Reverse Osmosis Concentrate: Physicochemical Characteristics, Environmental Impact, and Technologies
Abstract
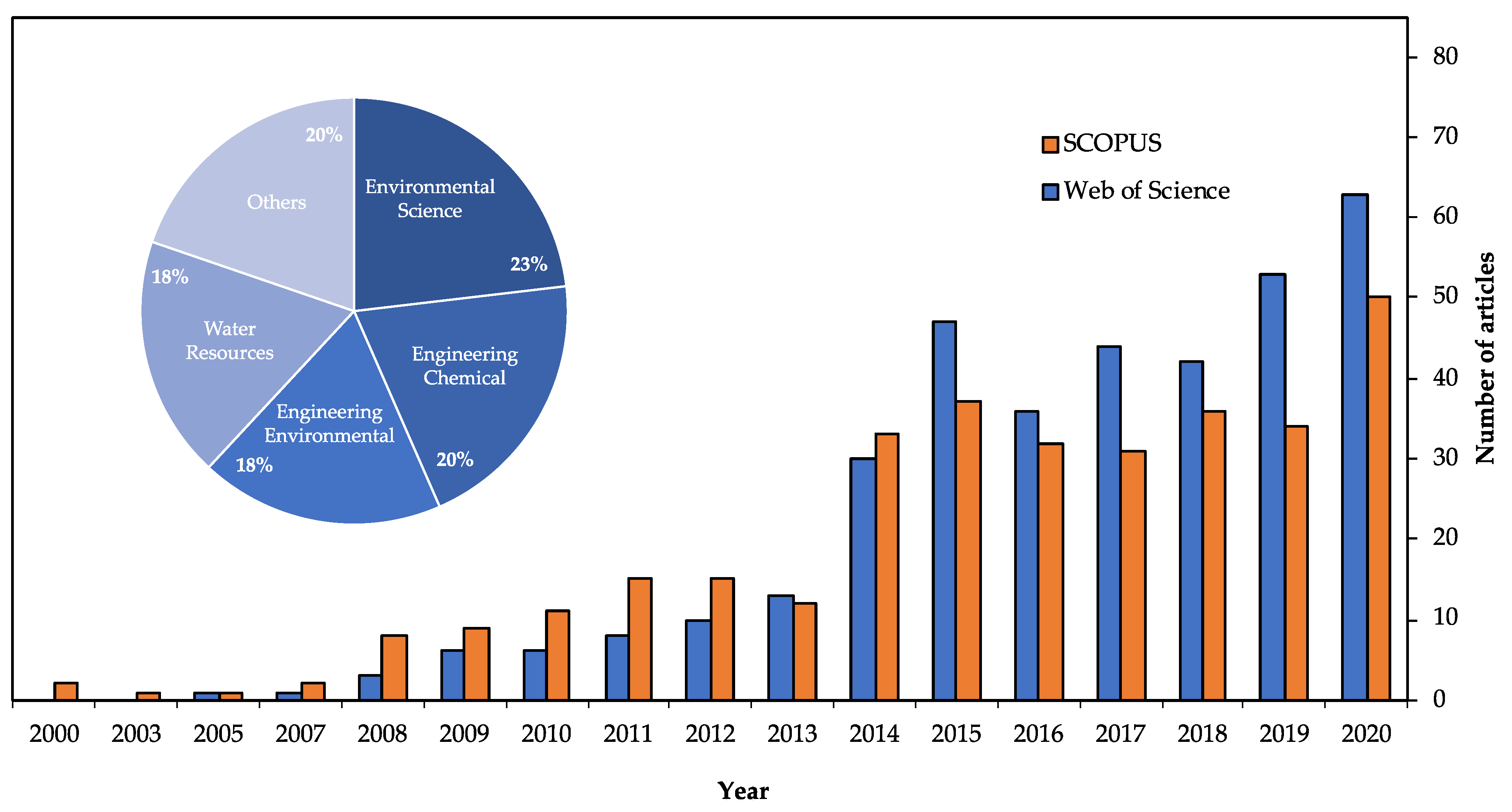
1. Introduction
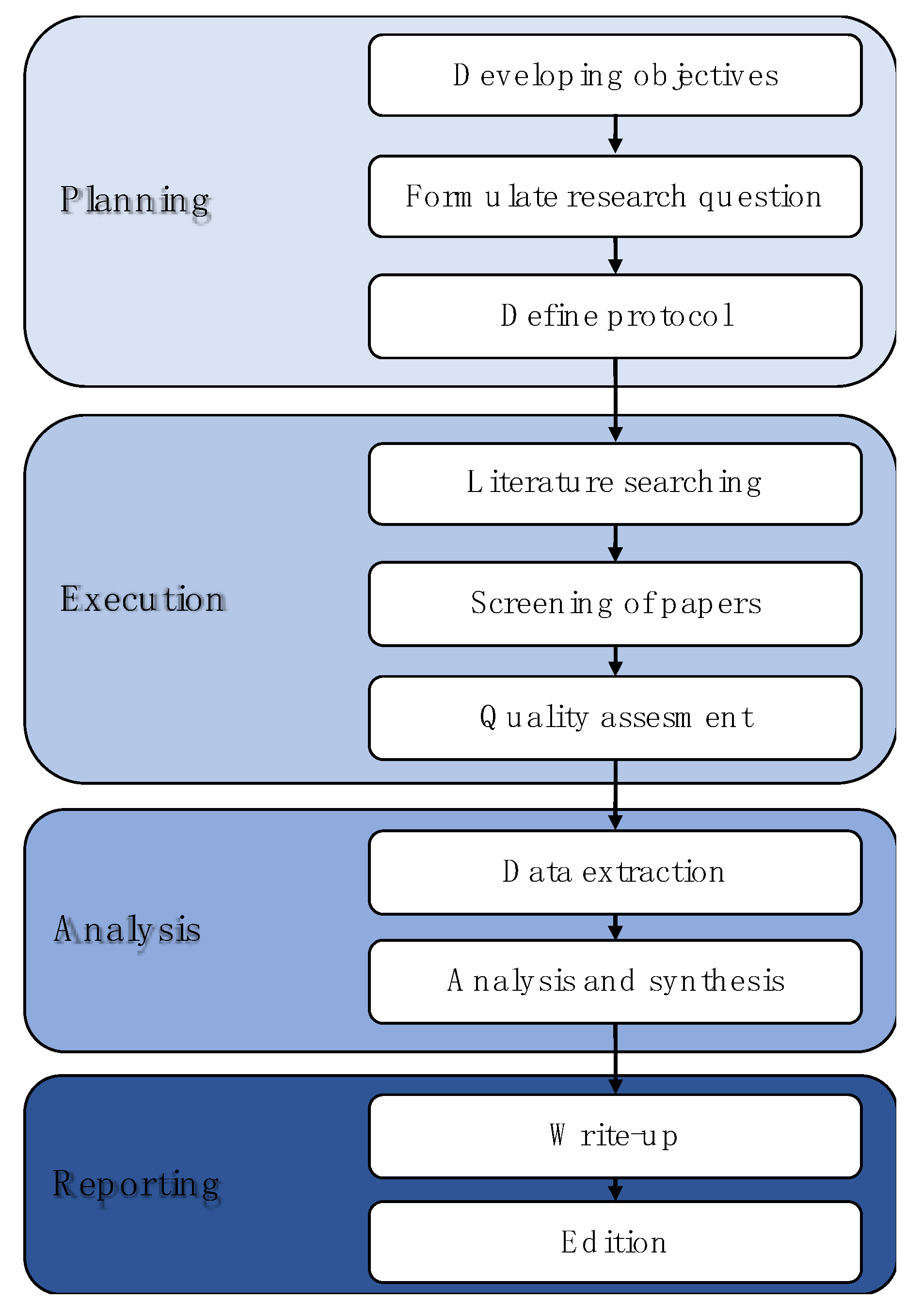
2. Methodology
- RQ1: What characteristics of ROC generate an environmental impact on the receiving water body?
- RQ2: What technologies mitigate the environmental impact of ROC and provide a revalue to this by product of desalination?
3. Results and Discussion
3.1. Physicochemical Characteristics of the ROC
3.2. Environmental Impact of ROC
- ○
- Salinity and temperature are the major parameters that impact the marine environment, as brine salinity can go up to 65,000–85,000 mg/L (twice the regular seawater concentration), and temperature up to 45–50 °C [76,77,78]. Changes in biota (mainly in plankton and fish species, and pelagic microbial communities) and water quality can occur in the ROC discharge area due to great variations in salinity and temperature [79,80]. These changes are concentrated in the water column and near the seabed, both associated with the discharge point [47].
- ○
- The load of chemical products used during pretreatment as biocides and biocide scavengers, alongside the by-products of the disinfection process, can present ecotoxicity in the marine environment [81,82]. The disinfection by-products (DBPs), upon reaction with natural organic matter present in feedwater, have some ecotoxic effects on aquatic life [83,84,85]. Antiscalant is added to control scaling due to poorly soluble salts, hence maintaining plant productivity—especially at increased recovery [86]. Antiscalants have relatively low toxicity and their environmental fate is defined by their dilution, which further reduces any risk of negative effects; however, their poor degradability is a major drawback [26,87]. Coagulants such as aluminum sulfate, ferric chloride and flocculants are added during pretreatment to enhance the removal of suspended and very fine particles, ending with a filter wash that is disposed of into the brine stream [14] containing iron and aluminum salts with large particles from coagulation and flocculation, which induce some coloring and turbidity effects in receiving waters [88].
