Self-Humidifying Membrane for High-Performance Fuel Cells Operating at Harsh Conditions: Heterojunction of Proton and Anion Exchange Membranes Composed of Acceptor-Doped SnP2O7 Composites
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Fabrication of Hydrogen/Air Fuel Cell
2.3. Characterization
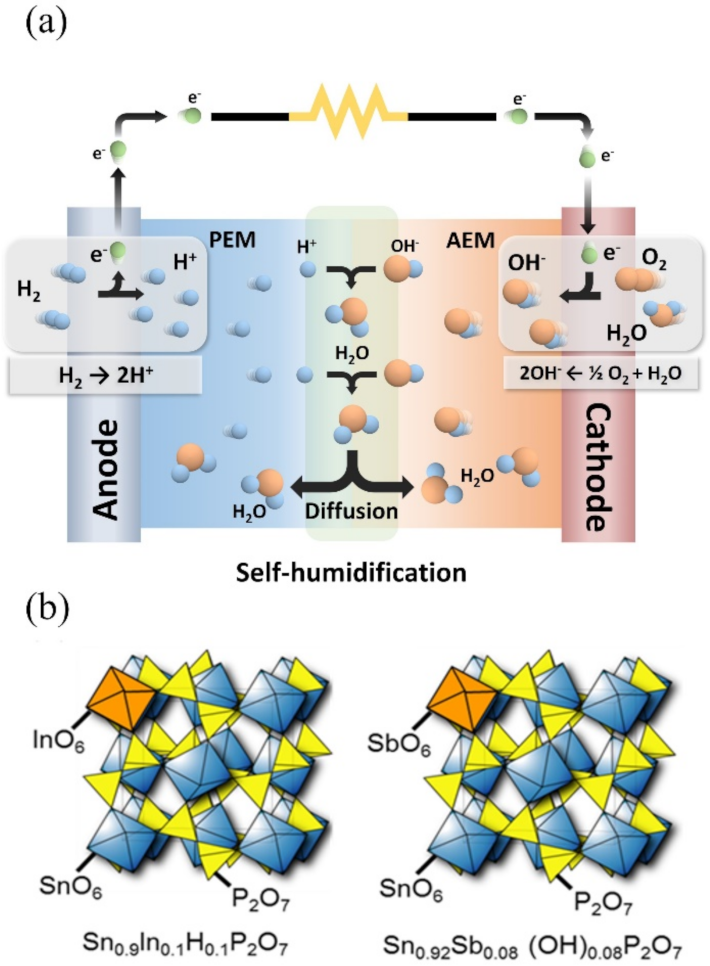
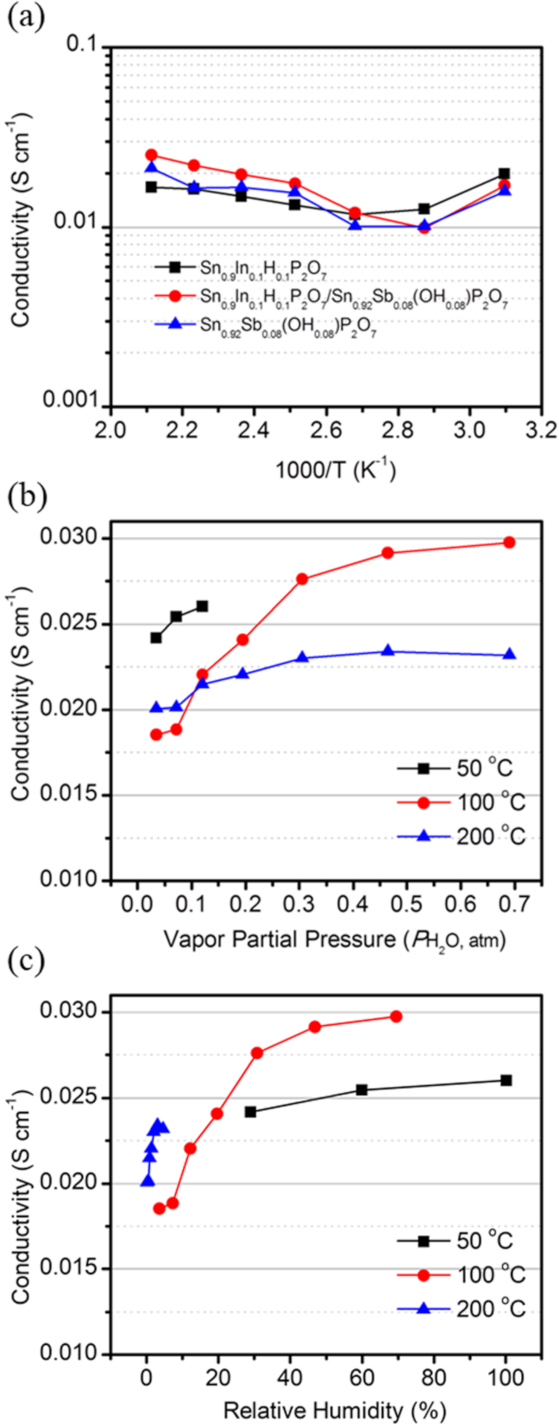
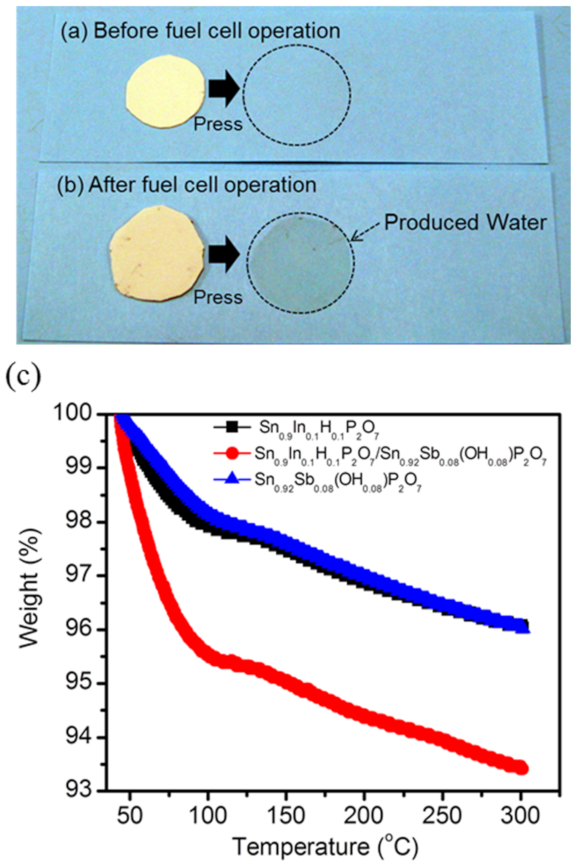
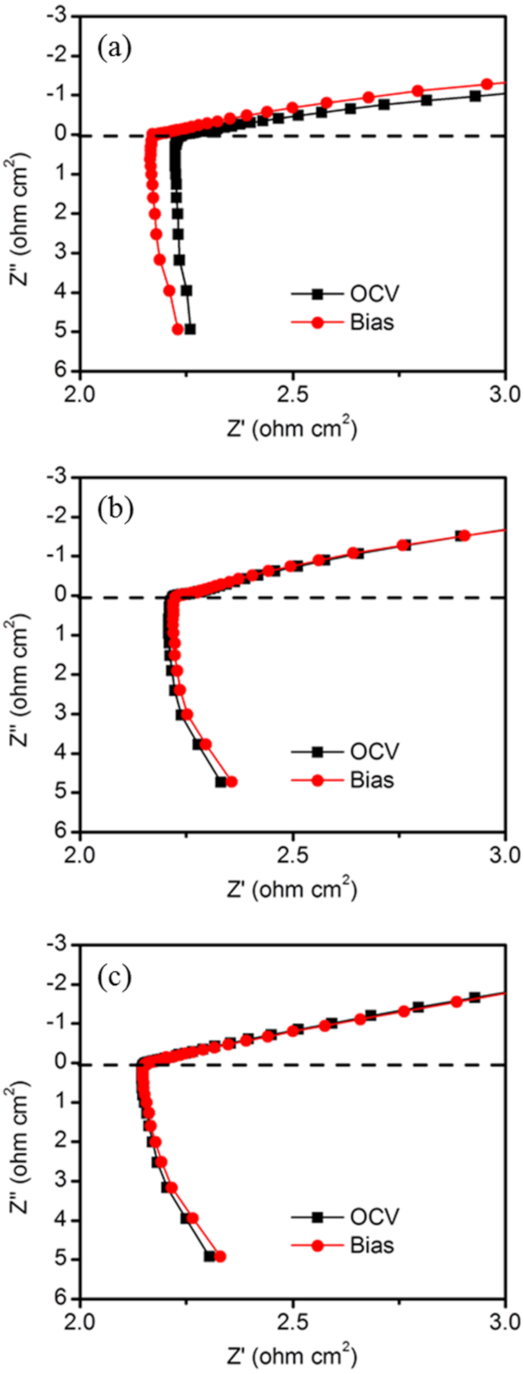
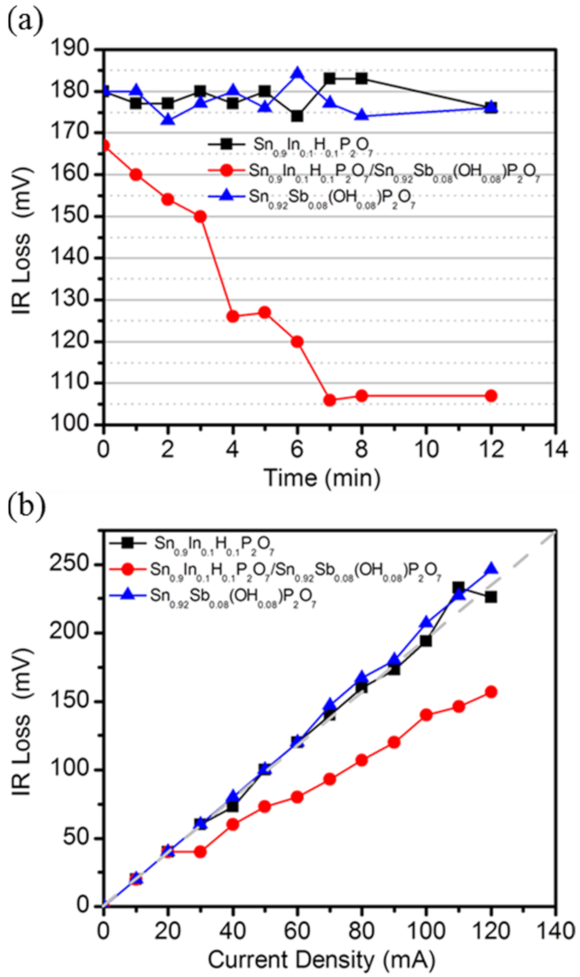
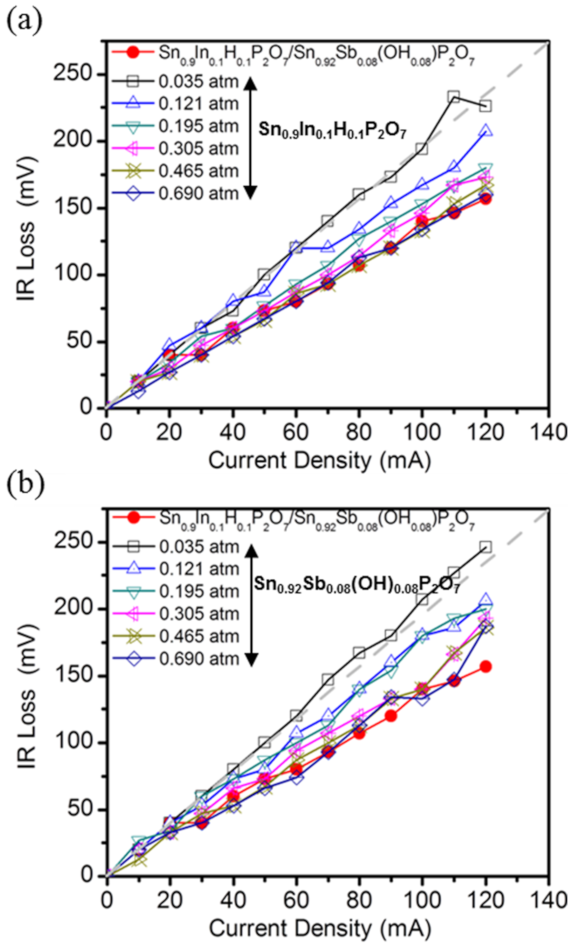
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Goñi-Urtiaga, A.; Presvytes, D.; Scott, K. Solid acids as electrolyte materials for proton exchange membrane (PEM) electrolysis. Int. J. Hydrog. Energy 2012, 37, 3358–3372. [Google Scholar] [CrossRef]
- Kim, K.; Heo, P.; Ko, T.; Lee, J.-C. Semi-interpenetrating network electrolyte membranes based on sulfonated poly (arylene ether sulfone) for fuel cells at high temperature and low humidity conditions. Electrochem. Commun. 2014, 48, 44–48. [Google Scholar] [CrossRef]
- Marcinkoski, J.; Kopasz, J.P.; Benjamin, T.G. Progress in the US DOE fuel cell subprogram efforts in polymer electrolyte fuel cells. Int. J. Hydrog. Energy 2008, 33, 3894–3902. [Google Scholar] [CrossRef]
- Kim, K.; Choi, S.-W.; Park, J.O.; Kim, S.-K.; Lim, M.-Y.; Kim, K.-H.; Ko, T.; Lee, J.-C. Proton conductive cross-linked benzoxazine-benzimidazole copolymers as novel porous substrates for reinforced pore-filling membranes in fuel cells operating at high temperatures. J. Membr. Sci. 2017, 536, 76–85. [Google Scholar]
