In Vivo Secretion of β-Lactamase-Carrying Outer Membrane Vesicles as a Mechanism of β-Lactam Therapy Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient and Microbiological Examinations
2.2. Ethical Approval
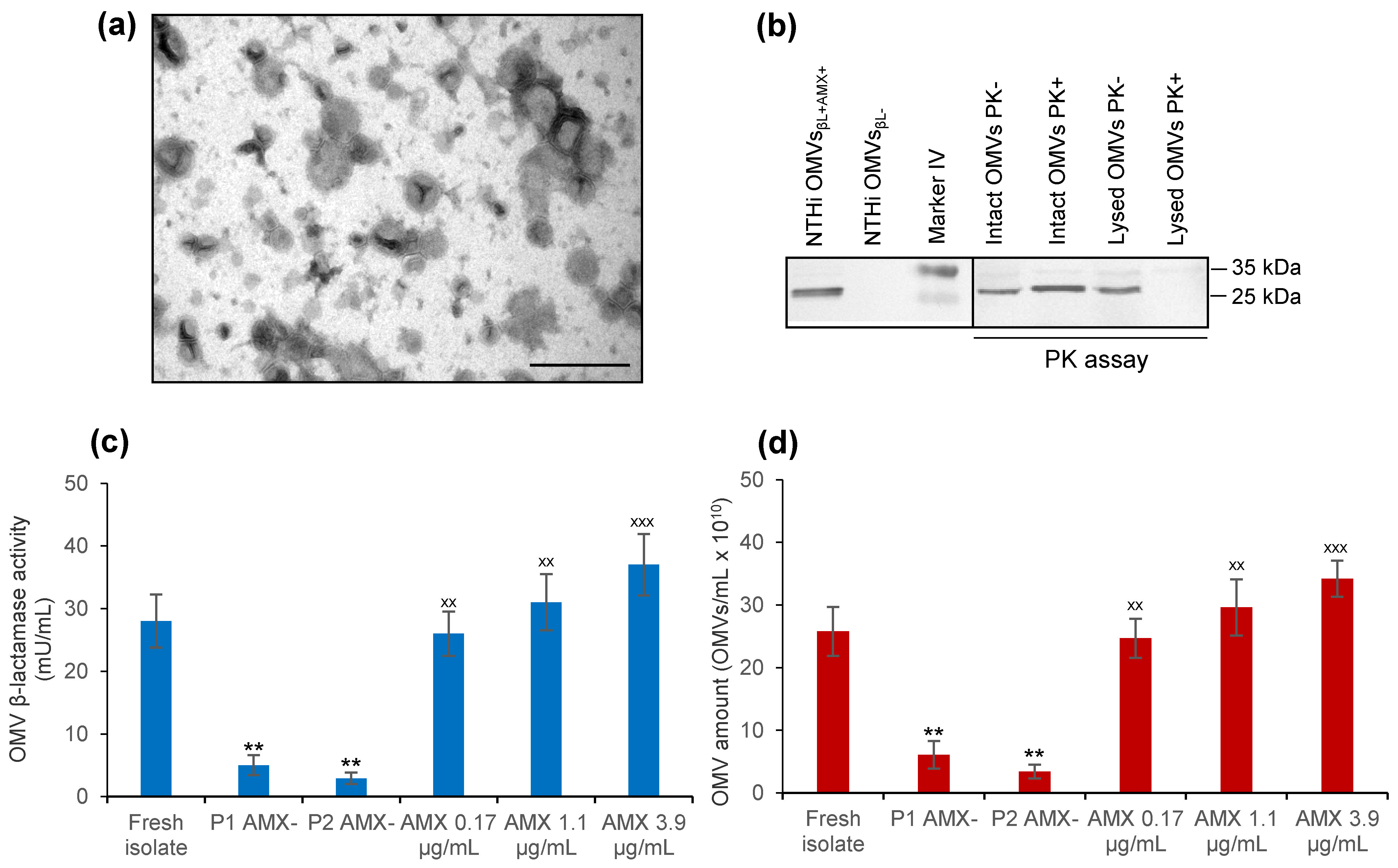
2.3. Isolation and Characterization of OMVs Produced by Nontypeable Haemophilus Influenzae (NTHi) patient’s Isolate
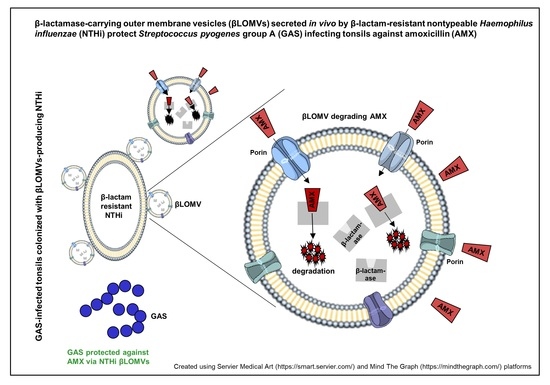
2.4. GAS Protection against Amoxicillin via β-Lactamase-Carrying, Amoxicillin-Induced NTHi OMVs
2.5. The Influence of NTHi OMVsβL+AMX+ on Amoxicillin MIC for GAS
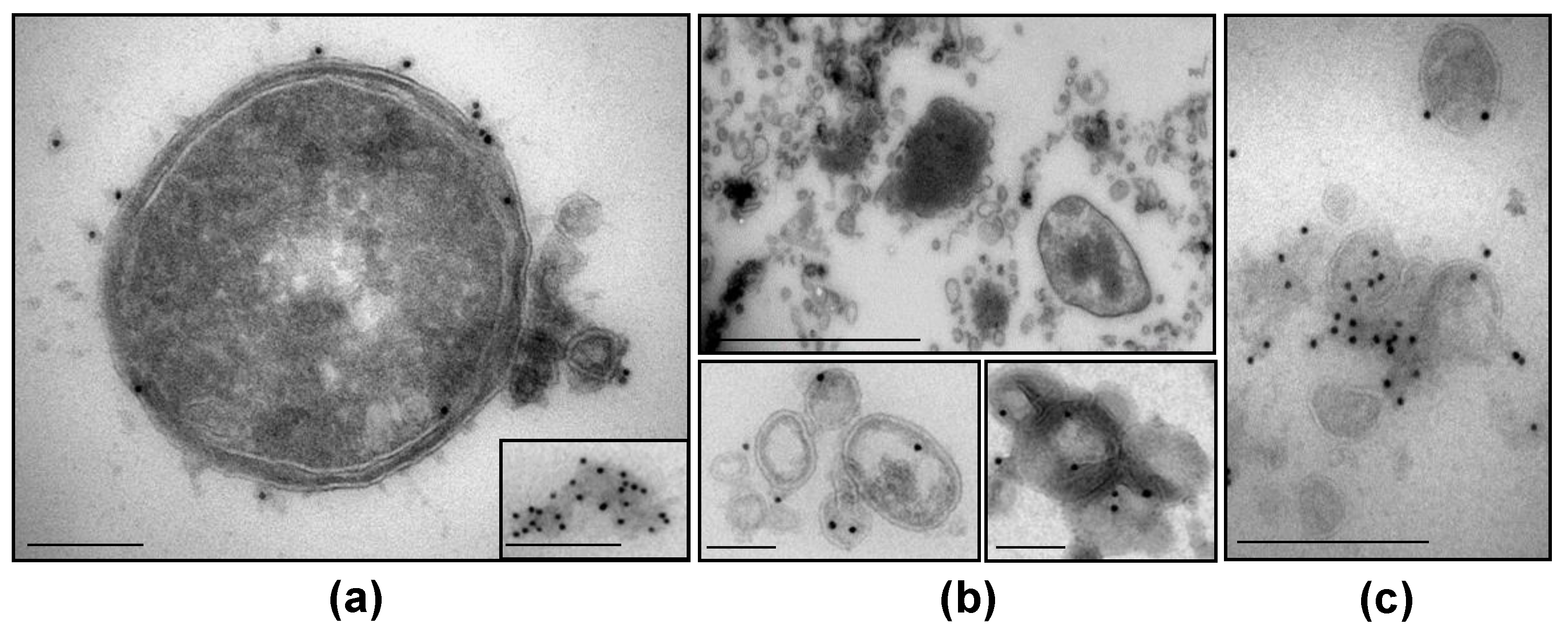
2.6. In Vivo Detection of OMV-Producing H. Influenzae and β-Lactamase-Carrying OMVs
2.7. Statistical Analysis
3. Results
3.1. Amoxicillin Treatment Failure in a Patient with GAS Pharyngotonsillitis
3.2. NTHi patient’s Isolate Secretes β-Lactamase-Carrying OMVs That Are Induced by Amoxicillin
3.3. NTHi OMVsβL+AMX+ Protect GAS against Bactericidal Concentrations of Amoxicillin
3.4. NTHi OMVsβL+AMX+ Increase Amoxicillin MIC for GAS
3.5. NTHi β-Lactamase-Carrying OMVs Are Secreted In Vivo at the Infection Site
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shulman, S.T.; Bisno, A.L.; Clegg, H.W.; Gerber, M.A.; Kaplan, E.L.; Lee, G.; Martin, J.M.; Van Beneden, C. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2012, 55, e86–e102. [Google Scholar] [CrossRef] [Green Version]
- Brook, I. Overcoming penicillin failures in the treatment of Group A streptococcal pharyngo-tonsillitis. Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 1501–1508. [Google Scholar] [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Mandujano, A.; Hernández-Cortez, C.; Ibarra, J.A.; Castro-Escarpulli, G. The outer membrane vesicles: Secretion system type zero. Traffic 2017, 18, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R.; et al. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 2016, 7, 10515. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, I.A.; Kuehn, M.J. Offense and defense: Microbial membrane vesicles play both ways. Res. Microbiol. 2012, 163, 607–618. [Google Scholar] [CrossRef] [Green Version]
- Caruana, J.C.; Walper, S.A. Bacterial membrane vesicles as mediators of microbe—Microbe and microbe—Host community interactions. Front. Microbiol. 2020, 11, 432. [Google Scholar] [CrossRef] [Green Version]
- Rueter, C.; Bielaszewska, M. Secretion and delivery of intestinal pathogenic Escherichia coli virulence factors via outer membrane vesicles. Front. Cell. Infect. Microbiol. 2020, 10, 91. [Google Scholar] [CrossRef]