3.2.1. Regulations Related to ROC
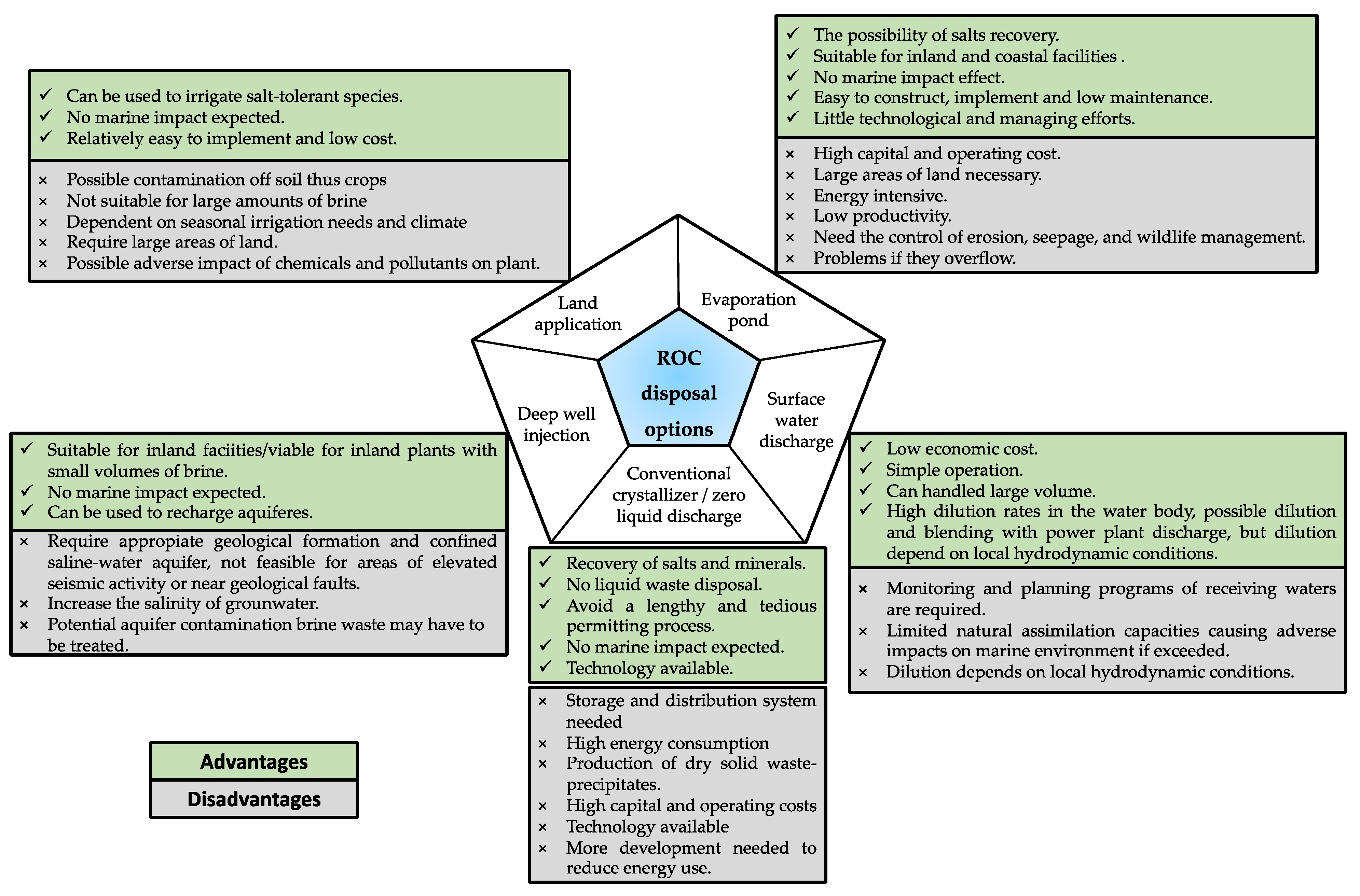
3.2.2. Mitigation and Control Strategies
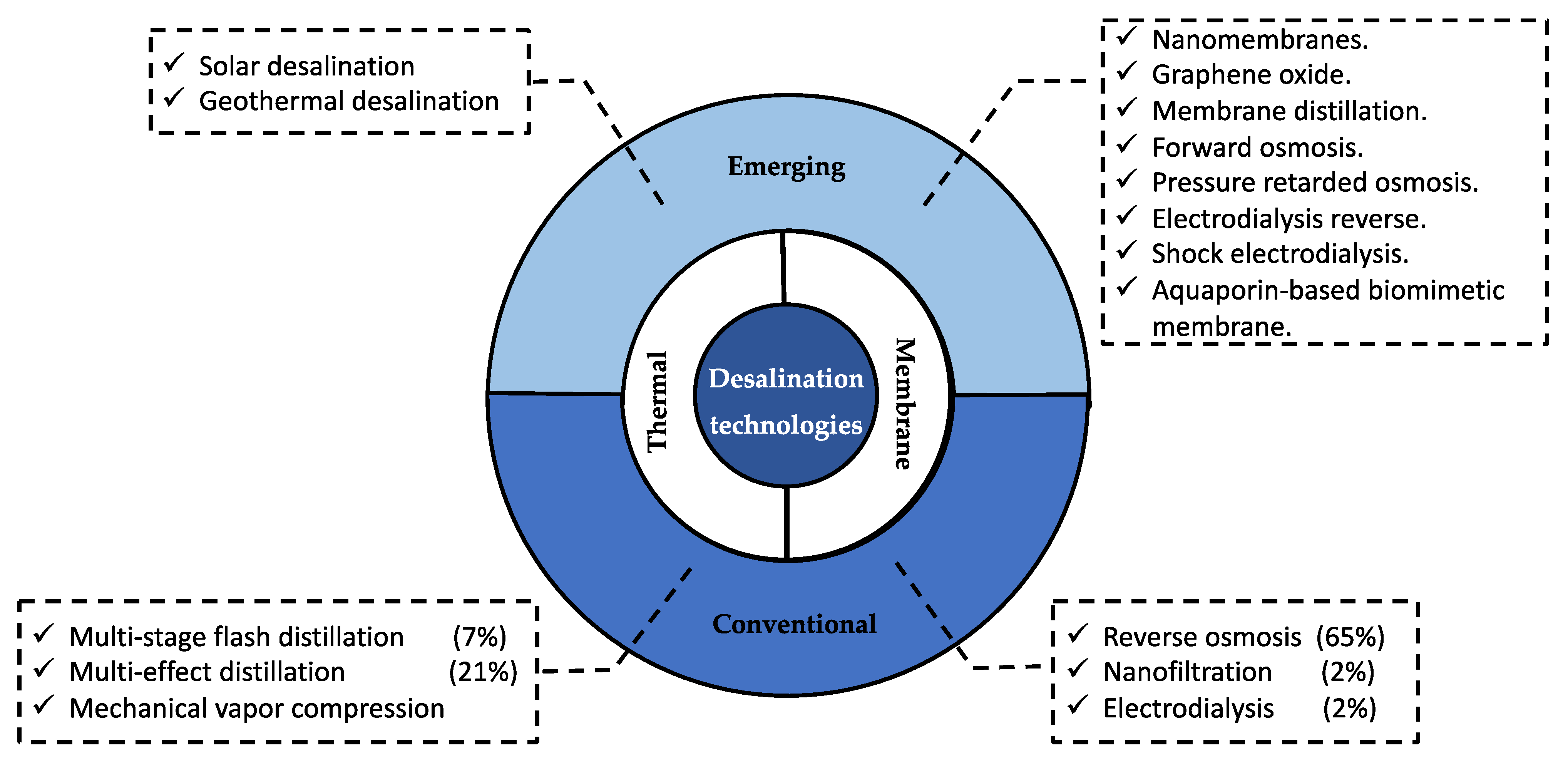
3.3. Technologies for ROC Treatment
3.3.1. Conventional Technologies
3.3.2. Emerging Technologies
- ○
- Forward osmosis drives water through membranes due to osmotic pressure differences (differences in salt concentration) that are inherently present in the system. Water moves from the feed (low salt concentration) to the draw solution (high salt concentration) [158,159]. The key benefits of using forward osmosis for ROC treatment are: (1) the low energy consumption that comes with it, (2) that high TDS water can be treated, and (3) the lower fouling propensity of the membranes compared to pressure-driven membrane processes [12,160]. However, water flux can be lower than expected in the forward osmosis process due to the existence of internal concentration polarization [161].
- ○
- Membrane distillation is based on the fundamentals of evaporation, and vapor distillate may be produced by temperature, partial pressure, or vacuum gradients [12]. A gas–liquid interface is created as volatile constituents are transferred through a microporous hydrophobic membrane. When water vapor evaporates from the hot brine at the periphery of the brine–membrane interface, it diffuses through hydrophobic membrane pores filled with gas. The water vapor then condenses in the membrane interface at the side, whereby the cooler distillate flows. By heating the feedwater, vapor pressure is increased, thus enhancing the driving gradient for vapor production. The key benefits of using membrane distillation for ROC treatment are: (1) it is operated at low temperatures; (2) it can be retrofitted with heat sources, such as renewable solar energy, geothermal energy, or waste heat sources; and (3) its efficacy is barely affected by the concentration polarization phenomenon, which enables high salt concentrations nearing saturation limits to be fed into the process [162].