- Kim, K.; Heo, P.; Ko, T.; Kim, K.-h.; Kim, S.-K.; Pak, C.; Lee, J.-C. Poly (arlyene ether sulfone) based semi-interpenetrating polymer network membranes containing cross-linked poly (vinyl phosphonic acid) chains for fuel cell applications at high temperature and low humidity conditions. J. Power Sources 2015, 293, 539–547. [Google Scholar] [CrossRef]
- Lee, H.; Han, J.; Ahn, S.M.; Jeong, H.Y.; Kim, J.; Kim, H.; Kim, T.-H.; Kim, K.; Lee, J.-C. Simple and effective cross-linking technology for the preparation of cross-linked membranes composed of highly sulfonated poly (ether ether ketone) and poly (arylene ether sulfone) for fuel cell applications. J. ACS Appl. Energy Mater. 2020, 3, 10495–10505. [Google Scholar] [CrossRef]
- Han, J.; Lee, H.; Kim, J.; Kim, S.; Kim, H.; Kim, E.; Sung, Y.-E.; Kim, K.; Lee, J.-C. Sulfonated poly (arylene ether sulfone) composite membrane having sulfonated polytriazole grafted graphene oxide for high-performance proton exchange membrane fuel cells. J. Membr. Sci. 2020, 612, 118428. [Google Scholar] [CrossRef]
- Han, J.; Kim, K.; Kim, J.; Kim, S.; Choi, S.-W.; Lee, H.; Kim, J.-j.; Kim, T.-H.; Sung, Y.-E.; Lee, J.-C. Cross-linked highly sulfonated poly (arylene ether sulfone) membranes prepared by in-situ casting and thiol-ene click reaction for fuel cell application. J. Membr. Sci. 2019, 579, 70–78. [Google Scholar] [CrossRef]
