Surface Design of Liquid Separation Membrane through Graft Polymerization: A State of the Art Review
Abstract
:1. Introduction
2. Brief Overview on Membrane Technology
3. Surface Chemical Grafting on Polymeric Substrates
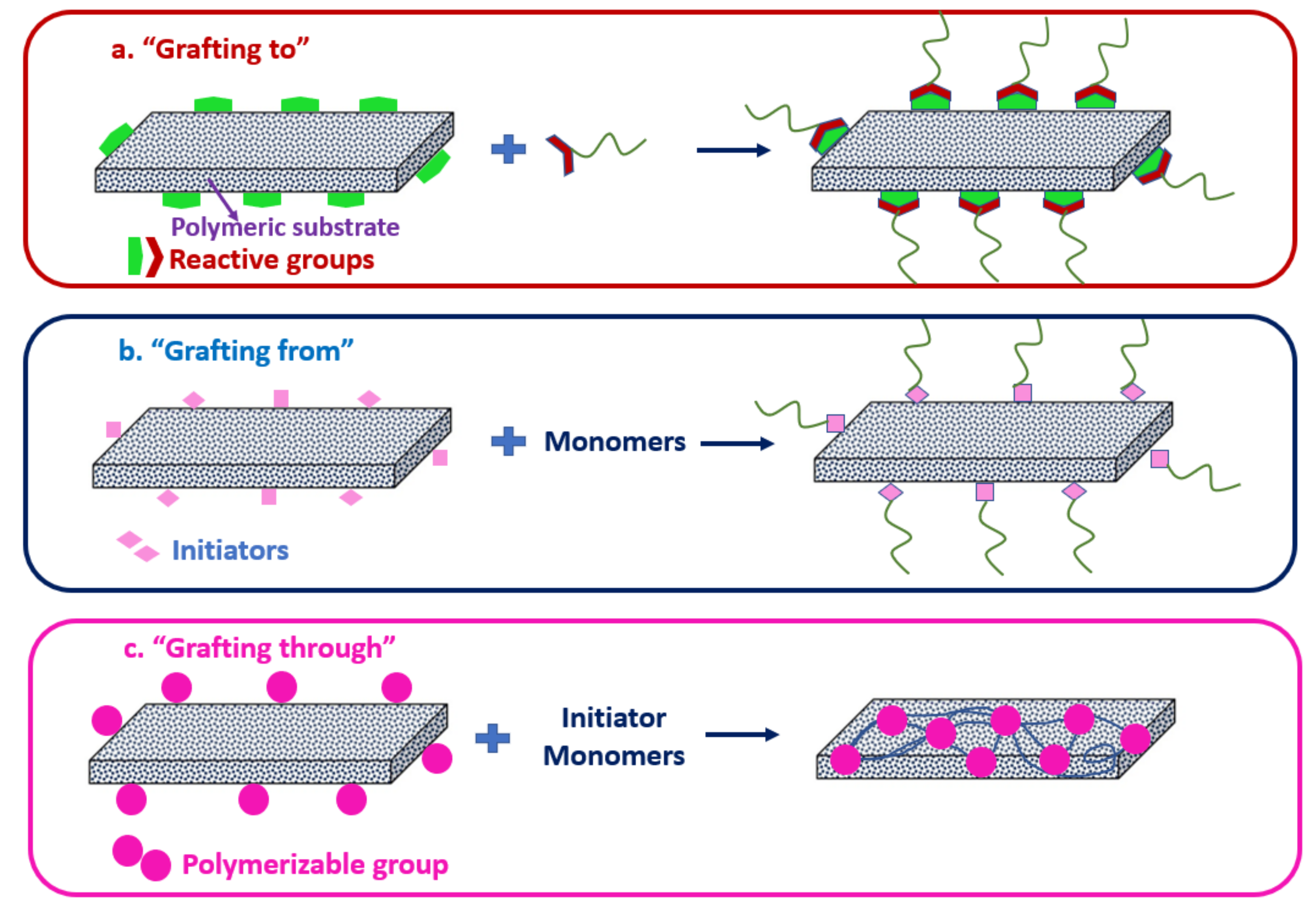
3.1. Classification of Chemical Grafting
3.2. Chemical-Induced Graft Polymerization
3.3. Plasma-Induced Graft Polymerization
3.4. Irradiation-Induced Graft Polymerization

4. Liquid Separation Membrane with Chemically Grafted Architecture: Performance Evaluation
4.1. Asymmetric Integrally Skin Membrane
4.2. Polyamide Thin Film Membrane
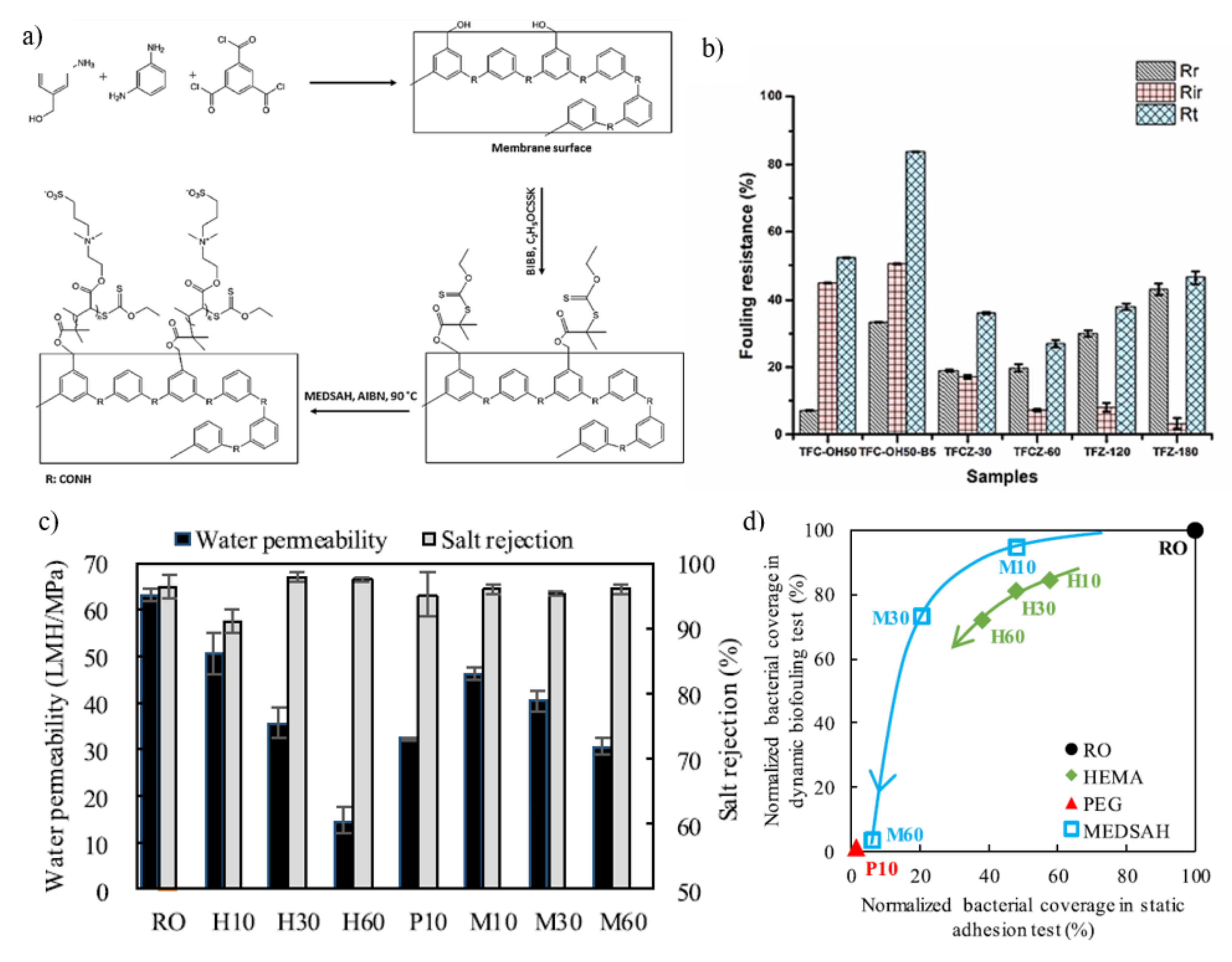
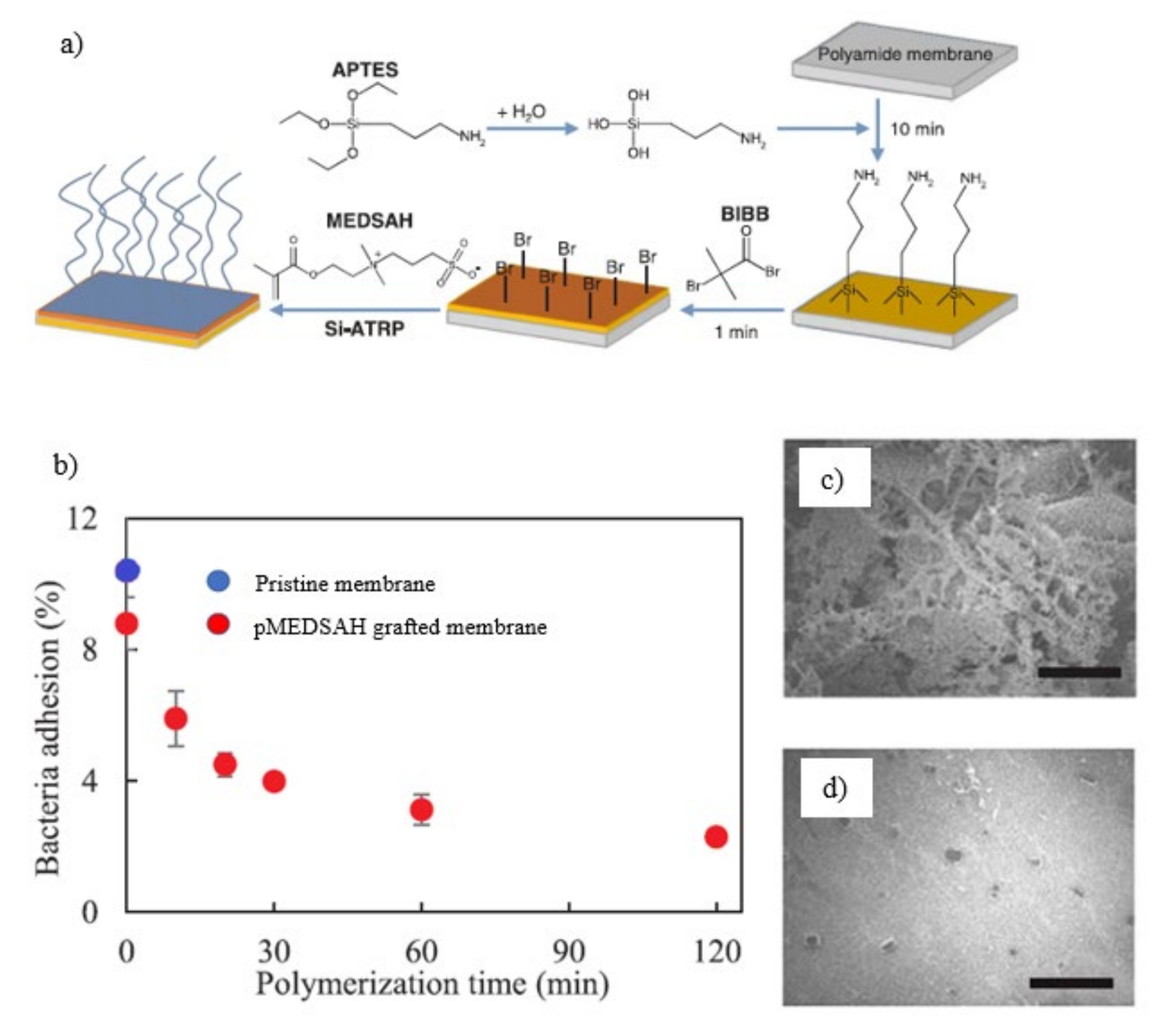
5. Challenges and Future Direction
6. Concluding Remark
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jawad, J.; Hawari, A.H.; Javaid Zaidi, S. Artificial neural network modeling of wastewater treatment and desalination using membrane processes: A review. Chem. Eng. J. 2021, 419, 129540. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef] [Green Version]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Darwish, N.N.; Mhiyo, S.; Darwish, N.A.; Hilal, N. The use of ultrasound to mitigate membrane fouling in desalination and water treatment. Desalination 2018, 443, 143–164. [Google Scholar] [CrossRef] [Green Version]
- Bokhary, A.; Tikka, A.; Leitch, M.; Liao, B. Membrane fouling prevention and control strategies in pulp and paper industry applications: A review. J. Membr. Sci. Res. 2018, 4, 181–197. [Google Scholar] [CrossRef]
- Heidari, A.; Abdollahi, E.; Mohammadi, T.; Asadi, A.A. Improving permeability, hydrophilicity and antifouling characteristic of PES hollow fiber UF membrane using carboxylic PES: A promising substrate to fabricate NF layer. Sep. Purif. Technol. 2021, 270, 118811. [Google Scholar] [CrossRef]
- Fadillah, G.; Saputra, O.A.; Saleh, T.A. Trends in polymers functionalized nanostructures for analysis of environmental pollutants. Trends Environ. Anal. Chem. 2020, 26, e00084. [Google Scholar] [CrossRef]
- Shao, F.; Su, X.; Shen, X.; Ren, S.; Wang, H.; Yi, Z.; Xu, C.; Yu, L.; Dong, L. Highly improved chlorine resistance of polyamide reverse membrane by grafting layers of graphene oxide. Sep. Purif. Technol. 2021, 254, 117586. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, H.; Xu, Z.; Li, F. Surface Modification of Polyacrylonitrile Membrane by Chemical Reaction and Physical Coating: Comparison between Static and Pore-Flowing Procedures. ACS Omega 2018, 3, 4231–4241. [Google Scholar] [CrossRef]
- Jiang, Z.; Chu, L.; Wu, X.; Wang, Z.; Jiang, X.; Ju, X.; Ruan, X.; He, G. Membrane-based separation technologies: From polymeric materials to novel process: An outlook from China. Rev. Chem. Eng. 2019, 36, 67–105. [Google Scholar] [CrossRef]
- Shin, H.; Park, C.; Lee, C.K.; Lee, Y.S.; Kim, J.O. Mitigating biofouling with a vanillin coating on thin film composite reverse osmosis membranes. Environ. Sci. Pollut. Res. 2020, 27, 1677–1685. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Wong, K.C.; Zulhairun, A.K.; Ismail, A.F. Enhancing desalination performance of thin film composite membrane through layer by layer assembly of oppositely charged titania nanosheet. Desalination 2020, 476, 114167. [Google Scholar] [CrossRef]
- Dhar, J.; Patil, S. Self-assembly and catalytic activity of metal nanoparticles immobilized in polymer membrane prepared via layer-by-layer approach. ACS Appl. Mater. Interfaces 2012, 4, 1803–1812. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coatings 2020, 142, 105557. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Gray, S.R.; Matsuura, T.; Jamshidi Gohari, R.; Subramanian, M.N.; Lai, S.O.; Ong, C.S.; Ismail, A.F.; Emazadah, D.; et al. A practical approach to synthesize polyamide thin film nanocomposite (TFN) membranes with improved separation properties for water/wastewater treatment. J. Mater. Chem. A 2016, 4, 4134–4144. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Chen, B.; Zhu, X. Membrane hydrophilicity switching via molecular design and re-construction of the functional additive for enhanced fouling resistance. J. Memb. Sci. 2019, 588, 117222. [Google Scholar] [CrossRef]
- Saqib, J.; Aljundi, I.H. Membrane fouling and modification using surface treatment and layer-by-layer assembly of polyelectrolytes: State-of-the-art review. J. Water Process Eng. 2016, 11, 68–87. [Google Scholar] [CrossRef]
- Kochameshki, M.G.; Marjani, A.; Mahmoudian, M.; Farhadi, K. Grafting of diallyldimethylammonium chloride on graphene oxide by RAFT polymerization for modification of nanocomposite polysulfone membranes using in water treatment. Chem. Eng. J. 2017, 309, 206–221. [Google Scholar] [CrossRef]
- Wang, X.H.; Song, R.H.; Yang, H.C.; Shi, Y.J.; Dang, G.B.; Yang, S.; Zhao, Y.; Sun, X.F.; Wang, S.G. Fluoride adsorption on carboxylated aerobic granules containing Ce(III). Bioresour. Technol. 2013, 127, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Galina, H.; Lechowicz, J.B. Grafting/Characterization Techniques/Kinetic Modeling; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 3540696857. [Google Scholar]
- Li, Q.; Imbrogno, J.; Belfort, G.; Wang, X.L. Making polymeric membranes antifouling via “grafting from” polymerization of zwitterions. J. Appl. Polym. Sci. 2015, 132, 1–12. [Google Scholar] [CrossRef]
- Lee, X.J.; Show, P.L.; Katsuda, T.; Chen, W.H.; Chang, J.S. Surface grafting techniques on the improvement of membrane bioreactor: State-of-the-art advances. Bioresour. Technol. 2018, 269, 489–502. [Google Scholar] [CrossRef]