- ○
- The benefits of membrane distillation have allowed for the emergence of membrane crystallization (simultaneous production of water and precious crystalline salts) [163,164]. The key benefits of using membrane crystallization for ROC treatment are as follows: (1) higher than average crystallization rates, (2) well-controlled crystal nucleation, and (3) known growth kinetics [165]. Therefore, membrane crystallization is a technology that should be widely addressed in the coming years for ROC treatment.
- ○
- Currently, electrodialysis has been reported to be an efficient method for treating ROC, improving overall RO water recovery to above 90%, and reaching a “near-zero liquid discharge approach” [110,166]. Electrodialysis enables ion transport through an ion exchange membrane using electrical energy as the driving force. These membranes have a high density of ionic groups fixed on them, which allow the selective transport of ions through the membrane depending on their charge. The passage of counter-ions (opposite charge) is allowed, while the passage of co-ions (same charge) is prevented due to Donnan repulsion. Electrodialysis is suitable for ROC treatment since applied electrical energy allows the ions to transfer from the less concentrated solution (water or seawater) to the more concentrated solution (brine). The benefits of using electrodialysis to treat ROC are as follows: (1) low rejection amount, (2) low sensitivity to suspended solids, (3) longer membrane life compared to other applications (e.g., RO), (4) complex pretreatment is not required, (5) ease of operation, and (6) low energy consumption [167,168,169].
- ○
- Another emerging technology related to the electrical charge of the components is capacitive deionization. This technology has received significant attention as an energy-efficient technology for brackish water desalination [170]. Capacitive deionization is an electrochemically induced alternative approach for removing ions from concentrated aqueous solutions by forcing charged ions into the electrical double layer at the electrode–solution interface, where the electrode is connected to an external power supply [55]. The key benefits of using capacitive deionization for ROC treatment are as follows: (1) low operating costs, (2) reduced pretreatment, (3) high recovery, and (4) reduced fouling due to the reversal charge—where the most critical component is that the carbon electrode materials, due to their electrosorptive capacity, depend strongly on physical properties such as the surface area and conductivity of the electrode [22,55].
- ○
- Nanomembranes are membranes that contain nanoparticles (zeolitic type or metal oxide) in the active layer of the polymer matrix, e.g., polymerized polyamide, aiming at improving hydrophilicity, productivity, and salt rejection [171]. Nanomembranes are also known as thin film nanocomposite membranes. Yacou et al. [172] achieved high water fluxes, 10.5 kg/m2 h for brackish water at 0.3 wt% salt concentration and up to 4.0–6.0 kg/m2 h for 10 wt% salt concentration in reject brine. However, the use of nanomembranes for commercial and industrial RO applications remains underdeveloped, as their scalability remains a challenge [173].
- ○
- Aquaporins are pore-forming proteins in biological cells. Under the right conditions, aquaporin forms a water channel that selectively transports water molecules across while excluding ionic species or other polar molecules. Amy et al. [13] reported that aquaporin-based biomimetic membranes are being developed as ultrahigh permeability RO membranes; with impregnation of aquaporins into a polymeric matrix, aquaporin can provide water channeling/gating, leading to controlled water permeability and ion selectivity [12]. This technology promises high efficiency in ROC treatment since the movement of water in aquaporins is facilitated by “selective rapid diffusion” and an osmotic gradient. The major advantage of aquaporin-based biomimetic membranes is that they don’t require a compromise between selectivity and water permeability. Most applications of aquaporin-based biomimetic membrane technology for water treatment have been conducted using forward osmosis [174].
- ○
- Currently, desalination has high energy demands; hence, integrating renewable energy sources into its process is imperative. However, there are challenges for reducing energy demands and in the use of renewable energy in managing ROC. Okampo and Nwulu [175] explored efficient energy acquirement from renewable energy sources, and brine management in the production of freshwater by synergizing RO, electrodialysis, and crystallization methods. In this case, the brine produced from the RO unit is further desalinated by electrodialysis, leaving a very high concentration to crystallize into soluble salts, thereby achieving a ZLD. The results show that renewable energy sources are more cost-effective and environmentally friendly. Furthermore, the average cost of energy is within the average range of standalone desalination units, suggesting a similar cost of energy for standalone desalination units and combined desalination–brine treatment units.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AQUASTAT Website. FAO’s Global Information System on Water and Agriculture. Food and Agriculture Organization (FAO). 2021. Available online: http://fao.org/aquastat/statistics/query/index.html?lang=es (accessed on 2 August 2021).
- WWAP (UNESCO World Water Assessment Programme). The United Nations World Water Development Report 2019: Leaving No One Behind; UNESCO: Paris, France, 2019; ISBN 978-92-3-300108-4. [Google Scholar]
- IDA (International Desalination Association). IDA Water Security Handbook: 2019–2020; IDA and GWI DesalData; IDA: Topsfield, MA, USA, 2019; ISBN 978-1-907467-57-8. [Google Scholar]
- IWA (International Water Association). Three Steps to Solving Water Scarcity and Creating Climate Resilience; IWA Publishing: London, UK, 2016; Available online: https://iwa-network.org/three-steps-to-solving-water-scarcity-and-creating-climate-resilience/ (accessed on 2 August 2021).
- Akhatov, J.S. Desalination of Saline Water with the Use of RES: Demand, Current Situation, Development Trends, Forecasts for the Future (Review). Appl. Sol. Energy 2019, 55, 133–148. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Morote, Á.F.; Rico, A.M.; Moltó, E. Critical review of desalination in Spain: A resource for the future? Geogr. Res. 2017, 55, 412–423. [Google Scholar] [CrossRef]
- Schunke, A.J.; Hernandez-Herrera, G.A.; Padhye, L.; Berry, T.A. Energy recovery in SWRO desalination: Current status and new possibilities. Front. Sustain. Cities 2020, 2, 9. [Google Scholar] [CrossRef]
- Widiasa, I.N.; Yoshi, L.A. Techno-Economy Analysis A Small Scale Reverse Osmosis System for Brackish Water Desalination. Int. J. Sci. Eng. 2016, 10, 51–57. [Google Scholar] [CrossRef]
- Chandwankar, R.; Nowak, J. Thermal Processes for Seawater Desalination: Multi-effect Distillation, Thermal Vapor Compression, Mechanical Vapor Compression, and Multistage Flash. In Handbook of Water and Used Water Purification; Springer: Cham, Switzerland, 2019; pp. 1–38. [Google Scholar] [CrossRef]
- Ismail, A.; Matsuura, T. Progress in transport theory and characterization method of Reverse Osmosis (RO) membrane in past fifty years. Desalination 2018, 434, 2–11. [Google Scholar] [CrossRef]
- Saavedra, A.; Valdés, H.; Mahn, A.; Acosta, O. Comparative Analysis of Conventional and Emerging Technologies for Seawater Desalination: Northern Chile as A Case Study. Membranes 2021, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-based seawater desalination: Present and future prospects. Desalination 2017, 40, 16–21. [Google Scholar] [CrossRef]
- Shenvi, S.S.; Isloor, A.M.; Ismail, A.F. A review on RO membrane technology: Developments and challenges. Desalination 2015, 368, 10–26. [Google Scholar] [CrossRef]
- Elsaid, K.; Sayed, E.T.; Abdelkareem, M.A.; Baroutaji, A.; Olabi, A.G. Environmental Impact of Desalination Processes: Mitigation and Control Strategies. Sci. Total Environ. 2020, 740, 140125. [Google Scholar] [CrossRef]
- Ghernaout, D. Desalination Engineering: Environmental Impacts of the Brine Disposal and Their Control. Open Access Libr. J. 2020, 7, 1. [Google Scholar] [CrossRef]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Shu, L.; Jegatheesan, V. A review of the management and treatment of brine solutions. Environ. Sci. Water Res. Technol. 2017, 3, 625–658. [Google Scholar] [CrossRef]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy consumption and environmental impact assessment of desalination plants and brine disposal strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Amma, L.V.; Ashraf, F. Brine Management in Reverse Osmosis Desalination: A UAE Perspective. In 2020 Advances in Science and Engineering Technology International Conferences (ASET); IEEE: Piscataway, NJ, USA, 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Luukkonen, T.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. Sustainable batching water options for one-part alkali-activated slag mortar: Sea water and reverse osmosis reject water. PLoS ONE 2020, 15, e0242462. [Google Scholar] [CrossRef]