- Kim, K.; Jung, B.-K.; Ko, T.; Kim, T.-H.; Lee, J.-C. Comb-shaped polysulfones containing sulfonated polytriazole side chains for proton exchange membranes. J. Membr. Sci. 2018, 554, 232–243. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Ko, T.; Han, J.; Lee, J.-C. Polybenzimidazole composite membranes containing imidazole functionalized graphene oxide showing high proton conductivity and improved physicochemical properties. Int. J. Hydrog. Energy 2021, 46, 12254–12262. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Nam, S.Y. Research Trends of Polybenzimidazole-based Membranes for Hydrogen Purification Applications. Appl. Chem. Eng. 2020, 31, 453–466. [Google Scholar]
- Zelenay, P.; Scharifker, B.; Bockris, J.M.; Gervasio, D. A Comparison of the Properties of CF 3 SO 3 H and H 3 PO 4 in Relation to Fuel Cells. J. Electrochem. Soc. 1986, 133, 2262. [Google Scholar] [CrossRef]
- Watanabe, M.; Uchida, H.; Seki, Y.; Emori, M.; Stonehart, P. Self-humidifying polymer electrolyte membranes for fuel cells. J. Electrochem. Soc. 1996, 143, 3847. [Google Scholar] [CrossRef]
- Amjadi, M.; Rowshanzamir, S.; Peighambardoust, S.; Hosseini, M.; Eikani, M. Investigation of physical properties and cell performance of Nafion/TiO2 nanocomposite membranes for high temperature PEM fuel cells. Int. J. Hydrog. Energy 2010, 35, 9252–9260. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, B.; Xu, Y. Composite membranes of sulfonated poly (phthalazinone ether ketone) doped with 12-phosphotungstic acid (H3PW12O40) for proton exchange membranes. Solid State Ion. 2006, 177, 1123–1128. [Google Scholar] [CrossRef]
- Lee, H.-K.; Kim, J.-I.; Park, J.-H.; Lee, T.-H. A study on self-humidifying PEMFC using Pt–ZrP–Nafion composite membrane. Electrochim. Acta 2004, 50, 761–768. [Google Scholar] [CrossRef]