- Pinem, J.A.; Wardani, A.K.; Aryanti, P.T.P.; Khoiruddin, K.; Wenten, I.G. Hydrophilic Modification of Polymeric Membrane using Graft Polymerization Method: A Mini Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012054. [Google Scholar] [CrossRef]
- Nazari, N.; Torki, M. Evaluation of Biological Fouling of RO/MF Membrane and Methods to prevent it. Eur. J. Adv. Eng. Technol. 2017, 4, 707–711. [Google Scholar]
- Krüger, R.; Vial, D.; Arifin, D.; Weber, M.; Heijnen, M. Novel ultrafiltration membranes from low-fouling copolymers for RO pretreatment applications. Desalin. Water Treat. 2016, 57, 23185–23195. [Google Scholar] [CrossRef]
- Koelmel, J.; Prasad, M.N.V.; Velvizhi, G.; Butti, S.K.; Mohan, S.V. Metalliferous Waste in India and Knowledge Explosion in Metal Recovery Techniques and Processes for the Prevention of Pollution; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128039069. [Google Scholar]
- Ahmad, A.L.; Abdulkarim, A.A.; Ooi, B.S.; Ismail, S. Recent development in additives modifications of polyethersulfone membrane for flux enhancement. Chem. Eng. J. 2013, 223, 246–267. [Google Scholar] [CrossRef]
- Liu, C.; Lee, J.; Ma, J.; Elimelech, M. Antifouling Thin-Film Composite Membranes by Controlled Architecture of Zwitterionic Polymer Brush Layer. Environ. Sci. Technol. 2017, 51, 2161–2169. [Google Scholar] [CrossRef]
- Ong, C.S.; Al-Anzi, B.; Lau, W.J.; Goh, P.S.; Lai, G.S.; Ismail, A.F.; Ong, Y.S. Anti-Fouling Double-Skinned Forward Osmosis Membrane with Zwitterionic Brush for Oily Wastewater Treatment. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Suwaileh, W.A.; Johnson, D.J.; Sarp, S.; Hilal, N. Advances in forward osmosis membranes: Altering the sub-layer structure via recent fabrication and chemical modification approaches. Desalination 2018, 436, 176–201. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Chung, T.S.; Toriida, M.; Tamai, S. Thin-film composite forward osmosis membranes with novel hydrophilic supports for desalination. J. Memb. Sci. 2012, 423–424, 543–555. [Google Scholar] [CrossRef]
- Yasukawa, M.; Mishima, S.; Shibuya, M.; Saeki, D.; Takahashi, T.; Miyoshi, T.; Matsuyama, H. Preparation of a forward osmosis membrane using a highly porous polyketone microfiltration membrane as a novel support. J. Memb. Sci. 2015, 487, 51–59. [Google Scholar] [CrossRef]
- Reza Shirzad Kebria, M.; Rahimpour, A. Membrane Distillation: Basics, Advances, and Applications. Adv. Membr. Technol. 2020, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Attia, H.; Alexander, S.; Wright, C.J.; Hilal, N. Superhydrophobic electrospun membrane for heavy metals removal by air gap membrane distillation (AGMD). Desalination 2017, 420, 318–329. [Google Scholar] [CrossRef] [Green Version]
- Al-Obaidani, S.; Curcio, E.; Macedonio, F.; Di Profio, G.; Al-Hinai, H.; Drioli, E. Potential of membrane distillation in seawater desalination: Thermal efficiency, sensitivity study and cost estimation. J. Memb. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Memb. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Li, C.; Goswami, Y.; Stefanakos, E. Solar assisted sea water desalination: A review. Renew. Sustain. Energy Rev. 2013, 19, 136–163. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Fouling and its control in membrane distillation-A review. J. Memb. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Memb. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Li, J.; Li, L.; Hu, W. Surface-grafting polymers: From chemistry to organic electronics. Mater. Chem. Front. 2020, 4, 692–714. [Google Scholar] [CrossRef]
- Liu, T.; Chen, D.; Yang, F.; Chen, J.; Cao, Y.; Xiang, M.; Kang, J.; Xu, R. Enhancing the permeability and anti-fouling properties of a polyamide thin-film composite reverse osmosis membrane: Via surface grafting of l-lysine. RSC Adv. 2019, 9, 20044–20052. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Shao, F.; Yu, L.; Song, N.; Dong, H.; Pang, B.; Yu, J.; Feng, J.; Dong, L. Chemical grafting N-GOQD of polyamide reverse osmosis membrane with improved chlorine resistance, water flux and NaCl rejection. Desalination 2020, 479, 114341. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Low, S.C.; Ng, Q.H. Progress of Stimuli Responsive Membranes in Water Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128145043. [Google Scholar]
- Liu, Z.M.; Xu, Z.K.; Wan, L.S.; Wu, J.; Ulbricht, M. Surface modification of polypropylene microfiltration membranes by the immobilization of poly(N-vinyl-2-pyrrolidone): A facile plasma approach. J. Memb. Sci. 2005, 249, 21–31. [Google Scholar] [CrossRef]
- Fan, X.; Su, Y.; Zhao, X.; Li, Y.; Zhang, R.; Zhao, J.; Jiang, Z.; Zhu, J.; Ma, Y.; Liu, Y. Fabrication of polyvinyl chloride ultrafiltration membranes with stable antifouling property by exploring the pore formation and surface modification capabilities of polyvinyl formal. J. Memb. Sci. 2014, 464, 100–109. [Google Scholar] [CrossRef]
- Datta, P.; Genzer, J. “Grafting Through” polymerization involving surface-bound monomers. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 263–274. [Google Scholar] [CrossRef]
- Ito, K.; Tsuchida, H.; Hayashi, A.; Yamada, E.; Matsumoto, T. Reactivity of poly(ethylene oxide) macromonomers in radical copolymerization. Polym. J. 1985, 17, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Merlet, R.B.; Pizzoccaro-Zilamy, M.A.; Nijmeijer, A.; Winnubst, L. Hybrid ceramic membranes for organic solvent nanofiltration: State-of-the-art and challenges. J. Memb. Sci. 2020, 599, 117839. [Google Scholar] [CrossRef]
- Deng, H.T.; Xu, Z.K.; Wu, J.; Ye, P.; Liu, Z.M.; Seta, P. A comparative study on lipase immobilized polypropylene microfiltration membranes modified by sugar-containing polymer and polypeptide. J. Mol. Catal. B Enzym. 2004, 28, 95–100. [Google Scholar] [CrossRef]