- Maheshwari, K.; Agrawal, M. Advances in capacitive deionization as an effective technique for reverse osmosis reject stream treatment. J. Environ. Chem. Eng. 2020, 8, 104413. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, D.; Song, H.; Sim, S.W.; Kim, D.; Yu, J.; Cho, K.H.; Oh, J.E. Recycling of Reverse Osmosis (RO) Reject Water as a Mixing Water of Calcium Sulfoaluminate (CSA) Cement for Brick Production. Appl. Sci. 2019, 9, 5044. [Google Scholar] [CrossRef]
- Rana, M.S.; Sharma, A.K.; Parambil, J.V.; Prajapati, S.K. Potential of reverse osmosis reject water as a growth medium for the production of algal metabolites–A state-of-the-art review. J. Water Process. Eng. 2020, 40, 101849. [Google Scholar] [CrossRef]
- Jeppesen, T.; Shu, L.; Keir, G.; Jegatheesan, V. Metal recovery from reverse osmosis concentrate. J. Clean. Prod. 2009, 17, 703–707. [Google Scholar] [CrossRef]
- Drenkova-Tuhtan, A.; Sheeleigh, E.K.; Rott, E.; Meyer, C.; Sedlak, D.L. Sorption of recalcitrant phosphonates in reverse osmosis concentrates and wastewater effluents–influence of metal ions. Water Sci. Technol. 2021, 83, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Scholes, R.C.; Vega, M.A.; Sharp, J.O.; Sedlak, D.L. Nitrate removal from reverse osmosis concentrate in pilot-scale open-water unit process wetlands. Environ. Sci. Water Res. Technol. 2021, 7, 650–661. [Google Scholar] [CrossRef]
- Boland, A.; Cherry, G.; Dickson, R. (Eds.) Doing a Systematic Review: A Student’s Guide, 2nd ed.; Sage Publications Ltd.: London, UK, 2017. [Google Scholar]
- Tranfield, D.; Denyer, D.; Smart, P. Towards a methodology for developing evidence-informed management knowledge by means of systematic review. Br. J. Manag. 2003, 14, 207–222. [Google Scholar] [CrossRef]
- Andreini, D.; Bettinelli, C. Systematic Literature Review. In Business Model Innovation; International Series in Advanced Management Studies; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Mohamed-Shaffril, H.A.; Samsuddin, S.F.; Abu Samah, A. The ABC of systematic literature review: The basic methodological guidance for beginners. Qual. Quant. 2021, 55, 1319–1346. [Google Scholar] [CrossRef]
- Torres-Carrión, P.V.; González-González, C.S.; Aciar, S.; Rodríguez-Morales, G. Methodology for systematic literature review applied to engineering and education. In Proceedings of the 2018 IEEE Global Engineering Education Conference (EDUCON), Santa Cruz de Tenerife, Spain, 17–20 April 2008; IEEE: Piscataway, NJ, USA, 2018; pp. 1364–1373. [Google Scholar] [CrossRef]
- Stern, C.; Jordan, Z.; McArthur, A. Developing the review question and inclusion criteria. Am. J. Nurs. 2014, 114, 53–56. [Google Scholar] [CrossRef]
- Krippendorff, K. Content Analysis: An Introduction to Its Methodology, 4th ed.; SAGE Publications Ltd.: London, UK, 2018. Available online: https://lccn.loc.gov/2017050739 (accessed on 1 September 2021).
- Pellicer, E.; Correa, C.L.; Yepes, V.; Alarcón, L.F. Organizational improvement through standardization of the innovation process in construction firms. Eng. Manag. J. 2012, 24, 40–53. [Google Scholar] [CrossRef]
- Cemre-Birben, N.; Uyguner-Demirel, C.; Bekbolet, M. Organic matrix in reverse osmosis concentrate: Composition and treatment alternatives. Curr. Org. Chem. 2017, 21, 1084–1097. [Google Scholar] [CrossRef]
- Semblante, G.U.; Lee, J.Z.; Lee, L.Y.; Ong, S.L.; Ng, H.Y. Brine pre-treatment technologies for zero liquid discharge systems. Desalination 2018, 441, 96–111. [Google Scholar] [CrossRef]
- Sohn, J.; Valavala, R.; Han, J.; Her, N.; Yoon, Y. Pretreatment in reverse osmosis seawater desalination: A short review. Environ. Eng. Res. 2011, 16, 205–212. [Google Scholar] [CrossRef]
- Joo, S.H.; Tansel, B. Novel technologies for reverse osmosis concentrate treatment: A review. J. Environ. Manag. 2015, 150, 322–335. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; Poussade, Y.; Drewes, J.E.; Nghiem, L.D. N-Nitrosamine removal by reverse osmosis for indirect potable water reuse e a critical review based on observations from laboratory-, pilot- and full-scale studies. Sep. Purif. Technol. 2012, 98, 503–515. [Google Scholar] [CrossRef]
- Bruggen, B.V.D.; Lejon, L.; Vandecasteele, C. Reuse, treatment, and discharge of the concentrate of pressure-driven membrane processes. Environ. Sci. Technol. 2003, 37, 3733–3738. [Google Scholar] [CrossRef]
- Aljohani, N.S.; Al-Farawati, R.K.; Shabbaj, I.I.; Al-Mur, B.A.; Kavil, Y.N.; Abdel Salam, M. Environmental Remediation of Desalination Plant Outfall Brine Discharge from Heavy Metals and Salinity Using Halloysite Nanoclay. Water 2021, 13, 969. [Google Scholar] [CrossRef]
- Kang, D.; Yoo, Y.; Park, J. Accelerated chemical conversion of metal cations dissolved in seawater-based reject brine solution for desalination and CO2 utilization. Desalination 2020, 473, 114147. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Y.; Shi, L.; Li, H.; Li, R.; Hong, S.; Zhuo, S.; Tiejun Zhang, T.; Wang, P. Designing a next generation solar crystallizer for real seawater brine treatment with zero liquid discharge. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Sanmartino, J.A.; Khayet, M.; García-Payo, M.C.; El-Bakouri, H.; Riaza, A. Treatment of reverse osmosis brine by direct contact membrane distillation: Chemical pretreatment approach. Desalination 2017, 420, 79–90. [Google Scholar] [CrossRef]
- Ahmed, M.; Arakel, A.; Hoey, D.; Thumarukudy, M.R.; Goosen, M.F.; Al-Haddabi, M.; Al-Belushi, A. Feasibility of salt production from inland RO desalination plant reject brine: A case study. Desalination 2003, 158, 109–117. [Google Scholar] [CrossRef]
- Dawoud, M.A.; Al Mulla, M.M. Environmental impacts of seawater desalination: Arabian Gulf case study. Int. J. Environ. Sustain. 2012, 1. [Google Scholar] [CrossRef]
- Kang, N.W.; Lee, S.; Kim, D.; Hong, S.; Kweon, J.H. Analyses of calcium carbonate scale deposition on four RO membranes under a seawater desalination condition. Water Sci. Technol. 2011, 64, 1573–1580. [Google Scholar] [CrossRef][Green Version]
- Missimer, T.M.; Maliva, R.G. Environmental issues in seawater reverse osmosis desalination: Intakes and outfalls. Desalination 2018, 434, 198–215. [Google Scholar] [CrossRef]
- Kress, N.; Gertner, Y.; Shoham-Frider, E. Seawater quality at the brine discharge site from two mega size seawater reverse osmosis desalination plants in Israel (Eastern Mediterranean). Water Res. 2020, 171, 115402. [Google Scholar] [CrossRef]
- Xu, X.; Lin, L.; Ma, G.; Wang, H.; Jiang, W.; He, Q.; Nirmalakhandan, N.; Xu, P. Study of polyethyleneimine coating on membrane permselectivity and desalination performance during pilot-scale electrodialysis of reverse osmosis concentrate. Sep. Purif. Technol. 2018, 207, 396–405. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Song, X.; Yu, J. Separation of mono-and di-valent ions from seawater reverse osmosis brine using selective electrodialysis. Environ. Sci. Pollut. Res. 2021, 28, 18754–18767. [Google Scholar] [CrossRef] [PubMed]
- Hajbi, F.; Hammi, H.; M’nif, A. Reuse of RO desalination plant reject brine. J. Phase Equilibria Diffus. 2010, 31, 341–347. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, M.; Pan, J.; Sotto, A.; Shen, J.; Gao, C.; Van der Bruggen, B. Process economic evaluation of resource valorization of seawater concentrate by membrane technology. ACS Sustain. Chem. Eng. 2017, 5, 5820–5830. [Google Scholar] [CrossRef]
- Zarzo, D. Beneficial uses and valorization of reverse osmosis brines. In Emerging Technologies for Sustainable Desalination Handbook; Butterworth-Heinemann: Oxford, UK, 2018; pp. 365–397. [Google Scholar]
- Ji, X.; Curcio, E.; Al Obaidani, S.; Di Profio, G.; Fontananova, E.; Drioli, E. Membrane distillation-crystallization of seawater reverse osmosis brines. Sep. Purif. Technol. 2010, 71, 76–82. [Google Scholar] [CrossRef]
- Martinetti, C.R.; Childress, A.E.; Cath, T.Y. High recovery of concentrated RO brines using forward osmosis and membrane distillation. J. Membr. Sci. 2009, 331, 31–39. [Google Scholar] [CrossRef]
- Li, X.; Hasson, D.; Semiat, R.; Shemer, H. Intermediate concentrate demineralization techniques for enhanced brackish water reverse osmosis water recovery–A review. Desalination 2019, 466, 24–35. [Google Scholar] [CrossRef]