- Tan, T.T.; Morgelin, M.; Forsgren, A.; Riesbeck, K. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J. Infect. Dis. 2007, 195, 1661–1670. [Google Scholar] [CrossRef] [Green Version]
- Namork, E.; Brandtzaeg, P. Fatal meningococcal septicaemia with “blebbing” meningococcus. Lancet 2002, 360, 1741. [Google Scholar] [CrossRef]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Orench-Rivera, N.; Kuehn, M.J. Environmentally controlled bacterial vesicle-mediated export. Cell. Microbiol. 2016, 18, 1525–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauwens, A.; Kunsmann, L.; Marejková, M.; Zhang, W.; Karch, H.; Bielaszewska, M.; Mellmann, A. Intrahost milieu modulates production of outer membrane vesicles, vesicle-associated Shiga toxin 2a and cytotoxicity in Escherichia coli O157:H7 and O104:H4. Environ. Microbiol. Rep. 2017, 9, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, A.; Kunsmann, L.; Karch, H.; Mellmann, A.; Bielaszewska, M. Antibiotic-mediated modulations of outer membrane vesicles in enterohemorrhagic Escherichia coli O104:H4 and O157:H7. Antimicrob. Agents Chemother. 2017, 61, e00937-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devos, S.; Van Oudenhove, L.; Stremersch, S.; Van Putte, W.; De Rycke, R.; Van Driessche, G.; Vitse, J.; Raemdonck, K.; Devreese, B. The effect of imipenem and diffusible signaling factors on the secretion of outer membrane vesicles and associated Ax21 proteins in Stenotrophomonas maltophilia. Front. Microbiol. 2015, 6, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devos, S.; Stremersch, S.; Raemdonck, K.; Braeckmans, K.; Devreese, B. Intra- and interspecies effects of outer membrane vesicles from Stenotrophomonas maltophilia on β-lactam resistance. Antimicrob. Agents Chemother. 2016, 60, 2516–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maredia, R.; Devineni, N.; Lentz, P.; Dallo, S.F.; Yu, J.; Guentzel, N.; Chambers, J.; Arulanandam, B.; Haskins, W.E.; Weitao, T. Vesiculation from Pseudomonas aeruginosa under SOS. Sci. World J. 2012, 2012, 402919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, H.M.; Nagaraj, R.; Jagannadham, M.V. Protective role of E. coli outer membrane vesicles against antibiotics. Microbiol. Res. 2015, 181, 1–7. [Google Scholar] [CrossRef]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11, 258. [Google Scholar] [CrossRef] [Green Version]
- Schaar, V.; Nordström, T.; Mörgelin, M.; Riesbeck, K. Moraxella catarrhalis outer membrane vesicles carry β-lactamase and promote survival of Streptococcus pneumoniae and Haemophilus influenzae by inactivating amoxicillin. Antimicrob. Agents Chemother. 2011, 55, 3845–3853. [Google Scholar] [CrossRef] [Green Version]