- Liang, H.; Dang, D.; Xiong, W.; Song, H.; Liao, S. High-performance self-humidifying membrane electrode assembly prepared by simultaneously adding inorganic and organic hygroscopic materials to the anode catalyst layer. J. Power Sources 2013, 241, 367–372. [Google Scholar] [CrossRef]
- Liang, H.; Zheng, L.; Liao, S. Self-humidifying membrane electrode assembly prepared by adding PVA as hygroscopic agent in anode catalyst layer. Int. J. Hydrog. Energy 2012, 37, 12860–12867. [Google Scholar] [CrossRef]
- Su, H.; Xu, L.; Zhu, H.; Wu, Y.; Yang, L.; Liao, S.; Song, H.; Liang, Z.; Birss, V. Self-humidification of a PEM fuel cell using a novel Pt/SiO2/C anode catalyst. Int. J. Hydrog. Energy 2010, 35, 7874–7880. [Google Scholar] [CrossRef]
- Heo, P.; Ito, K.; Tomita, A.; Hibino, T. A Proton-Conducting Fuel Cell Operating with Hydrocarbon Fuels. Angew. Chem. 2008, 120, 7959–7962. [Google Scholar] [CrossRef]
- Jin, Y.; Shen, Y.; Hibino, T. Proton conduction in metal pyrophosphates (MP2O7) at intermediate temperatures. J. Mater. Chem. 2010, 20, 6214–6217. [Google Scholar] [CrossRef]
- Hibino, T.; Shen, Y.; Nishida, M.; Nagao, M. Hydroxide Ion Conducting Antimony (V)-Doped Tin Pyrophosphate Electrolyte for Intermediate-Temperature Alkaline Fuel Cells. Angew. Chem. 2012, 124, 10944–10948. [Google Scholar] [CrossRef]
- Shimura, T.; Egusa, G.; Iwahara, H.; Katahira, K.; Yamamoto, K. Electrochemical properties of junction between protonic conductor and oxide ion conductor. Solid State Ion. 1997, 97, 477–482. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.