The Distribution and Role of the CFTR Protein in the Intracellular Compartments
Abstract
:1. Introduction
2. Structure and Function of CFTR in Cystic Fibrosis
3. Intracellular Trafficking of CFTR
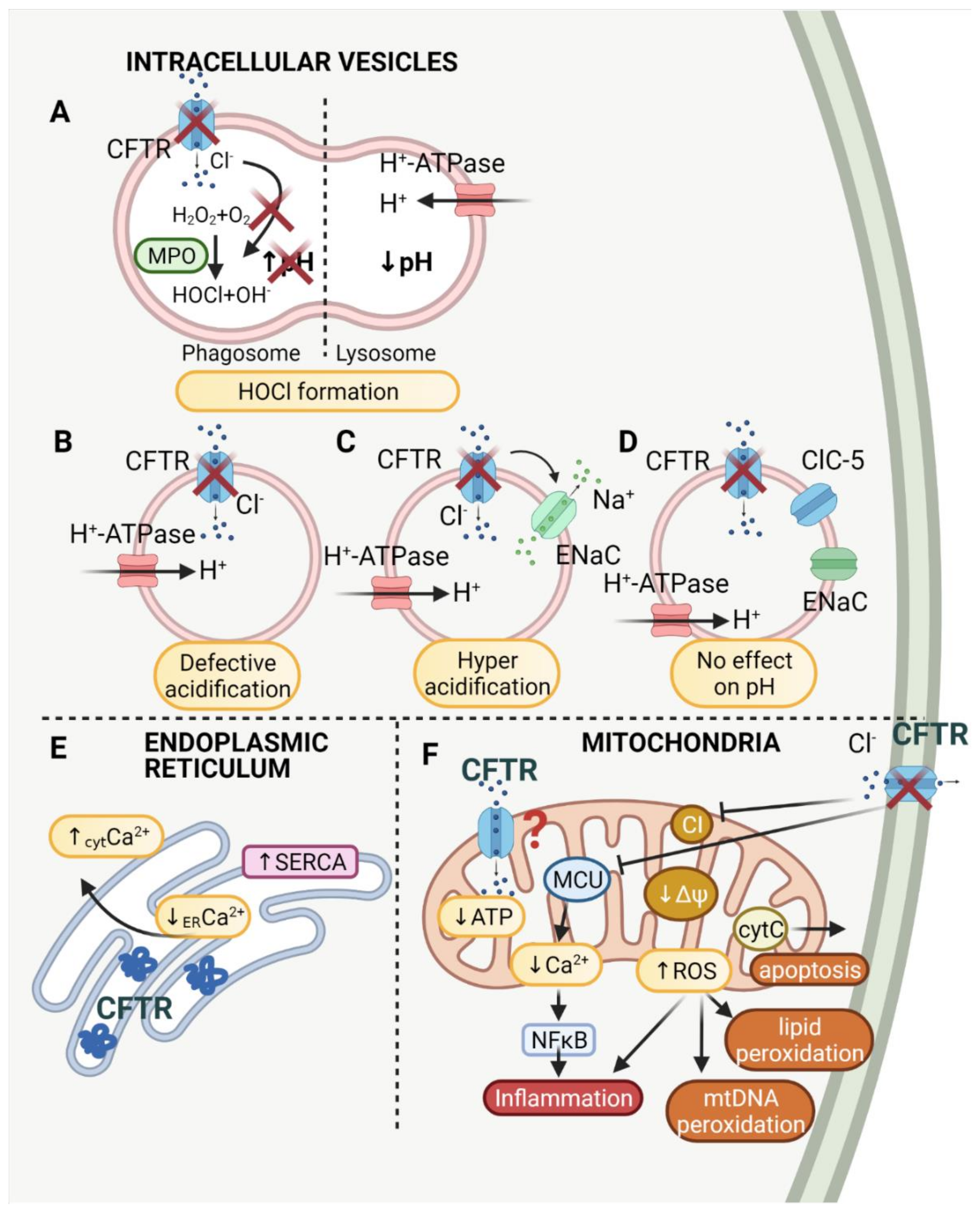
4. CFTR Intracellular Vesicles: Focus on Endosomes, Golgi, Lysosomes and pH
| Function | Organelle | Material | Reference |
|---|---|---|---|
| CFTR sustains lower pH | Golgi network Lysosomes Endosomes Phagosomes Lysosomes | CF nasal polyps HBE, CFT1, HeLa, JME T84 (human colon carcinoma) cells; Swiss 3T3 fibroblasts Primary monocyte-derived macrophages (MDMs); human isolated neutrophils; isolated murine alveolar macrophages; J774 (murine macrophage-like cell line); raw 264.7 (murine macrophage cells) RPE (retinal pigmented epithelial cells); ARPE−19 cells; murine bronchial epithelial cells; pancreatic adenocarcinoma cell line (CFPAC−1) | [82] [92] [100] [107,108,109] [110] [88,89,94,111] |
| CFTR sustains higher pH | Golgi network Endosomes | IB3−1 (human bronchial epithelial cell line derived from CF patient); C38, S9 (control cell lines); CFT−1 (cell line derived from tracheal epithelium of a CF patient) | [101,102,103] |
| No effect of CFTR on pH | Golgi network Endosomes Phagosomes Lysosomes | HeLa; airway epithelial cells (CFT1, CFT1−CFTR), Madin–Darby canine kidney (MDCK) cells, Swiss 3T3 fibroblasts, Calu−3 cells, SK−MES−1 cells; mouse alveolar macrophages, baby hamster kidney (BHK) cells; pancreatic adenocarcinoma cell lines (CFPAC−1); intestinal endocrine cells (L cells); monocytes derived from PBMC; murine alveolar macrophages; J774A.1 cell line; human and murine isolated epithelial cells; primary cultures of human airway epithelial cells; murine macrophages: RAW264.7; J774 | [93,95,96,97,98,99,112,113,114,115] |
| ROS production | Phagosomes | Isolated murine alveolar macrophages; J774 (murine macrophage-like cell line) | [109] |
| Cl− transport into vesicle lumen HOCl formation | Phagosomes | Human PMN (polymorphonuclear) neutrophils; human peripheral blood neutrophils; murine peripheral blood neutrophils | [116,117,118,119,120,121] |
| Ca2+ homeostasis | Endoplasmic reticulum | CFBE, HBE (human bronchial epithelial cells) | [122,123] |
5. CFTR in Phagolysosomes: pH Implications for Bacteria Killing
6. CFTR in Phagolysosomes: HOCl Production
7. CFTR and Mitochondria
8. CFTR and the Endoplasmic Reticulum
9. Intracellular Crosstalk and Interdependencies
10. Therapies
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaczmarek, L.K.; Jonas, E.A. Ion Channels on Intracellular Organelles. In Molecular Insights into Ion Channel Biology in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2004; Volume 32, pp. 433–458. [Google Scholar] [CrossRef]
- Takeshima, H.; Venturi, E.; Sitsapesan, R. New and Notable Ion-Channels in the Sarcoplasmic/Endoplasmic Reticulum: Do They Support the Process of Intracellular Ca2+ Release? J. Physiol. 2015, 593, 3241–3251. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Gu, M.; Xu, H. Lysosomal Ion Channels as Decoders of Cellular Signals. Trends Biochem. Sci. 2019, 44, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Matzke, A.J.M.; Weiger, T.M.; Matzke, M. Ion Channels at the Nucleus: Electrophysiology Meets the Genome. Mol. Plant 2010, 3, 642–652. [Google Scholar] [CrossRef]
- O’Rourke, B. Mitochondrial Ion Channels. Annu. Rev. Physiol. 2007, 69, 19–49. [Google Scholar] [CrossRef] [Green Version]
- Bednarczyk, P.; Kicinska, A.; Laskowski, M.; Kulawiak, B.; Kampa, R.; Walewska, A.; Krajewska, M.; Jarmuszkiewicz, W.; Szewczyk, A. Evidence for a Mitochondrial ATP-Regulated Potassium Channel in Human Dermal Fibroblasts. Biochim. Biophys. Acta. Bioenerg. 2018, 1859, 309–318. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Koziel, A.; Jarmuszkiewicz, W.; Szewczyk, A. Large-Conductance Ca2+-Activated Potassium Channel in Mitochondria of Endothelial EA.Hy926 Cells. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1415-27. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Kowalczyk, J.E.; Beresewicz, M.; Dołowy, K.; Szewczyk, A.; Zabłocka, B. Identification of a Voltage-Gated Potassium Channel in Gerbil Hippocampal Mitochondria. Biochem. Biophys. Res. Commun. 2010, 397, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Ponnalagu, D.; Singh, H. Anion Channels of Mitochondria. Handb. Exp. Pharmacol. 2017, 240, 71–101. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.H.; Mohiuddin, S.S. Biochemistry, Chloride Channels; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A. Molecular Structure and Physiological Function of Chloride Channels. Physiol. Rev. 2002, 82, 503–568. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, T.J.; Pusch, M. CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol. Rev. 2018, 98, 1493–1590. [Google Scholar] [CrossRef]
- Al Khamici, H.; Brown, L.J.; Hossain, K.R.; Hudson, A.L.; Sinclair-Burton, A.A.; Ng, J.P.M.; Daniel, E.L.; Hare, J.E.; Cornell, B.A.; Curmi, P.M.G.; et al. Members of the Chloride Intracellular Ion Channel Protein Family Demonstrate Glutaredoxin-like Enzymatic Activity. PLoS ONE 2015, 10, e115699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, R.H. Challenging Accepted Ion Channel Biology: P64 and the CLIC Family of Putative Intracellular Anion Channel Proteins (Review). Mol. Membr. Biol. 2003, 20, 1–11. [Google Scholar] [CrossRef]
- Littler, D.R.; Harrop, S.J.; Goodchild, S.C.; Phang, J.M.; Mynott, A.V.; Jiang, L.; Valenzuela, S.M.; Mazzanti, M.; Brown, L.J.; Breit, S.N.; et al. The Enigma of the CLIC Proteins: Ion Channels, Redox Proteins, Enzymes, Scaffolding Proteins? FEBS Lett. 2010, 584, 2093–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H. Two Decades with Dimorphic Chloride Intracellular Channels (CLICs). FEBS Lett. 2010, 584, 2112–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gururaja Rao, S.; Ponnalagu, D.; Patel, N.J.; Singh, H. Three Decades of Chloride Intracellular Channel Proteins: From Organelle to Organ Physiology. Curr. Protoc. Pharmacol. 2018, 80, 11.21.1–11.21.17. [Google Scholar] [CrossRef] [PubMed]