- Schaar, V.; Uddbäck, I.; Nordström, T.; Riesbeck, K. Group A streptococci are protected from amoxicillin-mediated killing by vesicles containing β-lactamase derived from Haemophilus influenzae. J. Antimicrob. Chemother. 2013, 69, 117–120. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, S.B.; Im, S.P.; Lee, J.S.; Jung, J.W.; Gong, T.W.; Lazarte, J.M.S.; Kim, J.; Seo, J.S.; Kim, J.H.; et al. Outer membrane vesicles from beta-lactam-resistant Escherichia coli enable the survival of beta-lactam-susceptible E. coli in the presence of beta-lactam antibiotics. Sci. Rep. 2018, 8, 5402. [Google Scholar] [CrossRef] [Green Version]
- Stentz, R.; Horn, N.; Cross, K.; Salt, L.; Brearley, C.; Livermore, D.M.; Carding, S.R. Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp. protect gut pathogens and commensals against beta-lactam antibiotics. J. Antimicrob. Chemother. 2015, 70, 701–709. [Google Scholar] [CrossRef] [Green Version]
- González, L.J.; Bahr, G.; Nakashige, T.G.; Nolan, E.M.; Bonomo, R.A.; Vila, A.J. Membrane anchoring stabilizes and favors secretion of New Delhi metallo-beta-lactamase. Nat. Chem. Biol. 2016, 12, 516–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, J.H.; Pfaller, M.A.; Carroll, K.C.; Landry, M.L.; Funke, G.; Richter, S.S.; Warnock, D.W. Manual of Clinical Microbiology, 11th ed.; ASM Press: Washinghton, DC, USA, 2015; pp. 383–402, 667–684. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 25th ed.; M100; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Scriver, S.R.; Walmsley, S.L.; Kau, C.L.; Hoban, D.J.; Brunton, J.; McGeer, A.; Moore, T.C.; Witwicki, E.; Canadian Haemophilus Study Group; Low, D.E. Determination of antimicrobial susceptibilities of Canadian isolates of Haemophilus influenzae and characterization of their beta-lactamases. Antimicrob. Agents Chemother. 1994, 38, 1678–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blenk, H.; Simm, K.; Blenk, B.; Jahneke, G. Concentrations of erythromycin and amoxicillin in tonsil and sinus tissues of patients with tonsillitis and sinusitis. A comparison. Infection 1982, 10 (Suppl. 2), S108–S112. [Google Scholar] [CrossRef]