- Rodrigues, P.R.; Vieira, R.P. Advances in atom-transfer radical polymerization for drug delivery applications. Eur. Polym. J. 2019, 115, 45–58. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Ginic-Markovic, M.; Barclay, T.; Constantopoulos, K.T.; Al-Ghamdi, T.; Blok, A.; Markovic, E.; Ellis, A.V. A versatile approach to grafting biofouling resistant coatings from polymeric membrane surfaces using an adhesive macroinitiator. RSC Adv. 2015, 5, 63017–63024. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Yang, P.; Li, J.; Li, S.; Li, P.; Zhao, Y.; Huang, N. Immobilization of poly(MPC) brushes onto titanium surface by combining dopamine self-polymerization and ATRP: Preparation, characterization and evaluation of hemocompatibility in vitro. Appl. Surf. Sci. 2015, 349, 445–451. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Inner Sphere and Outer Sphere Electron Transfer Reactions in Atom Transfer Radical Polymerization. Macromol. Symp. 1998, 134, 105–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Lin, W.; Sun, H.; Wu, L.; Chen, S. A facile method for polyamide membrane modification by poly(sulfobetaine methacrylate) to improve fouling resistance. J. Memb. Sci. 2013, 446, 164–170. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Wang, X.; Kong, M.; Zhang, Q.; Li, G. Synthesis of PVDF-g-PSSA proton exchange membrane by ozone-induced graft copolymerization and its application in microbial fuel cells. J. Memb. Sci. 2017, 527, 35–42. [Google Scholar] [CrossRef]
- Schröder, K.; Konkolewicz, D.; Poli, R.; Matyjaszewski, K. Formation and possible reactions of organometallic intermediates with active copper(I) catalysts in ATRP. Organometallics 2012, 31, 7994–7999. [Google Scholar] [CrossRef]
- Dong, H.; Tang, W.; Matyjaszewski, K. Well-defined high-molecular-weight polyacrylonitrile via activators regenerated by electron transfer ATRP. Macromolecules 2007, 40, 2974–2977. [Google Scholar] [CrossRef]
- Mueller, L.; Jakubowski, W.; Tang, W.; Matyjaszewski, K. Successful chain extension of polyacrylate and polystyrene macroinitiators with methacrylates in an ARGET and ICAR ATRP. Macromolecules 2007, 40, 6464–6472. [Google Scholar] [CrossRef]
- Anderson, D.L. References and notes. New Theory Earth 2012, 332, 356–374. [Google Scholar] [CrossRef]
- Krys, P.; Matyjaszewski, K. Kinetics of Atom Transfer Radical Polymerization. Eur. Polym. J. 2017, 89, 482–523. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Song, H.; Li, D.; Zhao, W.; Zhang, W.; Kang, L.; Ran, F.; Zhao, C. A effective approach for surface modification of polymer membrane via SI-eATRP in an electrochemical cell with a three electrode system. Surf. Interfaces 2017, 8, 119–126. [Google Scholar] [CrossRef]
- Song, C.; Wang, M.; Liu, X.; Wang, H.; Chen, X.; Dai, L. Fabrication of high-capacity polyelectrolyte brush-grafted porous AAO-silica composite membrane via RAFT polymerization. Mater. Sci. Eng. C 2017, 78, 748–755. [Google Scholar] [CrossRef]
- Cadena, P.G.; Jeronimo, R.A.S.; Melo, J.M.; Silva, R.A.; Filho, J.L.L.; Pimentel, M.C.B. Covalent immobilization of invertase on polyurethane, plast-film and ferromagnetic Dacron. Bioresour. Technol. 2010, 101, 1595–1602. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, J.H.; Choo, K.H.; Lee, Y.S.; Lee, C.H. Hydrophilic modification of polypropylene microfiltration membranes by ozone-induced graft polymerization. J. Memb. Sci. 2000, 169, 269–276. [Google Scholar] [CrossRef]
- Sainbayar, A.; Kim, J.S.; Jung, W.J.; Lee, Y.S.; Lee, C.H. Application of surface modified polypropylene membranes to an anaerobic membrane bioreactor. Environ. Technol. 2001, 22, 1035–1042. [Google Scholar] [CrossRef]
- Dai, N.T.; Williamson, M.R.; Khammo, N.; Adams, E.F.; Coombes, A.G.A. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials 2004, 25, 4263–4271. [Google Scholar] [CrossRef]
- Duann, Y.F.; Chen, Y.C.; Shen, J.T.; Lin, Y.H. Thermal induced graft polymerization using peroxide onto polypropylene fiber. Polymer 2004, 45, 6839–6843. [Google Scholar] [CrossRef]
- Mohd Hidzir, N.; Hill, D.J.T.; Taran, E.; Martin, D.; Grøndahl, L. Argon plasma treatment-induced grafting of acrylic acid onto expanded poly(tetrafluoroethylene) membranes. Polymer 2013, 54, 6536–6546. [Google Scholar] [CrossRef]
- Kato, K.; Uchida, E.; Kang, E.T.; Uyama, Y.; Ikada, Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Modification of porous poly(ether sulfone) membranes by low-temperature CO2-plasma treatment. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2473–2488. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Plasma-assisted surface modification of organic biopolymers to prevent bacterial attachment. Acta Biomater. 2011, 7, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Pandiyaraj, K.N.; Selvarajan, V.; Deshmukh, R.R.; Gao, C. Modification of surface properties of polypropylene (PP) film using DC glow discharge air plasma. Appl. Surf. Sci. 2009, 255, 3965–3971. [Google Scholar] [CrossRef]
- Hu, J.; Shao, D.; Chen, C.; Sheng, G.; Li, J.; Wang, X.; Nagatsu, M. Plasma-induced grafting of cyclodextrin onto multiwall carbon nanotube/iron oxides for adsorbent application. J. Phys. Chem. B 2010, 114, 6779–6785. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Grant, D.; Kumar, A.; Bazaka, K.; Jacob, M.V. Plasma Treatment of Polymeric Membranes; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128131527. [Google Scholar]
- Yuenyao, C.; Tirawanichakul, Y.; Chittrakarn, T. Asymmetric polysulfone gas separation membranes treated by low pressure DC glow discharge plasmas. J. Appl. Polym. Sci. 2015, 132, 42116. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Shen, L.; Men, D.; Xu, Y. Microporous polypropylene hollow fiber membrane. Part I. Surface modification by the graft polymerization of acrylic acid. J. Memb. Sci. 2002, 196, 221–229. [Google Scholar] [CrossRef]