- Petersková, M.; Valderrama, C.; Gibert, O.; Cortina, J.L. Extraction of valuable metal ions (Cs, Rb, Li, U) from reverse osmosis concentrate using selective sorbents. Desalination 2012, 286, 316–323. [Google Scholar] [CrossRef]
- Arroyo, F.; Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H. Lithium recovery from desalination brines using specific ion-exchange resins. Desalination 2019, 468, 114073. [Google Scholar] [CrossRef]
- Kim, S.; Joo, H.; Moon, T.; Kim, S.H.; Yoon, J. Rapid and selective lithium recovery from desalination brine using an electrochemical system. Environ. Sci. Process Impacts 2019, 21, 667–676. [Google Scholar] [CrossRef]
- Le Dirach, J.; Nisan, S.; Poletiko, C. Extraction of strategic materials from the concentrated brine rejected by integrated nuclear desalination systems. Desalination 2005, 182, 449–460. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.C.; Xie, J.; Zhao, J.; Wu, T.; Wang, H.; Liu, W.; Zhang, J.; Chu, S.; Cui, Y. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Loganathan, K.; Chelme-Ayala, P.; El-Din, M.G. Treatment of basal water using a hybrid electrodialysis reversal–reverse osmosis system combined with a low-temperature crystallizer for near-zero liquid discharge. Desalination 2015, 363, 92–98. [Google Scholar] [CrossRef]
- Naidu, G.; Zhong, X.; Vigneswaran, S. Comparison of membrane distillation and freeze crystallizer as alternatives for reverse osmosis concentrate treatment. Desalination 2018, 427, 10–18. [Google Scholar] [CrossRef]
- Bello, A.S.; Zouari, N.; Da’ana, D.A.; Hahladakis, J.N.; Al-Ghouti, M.A. An overview of brine management: Emerging desalination technologies, life cycle assessment, and metal recovery methodologies. J. Environ. Manag. 2021, 288, 112358. [Google Scholar] [CrossRef]
- Korngold, E.; Aronov, L.; Daltrophe, N. Electrodialysis of brine solutions discharged from an RO plant. Desalination 2009, 242, 215–227. [Google Scholar] [CrossRef]
- Gude, G. Emerging Technologies for Sustainable Desalination Handbook; Butterworth-Heinemann: Oxford, UK, 2018. [Google Scholar]
- Fard, A.K.; Rhadfi, T.; Khraisheh, M.; Atieh, M.A.; Khraisheh, M.; Hilal, N. Reducing flux decline and fouling of direct contact membrane distillation by utilizing thermal brine from MSF desalination plant. Desalination 2016, 379, 172–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghyselbrecht, K.; Vanherpe, R.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. RO concentrate minimization by electrodialysis: Techno-economic analysis and environmental concerns. J. Environ. Manag. 2012, 107, 28–36. [Google Scholar] [CrossRef]
- Ariono, D.; Purwasasmita, M.; Wenten, I.G. Brine Effluents: Characteristics, Environmental Impacts, and Their Handling. J. Eng. Technol. 2016, 48. [Google Scholar] [CrossRef]
- Herrero-Gonzalez, M.; Admon, N.; Dominguez-Ramos, A.; Ibañez, R.; Wolfson, A.; Irabien, A. Environmental sustainability assessment of seawater reverse osmosis brine valorization by means of electrodialysis with bipolar membranes. Environ. Sci. Pollut. Res. 2020, 27, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Dolnicar, S.; Schäfer, A.I. Desalinated versus recycled water: Public perceptions and profiles of the accepters. J. Environ. Manag. 2009, 90, 888–900. [Google Scholar] [CrossRef]
- Kress, N. Marine Environmental Impact of Seawater Desalination Science, Management, and Policy; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-811953-2. [Google Scholar]
- Mannan, M.; Alhaj, M.; Mabrouk, A.N.; Al-Ghamdi, S.G. Examining the life-cycle environmental impacts of desalination: A case study in the State of Qatar. Desalination 2019, 452, 238–246. [Google Scholar] [CrossRef]
- Alharbi, O.A.; Phillips, M.R.; Williams, A.T.; Gheith, A.M.; Bantan, R.A.; Rasul, N.M. Desalination impacts on the coastal environment: Ash Shuqayq, Saudi Arabia. Sci. Total Environ. 2012, 421–422, 163–172. [Google Scholar] [CrossRef]
- Tarnacki, K.; Meneses, M.; Melin, T.; van Medevoort, J.; Jansen, A. Environmental assessment of desalination processes: Reverse osmosis and Memstill®. Desalination 2012, 296, 69–80. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, V.W.C.; Fane, A.G. An improved life cycle impact assessment (LCIA) approach for assessing aquatic eco-toxic impact of brine disposal from seawater desalination plants. Desalination 2013, 308, 233–241. [Google Scholar] [CrossRef]
- Hiscock, K.; Southward, A.J.; Tittley, I.; Hawkins, S.J. Effect of changing temperature on benthic marine life in Britain and Ireland. Aquat. Conserv. 2004, 14, 333–362. [Google Scholar] [CrossRef]
- Al-Sanea, S.; Orfia, J.; Najiba, A. Numerical study of flow, temperature, and salinity distributions of a brine discharge problem. Desalin. Water Treat. 2015, 55, 3218–3230. [Google Scholar] [CrossRef]
- Kim, D.; Amy, G.L.; Karanfil, T. Disinfection by-product formation during seawater desalination: A review. Water Res. 2015, 81, 343–355. [Google Scholar] [CrossRef]
- Yu, H.W.; Oh, S.G.; Kim, I.S.; Pepper, I.; Snyder, S.; Jang, A. Formation and speciation of haloacetic acids in seawater desalination using chlorine dioxide as disinfectant. J. Ind. Eng. Chem. 2015, 26, 193–201. [Google Scholar] [CrossRef]
- Elsaid, K.; Batchelor, B.; Abdel-Wahab, A. Kinetics of Halogenated Disinfection By-Products Formation in Chlorinated Seawater. In Encyclopedia of Water: Science, Technology, and Society; Maurice, P.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.X.; Ye, T.; Shi, N.; Tang, F.; Hu, H.Y. Characterization of trihalomethane, haloacetic acid, and haloacetonitrile precursors in a seawater reverse osmosis system. Sci. Total Environ. 2017, 576, 391–397. [Google Scholar] [CrossRef]
- Hang, C.; Zhang, B.; Gong, T.; Xian, Q. Occurrence and health risk assessment of halogenated disinfection byproducts in indoor swimming pool water. Sci. Total Environ. 2016, 543, 425–431. [Google Scholar] [CrossRef]
- Peñate, B.; García-Rodríguez, L. Current trends and future prospects in the design of seawater reverse osmosis desalination technology. Desalination 2012, 284, 1–8. [Google Scholar] [CrossRef]
- Hoepner, T.; Lattemann, S. Chemical impacts from seawater desalination plants—A case study of the northern Red Sea. Desalination 2003, 152, 133–140. [Google Scholar] [CrossRef]
- Belkin, N.; Rahav, E.; Elifantz, H.; Kress, N.; Berman-Frank, I. The effect of coagulants and antiscalants discharged with seawater desalination brines on coastal microbial communities: A laboratory and in situ study from the southeastern Mediterranean. Water Res. 2017, 110, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Tularam, G.A.; Ilahee, M. Environmental concerns of desalinating seawater using reverse osmosis. J. Environ. Monit. 2007, 9, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, B. Short Review of Salt Recovery from Reverse Osmosis Rejects. In Salt in the Earth; IntechOpen: London, UK, 2019; pp. 64–80. [Google Scholar]
- Hagmeyer, G.; Gimbel, R. Modelling the rejection of nanofiltration membranes using zeta potential measurements. Sep. Purif. Technol. 1999, 15, 19–30. [Google Scholar] [CrossRef]
- Cornejo-Poncea, L.; Moraga-Contrerasc, C.; Vilca-Salinasb, P. Analysis of Chilean legal regime for brine obtained from desalination processes. Desalin. Water Treat. 2020, 203, 91–103. [Google Scholar] [CrossRef]
- Kress, N. Chapter 7: Policy and Regulations for Seawater Desalination. In Marine Impacts of Seawater Desalination, Science, Management, and Policy; Fisher, M., Munri, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–163. [Google Scholar]
- Palomar, P.; Ruiz-Mateo, A.; Losada, I.J.; Lara, J.L.; Lloret, A.; Castanedo, S.; Alvárez, A.; Méndez, F.; Rodrigo, M.; Camus, P.; et al. MEDVSA: A methodology for the design of brine discharges into the seawater. J. Water Reuse Desal. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- General Environmental Regulations and Rules for Implementation (GERRI). 28 Rajab 1422 H. p. Kingdom of Saudi Arabia Presidency of Meteorology and Environment. 2001. Available online: http://extwprlegs1.fao.org/docs/pdf/sau138926E.pdf (accessed on 1 September 2021).
- Royal Legislative Decree 1302/1986, Environmental Impact Note. BOE, 155 del 30 de Junio de. 1986. Available online: http://istas.net/descargas/RD%20legislativo%201302.%201986.pdf (accessed on 1 September 2021).
- Clean Water Act, National Pollutant Discharge Elimination System Compliance Monitoring Strategy. U.S. Environmental Protection Agency Office of Enforcement and Compliance Assurance Office of Compliance, 2014; pp. 1–38. Available online: https://www.epa.gov/sites/default/files/2013-09/documents/npdescms.pdf (accessed on 1 September 2021).