- Averono, G.; Vidali, M.; Olina, M.; Basile, M.; Bagnati, M.; Bellomo, G.; Aluffi, P. Evaluation of amoxicillin plasma and tissue levels in pediatric patients undergoing tonsillectomy. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Chomarat, M.; Panteix, G.; Guillaumond, B.; Dubreuil, C. Tonsillar diffusion kinetics of amoxycillin after oral administration of 1 g to adults. Eur. J. Drug Metab. Pharmacokinet. 1997, 22, 141–144. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Daniel, O.; Karch, H.; Mellmann, A. Dissemination of the blaCTX-M-15 gene among Enterobacteriaceae via outer membrane vesicles. J. Antimicrob. Chemother. 2020, 75, 2442–2451. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Greune, L.; Bauwens, A.; Dersch, P.; Mellmann, A.; Rüter, C. Virulence factor cargo and host cell interactions of Shiga toxin-producing Escherichia coli outer membrane vesicles. Methods Mol. Biol. 2021, 2291, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Willysson, A.; Stahl, A.; Karpman, D. Isolation and characterization of Shiga toxin-associated microvesicles. Methods Mol. Biol. 2021, 2291, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Kunsmann, L.; Rüter, C.; Bauwens, A.; Greune, L.; Glüder, M.; Kemper, B.; Fruth, A.; Wai, S.N.; He, X.; Lloubes, R.; et al. Virulence from vesicles: Novel mechanisms of host cell injury by Escherichia coli O104:H4 outbreak strain. Sci. Rep. 2015, 5, 13252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brook, I.; Gober, A.E. Increased recovery of Moraxella catarrhalis and Haemophilus influenzae in association with group A β-haemolytic streptococci in healthy children and those with pharyngo-tonsillitis. J. Med. Microbiol. 2006, 55, 989–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Lee, J.S.; Park, S.B.; Lee, A.R.; Jung, J.W.; Chun, J.H.; Lazarte, J.M.S.; Kim, J.; Seo, J.S.; Kim, J.H.; et al. The importance of porins and beta-lactamase in outer membrane vesicles on the hydrolysis of beta-lactam antibiotics. Int. J. Mol. Sci. 2020, 21, 2822. [Google Scholar] [CrossRef]
- Marchant, P.; Carreño, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderón, I.L.; Fuentes, J.A. “One for All”: Functional transfer of OMV-mediated polymyxin B resistance from Salmonella enterica sv. Typhi ΔtolR and ΔdegS to susceptible bacteria. Front. Microbiol. 2021, 12, 672467. [Google Scholar] [CrossRef] [PubMed]
- Rumbo, C.; Fernández-Moreira, E.; Merino, M.; Poza, M.; Mendez, J.A.; Soares, N.C.; Mosquera, A.; Chaves, F.; Bou, G. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: A new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2011, 55, 3084–3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Mondal, A.; Mitra, S.; Basu, S. Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles. J. Antimicrob. Chemother. 2017, 72, 2201–2207. [Google Scholar] [CrossRef] [Green Version]