- Stauber, T.; Jentsch, T.J. Chloride in Vesicular Trafficking and Function. Annu. Rev. Physiol. 2013, 75, 453–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdivieso, A.G.; Santa-Coloma, T.A. CFTR Activity and Mitochondrial Function. Redox Biol. 2013, 1, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Ashley, R.H. Redox Regulation of CLIC1 by Cysteine Residues Associated with the Putative Channel Pore. Biophys. J. 2006, 90, 1628–1638. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Ashley, R.H. CLIC4 (P64H1) and Its Putative Transmembrane Domain Form Poorly Selective, Redox-Regulated Ion Channels. Mol. Membr. Biol. 2007, 24, 41–52. [Google Scholar] [CrossRef]
- Duan, D.D. Phenomics of Cardiac Chloride Channels. Compr. Physiol. 2013, 3, 667–692. [Google Scholar] [CrossRef] [Green Version]
- Duan, D.D. The ClC-3 Chloride Channels in Cardiovascular Disease. Acta Pharmacol. Sin. 2011, 32, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Gururaja Rao, S.; Patel, N.J.; Singh, H. Intracellular Chloride Channels: Novel Biomarkers in Diseases. Front. Physiol. 2020, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, S.Y.; Ye, L.L.; Duan, L.L.M.; Liu, L.H.; Ge, Z.D.; Auchampach, J.A.; Gross, G.J.; Duan, D.D. Characterization of a Critical Role for CFTR Chloride Channels in Cardioprotection against Ischemia/Reperfusion Injury. Acta Pharmacol. Sin. 2011, 32, 824–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ntimbane, T.; Comte, B.; Mailhot, G.; Berthiaume, Y.; Poitout, V.; Prentki, M.; Rabasa-Lhoret, R.; Levy, E. Cystic Fibrosis-Related Diabetes: From CFTR Dysfunction to Oxidative Stress. Clin. Biochem. Rev. 2009, 30, 153–177. [Google Scholar]
- Bradbury, N.A. Intracellular CFTR: Localization and Function. Physiol. Rev. 1999, 79 (Suppl. 1), S175–S191. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC Transporters: The Power to Change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadsby, D.C.; Vergani, P.; Csanády, L. The ABC Protein Turned Chloride Channel Whose Failure Causes Cystic Fibrosis. Nature 2006, 440, 477–483. [Google Scholar] [CrossRef]
- Linsdell, P.; Evagelidis, A.; Hanrahan, J.W. Molecular Determinants of Anion Selectivity in the Cystic Fibrosis Transmembrane Conductance Regulator Chloride Channel Pore. Biophys. J. 2000, 78, 2973–2982. [Google Scholar] [CrossRef] [Green Version]
- Davidson, D.J.; Porteous, D.J. Genetics and Pulmonary Medicine. 1. The Genetics of Cystic Fibrosis Lung Disease. Thorax 1998, 53, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L. Identification of the Cystic Fibrosis Gene: Cloning and Characterization of Complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Vankeerberghen, A.; Cuppens, H.; Cassiman, J.-J. The Cystic Fibrosis Transmembrane Conductance Regulator: An Intriguing Protein with Pleiotropic Functions. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2002, 1, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Moran, O. The Gating of the CFTR Channel. Cell. Mol. Life Sci. 2017, 74, 85–92. [Google Scholar] [CrossRef]
- Berger, H.A.; Anderson, M.P.; Gregory, R.J.; Thompson, S.; Howard, P.W.; Maurer, R.A.; Mulligan, R.; Smith, A.E.; Welsh, M.J. Identification and Regulation of the Cystic Fibrosis Transmembrane Conductance Regulator-Generated Chloride Channel. J. Clin. Investig. 1991, 88, 1422–1431. [Google Scholar] [CrossRef] [Green Version]
- Corradi, V.; Vergani, P.; Tieleman, D.P. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR): CLOSED AND OPEN STATE CHANNEL MODELS. J. Biol. Chem. 2015, 290, 22891–22906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegedus, T.; Aleksandrov, A.; Mengos, A.; Cui, L.; Jensen, T.J.; Riordan, J.R. Role of Individual R Domain Phosphorylation Sites in CFTR Regulation by Protein Kinase, A. Biochim. Biophys. Acta 2009, 1788, 1341–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Zhang, Z.; Csanády, L.; Gadsby, D.C.; Chen, J. Molecular Structure of the Human CFTR Ion Channel. Cell 2017, 169, 85–95.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabcharani, J.A.; Linsdell, P.; Hanrahan, J.W. Halide Permeation in Wild-Type and Mutant Cystic Fibrosis Transmembrane Conductance Regulator Chloride Channels. J. Gen. Physiol. 1997, 110, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Linsdell, P. Relationship between Anion Binding and Anion Permeability Revealed by Mutagenesis within the Cystic Fibrosis Transmembrane Conductance Regulator Chloride Channel Pore. J. Physiol. 2001, 531 Pt 1, 51–66. [Google Scholar] [CrossRef]
- Linsdell, P.; Tabcharani, J.A.; Rommens, J.M.; Hou, Y.X.; Chang, X.B.; Tsui, L.C.; Riordan, J.R.; Hanrahan, J.W. Permeability of Wild-Type and Mutant Cystic Fibrosis Transmembrane Conductance Regulator Chloride Channels to Polyatomic Anions. J. Gen. Physiol. 1997, 110, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridges, R.J. Mechanisms of Bicarbonate Secretion: Lessons from the Airways. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Frizzell, R.A.; Hanrahan, J.W. Physiology of Epithelial Chloride and Fluid Secretion. Cold Spring Harb. Perspect. Med. 2012, 2, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Novak, I.; Wang, J.; Henriksen, K.L.; Haanes, K.A.; Krabbe, S.; Nitschke, R.; Hede, S.E. Pancreatic Bicarbonate Secretion Involves Two Proton Pumps. J. Biol. Chem. 2011, 286, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinton, P.M. Cystic Fibrosis: Impaired Bicarbonate Secretion and Mucoviscidosis. Lancet 2008, 372, 415–417. [Google Scholar] [CrossRef] [Green Version]
- Zajac, M.; Dreano, E.; Edwards, A.; Planelles, G.; Sermet-Gaudelus, I. Airway Surface Liquid PH Regulation in Airway Epithelium Current Understandings and Gaps in Knowledge. Int. J. Mol. Sci. 2021, 22, 3384. [Google Scholar] [CrossRef]