- Water Quality Control Plan for the Ocean Waters of California, State Water Resources Control Board California Environmental Protection Agency. 2015. Available online: https://www.epa.gov/sites/production/files/2017–01/documents/ca-cop2012.pdf (accessed on 1 September 2021).
- Law 21/2013, Environmental Note, State Official Newsletter of España. 2013. Available online: https://www.boe.es/eli/es/l/2013/12/09/21/dof/spa/pdf (accessed on 1 September 2021).
- Zhu, Z.; Peng, D.; Wang, H. Seawater desalination in China: An overview. J. Water Reuse Desal. 2019, 9, 115–132. [Google Scholar] [CrossRef]
- Katal, R.; Ying Shen, T.; Jafari, I.; Masudy-Panah, S.; Hossein Davood Abadi Farahani, M. An Overview on the Treatment and Management of the Desalination Brine Solution. In Desalination-Challenges and Opportunities; Farahani, M.H.D.A., Vahid Vatanpour, V., Taheri, A.H., Eds.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/72467 (accessed on 1 September 2021). [CrossRef]
- Zarzo, D. La “Mineria de la Salmuera” para Aprovechar los Procesos de Desalación. Desalación. Sacyr S.A. Available online: https://www.sacyr.com/-/la-mineria-de-la-salmuera-para-aprovechar-los-procesos-de-desalacion (accessed on 17 September 2021). (In Spanish).
- Dehwah, A.H.A.; Li, S.; Al-Mashharawi, S.; Winters, H.; Missimer, T.M. Changes in feedwater organic matter concentrations based on intake type and pretreatment processes at SWRO facilities, Red Sea, Saudi Arabia. Desalination 2015, 360, 19–27. [Google Scholar] [CrossRef]
- Dehwah, A.H.A.; Missimer, T.M. Seabed gallery intakes: Investigation of the water pretreatment effectiveness of the active layer using a long-term column experiment. Water Res. 2017, 121, 95–108. [Google Scholar] [CrossRef]
- Dehwah, A.H.A.; Missimer, T.M. Subsurface intake systems: Green choice for improving feed water quality at SWRO desalination plants, Jeddah, Saudi Arabia. Water Res. 2016, 88, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Del-Pilar-Ruso, Y.; Martinez-Garcia, E.; Giménez-Casalduero, F.; Loya-Fernández, A.; Ferrero-Vicente, L.M.; Marco-Méndez, C.; de-la-Ossa-Carretero, J.A.; Sánchez-Lizaso, J.L. Benthic community recovery from brine impact after the implementation of mitigation measures. Water Res. 2015, 70, 325–336. [Google Scholar] [CrossRef]
- Malcangio, D.; Petrillo, A.F. Modeling of brine outfall at the planning stage of desalination plants. Desalination 2010, 254, 114–125. [Google Scholar] [CrossRef]
- Maalouf, S.; Rosso, D.; Yeh, W.W.G. Optimal planning and design of seawater RO brine outfalls under environmental uncertainty. Desalination 2014, 333, 134–145. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Oren, Y.; Korngold, E.; Daltrophe, N.; Messalem, R.; Volkman, Y.; Aronov, L.; Weismann, M.; Bouriakov, N.; Glueckstern, P.; Gilron, J. Pilot studies on high recovery BWRO-EDR for near zero liquid discharge approach. Desalination 2010, 261, 321–330. [Google Scholar] [CrossRef]
- Xevgenos, D.; Moustakas, K.; Malamis, D.; Loizidou, M. An overview on desalination & sustainability: Renewable energy-driven desalination and brine management. Desalin. Water Treat. 2016, 57, 2304–2314. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The global rise of zero liquid discharge for wastewater management: Drivers, technologies, and future directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef]
- Radziemska, M.; Vaverková, M.D.; Adamcová, D.; Brtnický, M.; Mazur, Z. Valorization of fish waste compost as a fertilizer for agricultural use. Waste Biomass Valori. 2019, 10, 2537–2545. [Google Scholar] [CrossRef]
- Kavanagh, L.; Keohane, J.; Garcia Cabellos, G.; Lloyd, A.; Cleary, J. Global lithium sources—Industrial use and future in the electric vehicle industry: A review. Resources 2018, 7, 57. [Google Scholar] [CrossRef]
- Shanks, W., III; Kimball, B.; Tolcin, A.; Guberman, D. Germanium and indium, chap. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey Professional Paper 1802; U.S. Geological Survey: Reston, VA, USA, 2017; pp. I1–I27. [Google Scholar] [CrossRef]
- Ramanujam, J.; Singh, U.P. Copper indium gallium selenide based solar cells—A review. Energy Environ. Sci. 2017, 10, 1306–1319. [Google Scholar] [CrossRef]
- Romanchuk, A.Y.; Vlasova, I.E.; Kalmykov, S.N. Speciation of uranium and plutonium from nuclear legacy sites to the environment: A mini review. Front. Chem. 2020, 8, 630. [Google Scholar] [CrossRef]
- Rest, J.; Cooper, M.W.D.; Spino, J.; Turnbull, J.A.; Van Uffelen, P.; Walker, C.T. Fission gas release from UO2 nuclear fuel: A review. J. Nucl. Mater. 2019, 513, 310–345. [Google Scholar] [CrossRef]
- Ogunbiyi, O.; Saththasivam, J.; Al-Masri, D.; Manawi, Y.; Lawler, J.; Zhang, X.; Liu, Z. Sustainable brine management from the perspectives of water, energy and mineral recovery: A comprehensive review. Desalination 2021, 513, 115055. [Google Scholar] [CrossRef]
- Roberts, D.A.; Johnston, E.L.; Knott, N.A. Impacts of desalination plant discharges on the marine environment: A critical review of published studies. Water Res. 2010, 44, 5117–5128. [Google Scholar] [CrossRef]
- Liyanaarachchi, S.; Jegatheesan, V.; Shu, L.; Shon, H.K.; Muthukumaran, S.; Li, C.Q. Evaluating the Feasibility of Forward Osmosis in Diluting RO Concentrate Using Pretreatment Backwash Water. Membranes 2020, 10, 35. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Oppenheimer, J.; Adham, S.; Kumar, M. Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electro-chlorination processes. J. Membr. Sci. 2009, 326, 392–399. [Google Scholar] [CrossRef]
- Fernandez, C.; Dominguez, A.; Ibanez, R.; Irabien, A. Electrodialysis with bipolar membranes for valorization of brines. Sep. Purif. Rev. 2016, 45, 275–287. [Google Scholar] [CrossRef]
- Chung, H.W.; Nayar, K.G.; Swaminathan, J.; Chehayeb, K.M.; Lienhard, J.H. Thermodynamic analysis of brine management methods: Zero-discharge desalination and salinity-gradient power production. Desalination 2017, 404, 291–303. [Google Scholar] [CrossRef]
- Khan, Q.; Maraqa, M.A.; Mohamed, A.M.O. Inland desalination: Techniques, brine management, and environmental concerns. Pollut. Assess. Sustain. Pract. Appl. Sci. Eng. 2021, 2021, 871–918. [Google Scholar] [CrossRef]
- Laspidou, C.; Hadjibiros, K.; Gialis, S. Minimizing the environmental impact of sea brine disposal coupling desalination plants with solar saltworks: A case study for Greece. Water 2010, 2, 75–84. [Google Scholar] [CrossRef]
- Collares-Pereira, M.; Mendes, J.F.; Horta, P.; Korovessis, N. Final design of an advanced solar dryer for salt recovery from brine effluent of an MED desalination plant. Desalination 2007, 211, 222–231. [Google Scholar] [CrossRef]
- Pérez-González, A.; Ibañez, R.; Gomez, P.; Urtiaga, A.M.; Ortiz, I.; Irabien, J.A. Recovery of desalination brines: Separation of calcium, magnesium and sulfate as a pretreatment step. Desalin. Water Treat. 2015, 56, 3617–3625. [Google Scholar] [CrossRef]
- El-Sayed, M.M.H.; Hani, H.A.; Mohamed, H. Polymeric ion exchangers for the recovery of ions from brine and seawater. Chem. Eng. Process Tech. 2014, 2, 1020. Available online: https://www.jscimedcentral.com/ChemicalEngineering/chemicalengineering-2-1020.php (accessed on 1 September 2021).
- Leusbrock, I. Removal of Inorganic Compounds via Supercritical Water: Fundamentals and Applications. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2011. Available online: https://pure.rug.nl/ws/portalfiles/portal/14565912/thesis.pdf (accessed on 1 September 2021).