- Kochkodan, V.M.; Sharma, V.K. Graft polymerization and plasma treatment of polymer membranes for fouling reduction: A review. J. Environ. Sci. Health Part A 2012, 47, 1713–1727. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Li, M.S.; Li, N.; Wang, M.X.; Zhang, Y. Controllable modification of polymer membranes by long-distance and dynamic low-temperature plasma flow: AA grafting penetrated through electrospun PP fibrous membranes. J. Memb. Sci. 2013, 440, 9–19. [Google Scholar] [CrossRef]
- Kim, S.; Rahardianto, A.; Walker, J.S.; Wolfe, T.; Coleman, K.; Cohen, Y. Upgrading polyamide TFC BWRO and SWRO membranes to higher SWRO membrane performance via surface nano-structuring with tethered poly(acrylic acid). J. Memb. Sci. 2020, 597, 117736. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Földváry, C.M.; Takács, E. Radiation-induced grafting of cellulose for adsorption of hazardous water pollutants: A review. Radiat. Phys. Chem. 2010, 79, 848–862. [Google Scholar] [CrossRef]
- Nasef, M.M.; Hegazy, E.S.A. Preparation and applications of ion exchange membranes by radiation-induced graft copolymerization of polar monomers onto non-polar films. Prog. Polym. Sci. 2004, 29, 499–561. [Google Scholar] [CrossRef]
- Irwan, G.S.; Aoyama, Y.; Kuroda, S.I.; Kubota, H.; Kondo, T. Photografting of N-isopropylacrylamide and glycidyl methacrylate binary monomers on polyethylene film: Effect of mixed solvent consisting of water and organic solvent. J. Appl. Polym. Sci. 2005, 97, 2469–2475. [Google Scholar] [CrossRef]
- Liu, Z.M.; Xu, Z.K.; Wang, J.Q.; Wu, J.; Fu, J.J. Surface modification of polypropylene microfiltration membranes by graft polymerization of N-vinyl-2-pyrrolidone. Eur. Polym. J. 2004, 40, 2077–2087. [Google Scholar] [CrossRef]
- Carroll, T.; Booker, N.A.; Meier-Haack, J. Polyelectrolyte-grafted microfiltration membranes to control fouling by natural organic matter in drinking water. J. Memb. Sci. 2002, 203, 3–13. [Google Scholar] [CrossRef]
- Abuhabib, A.A.; Mohammad, A.W.; Hilal, N.; Rahman, R.A.; Shafie, A.H. Nanofiltration membrane modification by UV grafting for salt rejection and fouling resistance improvement for brackish water desalination. Desalination 2012, 295, 16–25. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Akbari, A.; Mehrnia, M.R. Preparation of polysulfone nanofiltration membranes by UV-assisted grafting polymerization for water softening. Desalination 2010, 263, 217–225. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Huang, S.H.; Chang, M.W.; Lai, C.L.; Tsai, H.A.; Hung, W.S.; Hu, C.C.; Lee, K.R. Ultraviolet-initiated graft polymerization of acrylic acid onto thin-film polyamide surface for improved ethanol dehydration performance of pervaporation membranes. Sep. Purif. Technol. 2020, 235, 116155. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Wang, L.; Yu, S.; Gao, C. Improving fouling resistance and chlorine stability of aromatic polyamide thin-film composite RO membrane by surface grafting of polyvinyl alcohol (PVA). Desalination 2015, 367, 11–20. [Google Scholar] [CrossRef]
- Yu, H.; Cao, Y.; Kang, G.; Liu, J.; Li, M.; Yuan, Q. Enhancing antifouling property of polysulfone ultrafiltration membrane by grafting zwitterionic copolymer via UV-initiated polymerization. J. Memb. Sci. 2009, 342, 6–13. [Google Scholar] [CrossRef]
- Cheng, Q.; Zheng, Y.; Yu, S.; Zhu, H.; Peng, X.; Liu, J.; Liu, J.; Liu, M.; Gao, C. Surface modification of a commercial thin-film composite polyamide reverse osmosis membrane through graft polymerization of N-isopropylacrylamide followed by acrylic acid. J. Memb. Sci. 2013, 447, 236–245. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Mousavi, S.A.; Heydari, H.; Mousavi, D.V. Improvement of performance and fouling resistance of polyamide reverse osmosis membranes using acrylamide and TiO2 nanoparticles under UV irradiation for water desalination. J. Appl. Polym. Sci. 2020, 137, 1–16. [Google Scholar] [CrossRef]
- Ariono, D.; Wardani, A.K. Modification and Applications of Hydrophilic Polypropylene Membrane. IOP Conf. Ser. Mater. Sci. Eng. 2017, 214, 012014. [Google Scholar] [CrossRef] [Green Version]
- Dessouki, A.M.; El-Tahawy, M.; El-Boohy, H.; El-Mongy, S.A.; Badawy, S.M. Chemical reactive filter paper prepared by radiation-induced graft polymerization—I. Radiat. Phys. Chem. 1999, 54, 627–635. [Google Scholar] [CrossRef]
- Shim, J.K.; Na, H.S.; Lee, Y.M.; Huh, H.; Nho, Y.C. Surface modification of polypropylene membranes by γ-ray induced graft copolymerization and their solute permeation characteristics. J. Memb. Sci. 2001, 190, 215–226. [Google Scholar] [CrossRef]
- Takács, E.; Mirzadeh, H.; Wojnárovits, L.; Borsa, J.; Mirzataheri, M.; Benke, N. Comparison of simultaneous and pre-irradiation grafting of N-vinylpyrrolidone to cotton-cellulose. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 265, 217–220. [Google Scholar] [CrossRef]
- Schulze, A.; Marquardt, B.; Kaczmarek, S.; Schubert, R.; Prager, A.; Buchmeiser, M.R. Electron beam-based functionalization of poly(ethersulfone) membranes. Macromol. Rapid Commun. 2010, 31, 467–472. [Google Scholar] [CrossRef]
- Kimura, Y.; Chen, J.; Asano, M.; Maekawa, Y.; Katakai, R.; Yoshida, M. Anisotropic proton-conducting membranes prepared from swift heavy ion-beam irradiated ETFE films. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 263, 463–467. [Google Scholar] [CrossRef]