- Lorentzen, D.; Durairaj, L.; Pezzulo, A.A.; Nakano, Y.; Launspach, J.; Stoltz, D.A.; Zamba, G.; McCray, P.B.J.; Zabner, J.; Welsh, M.J.; et al. Concentration of the Antibacterial Precursor Thiocyanate in Cystic Fibrosis Airway Secretions. Free Radic. Biol. Med. 2011, 50, 1144–1150. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.J.; Soong, G.; Bryan, R.; Saba, S.; Prince, A. Activation of NF-KappaB in Airway Epithelial Cells Is Dependent on CFTR Trafficking and Cl- Channel Function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L71–L78. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Bilodeau, G.; Ouellet, C.; Liao, J.; Hanrahan, J.W. Oxidant Stress Suppresses CFTR Expression. Am. J. Physiol. Cell Physiol. 2006, 290, C262–C270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, Y.; Sun, L.; Yuan, C.; Jiang, M.; Lou, Q.; Xu, Y. CFTR Ameliorates High Glucose-Induced Oxidative Stress and Inflammation by Mediating the NF-κB and MAPK Signaling Pathways in Endothelial Cells. Int. J. Mol. Med. 2018, 41, 3501–3508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.M.; Quinton, P.M. Functional Interaction of CFTR and ENaC in Sweat Glands. Pflug. Arch. 2003, 445, 499–503. [Google Scholar] [CrossRef]
- Benedetto, R.; Ousingsawat, J.; Wanitchakool, P.; Zhang, Y.; Holtzman, M.J.; Amaral, M.; Rock, J.R.; Schreiber, R.; Kunzelmann, K. Epithelial Chloride Transport by CFTR Requires TMEM16A. Sci. Rep. 2017, 7, 12397. [Google Scholar] [CrossRef] [Green Version]
- Fong, P. CFTR-SLC26 Transporter Interactions in Epithelia. Biophys. Rev. 2012, 4, 107–116. [Google Scholar] [CrossRef]
- Schrijver, I.; Pique, L.; Graham, S.; Pearl, M.; Cherry, A.; Kharrazi, M. The Spectrum of CFTR Variants in Nonwhite Cystic Fibrosis Patients: Implications for Molecular Diagnostic Testing. J. Mol. Diagn. 2016, 18, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2019, 10, 1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boeck, K.; Zolin, A.; Cuppens, H.; Olesen, H.V.; Viviani, L. The Relative Frequency of CFTR Mutation Classes in European Patients with Cystic Fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2014, 13, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elborn, J.S. Cystic Fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- De Boeck, K.; Amaral, M.D. Progress in Therapies for Cystic Fibrosis. Lancet. Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef]
- Marson, F.A.L.; Bertuzzo, C.S.; Ribeiro, J.D. Classification of CFTR Mutation Classes. Lancet. Respir. Med. 2016, e37–e38. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, D.N.; Welsh, M.J. Structure and Function of the CFTR Chloride Channel. Physiol. Rev. 1999, 79 (Suppl. 1), 23–45. [Google Scholar] [CrossRef]
- Yoshimura, K.; Nakamura, H.; Trapnell, B.C.; Chu, C.S.; Dalemans, W.; Pavirani, A.; Lecocq, J.P.; Crystal, R.G. Expression of the Cystic Fibrosis Transmembrane Conductance Regulator Gene in Cells of Non-Epithelial Origin. Nucleic Acids Res. 1991, 19, 5417–5423. [Google Scholar] [CrossRef] [PubMed]
- Levesque, P.C.; Hart, P.J.; Hume, J.R.; Kenyon, J.L.; Horowitz, B. Expression of Cystic Fibrosis Transmembrane Regulator Cl- Channels in Heart. Circ. Res. 1992, 71, 1002–1007. [Google Scholar] [CrossRef] [Green Version]
- Mulberg, A.E.; Wiedner, E.B.; Bao, X.; Marshall, J.; Jefferson, D.M.; Altschuler, S.M. Cystic Fibrosis Transmembrane Conductance Regulator Protein Expression in Brain. Neuroreport 1994, 5, 1684–1688. [Google Scholar] [CrossRef]
- Kulka, M.; Gilchrist, M.; Duszyk, M.; Befus, A.D. Expression and Functional Characterization of CFTR in Mast Cells. J. Leukoc. Biol. 2002, 71, 54–64. [Google Scholar] [PubMed]
- Lange, T.; Jungmann, P.; Haberle, J.; Falk, S.; Duebbers, A.; Bruns, R.; Ebner, A.; Hinterdorfer, P.; Oberleithner, H.; Schillers, H. Reduced Number of CFTR Molecules in Erythrocyte Plasma Membrane of Cystic Fibrosis Patients. Mol. Membr. Biol. 2006, 23, 317–323. [Google Scholar] [CrossRef]
- Thul, P.J.; Lindskog, C. The Human Protein Atlas: A Spatial Map of the Human Proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Cabrini, G.; Rimessi, A.; Borgatti, M.; Lampronti, I.; Finotti, A.; Pinton, P.; Gambari, R. Role of Cystic Fibrosis Bronchial Epithelium in Neutrophil Chemotaxis. Front. Immunol. 2020, 11, 1438. [Google Scholar] [CrossRef] [PubMed]
- Koivula, F.N.M.; McClenaghan, N.H.; Harper, A.G.S.; Kelly, C. Islet-Intrinsic Effects of CFTR Mutation. Diabetologia 2016, 59, 1350–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Hanukoglu, I. Mapping the Sites of Localization of Epithelial Sodium Channel (ENaC) and CFTR in Segments of the Mammalian Epididymis. J. Mol. Histol. 2019, 50, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xiong, X.; Helm, A.; Kimani, K.; Bragin, A.; Skach, W.R. Co- and Posttranslational Translocation Mechanisms Direct Cystic Fibrosis Transmembrane Conductance Regulator N Terminus Transmembrane Assembly. J. Biol. Chem. 1998, 273, 568–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Janich, S.; Cohn, J.A.; Wilson, J.M. The Common Variant of Cystic Fibrosis Transmembrane Conductance Regulator Is Recognized by Hsp70 and Degraded in a Pre-Golgi Nonlysosomal Compartment. Proc. Natl. Acad. Sci. USA 1993, 90, 9480–9484. [Google Scholar] [CrossRef] [Green Version]
- Pranke, I.M.; Sermet-Gaudelus, I. Biosynthesis of Cystic Fibrosis Transmembrane Conductance Regulator. Int. J. Biochem. Cell Biol. 2014, 52, 26–38. [Google Scholar] [CrossRef]
- Lukacs, G.L.; Mohamed, A.; Kartner, N.; Chang, X.B.; Riordan, J.R.; Grinstein, S. Conformational Maturation of CFTR but Not Its Mutant Counterpart (Delta F508) Occurs in the Endoplasmic Reticulum and Requires ATP. EMBO J. 1994, 13, 6076–6086. [Google Scholar] [CrossRef] [PubMed]
