferentially permeating component (propylene) in feed (
Figure 3b (top)). The permeability of both components increases with the propylene concentration in the feed (
Figure 3b (bottom)). Permeability behavior in mixed-gas conditions follows the first inequality (45): for more mobile gas C
3H
6 permeability decreases compared to pure gas one, while for less mobile gas C
3H
8 there is an increase in permeability compared to a pure gas one.
Generally, one can state a semi-quantitative agreement with the experiment for both penetrants/polymer systems. As can be seen in
Figure 3, the agreement between the calculated and experimental selectivities improves at non-zero permeate pressure. The calculation results depend on the Peclét number. It was evaluated using Equation (49) with fixed parameters
atm,
cm
3/mol, and
. Given the crude approximation (48) for 1–2 diffusivity, the developed model shows more than satisfactory performance.
In closing, it should be pointed out the following. It has been known since [
35] that the mixed-gas
n-C
4H
10/CH
4 selectivity of PTMSP membranes significantly exceeds the ideal selectivity, by a factor of 6–18 according to various experimental data [
31]. The increase in selectivity is associated with a marked (3–9 times) decrease in CH
4 permeability under mixed-gas conditions compared to pure gas conditions [
31]. The physical reason for depressing the CH
4 permeability is commonly attributed to a blocking mechanism: light gas CH
4 diffusion in free volume elements of ”microporous” PTMSP is hindered, blocked by the sorbed molecules of heavier, condensable
n-C
4H
10 [
11,
35]. Along with this basic reason, one should also take into account the effect of competitive sorption, which can lead to a change (increase) of solubility selectivity [
20,
33]. In terms of the developed model, the blocking of methane transport by
n-butane is a consequence of frictional interaction between penetrants, in other words, the blocking mechanism is a manifestation of coupling between methane and
n-butane fluxes.