- Almarsi, D.; Mahmoud, K.A.; Abdel-Wahab, A. Two-stage sulfate removal from reject brine in inland desalination with zero-liquid discharge. Desalination 2015, 362, 52–58. [Google Scholar] [CrossRef]
- Garcia, C.; Molina, F.; Zarzo, D. 7 year operation of a BWRO plant with raw water from a coastal aquifer for agricultural irrigation. Desalin. Water Treat. 2011, 31, 331–338. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Hybrid technologies: The future of energy efficient desalination—A review. Desalination 2020, 495, 114659. [Google Scholar] [CrossRef]
- Makabe, R.; Ueyama, T.; Sakai, H.; Tanioka, A. Commercial Pressure Retarded Osmosis Systems for Seawater Desalination Plants. Membranes 2021, 11, 69. [Google Scholar] [CrossRef]
- Parsa, S.M.; Majidniya, M.; Alawee, W.H.; Dhahad, H.A.; Ali, H.M.; Afrand, M.; Amidpour, M. Thermodynamic, economic, and sensitivity analysis of salt gradient solar pond (SGSP) integrated with a low-temperature multi effect desalination (MED): Case study, Iran. Sustain. Energy Technol. 2021, 47, 101478. [Google Scholar] [CrossRef]
- Ahdab, Y.D.; Lienhard, J.H. Desalination of brackish groundwater to improve water quality and water supply. In Global Groundwater; Elsevier: Amsterdam, The Netherlands, 2021; pp. 559–575. [Google Scholar] [CrossRef]
- Torquemada, Y.; Sanchez, J.L. Monitoring of brine discharges from seawater desalination plants in the Mediterranean. Int. J. Environ. Health. 2007, 1, 1–13. [Google Scholar] [CrossRef]
- Zarzo, D.; Campos, E.; Prats, D.; Hernandez, P.; Garcia, A. Microalgae production for nutrient removal in desalination brines. IDA J. Desalin. Water Reuse 2014, 6, 61–68. [Google Scholar] [CrossRef]
- Khan, S.J.; Murchland, D.; Rhodes, M.; Waite, T.D. Management of concentrated waste streams from high-pressure membrane water treatment systems. Crit. Rev. Environ. Sci. Technol. 2009, 39, 367–415. [Google Scholar] [CrossRef]
- De Souza, F.I.; Da Silva, N.; De Sousa, O.N.; Cruz, J.; Medeiros, A.C.; Nascimento, V.C.; da Silva, C.; de Sousa-Junior, F. Agricultural potential of reject brine from water desalination. Afr. J. Agric. Res. 2015, 10, 4713–4717. [Google Scholar] [CrossRef]
- Donnelly, K. The Red Sea-Dead Sea Project Update. In The World’s Water; Island Press: Washington, DC, USA, 2014; pp. 153–158. [Google Scholar]
- Sánchez-Carceller, E. Concentrate Treatments in Reverse Osmosis Desalination Plants: Status and Innovative Proposals. Master’s Thesis, Universidad de Sevilla, Sevilla, Spain, 2020. Available online: https://hdl.handle.net/11441/108816 (accessed on 1 September 2021).
- Melián-Martel, N.; Sadhwani, J.J.; Báez, S.O.P. Saline waste disposal reuse for desalination plants for the chlor-alkali industry: The particular case of pozo izquierdo SWRO desalination plant. Desalination 2011, 281, 35–41. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Hassan, A.A.; El Badawy, A. Bio-desalination of brackish and seawater using halophytic algae. Desalination 2019, 465, 104–113. [Google Scholar] [CrossRef] [PubMed]
- De Vito, C.; Ferrini, V.; Mignardi, S.; Cagnetti, M.; Leccese, F. Progress in carbon dioxide sequestration via carbonation of aqueous saline wastes. Period. Mineral. 2012, 81, 333–344. [Google Scholar] [CrossRef]
- Parker, D.M.; Tatum, T.C. Is the Use of Road Salt and Chemical Deicers Worth the Costs? A Call for Environmentally Sustainable Winter Road Operations. J. Strateg. Innov. Sustain. 2021, 16. [Google Scholar] [CrossRef]
- Ayoub, G.M.; Korban, L.; Al-Hindi, M.; Zayyat, R. Removal of fouling species from brackish water reverse osmosis reject stream. Environ. Technol. 2018, 39, 804–813. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Williams, M.D.; Rahardianto, A.; Franklin, J.C.; Cohen, Y. High-recovery reverse osmosis desalination using intermediate chemical demineralization. J. Membr. Sci. 2007, 301, 131–141. [Google Scholar] [CrossRef]
- Liu, Z.Q.; You, L.; Xiong, X.; Wang, Q.; Yan, Y.; Tu, J.; Cui, Y.H.; Li, X.Y.; Gen, W.; Wu, X. Potential of the integration of coagulation and ozonation as a pretreatment of reverse osmosis concentrate from coal gasification wastewater reclamation. Chemosphere 2019, 222, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, J.; Ye, W.; Zhou, W.; Jia, X.; Zhao, S.; Ye, C. Potential of coagulation/GAC adsorption combined with UV/H2O2 and ozonation for removing dissolved organic matter from secondary RO concentrate. J. Chem. Technol. Biot. 2019, 94, 1091–1099. [Google Scholar] [CrossRef]
- Chen, Y.; Baygents, J.C.; Farrell, J. Evaluating electrocoagulation and chemical coagulation for removing dissolved silica from high efficiency reverse osmosis (HERO) concentrate solutions. J. Water Process. Eng. 2017, 16, 50–55. [Google Scholar] [CrossRef]
- Ashraf, S.N.; Rajapakse, J.; Dawes, L.A.; Millar, G.J. Electrocoagulation for the purification of highly concentrated brine produced from reverse osmosis desalination of coal seam gas associated water. J. Water Process. Eng. 2019, 28, 300–310. [Google Scholar] [CrossRef]
- Lalia, B.S.; Hashaikeh, R. Electrochemical precipitation to reduce waste brine salinity. Desalination 2021, 498, 114796. [Google Scholar] [CrossRef]
- Zhang, A.; Gu, Z.; Chen, W.; Li, Q.; Jiang, G. Removal of refractory organic pollutants in reverse-osmosis concentrated leachate by Microwave–Fenton process. Environ. Sci. Pollut. Res. 2018, 25, 28907–28916. [Google Scholar] [CrossRef]
- Zhou, M.; Tan, Q.; Wang, Q.; Jiao, Y.; Oturan, N.; Oturan, M.A. Degradation of organics in reverse osmosis concentrate by electro-Fenton process. J. Hazard. Mater. 2012, 215, 287–293. [Google Scholar] [CrossRef]
- Zeng, D.; Liang, K.; Guo, F.; Wu, Y.; Wu, G. Denitrification performance and microbial community under salinity and MIT stresses for reverse osmosis concentrate treatment. Sep. Purif. Technol. 2020, 242, 116799. [Google Scholar] [CrossRef]
- Zhang, Z.; King, J.F.; Szczuka, A.; Chuang, Y.H.; Mitch, W.A. Pilot-scale ozone/biological activated carbon treatment of reverse osmosis concentrate: Potential for synergism between nitrate and contaminant removal and potable reuse. Environ. Sci. Water Res. Technol. 2020, 6, 1421–1431. [Google Scholar] [CrossRef]
- Mohammadifakhr, M.; de Grooth, J.; Roesink, H.D.W.; Kemperman, A.J.B. Forward Osmosis: A Critical Review. Processes 2020, 8, 404. [Google Scholar] [CrossRef]
- Wu, X.; Lau, C.H.; Pramanik, B.K.; Zhang, J.; Xie, Z. State-of-the-Art and Opportunities for Forward Osmosis in Sewage Concentration and Wastewater Treatment. Membranes 2021, 11, 305. [Google Scholar] [CrossRef]
- Akther, N.; Daer, S.; Hasan, S.W. Effect of flow rate, draw solution concentration and temperature on the performance of TFC FO membrane, and the potential use of RO reject brine as a draw solution in FO–RO hybrid systems. Desalin. Water Treat. 2018, 136, 65–71. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Song, X.; Yu, J. Forward osmosis for multi-effect distillation brine treatment: Performance and concentration polarization evaluation. Can. J. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Thomas, N.; Mavukkandy, M.O.; Loutatidou, S.; Arafat, H.A. Membrane distillation research & implementation: Lessons from the past five decades. Sep. Purif. Technol. 2017, 189, 108–127. [Google Scholar] [CrossRef]
- Das, P.; Dutta, S.; Singh, K.K. Insights into membrane crystallization: A sustainable tool for value added product recovery from effluent streams. Sep. Purif. Technol. 2020, 257, 117666. [Google Scholar] [CrossRef]
- Salmón, I.R.; Luis, P.J.C.E. Membrane crystallization via membrane distillation. Chem. Eng. Process. 2018, 123, 258–271. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L.; Fontananova, E. (Eds.) Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherland, 2017; pp. 297–317. [Google Scholar]