- Estabrooks, S.; Brodsky, J.L. Regulation of CFTR Biogenesis by the Proteostatic Network and Pharmacological Modulators. Int. J. Mol. Sci. 2020, 21, 452. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, E.L.; Rosser, M.F.N.; Cyr, D.M. The Role of the UPS in Cystic Fibrosis. BMC Biochem. 2007, 8 (Suppl. 1), 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, S.H.; Gee, H.Y.; Kim, Y.; Piao, H.; Kim, J.; Kang, C.M.; Lee, G.; Mook-Jung, I.; Lee, Y.; Cho, J.W.; et al. Specific Autophagy and ESCRT Components Participate in the Unconventional Secretion of CFTR. Autophagy 2018, 14, 1761–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Gee, H.Y.; Lee, M.G. Unconventional Protein Secretion—New Insights into the Pathogenesis and Therapeutic Targets of Human Diseases. J. Cell Sci. 2018, 131, jcs213686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gee, H.Y.; Noh, S.H.; Tang, B.L.; Kim, K.H.; Lee, M.G. Rescue of ΔF508-CFTR Trafficking via a GRASP-Dependent Unconventional Secretion Pathway. Cell 2011, 146, 746–760. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, C.A.; Frizzell, R.A. The Role of Regulated CFTR Trafficking in Epithelial Secretion. Am. J. Physiol.-Cell Physiol. 2003, 285, C1–C18. [Google Scholar] [CrossRef] [Green Version]
- Lukacs, G.L.; Segal, G.; Kartner, N.; Grinstein, S.; Zhang, F. Constitutive Internalization of Cystic Fibrosis Transmembrane Conductance Regulator Occurs via Clathrin-Dependent Endocytosis and Is Regulated by Protein Phosphorylation. Biochem. J. 1997, 328, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barasch, J.; Gershon, M.D.; Nunez, E.A.; Tamir, H.; al-Awqati, Q. Thyrotropin Induces the Acidification of the Secretory Granules of Parafollicular Cells by Increasing the Chloride Conductance of the Granular Membrane. J. Cell Biol. 1988, 107 Pt 1, 2137–2147. [Google Scholar] [CrossRef] [Green Version]
- Barasch, J.; Kiss, B.; Prince, A.; Saiman, L.; Gruenert, D.; al-Awqati, Q. Defective Acidification of Intracellular Organelles in Cystic Fibrosis. Nature 1991, 352, 70–73. [Google Scholar] [CrossRef]
- Souza-Menezes, J.; da Silva Feltran, G.; Morales, M.M. CFTR and TNR-CFTR Expression and Function in the Kidney. Biophys. Rev. 2014, 6, 227–236. [Google Scholar] [CrossRef]
- Jouret, F.; Bernard, A.; Hermans, C.; Dom, G.; Terryn, S.; Leal, T.; Lebecque, P.; Cassiman, J.-J.; Scholte, B.J.; de Jonge, H.R.; et al. Cystic Fibrosis Is Associated with a Defect in Apical Receptor-Mediated Endocytosis in Mouse and Human Kidney. J. Am. Soc. Nephrol. 2007, 18, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Devuyst, O.; Luciani, A. Chloride Transporters and Receptor-Mediated Endocytosis in the Renal Proximal Tubule. J. Physiol. 2015, 593, 4151–4164. [Google Scholar] [CrossRef] [Green Version]
- Jouret, F.; Courtoy, P.J.; Devuyst, O. Segmental and Subcellular Distribution of CFTR in the Kidney. Methods Mol. Biol. 2011, 741, 285–299. [Google Scholar] [CrossRef]
- Mindell, J.A. Lysosomal Acidification Mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Lu, W.; Guha, S.; Baltazar, G.C.; Coffey, E.E.; Laties, A.M.; Rubenstein, R.C.; Reenstra, W.W.; Mitchell, C.H. Cystic Fibrosis Transmembrane Conductance Regulator Contributes to Reacidification of Alkalinized Lysosomes in RPE Cells. Am. J. Physiol.-Cell Physiol. 2012, 303. [Google Scholar] [CrossRef] [Green Version]
- Guha, S.; Liu, J.; Baltazar, G.; Laties, A.M.; Mitchell, C.H. Rescue of Compromised Lysosomes Enhances Degradation of Photoreceptor Outer Segments and Reduces Lipofuscin-like Autofluorescence in Retinal Pigmented Epithelial Cells. Adv. Exp. Med. Biol. 2014, 801, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Sonawane, N.D.; Thiagarajah, J.R.; Verkman, A.S. Chloride Concentration in Endosomes Measured Using a Ratioable Fluorescent Cl- Indicator: Evidence for Chloride Accumulation during Acidification. J. Biol. Chem. 2002, 277, 5506–5513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukacs, G.L.; Chang, X.B.; Kartner, N.; Rotstein, O.D.; Riordan, J.R.; Grinstein, S. The Cystic Fibrosis Transmembrane Regulator Is Present and Functional in Endosomes. Role as a Determinant of Endosomal PH. J. Biol. Chem. 1992, 267, 14568–14572. [Google Scholar] [CrossRef]
- Chandy, G.; Grabe, M.; Moore, H.P.H.; Machen, T.E. Proton Leak and CFTR in Regulation of Golgi PH in Respiratory Epithelial Cells. Am. J. Physiol.-Cell Physiol. 2001, 281, 908–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Root, K.V.; Engelhardt, J.F.; Post, M.; Wilson, J.W.; Van Dyke, R.W. CFTR Does Not Alter Acidification of L Cell Endosomes. Biochem. Biophys. Res. Commun. 1994, 205, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Van Dyke, R.W.; Root, K.V.; Schreiber, J.H.; Wilson, J.M. Role of CFTR in Lysosome Acidification. Biochem. Biophys. Res. Commun. 1992, 184, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Dunn, K.W.; Park, J.; Semrad, C.E.; Gelman, D.L.; Shevell, T.; McGraw, T.E. Regulation of Endocytic Trafficking and Acidification Are Independent of the Cystic Fibrosis Transmembrane Regulator. J. Biol. Chem. 1994, 269, 5336–5345. [Google Scholar] [CrossRef]
- Seksek, O.; Biwersi, J.; Verkman, A.S. Evidence against Defective Trans-Golgi Acidification in Cystic Fibrosis. J. Biol. Chem. 1996, 271, 15542–15548. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.A.; Hill, W.G.; Weisz, O.A. Evidence against the Acidification Hypothesis in Cystic Fibrosis. Am. J. Physiol. Cell Physiol. 2000, 279, C1088–C1099. [Google Scholar] [CrossRef]
- Machen, T.E.; Chandy, G.; Wu, M.; Grabe, M.; Moore, H.P. Cystic Fibrosis Transmembrane Conductance Regulator and H+ Permeability in Regulation of Golgi PH. J. Pancreas 2001, 2, 229–236. [Google Scholar]
- Barriere, H.; Bagdany, M.; Bossard, F.; Okiyoneda, T.; Wojewodka, G.; Gruenert, D.; Radzioch, D.; Lukacs, G.L. Revisiting the Role of Cystic Fibrosis Transmembrane Conductance Regulator and Counterion Permeability in the PH Regulation of Endocytic Organelles. Mol. Biol. Cell 2009, 20, 3125–3141. [Google Scholar] [CrossRef] [Green Version]