- Liu, S.X.; Kim, J.T.; Kim, S. Effect of polymer surface modification on polymer-protein interaction via hydrophilic polymer grafting. J. Food Sci. 2008, 73, 143–150. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Memb. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, J.; Dai, J.; Yang, Y.; Chen, X.; Wang, Y. Hydrophilization of porous polypropylene membranes by atomic layer deposition of TiO2 for simultaneously improved permeability and selectivity. J. Memb. Sci. 2013, 448, 215–222. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Hydrophilic modification of polyethersulfone membranes by low temperature plasma-induced graft polymerization. J. Memb. Sci. 2002, 209, 255–269. [Google Scholar] [CrossRef]
- Meng, J.; Li, J.; Zhang, Y.; Ma, S. A novel controlled grafting chemistry fully regulated by light for membrane surface hydrophilization and functionalization. J. Memb. Sci. 2014, 455, 405–414. [Google Scholar] [CrossRef]
- Rahimpour, A. UV photo-grafting of hydrophilic monomers onto the surface of nano-porous PES membranes for improving surface properties. Desalination 2011, 265, 93–101. [Google Scholar] [CrossRef]
- Yamagishi, H.; Crivello, J.V.; Belfort, G. Development of a novel photochemical technique for modifying poly (arylsulfone) ultrafiltration membranes. J. Memb. Sci. 1995, 105, 237–247. [Google Scholar] [CrossRef]
- Zhang, M.; Nguyen, Q.T.; Ping, Z. Hydrophilic modification of poly (vinylidene fluoride) microporous membrane. J. Memb. Sci. 2009, 327, 78–86. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, C.; Jiang, W.; Luan, S.; Yang, H.; Yin, J.; Stagnaro, P. Melting grafting polypropylene with hydrophilic monomers for improving hemocompatibility. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 407, 141–149. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, J.; Oguzlu, H.; Zheng, Y.; Jiang, F. Antifouling, antibacterial and non-cytotoxic transparent cellulose membrane with grafted zwitterion and quaternary ammonium copolymers. Carbohydr. Polym. 2020, 250, 116960. [Google Scholar] [CrossRef]
- Pourziad, S.; Omidkhah, M.R.; Abdollahi, M. Improved antifouling and self-cleaning ability of PVDF ultrafiltration membrane grafted with polymer brushes for oily water treatment. J. Ind. Eng. Chem. 2020, 83, 401–408. [Google Scholar] [CrossRef]
- Seah, M.Q.; Lau, W.J.; Goh, P.S.; Tseng, H.H.; Wahab, R.A.; Ismail, A.F. Progress of interfacial polymerization techniques for polyamide thin film (Nano)composite membrane fabrication: A comprehensive review. Polymers 2020, 12, 2817. [Google Scholar] [CrossRef] [PubMed]
- Bhalani, D.V.; Trivedi, J.S.; Jewrajka, S.K. Selective grafting of morphologically modified poly(vinylidene fluoride) ultrafiltration membrane by poly(acrylic acid) for inducing antifouling property. Appl. Surf. Sci. 2021, 544, 148905. [Google Scholar] [CrossRef]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Modification and characterization of ultrafiltration membranes for treatment of produced water. J. Memb. Sci. 2011, 373, 178–188. [Google Scholar] [CrossRef]
- Porter, C.J.; Werber, J.R.; Ritt, C.L.; Guan, Y.F.; Zhong, M.; Elimelech, M. Controlled grafting of polymer brush layers from porous cellulosic membranes. J. Memb. Sci. 2020, 596, 117719. [Google Scholar] [CrossRef]
- Ishihara, K.; Nomura, H.; Mihara, T.; Kurita, K.; Iwasaki, Y.; Nakabayashi, N. Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res. 1998, 39, 323–330. [Google Scholar] [CrossRef]
- Xie, W.; Tiraferri, A.; Ji, X.; Chen, C.; Bai, Y.; Crittenden, J.C.; Liu, B. Green and sustainable method of manufacturing anti-fouling zwitterionic polymers-modified poly(vinyl chloride) ultrafiltration membranes. J. Colloid Interface Sci. 2021, 591, 343–351. [Google Scholar] [CrossRef]
- Salimi, P.; Aroujalian, A.; Iranshahi, D. Graft copolymerization of zwitterionic monomer on the polyethersulfone membrane surface by corona air plasma for separation of oily wastewater. Sep. Purif. Technol. 2021, 258, 117939. [Google Scholar] [CrossRef]
- Khongnakorn, W.; Bootluck, W.; Jutaporn, P. Surface modification of FO membrane by plasma-grafting polymerization to minimize protein fouling. J. Water Process Eng. 2020, 38, 101633. [Google Scholar] [CrossRef]
- Wang, C.X.; Du, M.; Lv, J.C.; Zhou, Q.Q.; Ren, Y.; Liu, G.L.; Gao, D.W.; Jin, L.M. Surface modification of aramid fiber by plasma induced vapor phase graft polymerization of acrylic acid. I. Influence of plasma conditions. Appl. Surf. Sci. 2015, 349, 333–342. [Google Scholar] [CrossRef]
- Pejman, M.; Dadashi Firouzjaei, M.; Aghapour Aktij, S.; Zolghadr, E.; Das, P.; Elliott, M.; Sadrzadeh, M.; Sangermano, M.; Rahimpour, A.; Tiraferri, A. Effective strategy for UV-mediated grafting of biocidal Ag-MOFs on polymeric membranes aimed at enhanced water ultrafiltration. Chem. Eng. J. 2021, 426, 130704. [Google Scholar] [CrossRef]
- Carter, B.M.; Sengupta, A.; Qian, X.; Ulbricht, M.; Wickramasinghe, S.R. Controlling external versus internal pore modification of ultrafiltration membranes using surface-initiated AGET-ATRP. J. Memb. Sci. 2018, 554, 109–116. [Google Scholar] [CrossRef]
- Kassa, S.T.; Hu, C.C.; Keshebo, D.L.; Belle Marie Ang, M.; Lai, J.Y.; Chu, J.P. Surface modification of high-rejection ultrafiltration membrane with antifouling capability using activated oxygen treatment and metallic glass deposition. Appl. Surf. Sci. 2020, 529, 147131. [Google Scholar] [CrossRef]
- Akamatsu, K.; Noto, W.; Fukuzawa, H.; Hara, A.; Nakao, S. ichi Grafting of carboxybetaine polymers to polyethylene membranes via plasma graft polymerization to improve low-fouling properties and to tune the molecular weight cut-off. Sep. Purif. Technol. 2018, 204, 298–303. [Google Scholar] [CrossRef]