- Kosgodage, U.S.; Matewele, P.; Mastroianni, G.; Kraev, I.; Brotherton, D.; Awamaria, B.; Nicolas, A.P.; Lange, S.; Inal, J.M. Peptidylarginine deiminase inhibitors reduce bacterial membrane vesicle release and sensitize bacteria to antibiotic treatment. Front. Cell. Infect. Microbiol. 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Sauvage, E.; Terrak, M. Glycosyltransferases and transpeptidases/penicillin-binding proteins: Valuable targets for new antibacterials. Antibiotics 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]

| NTHi OMVs Produced under Conditions | OMV Protein Concentration (µg/mL) |
|---|---|
| BHI broth without amoxicillin | 325 |
| BHI broth + amoxicillin 0.17 µg/mL 1 | 724 |
| BHI broth + amoxicillin 1.1 µg/mL 2 | 989 |
| BHI broth + amoxicillin 3.9 µg/mL 3 | 1200 |
| Isolate | Disc Diffusion Method | Broth Microdilution Method MIC (µg/mL) | PCR blaTEM-1 | ||
|---|---|---|---|---|---|
| Susceptible to | Resistant to | Amoxicillin | Amoxicillin/ Clavulanate | ||
| GAS 1 | Penicillin, Ampicillin Amoxicillin | None of tested | 0.016 | 0.016 | Negative |
| NTHi | Amoxicillin/ clavulanate | Ampicillin Amoxicillin | 16 | 0.5 | Positive |
| GAS Culture Tested for Amoxicillin MIC | Amoxicillin MIC for GAS (µg/mL) |
|---|---|
| GAS alone | 0.016 |
| GAS + NTHi OMVsβL+AMX+1 (724 µg/mL) | 4 |
| GAS + NTHi OMVsβL+AMX+ (1.2 mg/mL) | 16 |
| GAS + NTHi OMVsβL+AMX+ (1.2 mg/mL) + clavulanate | 0.032 |
| GAS + NTHi OMVsβL− 2 (1.2 mg/mL) | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielaszewska, M.; Daniel, O.; Nyč, O.; Mellmann, A. In Vivo Secretion of β-Lactamase-Carrying Outer Membrane Vesicles as a Mechanism of β-Lactam Therapy Failure. Membranes 2021, 11, 806. https://doi.org/10.3390/membranes11110806
Bielaszewska M, Daniel O, Nyč O, Mellmann A. In Vivo Secretion of β-Lactamase-Carrying Outer Membrane Vesicles as a Mechanism of β-Lactam Therapy Failure. Membranes. 2021; 11(11):806. https://doi.org/10.3390/membranes11110806
Chicago/Turabian StyleBielaszewska, Martina, Ondřej Daniel, Otakar Nyč, and Alexander Mellmann. 2021. "In Vivo Secretion of β-Lactamase-Carrying Outer Membrane Vesicles as a Mechanism of β-Lactam Therapy Failure" Membranes 11, no. 11: 806. https://doi.org/10.3390/membranes11110806
APA StyleBielaszewska, M., Daniel, O., Nyč, O., & Mellmann, A. (2021). In Vivo Secretion of β-Lactamase-Carrying Outer Membrane Vesicles as a Mechanism of β-Lactam Therapy Failure. Membranes, 11(11), 806. https://doi.org/10.3390/membranes11110806