- Biwersi, J.; Verkman, A.S. Functional CFTR in Endosomal Compartment of CFTR-Expressing Fibroblasts and T84 Cells. Am. J. Physiol. 1994, 266 Pt 1, C149–C156. [Google Scholar] [CrossRef]
- Poschet, J.F.; Boucher, J.C.; Tatterson, L.; Skidmore, J.; Van Dyke, R.W.; Deretic, V. Molecular Basis for Defective Glycosylation and Pseudomonas Pathogenesis in Cystic Fibrosis Lung. Proc. Natl. Acad. Sci. USA 2001, 98, 13972–13977. [Google Scholar] [CrossRef] [Green Version]
- Poschet, J.F.; Skidmore, J.; Boucher, J.C.; Firoved, A.M.; Van Dyke, R.W.; Deretic, V. Hyperacidification of Cellubrevin Endocytic Compartments and Defective Endosomal Recycling in Cystic Fibrosis Respiratory Epithelial Cells. J. Biol. Chem. 2002, 277, 13959–13965. [Google Scholar] [CrossRef] [Green Version]
- Poschet, J.F.; Fazio, J.A.; Timmins, G.S.; Ornatowski, W.; Perkett, E.; Delgado, M.; Deretic, V. Endosomal Hyperacidification in Cystic Fibrosis Is Due to Defective Nitric Oxide-Cylic GMP Signalling Cascade. EMBO Rep. 2006, 7, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Jakab, R.L.; Collaco, A.M.; Ameen, N.A. Physiological Relevance of Cell-Specific Distribution Patterns of CFTR, NKCC1, NBCe1, and NHE3 along the Crypt-Villus Axis in the Intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G82–G98. [Google Scholar] [CrossRef] [Green Version]
- Ameen, N.A.; van Donselaar, E.; Posthuma, G.; de Jonge, H.; McLaughlin, G.; Geuze, H.J.; Marino, C.; Peters, P.J. Subcellular Distribution of CFTR in Rat Intestine Supports a Physiologic Role for CFTR Regulation by Vesicle Traffic. Histochem. Cell Biol. 2000, 114, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Collaco, A.M.; Geibel, P.; Lee, B.S.; Geibel, J.P.; Ameen, N.A. Functional Vacuolar ATPase (V-ATPase) Proton Pumps Traffic to the Enterocyte Brush Border Membrane and Require CFTR. Am. J. Physiol. Cell Physiol. 2013, 305, C981–C996. [Google Scholar] [CrossRef] [Green Version]
- Poerio, N.; De Santis, F.; Rossi, A.; Ranucci, S.; De Fino, I.; Henriquez, A.; D’Andrea, M.M.; Ciciriello, F.; Lucidi, V.; Nisini, R.; et al. Liposomes Loaded With Phosphatidylinositol 5-Phosphate Improve the Antimicrobial Response to Pseudomonas Aeruginosa in Impaired Macrophages From Cystic Fibrosis Patients and Limit Airway Inflammatory Response. Front. Immunol. 2020, 11, 532225. [Google Scholar] [CrossRef]
- Hayes, E.; Murphy, M.P.; Pohl, K.; Browne, N.; McQuillan, K.; Saw, L.E.; Foley, C.; Gargoum, F.; McElvaney, O.J.; Hawkins, P.; et al. Altered Degranulation and PH of Neutrophil Phagosomes Impacts Antimicrobial Efficiency in Cystic Fibrosis. Front. Immunol. 2020, 11, 600033. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Grassmé, H.; Döring, G.; Gulbins, E. Alterations in Ceramide Concentration and PH Determine the Release of Reactive Oxygen Species by Cftr-Deficient Macrophages on Infection. J. Immunol. 2010, 184, 5104–5111. [Google Scholar] [CrossRef] [Green Version]
- Lamothe, J.; Valvano, M.A. Burkholderia Cenocepacia-Induced Delay of Acidification and Phagolysosomal Fusion in Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)-Defective Macrophages. Microbiology 2008, 154 Pt 12, 3825–3834. [Google Scholar] [CrossRef] [Green Version]
- Teichgräber, V.; Ulrich, M.; Endlich, N.; Riethmüller, J.; Wilker, B.; De Oliveira-Munding, C.C.; van Heeckeren, A.M.; Barr, M.L.; von Kürthy, G.; Schmid, K.W.; et al. Ceramide Accumulation Mediates Inflammation, Cell Death and Infection Susceptibility in Cystic Fibrosis. Nat. Med. 2008, 14, 382–391. [Google Scholar] [CrossRef]
- Law, S.M.; Stanfield, S.J.; Hardisty, G.R.; Dransfield, I.; Campbell, C.J.; Gray, R.D. Human Cystic Fibrosis Monocyte Derived Macrophages Display No Defect in Acidification of Phagolysosomes When Measured by Optical Nanosensors. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2020, 19, 203–210. [Google Scholar] [CrossRef]
- Haggie, P.M.; Verkman, A.S. Cystic Fibrosis Transmembrane Conductance Regulator-Independent Phagosomal Acidification in Macrophages. J. Biol. Chem. 2007, 282, 31422–31428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haggie, P.M.; Verkman, A.S. Unimpaired Lysosomal Acidification in Respiratory Epithelial Cells in Cystic Fibrosis *. J. Biol. Chem. 2009, 284, 7681–7686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, B.E.; Huynh, K.K.; Brodovitch, A.; Jabs, S.; Stauber, T.; Jentsch, T.J.; Grinstein, S. A Cation Counterflux Supports Lysosomal Acidification. J. Cell Biol. 2010, 189, 1171–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Painter, R.G.; Valentine, V.G.; Lanson, N.A.J.; Leidal, K.; Zhang, Q.; Lombard, G.; Thompson, C.; Viswanathan, A.; Nauseef, W.M.; Wang, G.; et al. CFTR Expression in Human Neutrophils and the Phagolysosomal Chlorination Defect in Cystic Fibrosis. Biochemistry 2006, 45, 10260–10269. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.P.; Zhou, Y.; Song, K.; Hodges, C.A.; Drumm, M.L.; Wang, G. Neutrophil-Mediated Phagocytic Host Defense Defect in Myeloid Cftr-Inactivated Mice. PLoS ONE 2014, 9, e106813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, H.P.; Valentine, V.G.; Wang, G. CFTR Targeting during Activation of Human Neutrophils. J. Leukoc. Biol. 2016, 100, 1413–1424. [Google Scholar] [CrossRef]
- Dickerhof, N.; Isles, V.; Pattemore, P.; Hampton, M.B.; Kettle, A.J. Exposure of Pseudomonas Aeruginosa to Bactericidal Hypochlorous Acid during Neutrophil Phagocytosis Is Compromised in Cystic Fibrosis. J. Biol. Chem. 2019, 294, 13502–13514. [Google Scholar] [CrossRef] [PubMed]
- Painter, R.G.; Marrero, L.; Lombard, G.A.; Valentine, V.G.; Nauseef, W.M.; Wang, G. CFTR-Mediated Halide Transport in Phagosomes of Human Neutrophils. J. Leukoc. Biol. 2010, 87, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Aiken, M.L.; Painter, R.G.; Zhou, Y.; Wang, G. Chloride Transport in Functionally Active Phagosomes Isolated from Human Neutrophils. Free Radic. Biol. Med. 2012, 53, 2308–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippe, R.; Antigny, F.; Buscaglia, P.; Norez, C.; Becq, F.; Frieden, M.; Mignen, O. SERCA and PMCA Pumps Contribute to the Deregulation of Ca2+ Homeostasis in Human CF Epithelial Cells. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 892–903. [Google Scholar] [CrossRef]