- Bernstein, R.; Singer, C.E.; Singh, S.P.; Mao, C.; Arnusch, C.J. UV initiated surface grafting on polyethersulfone ultrafiltration membranes via ink-jet printing-assisted modification. J. Memb. Sci. 2018, 548, 73–80. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Misdan, N.; Kassim, M.A. A recent progress in thin film composite membrane: A review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Paul Chen, J.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef]
- Vatanpour, V.; Zoqi, N. Surface modification of commercial seawater reverse osmosis membranes by grafting of hydrophilic monomer blended with carboxylated multiwalled carbon nanotubes. Appl. Surf. Sci. 2017, 396, 1478–1489. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.L. In situ concentration-polarization-enhanced radical graft polymerization of NF270 for mitigating silica fouling and improving pharmaceutical and personal care product rejection. J. Memb. Sci. 2018, 552, 387–395. [Google Scholar] [CrossRef]
- Nadizadeh, Z.; Mahdavi, H. Grafting of zwitterion polymer on polyamide nanofiltration membranes via surface-initiated RAFT polymerization with improved antifouling properties as a new strategy. Sep. Purif. Technol. 2021, 254, 117605. [Google Scholar] [CrossRef]
- Yang, Z.; Saeki, D.; Wu, H.C.; Yoshioka, T.; Matsuyama, H. Effect of polymer structure modified on RO membrane surfaces via surface-initiated ATRP on dynamic biofouling behavior. J. Memb. Sci. 2019, 582, 111–119. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, Y.; Yu, H.; Zhang, X.; Tian, X.; Fu, S.; Zhang, H. Improving the biofouling resistance of polyamide thin-film composite membrane via grafting polyacrylamide brush on the surface by in-situ atomic transfer radical polymerization. J. Memb. Sci. 2021, 629, 119283. [Google Scholar] [CrossRef]
- Yang, Z.; Saeki, D.; Matsuyama, H. Zwitterionic polymer modification of polyamide reverse-osmosis membranes via surface amination and atom transfer radical polymerization for anti-biofouling. J. Memb. Sci. 2018, 550, 332–339. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Xie, M.; Wu, H.C.; Yoshioka, T.; Saeki, D.; Matsuyama, H. Antifouling thin-film composite membranes with multi-defense properties by controllably constructing amphiphilic diblock copolymer brush layer. J. Memb. Sci. 2020, 614, 118515. [Google Scholar] [CrossRef]
- Dimitriou, M.D.; Zhou, Z.; Yoo, H.S.; Killops, K.L.; Finlay, J.A.; Cone, G.; Sundaram, H.S.; Lynd, N.A.; Barteau, K.P.; Campos, L.M.; et al. A general approach to controlling the surface composition of poly(ethylene oxide)-based block copolymers for antifouling coatings. Langmuir 2011, 27, 13762–13772. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Yang, Z.; Saeki, D.; Takagi, R.; Matsuyama, H. Improved anti-biofouling performance of polyamide reverse osmosis membranes modified with a polyampholyte with effective carboxyl anion and quaternary ammonium cation ratio. J. Memb. Sci. 2020, 595, 117529. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Reis, R.; Duke, M.; Merenda, A.; Winther-Jensen, B.; Puskar, L.; Tobin, M.J.; Orbell, J.D.; Dumée, L.F. Customizing the surface charge of thin-film composite membranes by surface plasma thin film polymerization. J. Memb. Sci. 2017, 537, 1–10. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Safarpour, M.; Ghadimi, A.; Adabi, J. Synergistic effect of carboxylated-MWCNTs on the performance of acrylic acid UV-grafted polyamide nanofiltration membranes. React. Funct. Polym. 2019, 134, 74–84. [Google Scholar] [CrossRef]
- Abdul Rahman, A.F.H.B.; Abu Seman, M.N. Bin Polyacrylic-polyethersulfone membrane modified via UV photografting for forward osmosis application. J. Environ. Chem. Eng. 2018, 6, 4368–4379. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, Y.; Wang, C.; Hu, Z.; Zhang, C. High-hydrophilic and salt rejecting PA-g/co-PVP RO membrane via bionic sand-fixing grass for pharmaceutical wastewater treatment. Chem. Eng. J. 2019, 357, 269–279. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. Chemically functionalized polyamide thin film composite membranes: The art of chemistry. Desalination 2020, 495, 114655. [Google Scholar] [CrossRef]
- Liu, C.; Faria, A.F.; Ma, J.; Elimelech, M. Mitigation of Biofilm Development on Thin-Film Composite Membranes Functionalized with Zwitterionic Polymers and Silver Nanoparticles. Environ. Sci. Technol. 2017, 51, 182–191. [Google Scholar] [CrossRef]
- Liu, Z.; An, X.; Dong, C.; Zheng, S.; Mi, B.; Hu, Y. Modification of thin film composite polyamide membranes with 3D hyperbranched polyglycerol for simultaneous improvement in their filtration performance and antifouling properties. J. Mater. Chem. A 2017, 5, 23190–23197. [Google Scholar] [CrossRef]
- Kim, E.S.; Yu, Q.; Deng, B. Plasma surface modification of nanofiltration (NF) thin-film composite (TFC) membranes to improve anti organic fouling. Appl. Surf. Sci. 2011, 257, 9863–9871. [Google Scholar] [CrossRef]
- Zou, H.; Jin, Y.; Yang, J.; Dai, H.; Yu, X.; Xu, J. Synthesis and characterization of thin film composite reverse osmosis membranes via novel interfacial polymerization approach. Sep. Purif. Technol. 2010, 72, 256–262. [Google Scholar] [CrossRef]
- Abu Seman, M.N.; Khayet, M.; Bin Ali, Z.I.; Hilal, N. Reduction of nanofiltration membrane fouling by UV-initiated graft polymerization technique. J. Memb. Sci. 2010, 355, 133–141. [Google Scholar] [CrossRef]
- Ren, L.; Chen, J.; Lu, Q.; Han, J.; Wu, H. Anti-biofouling nanofiltration membrane constructed by in-situ photo-grafting bactericidal and hydrophilic polymers. J. Memb. Sci. 2021, 617, 118658. [Google Scholar] [CrossRef]