- Balcik-Canbolat, C.; Sengezer, C.; Sakar, H.; Karagunduz, A.; Keskinler, B. A study on near zero liquid discharge approach for the treatment of reverse osmosis membrane concentrate by electrodialysis. Environ. Technol. 2018. [Google Scholar] [CrossRef]
- Al-Anzi, B.S.; Al-Rashidi, A.; Abraham, L.; Fernandes, J.; Al-Sheikh, A.; Alhazza, A. Brine management from desalination plants for salt production utilizing high current density electrodialysis-evaporator hybrid system: A case study in Kuwait. Desalination 2021, 498, 114760. [Google Scholar] [CrossRef]
- Panagopoulos, A. A comparative study on minimum and actual energy consumption for the treatment of desalination brine. Energy 2020, 212, 118733. [Google Scholar] [CrossRef]
- Tristán, C.; Rumayor, M.; Dominguez-Ramos, A.; Fallanza, M.; Ibáñez, R.; Ortiz, I. Life cycle assessment of salinity gradient energy recovery by reverse electrodialysis in a seawater reverse osmosis desalination plant. Sustain. Energy Fuels 2020, 4, 4273–4284. [Google Scholar] [CrossRef]
- Sharan, P.; Yoon, T.J.; Jaffe, S.M.; Ju, T.; Currier, R.P.; Findikoglu, A.T. Can capacitive deionization outperform reverse osmosis for brackish water desalination? Clean. Eng. Technol. 2021, 3, 100102. [Google Scholar] [CrossRef]
- Zhao, D.; Japip, S.; Zhang, Y.; Weber, M.; Maletzko, C.; Chung, T. Emerging thin-film nanocomposite (TFN) membranes for reverse osmosis: A review. Water Res. 2020, 173, 115557. [Google Scholar] [CrossRef]
- Yacou, C.; Smart, S.; da Costa, J.C.D. Mesoporous TiO2 based membranes for water desalination and brine processing. Sep. Purif. Technol. 2015, 147, 166–171. [Google Scholar] [CrossRef]
- Giwa, A.; Akther, N.; Dufour, V.; Hasan, S.W. A critical review on recent polymeric and nano-enhanced membranes for reverse osmosis. Rsc. Adv. 2016, 6, 8134–8163. [Google Scholar] [CrossRef]
- Giwa, A.; Hasan, S.W.; Yousuf, A.; Chakraborty, S.; Johnson, D.J.; Hilal, N. Biomimetic membranes: A critical review of recent progress. Desalination 2017, 420, 403–424. [Google Scholar] [CrossRef]
- Okampo, E.J.; Nwulu, N. Optimal design and techno-economic evaluation of renewable energy powered combined reverse osmosis desalination and brine treatment unit. Desalin. Water Treat. 2020, 202, 27–37. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, H.; Bañuelos, G.; Yan, B.; Zhou, Q.; Yu, X.; Cheng, X. Constructed wetlands for saline wastewater treatment: A review. Ecol. Eng. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, H.; Bañuelos, G.; Xu, Y.; Yan, B.; Cheng, X. Preliminary study on the dynamics of heavy metals in saline wastewater treated in constructed wetland mesocosms or microcosms filled with porous slag. Environ. Sci. Pollut. Res. 2019, 26, 33804–33815. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, Z.; Bai, Z.; Zhuang, G.; Shim, H. Progress in decontamination by halophilic microorganisms in saline wastewater and soil. Environ. Pollut. 2010, 158, 1119–1126. [Google Scholar] [CrossRef]
- Scholes, R.C.; Stiegler, A.N.; Anderson, C.M.; Sedlak, D.L. Enabling Water Reuse by Treatment of Reverse Osmosis Concentrate: The Promise of Constructed Wetlands. ACS Environ. Au 2021. [Google Scholar] [CrossRef]

| Keywords | K1: Reverse Osmosis Concentrate K2: Reverse Osmosis Reject K3: Reverse Osmosis Brine K4: Component K5: Environmental Impact | K6: Regulation K7: Recovery K8: Treatment K9: Management K10: Zero Liquid Discharge | |
|---|---|---|---|
| Combinations for Search in TITLE-ABS-KEY | Number of Articles | ||
| WoS | Scopus | ||
| C1: K1 | 389 | 351 | |
| C2: K2 | 30 | 34 | |
| C3: K3 | 103 | 107 | |
| C4: (K1 OR K2 OR K3) AND K4 | 24 | 67 | |
| C5: (K1 OR K2 OR K3) AND K5 | 9 | 12 | |
| C6: (K1 OR K2 OR K3) AND K6 | 14 | 12 | |
| C7: (K1 OR K2 OR K3) AND K7 | 158 | 141 | |
| C8: (K1 OR K2 OR K3) AND K7 AND K8 | 102 | 95 | |
| C9: (K1 OR K2 OR K3) AND K7 AND K8 AND K9 | 22 | 26 | |
| C10: (K1 OR K2 OR K3) AND K7 AND K8 AND K9 AND K10 | 9 | 4 | |
| Parameter | Possible Range | Unit | References |
|---|---|---|---|
| pH | 6.2–8.2 | unit | [45,46,47,48,49] |
| Temperature | 24–28 | °C | [50,51,52] |
| Conductivity | 25,000–91,000 | µS/cm | [48,53,54,55] |
| Turbidity | 0.45 | NTU | [48] |
| TDS (1) | 10,000–70,000 | mg/L | [45,46,48,51,53,55,56,57] |
| Alkalinity (2) | 140–1500 | mg/L | [45,51,57,58] |
| TOC (3) | 1.5–142 | mg/L | [51,54,56] |
| Na+ | 3300–25,000 | mg/L | [45,47,48,51,52,53,54,56,57] |
| Mg2+ | 200–7600 | mg/L | [45,47,48,51,52,53,54,56,57] |
| K+ | 80–850 | mg/L | [46,51,52,53,54,59] |
| Ca2+ | 87–2800 | mg/L | [45,47,48,51,52,53,54,56,57,58] |
| B | 5.0–9.5 | mg/L | [55,59] |
| Li | 0.3–0.6 | mg/L | [59,60,61] |
| In | 0.02 | mg/L | [59,62] |
| Rb | 0.1–0.2 | mg/L | [59,62] |
| Cs | 0.0005–0.0008 | mg/L | [59,62] |
| U | 0.0039 | mg/L | [59,63] |
| Ge | 0.00007 | mg/L | [59,62] |
| Fe2+ | 0.001–0.4 | mg/L | [46,55] |
| Mn2+ | 0.1–0.3 | mg/L | [46,64] |
| Sr2+ | 9–18 | mg/L | [55,65,66] |
| Si | 9–11 | mg/L | [45,66] |
| SiO2 | 18–140 | mg/L | [47,51,57,66,67] |
| Cl− | 6500–42,000 | mg/L | [45,51,52,53,54,56,57,66] |
| Br− | 90–230 | mg/L | [52,54,59] |
| SO42− | 1600–8000 | mg/L | [45,47,51,52,53,54,56,57,59,65] |
| NO3− | 1.8–15 | mg/L | [46,55,66] |
| PO43- | 0.4–2.5 | mg/L | [57,68,69] |
| HCO3− | 140–3900 | mg/L | [47,55,56,57,68] |
| Anionic detergents | 112–126 | μg/L MBAS | [45] |
| Type of ROC valorization | Strategies | Reference |
|---|---|---|
| Uses inside the desalination plant | Source of water for pretreatment backwash | [121] |
| Generation of chlorine via electro-chlorination | [122] | |
| Production of acids (HCl) and basic compounds (NaOH) through electrodialysis | [123] | |
| As a source of minerals | Evaporation–crystallization (ZLD) | [124] |
| Evaporation ponds | [125] | |
| Desalination plants for combined water and salt production | [126] | |
| Salt solidification and sequestration | [46] | |
| Intensive evaporation processes | [127] | |
| Electrodialysis for salt recovery | [128] | |
| Ion exchangers for salt recovery | [129] | |
| Solvent extraction | [59] | |
| Supercritical water | [130] | |
| Hybrid processes including RO, NF, and precipitation | [131] | |
| For energy and energy production | Energy recovery | [132] |
| Energy production with turbines | [133] | |
| Energy production using the osmotic potential energy | [134] | |
| Technologies based on solar ponds | [135] | |
| Environmental applications | Land application | [136] |
| Regeneration of degraded areas | [137] | |
| In aquaculture and fish farming | Use of microalgae as biomass for removing certain salts | [138] |
| Inland saline aquaculture | [139] | |
| Other potential uses | Agriculture irrigation | [140] |
| Hydrotherapy | [141] | |
| Secondary recovery of oil through deep well injection of brine and/or CO2 | [142] | |
| Food industry | [143] | |
| Growing of halophiles | [144] | |
| CO2 retention technologies | [145] | |
| Deicing and dust suppression | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, H.; Saavedra, A.; Flores, M.; Vera-Puerto, I.; Aviña, H.; Belmonte, M. Reverse Osmosis Concentrate: Physicochemical Characteristics, Environmental Impact, and Technologies. Membranes 2021, 11, 753. https://doi.org/10.3390/membranes11100753
Valdés H, Saavedra A, Flores M, Vera-Puerto I, Aviña H, Belmonte M. Reverse Osmosis Concentrate: Physicochemical Characteristics, Environmental Impact, and Technologies. Membranes. 2021; 11(10):753. https://doi.org/10.3390/membranes11100753
Chicago/Turabian StyleValdés, Hugo, Aldo Saavedra, Marcos Flores, Ismael Vera-Puerto, Hector Aviña, and Marisol Belmonte. 2021. "Reverse Osmosis Concentrate: Physicochemical Characteristics, Environmental Impact, and Technologies" Membranes 11, no. 10: 753. https://doi.org/10.3390/membranes11100753
APA StyleValdés, H., Saavedra, A., Flores, M., Vera-Puerto, I., Aviña, H., & Belmonte, M. (2021). Reverse Osmosis Concentrate: Physicochemical Characteristics, Environmental Impact, and Technologies. Membranes, 11(10), 753. https://doi.org/10.3390/membranes11100753