- Philippe, R.; Antigny, F.; Buscaglia, P.; Norez, C.; Huguet, F.; Castelbou, C.; Trouvé, P.; Becq, F.; Frieden, M.; Férec, C.; et al. Calumenin Contributes to ER-Ca2+ Homeostasis in Bronchial Epithelial Cells Expressing WT and F508del Mutated CFTR and to F508del-CFTR Retention. Cell Calcium 2017, 62, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.R.; Doull, I.J.M.; Dewitt, S.; Hallett, M.B. Reduced IC3b-Mediated Phagocytotic Capacity of Pulmonary Neutrophils in Cystic Fibrosis. Clin. Exp. Immunol. 2005, 142, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Jarry, T.M.; Cheung, A.L. Staphylococcus Aureus Escapes More Efficiently from the Phagosome of a Cystic Fibrosis Bronchial Epithelial Cell Line than from Its Normal Counterpart. Infect. Immun. 2006, 74, 2568–2577. [Google Scholar] [CrossRef] [Green Version]
- Abdulrahman, B.A.; Khweek, A.A.; Akhter, A.; Caution, K.; Tazi, M.; Hassan, H.; Zhang, Y.; Rowland, P.D.; Malhotra, S.; Aeffner, F.; et al. Depletion of the Ubiquitin-Binding Adaptor Molecule SQSTM1/P62 from Macrophages Harboring Cftr ΔF508 Mutation Improves the Delivery of Burkholderia Cenocepacia to the Autophagic Machinery. J. Biol. Chem. 2013, 288, 2049–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Wu, Y.; Riehle, A.; Ma, J.; Kamler, M.; Gulbins, E.; Grassmé, H. Staphylococcus Aureus Survives in Cystic Fibrosis Macrophages, Forming a Reservoir for Chronic Pneumonia. Infect. Immun. 2017, 85, e00883-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Porto, P.; Cifani, N.; Guarnieri, S.; Di Domenico, E.G.; Mariggiò, M.A.; Spadaro, F.; Guglietta, S.; Anile, M.; Venuta, F.; Quattrucci, S.; et al. Dysfunctional Cftr Alters the Bactericidal Activity of Human Macrophages against Pseudomonas Aeruginosa. PLoS ONE 2011, 6, e19970. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Song, K.; Painter, R.G.; Aiken, M.; Reiser, J.; Stanton, B.A.; Nauseef, W.M.; Wang, G. CFTR Recruitment to Phagosomes in Neutrophils. J. Innate Immun. 2013, 5, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shrestha, C.L.; Kopp, B.T. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulators Have Differential Effects on Cystic Fibrosis Macrophage Function. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Swanson, J. CFTR: Helping to Acidify Macrophage Lysosomes. Nat. Cell Biol. 2006, 8, 908–909. [Google Scholar] [CrossRef]
- Di, A.; Brown, M.E.; Deriy, L.V.; Li, C.; Szeto, F.L.; Chen, Y.; Huang, P.; Tong, J.; Naren, A.P.; Bindokas, V.; et al. CFTR Regulates Phagosome Acidification in Macrophages and Alters Bactericidal Activity. Nat. Cell Biol. 2006, 8, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Deriy, L.V.; Gomez, E.A.; Zhang, G.; Beacham, D.W.; Hopson, J.A.; Gallan, A.J.; Shevchenko, P.D.; Bindokas, V.P.; Nelson, D.J. Disease-Causing Mutations in the Cystic Fibrosis Transmembrane Conductance Regulator Determine the Functional Responses of Alveolar Macrophages. J. Biol. Chem. 2009, 284, 35926–35938. [Google Scholar] [CrossRef] [Green Version]
- Haggie, P.M.; Verkman, A.S. Defective Organellar Acidification as a Cause of Cystic Fibrosis Lung Disease: Reexamination of a Recurring Hypothesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L859–L867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Weert-van Leeuwen, P.B.; Van Meegen, M.A.; Speirs, J.J.; Pals, D.J.; Rooijakkers, S.H.M.; Van Der Ent, C.K.; Terheggen-Lagro, S.W.J.; Arets, H.G.M.; Beekman, J.M. Optimal Complement-Mediated Phagocytosis of Pseudomonas Aeruginosa by Monocytes Is Cystic Fibrosis Transmembrane Conductance Regulator-Dependent. Am. J. Respir. Cell Mol. Biol. 2013, 49, 463–470. [Google Scholar] [CrossRef]
- Turton, K.B.; Ingram, R.J.; Valvano, M.A. Macrophage Dysfunction in Cystic Fibrosis: Nature or Nurture? J. Leukoc. Biol. 2021, 109, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Trudel, S.; Brouillard, F.; Bouillaud, F.; Colas, J.; Nguyen-Khoa, T.; Ollero, M.; Edelman, A.; Fritsch, J. Cystic Fibrosis Transmembrane Regulator Inhibitors CFTR(Inh)-172 and GlyH-101 Target Mitochondrial Functions, Independently of Chloride Channel Inhibition. J. Pharmacol. Exp. Ther. 2010, 333, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, D.; Winterbourn, C.C. Immunology. Lethal Weapons. Science 2002, 296, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; DeCoursey, T.E. Charge Compensation during the Phagocyte Respiratory Burst. Biochim. Biophys. Acta-Bioenerg. 2006, 1757, 996–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Painter, R.G.; Bonvillain, R.W.; Valentine, V.G.; Lombard, G.A.; LaPlace, S.G.; Nauseef, W.M.; Wang, G. The Role of Chloride Anion and CFTR in Killing of Pseudomonas Aeruginosa by Normal and CF Neutrophils. J. Leukoc. Biol. 2008, 83, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Duchen, M.R. Mitochondria in Health and Disease: Perspectives on a New Mitochondrial Biology. Mol. Asp. Med. 2004, 25, 365–451. [Google Scholar] [CrossRef]
- Duszynski, J.; Brutkowski, W.; Szczepanowska, J.; Zablocki, K. The Regulatory Role of Mitochondria in Capacitative Calcium Entry. (BBA)-Bioenerg. 2006, 1757, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.T. Mitochondria and Cellular Oxygen Sensing in the HIF Pathway. Biochem. J. 2008, 26, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.-Y.; Seol, D.-W. The Role of Mitochondria in Apoptosis. BMB Rep. 2008, 41, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Szewczyk, A.; Bednarczyk, P.; Jędraszko, J.; Kampa, R.P.; Koprowski, P.; Krajewska, M.; Kucman, S.; Rotko, D.; Sęk, A.; Żochowska, M.; et al. Mitochondrial Potassium Channels—An Overview. Postepy Biochem. 2018, 64, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Misak, A.; Grman, M.; Malekova, L.; Novotova, M.; Markova, J.; Krizanova, O.; Ondrias, K.; Tomaskova, Z. Mitochondrial Chloride Channels: Electrophysiological Characterization and PH Induction of Channel Pore Dilation. Eur. Biophys. J. 2013, 42, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.L.; Lam, L.F.; Feigal, R.J. Mitochondrial NADH Dehydrogenase in Cystic Fibrosis: Enzyme Kinetics in Cultured Fibroblasts. Am. J. Hum. Genet. 1982, 34, 846–852. [Google Scholar]