- El-Arnaouty, M.B.; Abdel Ghaffar, A.M.; Eid, M.; Aboulfotouh, M.E.; Taher, N.H.; Soliman, E.-S. Nano-modification of polyamide thin film composite reverse osmosis membranes by radiation grafting. J. Radiat. Res. Appl. Sci. 2018, 11, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Modi, A.; Bellare, J. Efficiently improved oil/water separation using high flux and superior antifouling polysulfone hollow fiber membranes modified with functionalized carbon nanotubes/graphene oxide nanohybrid. J. Environ. Chem. Eng. 2019, 7, 102944. [Google Scholar] [CrossRef]
- Ganj, M.; Asadollahi, M.; Mousavi, S.A.; Bastani, D.; Aghaeifard, F. Surface modification of polysulfone ultrafiltration membranes by free radical graft polymerization of acrylic acid using response surface methodology. J. Polym. Res. 2019, 26, 231. [Google Scholar] [CrossRef]
- Chung, Y.T.; Ng, L.Y.; Mohammad, A.W. Sulfonated-polysulfone membrane surface modification by employing methacrylic acid through UV-grafting: Optimization through response surface methodology approach. J. Ind. Eng. Chem. 2014, 20, 1549–1557. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Misra, B.N. Grafting: A versatile means to modify polymers: Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Dubé, M.A.; Salehpour, S. Applying the Principles of Green Chemistry to Polymer Production Technology. Macromol. React. Eng. 2014, 8, 7–28. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.; Ding, S. Catalyst separation in atom transfer radical polymerization. Prog. Polym. Sci. 2004, 29, 1053–1078. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, Z.; Sun, Y.; Cui, M.; Luo, Z.; Yu, B.; Zou, X.; Hu, H. Improved antifouling properties of PVA hydrogel via an organic semiconductor graphitic carbon nitride catalyzed surface-initiated photo atom transfer radical polymerization. Colloids Surfaces B Biointerfaces 2021, 203, 111718. [Google Scholar] [CrossRef]
- Vivaldo-Lima, E.; Mohammadi, Y.; Penlidis, A. Special issue: Modeling and simulation of polymerization processes. Processes 2021, 9, 821. [Google Scholar] [CrossRef]
- Vega-hernández, M.Á.; Cano-díaz, G.S.; Vivaldo-lima, E.; Rosas-aburto, A.; Hernández-luna, M.G.; Martinez, A.; Palacios-alquisira, J.; Mohammadi, Y.; Penlidis, A. A review on the synthesis, characterization and modeling of polymer grafting. Processes 2021, 9, 375. [Google Scholar] [CrossRef]
- Ueki, Y.; Seko, N.; Maekawa, Y. Machine learning approach for prediction of the grafting yield in radiation-induced graft polymerization. Appl. Mater. Today 2021, 101158. [Google Scholar] [CrossRef]


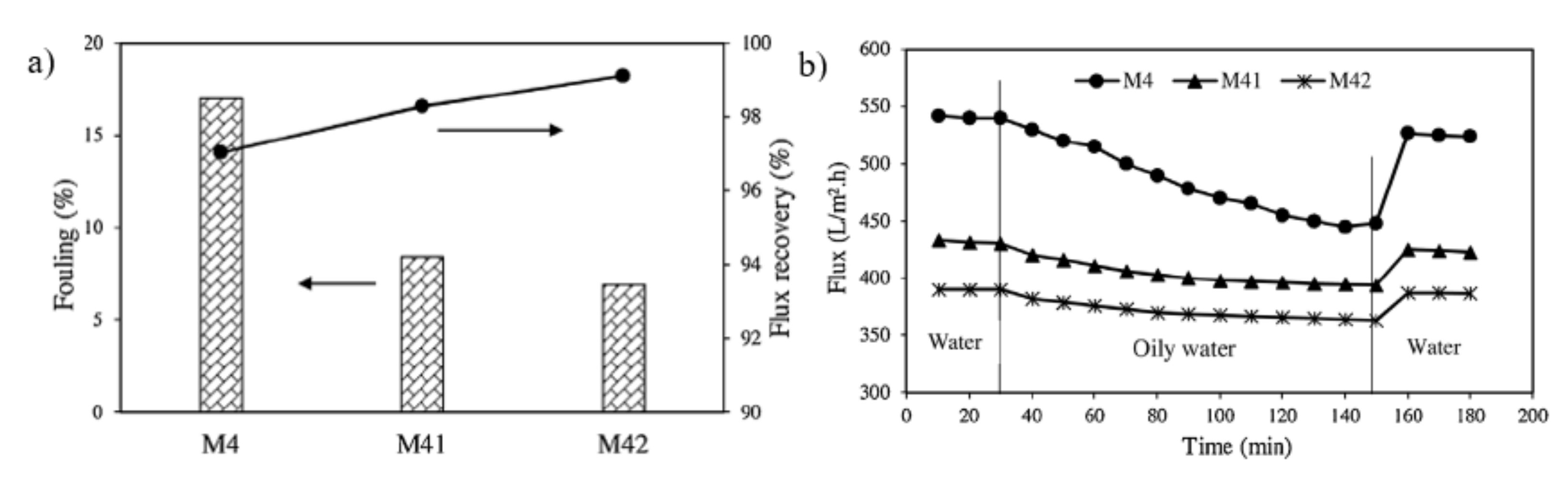



| Grafting Method | Membrane Material | Polymer/Additive | Induction | Characteristic Introduced to the Modified Surface | Ref. |
|---|---|---|---|---|---|
| Chemical-induced | PP | HEMA | Ozone | The HEMA grafted PP membrane surface became hydrophilic and less adsorbable to bovine serum albumin (BSA) proteins compared to pristine membrane. | [71] |
| PES | Poly (ethylene glycol) methacrylate (PEGMA) | Peroxydisulfate, Metabisulfite | The modified PES membrane showed additional absorption bands in the area of aliphatic stretching vibration, which was missing from the original membrane’s spectra. | [110] | |
| Plasma-induced | PP | TiO2 | Plasma: Air, O2 | Due to the increased hydrophilicity, modified membranes demonstrated greater resistance to protein fouling as compared to pristine membranes. | [111] |
| PES | AA | Plasma: Argon | The modified membranes are less susceptible to protein fouling than the pristine membranes, and plasma treatment greatly improved the modified membrane’s water flux. The modified membranes can be cleaned more easily and use less caustic to recover permeation flux. | [112] | |
| Irradiation-induced | PP | PEGMA, HEMA | UV | The grafted pHEMA and PEGMA surface shows tremendous increase in pure water flux while substantially reducing protein adsorptions. | [113] |
| PES | AA, Ethylene diamine, HEMA | UV | UV photo-grafting of hydrophilic monomers onto the membrane surface greatly improved the hydrophilicities of the membranes. | [114] | |
| PAS | Methacrylic acid, Glycidylmethacrylate (GMA), HEMA | UV | Modified membrane exhibited enhanced hydrophilicity compared to unmodified membrane. | [115] | |
| PVDF | PES | UV | BSA adsorption was reduced in the modified membrane, and flux recovery was improved. | [116] |
| Approach | Modification Materials/Membrane Material | Process | Surface—Grafted Membrane | Pristine Membrane | Ref. |
|---|---|---|---|---|---|
| SI-ATRP | PAA | UF | Rejection: 92% Flux: 230 Lm−2 h−1 | Rejection: 90% Flux: 310 Lm−2 h−1 | [122] |
| SI-ATRP | PNIPAAm PEGMA | UF | FRR: 99.1% Oil rejection: 98.2% | FRR: 97.1% Oil rejection: 91.1% | [119] |
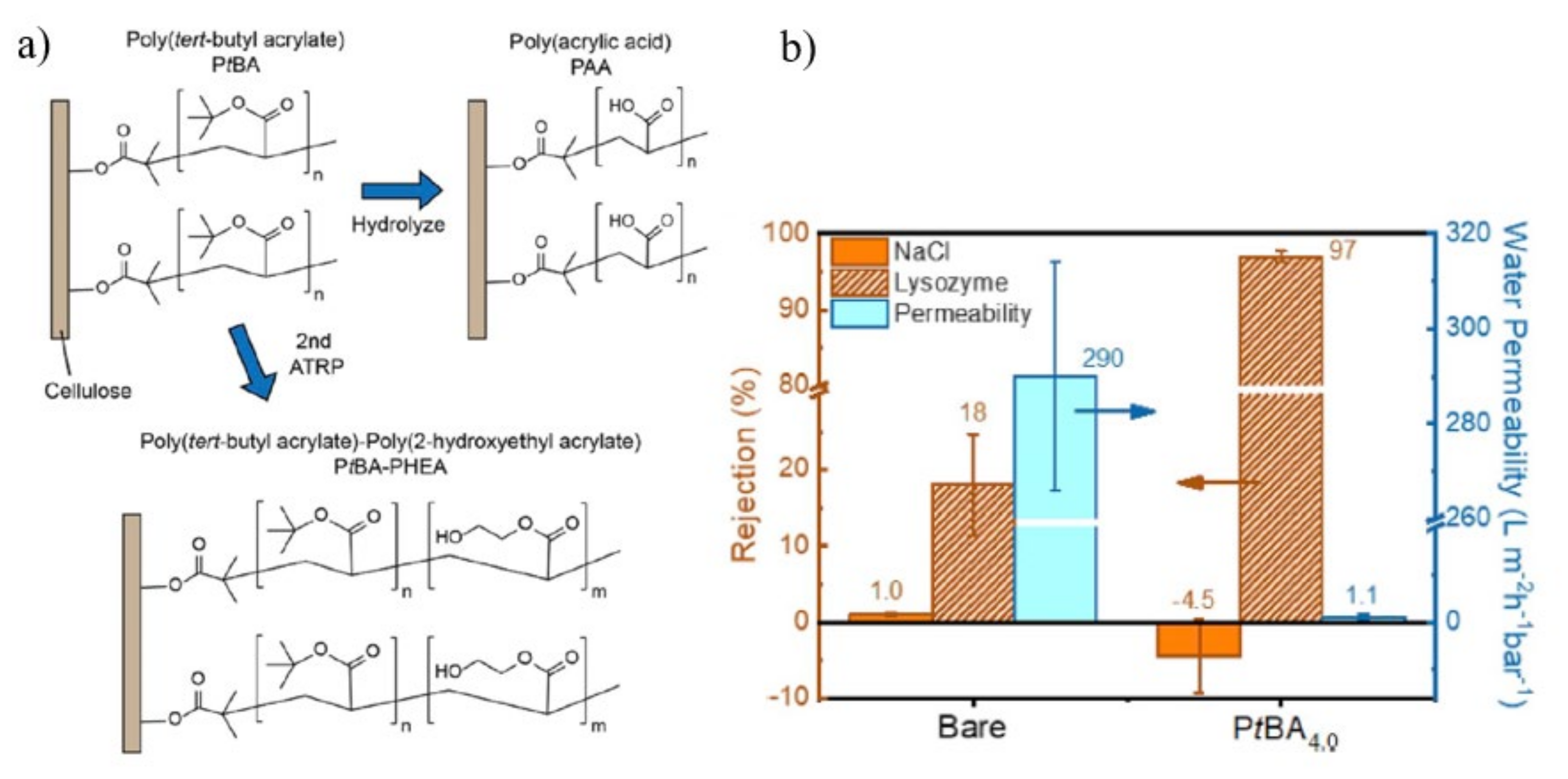
| SI-ATRP | PAA PtBA-PHEA copolymer | Cellulosic membrane | Lysozyme rejection: 97% Permeability: 1.1 Lm−2 h−1 bar−1 | Lysozyme rejection: 18% Permeability: 290 Lm−2 h−1 bar−1 | [123] |
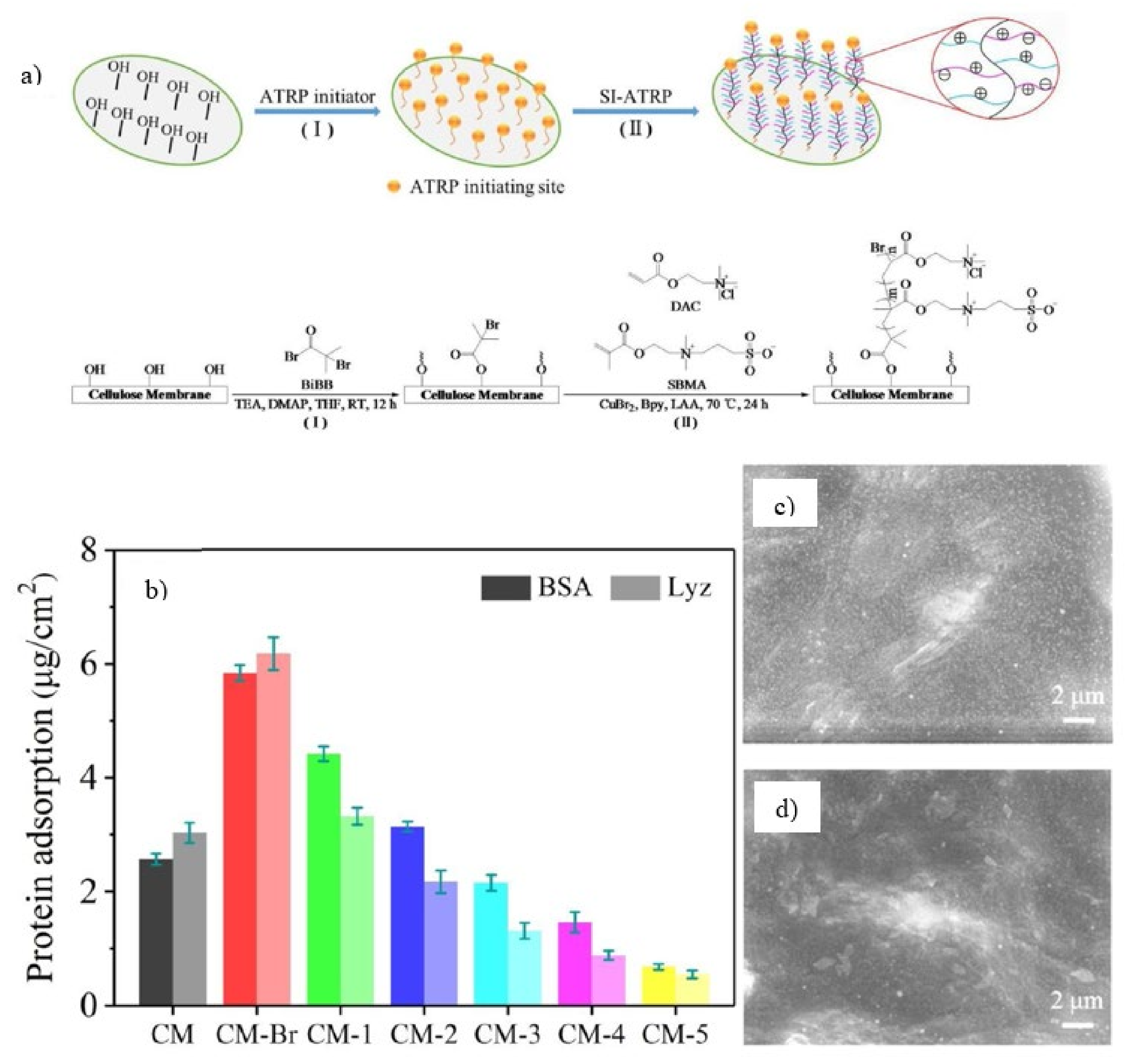
| SI-ATRP | SBMA DAC | Cellulose membrane | CFU Reduction S. aureus: 95.1% E. coli: 90.5% | CFU Reduction: S. aureus: 9.7% E. coli: 7.2% | [118] |
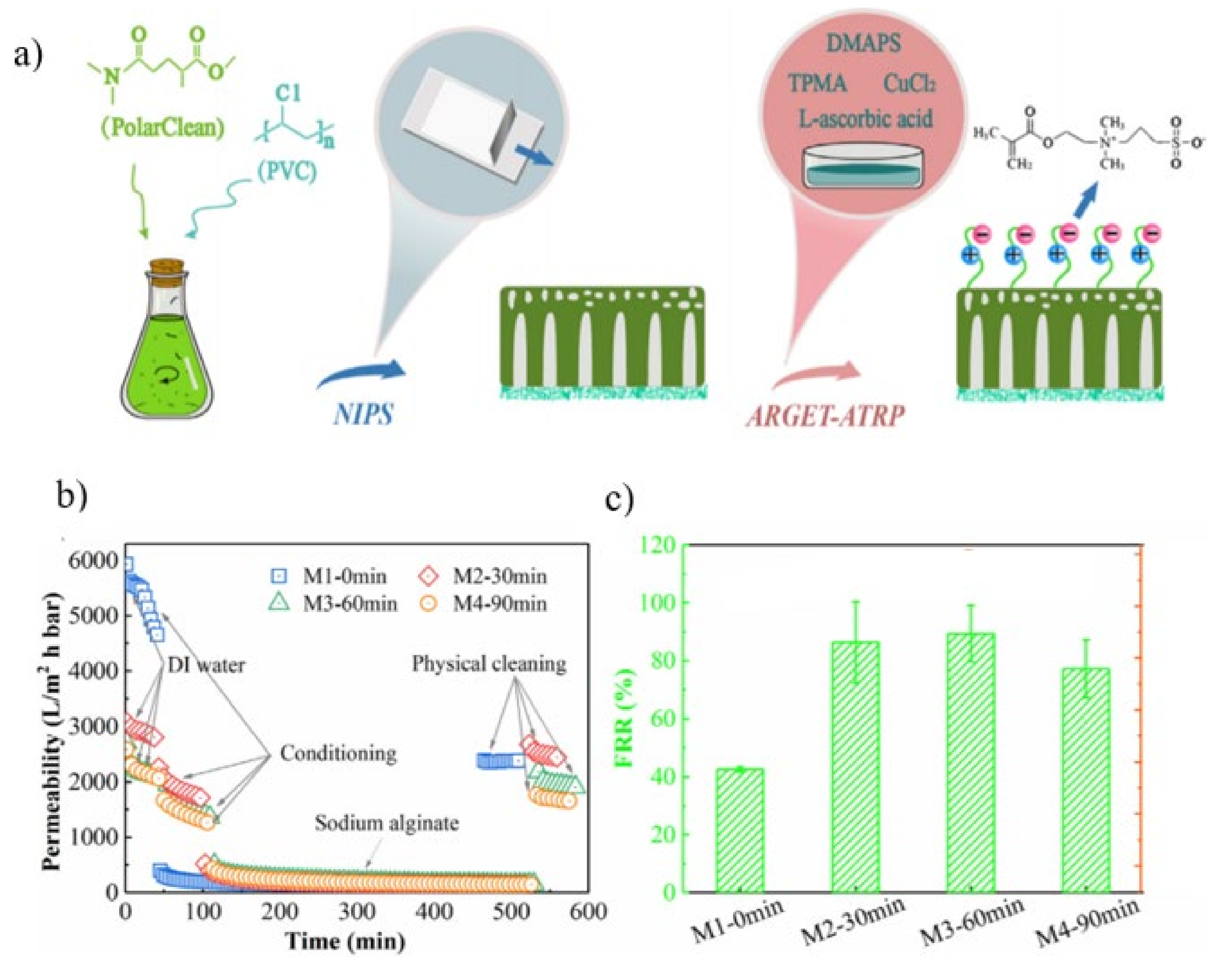
| ARGET-ATRP | DMAPS Zwitterionic | UF | Permeability: 2872.3 Lm−2 h−1 bar−1 FRR: 86.4% Rejection: 96% | Permeability: 500.0 Lm−2 h−1 bar−1 FRR: 42.6 ± 0.9% Rejection: 93.2 ± 2.4% | [125] |
| AGET-ATRP | HEMA | UF | Flux: 752.5 Lm−2 h−1 bar−1 | - | [130] |
| Plasma initiated grafting | SBMA zwitterionic monomers Corona air | UF | Flux: 800 Lm−2 h−1 | Flux: 198 Lm−2 h−1 | [126] |
| Plasma initiated grafting | Ar/O2 plasma assisted oxygen activation in PSF membrane | UF | Flux: 350.7 Lm−2 h−1 Fouling resistance: 82% BSA Rejection: 99.9% | Flux: 25.2 Lm−2 h−1 Fouling resistance: 50% BSA Rejection: 55% | [131] |