-K.; Park, J.O.; Choi, S.-W.; Kim, K.-H.; Ko, T.; Pak, C.; Lee, J.-C. Highly reinforced pore-filling membranes based on sulfonated poly (arylene ether sulfone) s for high-temperature/low-humidity polymer electrolyte membrane fuel cells. J. Membr. Sci. 2017, 537, 11–21. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, P.; Kim, M.; Ko, H.; Nam, S.Y.; Kim, K. Self-Humidifying Membrane for High-Performance Fuel Cells Operating at Harsh Conditions: Heterojunction of Proton and Anion Exchange Membranes Composed of Acceptor-Doped SnP2O7 Composites. Membranes 2021, 11, 776. https://doi.org/10.3390/membranes11100776
Heo P, Kim M, Ko H, Nam SY, Kim K. Self-Humidifying Membrane for High-Performance Fuel Cells Operating at Harsh Conditions: Heterojunction of Proton and Anion Exchange Membranes Composed of Acceptor-Doped SnP2O7 Composites. Membranes. 2021; 11(10):776. https://doi.org/10.3390/membranes11100776
Chicago/Turabian StyleHeo, Pilwon, Mijeong Kim, Hansol Ko, Sang Yong Nam, and Kihyun Kim. 2021. "Self-Humidifying Membrane for High-Performance Fuel Cells Operating at Harsh Conditions: Heterojunction of Proton and Anion Exchange Membranes Composed of Acceptor-Doped SnP2O7 Composites" Membranes 11, no. 10: 776. https://doi.org/10.3390/membranes11100776
APA StyleHeo, P., Kim, M., Ko, H., Nam, S. Y., & Kim, K. (2021). Self-Humidifying Membrane for High-Performance Fuel Cells Operating at Harsh Conditions: Heterojunction of Proton and Anion Exchange Membranes Composed of Acceptor-Doped SnP2O7 Composites. Membranes, 11(10), 776. https://doi.org/10.3390/membranes11100776