- Valdivieso, A.G.; Marcucci, F.; Taminelli, G.; Guerrico, A.G.; Alvarez, S.; Teiber, M.L.; Dankert, M.A.; Santa-Coloma, T.A. The Expression of the Mitochondrial Gene MT-ND4 Is Downregulated in Cystic Fibrosis. Biochem. Biophys. Res. Commun. 2007, 356, 805–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdivieso, A.G.; Clauzure, M.; Marín, M.C.; Taminelli, G.L.; Massip Copiz, M.M.; Sánchez, F.; Schulman, G.; Teiber, M.L.; Santa-Coloma, T.A. The Mitochondrial Complex I Activity Is Reduced in Cells with Impaired Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Function. PLoS ONE 2012, 7, e48059. [Google Scholar] [CrossRef] [PubMed]
- De Bari, L.; Favia, M.; Bobba, A.; Lassandro, R.; Guerra, L.; Atlante, A. Aberrant GSH Reductase and NOX Activities Concur with Defective CFTR to Pro-Oxidative Imbalance in Cystic Fibrosis Airways. J. Bioenerg. Biomembr. 2018, 50, 117–129. [Google Scholar] [CrossRef]
- Atlante, A.; Favia, M.; Bobba, A.; Guerra, L.; Casavola, V.; Reshkin, S.J. Characterization of Mitochondrial Function in Cells with Impaired Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Function. J. Bioenerg. Biomembr. 2016, 48, 197–210. [Google Scholar] [CrossRef]
- De Bari, F.; Atlante, B. An Intriguing Involvement of Mitochondria in Cystic Fibrosis. J. Clin. Med. 2019, 8, 1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favia, M.; de Bari, L.; Lassandro, R.; Atlante, A. Modulation of Glucose-Related Metabolic Pathways Controls Glucose Level in Airway Surface Liquid and Fight Oxidative Stress in Cystic Fibrosis Cells. J. Bioenerg. Biomembr. 2019, 51, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso, Á.G. Mitochondrial Alterations and Oxidative Stress in Cystic Fibrosis. In Oxidative Stress in Lung Diseases.; Chakraborti, S., Chakraborti, T., Das, S., Chattopadhyay, D., Eds.; Springer: Singapore, 2019; pp. 355–371. [Google Scholar] [CrossRef]
- Atlante, A. Mitochondria and Cystic Fibrosis Transmembrane Conductance Regulator Dialogue: Some News. J. Rare Dis. Res. Treat. 2018, 1, 23–29. [Google Scholar] [CrossRef]
- García, R.; Falduti, C.; Clauzure, M.; Jara, R.; Massip-Copiz, M.M.; de Los Ángeles Aguilar, M.; Santa-Coloma, T.A.; Valdivieso, Á.G. CFTR Chloride Channel Activity Modulates the Mitochondrial Morphology in Cultured Epithelial Cells. Int. J. Biochem. Cell Biol. 2021, 135, 105976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Meng, Y.H.; Chang, S.; Zhang, R.Y.; Shi, C. High Fructose Causes Cardiac Hypertrophy via Mitochondrial Signaling Pathway. Am. J. Transl. Res. 2016, 8, 4869–4880. [Google Scholar]
- Kleme, M.L.; Sané, A.; Garofalo, C.; Seidman, E.; Brochiero, E.; Berthiaume, Y.; Levy, E. CFTR Deletion Confers Mitochondrial Dysfunction and Disrupts Lipid Homeostasis in Intestinal Epithelial Cells. Nutrients 2018, 10, 836. [Google Scholar] [CrossRef] [Green Version]
- Kleme, M.-L.; Sané, A.T.; Garofalo, C.; Levy, E. Targeted CFTR Gene Disruption with Zinc-Finger Nucleases in Human Intestinal Epithelial Cells Induces Oxidative Stress and Inflammation. Int. J. Biochem. Cell Biol. 2016, 74, 84–94. [Google Scholar] [CrossRef]
- Zeng, J.W.; Zeng, X.L.; Li, F.Y.; Ma, M.M.; Yuan, F.; Liu, J.; Lv, X.F.; Wang, G.L.; Guan, Y.Y. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Prevents Apoptosis Induced by Hydrogen Peroxide in Basilar Artery Smooth Muscle Cells. Apoptosis 2014, 19, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Zhang, Y.; Xiao, Z.B.; Zhang, Y.B.; Zhang, J.; Li, Z.Q.; Zhu, Y. Bin. CFTR Prevents Neuronal Apoptosis Following Cerebral Ischemia Reperfusion via Regulating Mitochondrial Oxidative Stress. J. Mol. Med. 2018, 96, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Antigny, F.; Girardin, N.; Raveau, D.; Frieden, M.; Becq, F.; Vandebrouck, C. Dysfunction of Mitochondria Ca2+ Uptake in Cystic Fibrosis Airway Epithelial Cells. Mitochondrion 2009, 9, 232–241. [Google Scholar] [CrossRef]
- Rimessi, A.; Bezzerri, V.; Patergnani, S.; Marchi, S.; Cabrini, G.; Pinton, P. Mitochondrial Ca2+-Dependent NLRP3 Activation Exacerbates the Pseudomonas Aeruginosa-Driven Inflammatory Response in Cystic Fibrosis. Nat. Commun. 2015, 6, 6201. [Google Scholar] [CrossRef] [PubMed]
- Rimessi, A.; Pozzato, C.; Carparelli, L.; Rossi, A.; Ranucci, S.; de Fino, I.; Cigana, C.; Talarico, A.; Wieckowski, M.R.; Ribeiro, C.M.P.; et al. Pharmacological Modulation of Mitochondrial Calcium Uniporter Controls Lung Inflammation in Cystic Fibrosis. Sci. Adv. 2020, 6, eaax9093. [Google Scholar] [CrossRef] [PubMed]
- López-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Valcárcel-Ares, M.N. Mitochondrial Dysfunction and the Inflammatory Response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Tiku, V.; Tan, M.W.; Dikic, I. Mitochondrial Functions in Infection and Immunity. Trends Cell Biol. 2020, 30, 263–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patergnani, S.; Vitto, V.A.M.; Pinton, P.; Rimessi, A. Mitochondrial Stress Responses and “Mito-Inflammation” in Cystic Fibrosis. Front. Pharmacol. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Antigny, F.; Norez, C.; Becq, F.; Vandebrouck, C. CFTR and Ca2+ Signaling in Cystic Fibrosis. Front. Pharmacol. 2011, OCT, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Marklew, A.J.; Patel, W.; Moore, P.J.; Tan, C.D.; Smith, A.J.; Sassano, M.F.; Gray, M.A.; Tarran, R. Cigarette Smoke Exposure Induces Retrograde Trafficking of CFTR to the Endoplasmic Reticulum. Sci. Rep. 2019, 9, 13655. [Google Scholar] [CrossRef] [Green Version]