| Plasma initiated grafting | CMB | UF | BSA Adsorption: 0.088 mg cm−2 grafted CMB: 0.045 mg cm−2 0.023 mg cm−2 grafted CMB: 0.023 mg cm−2 | BSA Adsorption: 0.096 mg cm−2 | [132] |
| Plasma initiated grafting | AA with Ar and CO2 | CTA | FluxAr: 8.12 Lm−2 h−1 FluxCO2: 7.56 Lm−2 h−1 | Flux: 6.11 Lm−2 h−1 | [127] |
| UV initiated grafting | Zwitterionic acrylate monomer | UF | BSA Adsorption: 50% P. aeruginosa biofilm growth: 2 µm | BSA Adsorption: 80% P. aeruginosa biofilm growth: 5 µm | [133] |
| UV initiated grafting | AA Ag-MOFs | UF | PWP: 1200 ± 260 Lm−1 h−1 bar−1 Inactivation rate: E. coli: 90% S. aerus: 95% | PWP: 1500–2500 Lm−1 h−1 bar−1 Inactivation rate: E. coli: 0% S. aerus: 0% | [129] |
| Approach | Modification Materials/Membrane Material | Process | Surface-Grafted Membrane | Pristine Membrane | Ref. |
|---|---|---|---|---|---|
| RAFT | pMEDSAH | NF | Na2SO4 Rejection:70% Flux: 11.5 Lm−2 h−1 bar−1 Fouling resistance, Rr: 35% | Na2SO4 Rejection: 72% Flux: 1.1 Lm−2 h−1 bar−1 Fouling resistance, Rr: 8% | [138] |
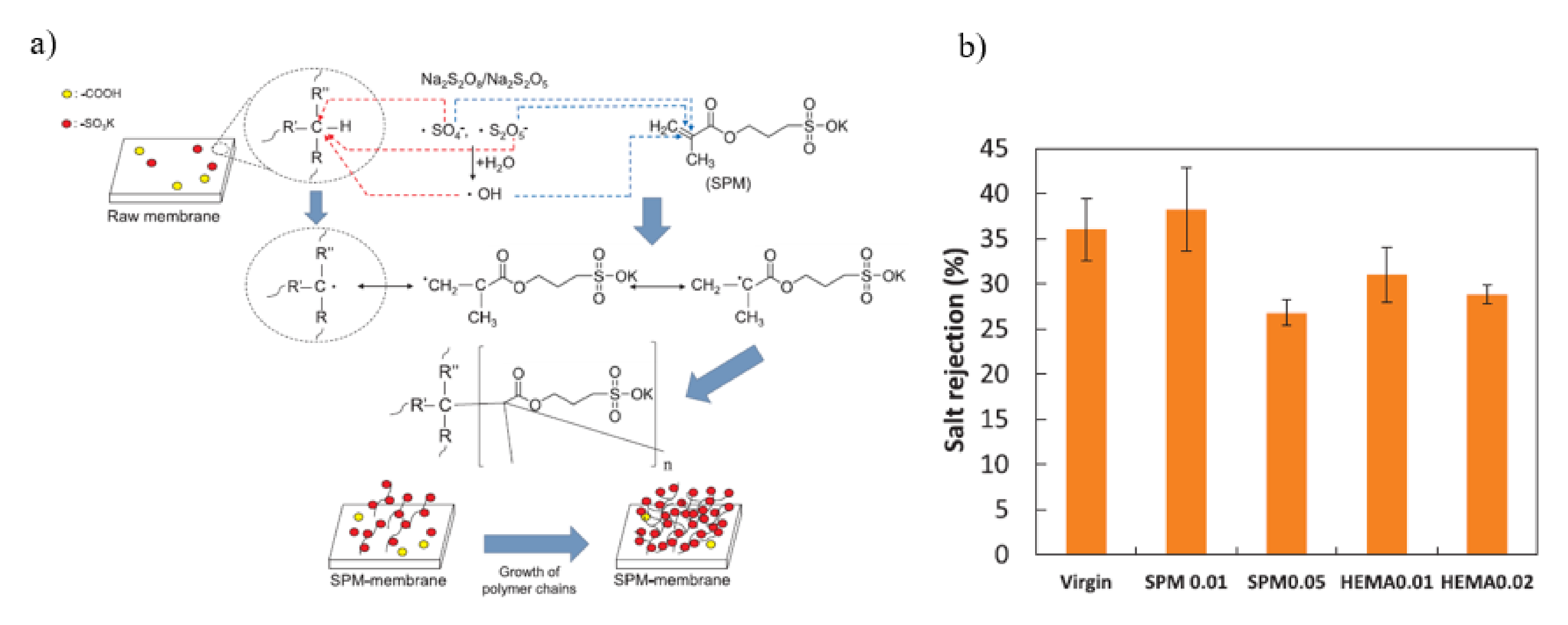
| ATRP | SPM HEMA | NF | 0.01 SPM Rejection: 38.2% Flux: 95 Lm−2 h−1 0.01 HEMA Rejection: 31.0% Flux: 105 Lm−2 h−1 | Rejection: 36.1% Flux: 80 Lm−2 h−1 | [137] |
| ATRP | PAAm | RO | Rejection: 99.2% PWP: 2.4 Lm−2 h−1 bar−1 | Rejection: 99.2% PWP: 2.5 Lm−2 h−1 bar−1 | [140] |
| ATRP | PVCIB | RO | Rejection: 98.3% PWP: 5.72 Lm−2 h−1 bar−1 E. coli mortality: 98.8% | Rejection: 97.3% PWP: 4.59 Lm−2 h−1 bar−1 E. coli mortality: 15.1% | [151] |
| SI-ATRP | pMEDSAH | RO | Rejection: 94.5% Permeability: 20 Lm−2 h−1/MPa Bacterial adhesion: 2.0% | Rejection: 96% Permeability: 68 Lm−2 h−1/MPa Bacterial adhesion: 9.5% | [141] |
| SI-ATRP | pMEDSAH pTFEMA | RO | RejectionpMEDSAH: 95.4% RejectionpMEDSAH-pTFEMA: 95.3% PermeabilitypMEDSAH: 3.5 Lm−2 h−1 bar−1 PermeabilitypMEDSAH-pTFEMA: 3.0 Lm−2 h−1 bar−1 | Rejection: 96.8% Permeability: 5.3 Lm−2 h−1 bar−1 | [142] |
| SI-ATRP | pHEMA | RO | Rejection: 97% Bacterial adhesion: 2.8% | Rejection: 97% Bacterial adhesion:7.6% | [139] |
| SI-ATRP | pPEG | RO | Rejection: 97% Bacterial adhesion: 0.5% | Rejection: 97% Bacterial adhesion:7.6% | [139] |
| SI-ATRP | pMEDSAH | RO | Rejection: 97% Bacterial adhesion: 0.1% | Rejection: 97% Bacterial adhesion:7.6% | [139] |
| SI-ATRP | CAA TMA | RO | Rejection: 80% Flux: 45 Lm−2 h−1/MPa | Rejection: 99% Flux: 60 Lm−2 h−1/MPa | [145] |
| ATRP | Ag NPs zwitterion | FO | Flux: 1.1 Lm−2 h−1 bar−1 Surface: Smoother EPS Biovolume: 10.7 ± 2.1 µm3 µm−2 | Flux: 1 Lm−2 h−1 bar−1 Surface: Rougher EPS Biovolume: 27.0 ± 3.4 µm3 µm−2 | [152] |
| ATRP | Silica NPs zwitterion | FO | Surface: Smoother Permeability: 4.8 Lm−2 h−1 bar−1 Attached E. coli: 0.1 × 105 cells/cm2 | Surface: Rougher Permeability: 5.9 Lm−2 h−1 bar−1 Attached E. coli: 1.4 × 105 cells/cm2 | [153] |
| ATRP | PVI AIBA | FO | Flux: 91.6 Lm−2 h−1 E. coli mortality rate: 98.8% | Flux: 68.7 Lm−2 h−1 E. coli mortality rate: 75.6% | [31] |
| Plasma initiated grafting | Low pressure NH3 plasma | NF | Rejection: 95% Flux: 1.4 Lm−2 h−1 bar−1 BSA Adsorption: 0.22 mg BSA/mg membrane | Rejection: 85% Flux: 1 Lm−2 h−1 bar−1 BSA Adsorption: 0.38 mg BSA/mg membrane | [154] |
| Plasma initiated grafting | Triglyme | RO | Rejection: 98.1% Flux: 45.5 Lm−2 h−1 | Rejection: 98.5% Flux: 47 Lm−2 h−1 | [155] |
| Plasma initiated grafting | MA VIM | RO | Rejection: 97% Flux: 49.2 Lm−2 h−1 | Rejection: 98% Flux: 44.9 Lm−2 h−1 | [147] |
| Plasma initiated grafting | Pure Helium Water | RO | Rejection: 98% Flux: 50 Lm−2 h−1 | Rejection: 98% Flux: 30 Lm−2 h−1 | [147] |
| Radiation initiated grafting (UV) | AA | NF | Rejection (5 g/L AA,5 min): 43% Flux (5 g/L AA,5 min): 2 Lm−2 h−1 | Rejection: 59% Flux: 0.75 Lm−2 h−1 | [149] |
| Radiation initiated grafting (UV) | AA | NF | Rejection: 95.8% Na2SO4 Rejection: 98.2% Flux: 39 Lm−2 h−1 | Rejection: 93% Na2SO4Rejection: 97.8% Flux: 29 Lm−2 h−1 | [148] |
| Radiation initiated grafting (UV) | AA | NF | Rejection: 98.5% Flux: 1.05 Lm−2 h−1 bar−1 Irreversible Fouling Factor, FRw: 8% (at pH 7) | Rejection: 98% Flux:1 Lm−2 h−1 bar−1 Irreversible Fouling Factor, FRw: 24% (at pH 7) | [156] |
| Radiation initiated grafting (UV) | PHMB PEG | NF | Na2SO4 Rejection: 99.5% FRR:70.8% Bacterial inhibition rate: 98.6% | Na2SO4 Rejection: 99.5% FRR:44.7% Bacterial inhibition rate:76% | [157] |
| Radiation initiated grafting (ᵞ-ray) | NVP PVP Cobalt-60 | RO | Rejection: 99.5% FRRBSA: 91.23% | Rejection: 98.3% FRRBSA: 62.28% | [150] |
| Radiation initiated grafting (ᵞ-ray) | NIPAM Cobalt-60 | RO | Rejection: 89% Flux: 8.14 Lm−2 h−1 bar −1 | Rejection: 94% Flux: 9.7 Lm−2 h−1 bar −1 | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suresh, D.; Goh, P.S.; Ismail, A.F.; Hilal, N. Surface Design of Liquid Separation Membrane through Graft Polymerization: A State of the Art Review. Membranes 2021, 11, 832. https://doi.org/10.3390/membranes11110832
Suresh D, Goh PS, Ismail AF, Hilal N. Surface Design of Liquid Separation Membrane through Graft Polymerization: A State of the Art Review. Membranes. 2021; 11(11):832. https://doi.org/10.3390/membranes11110832
Chicago/Turabian StyleSuresh, Deepa, Pei Sean Goh, Ahmad Fauzi Ismail, and Nidal Hilal. 2021. "Surface Design of Liquid Separation Membrane through Graft Polymerization: A State of the Art Review" Membranes 11, no. 11: 832. https://doi.org/10.3390/membranes11110832
APA StyleSuresh, D., Goh, P. S., Ismail, A. F., & Hilal, N. (2021). Surface Design of Liquid Separation Membrane through Graft Polymerization: A State of the Art Review. Membranes, 11(11), 832. https://doi.org/10.3390/membranes11110832