- Kiselyov, K.; Muallem, S. ROS and Intracellular Ion Channels. Cell Calcium 2016, 60, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Ehsan, Z.; Clancy, J.P. Management of Pseudomonas Aeruginosa Infection in Cystic Fibrosis Patients Using Inhaled Antibiotics with a Focus on Nebulized Liposomal Amikacin. Future Microbiol. 2015, 10, 1901–1912. [Google Scholar] [CrossRef] [Green Version]
- Hatziagorou, E.; Orenti, A.; Drevinek, P.; Kashirskaya, N.; Mei-Zahav, M.; De Boeck, K. Changing Epidemiology of the Respiratory Bacteriology of Patients with Cystic Fibrosis-Data from the European Cystic Fibrosis Society Patient Registry. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2020, 19, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and Management of Pulmonary Infections in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef]
- Davies, J.C.; Martin, I. New Anti-Pseudomonal Agents for Cystic Fibrosis- Still Needed in the Era of Small Molecule CFTR Modulators? Expert Opin. Pharmacother. 2018, 19, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Solomon, G.M.; Marshall, S.G.; Ramsey, B.W.; Rowe, S.M. Breakthrough Therapies: Cystic Fibrosis (CF) Potentiators and Correctors. Pediatr. Pulmonol. 2015, 50 (Suppl. 4), S3–S13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pranke, I.; Golec, A.; Hinzpeter, A.; Edelman, A.; Sermet-Gaudelus, I. Emerging Therapeutic Approaches for Cystic Fibrosis. From Gene Editing to Personalized Medicine. Front. Pharmacol. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic Fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef]
- Almughem, F.A.; Aldossary, A.M.; Tawfik, E.A.; Alomary, M.N.; Alharbi, W.S.; Alshahrani, M.Y.; Alshehri, A.A. Cystic Fibrosis: Overview of the Current Development Trends and Innovative Therapeutic Strategies. Pharmaceutics 2020, 12, 616. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Burton, B.; Huang, C.-J.; Worley, J.; Cao, D.; Johnson, J.P.J.; Urrutia, A.; Joubran, J.; Seepersaud, S.; Sussky, K.; et al. Ivacaftor Potentiation of Multiple CFTR Channels with Gating Mutations. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2012, 11, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF Airway Epithelial Cell Function in Vitro by a CFTR Potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef] [Green Version]
- Flume, P.A.; Liou, T.G.; Borowitz, D.S.; Li, H.; Yen, K.; Ordoñez, C.L.; Geller, D.E. Ivacaftor in Subjects with Cystic Fibrosis Who Are Homozygous for the F508del-CFTR Mutation. Chest 2012, 142, 718–724. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Baloch, N.U.-A.; Janahi, I.A. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 1783. [Google Scholar] [CrossRef] [Green Version]
- Rowe, S.M.; Daines, C.; Ringshausen, F.C.; Kerem, E.; Wilson, J.; Tullis, E.; Nair, N.; Simard, C.; Han, L.; Ingenito, E.P.; et al. Tezacaftor-Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N. Engl. J. Med. 2017, 377, 2024–2035. [Google Scholar] [CrossRef] [Green Version]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and Safety of the Elexacaftor plus Tezacaftor plus Ivacaftor Combination Regimen in People with Cystic Fibrosis Homozygous for the F508del Mutation: A Double-Blind, Randomised, Phase 3 Trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Antigny, F.; Norez, C.; Becq, F.; Vandebrouck, C. Calcium Homeostasis Is Abnormal in Cystic Fibrosis Airway Epithelial Cells but Is Normalized after Rescue of F508del-CFTR. Cell Calcium 2008, 43, 175–183. [Google Scholar] [CrossRef]
- Galli, F.; Battistoni, A.; Gambari, R.; Pompella, A.; Bragonzi, A.; Pilolli, F.; Iuliano, L.; Piroddi, M.; Dechecchi, M.C.; Cabrini, G. Oxidative Stress and Antioxidant Therapy in Cystic Fibrosis. Biochim. Biophys. Acta -Mol. Basis Dis. 2012, 1822, 690–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, B.H.; Evans, T.I.A.; Moll, S.R.; Gray, J.S.; Liang, B.; Sun, X.; Zhang, Y.; Jensen-Cody, C.W.; Swatek, A.M.; Zhou, W.; et al. Infection Is Not Required for Mucoinflammatory Lung Disease in CFTR-Knockout Ferrets. Am. J. Respir. Crit. Care Med. 2018, 197, 1308–1318. [Google Scholar] [CrossRef]
- Rogers, C.S.; Stoltz, D.A.; Meyerholz, D.K.; Ostedgaard, L.S.; Rokhlina, T.; Taft, P.J.; Rogan, M.P.; Pezzulo, A.A.; Karp, P.H.; Itani, O.A.; et al. Disruption of the CFTR Gene Produces a Model of Cystic Fibrosis in Newborn Pigs. Science 2008, 321, 1837–1841. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.Z.; Wagener, J.S.; Bost, T.; Martinez, J.; Accurso, F.J.; Riches, D.W. Early Pulmonary Inflammation in Infants with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 1995, 151, 1075–1082. [Google Scholar] [CrossRef]
- Nichols, D.; Chmiel, J.; Berger, M. Chronic Inflammation in the Cystic Fibrosis Lung: Alterations in Inter- and Intracellular Signaling. Clin. Rev. Allergy Immunol. 2008, 34, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.S.; Prince, A. Cystic Fibrosis: A Mucosal Immunodeficiency Syndrome. Nat. Med. 2012, 18, 509–519. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukasiak, A.; Zajac, M. The Distribution and Role of the CFTR Protein in the Intracellular Compartments. Membranes 2021, 11, 804. https://doi.org/10.3390/membranes11110804
Lukasiak A, Zajac M. The Distribution and Role of the CFTR Protein in the Intracellular Compartments. Membranes. 2021; 11(11):804. https://doi.org/10.3390/membranes11110804
Chicago/Turabian StyleLukasiak, Agnieszka, and Miroslaw Zajac. 2021. "The Distribution and Role of the CFTR Protein in the Intracellular Compartments" Membranes 11, no. 11: 804. https://doi.org/10.3390/membranes11110804
APA StyleLukasiak, A., & Zajac, M. (2021). The Distribution and Role of the CFTR Protein in the Intracellular Compartments. Membranes, 11(11), 804. https://doi.org/10.3390/membranes11110804






