Sulfonyl Imide Acid-Functionalized Membranes via Ni (0) Catalyzed Carbon-Carbon Coupling Polymerization for Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Poly(Benzophenone) Polymers (PBP)
2.3. Sulfonation of the PBP Polymer (SPBP)
2.4. Synthesis of Sulfonyl Imide Poly(Benzophenone)s Polymers (SI-PBP)
3. Results and Discussion
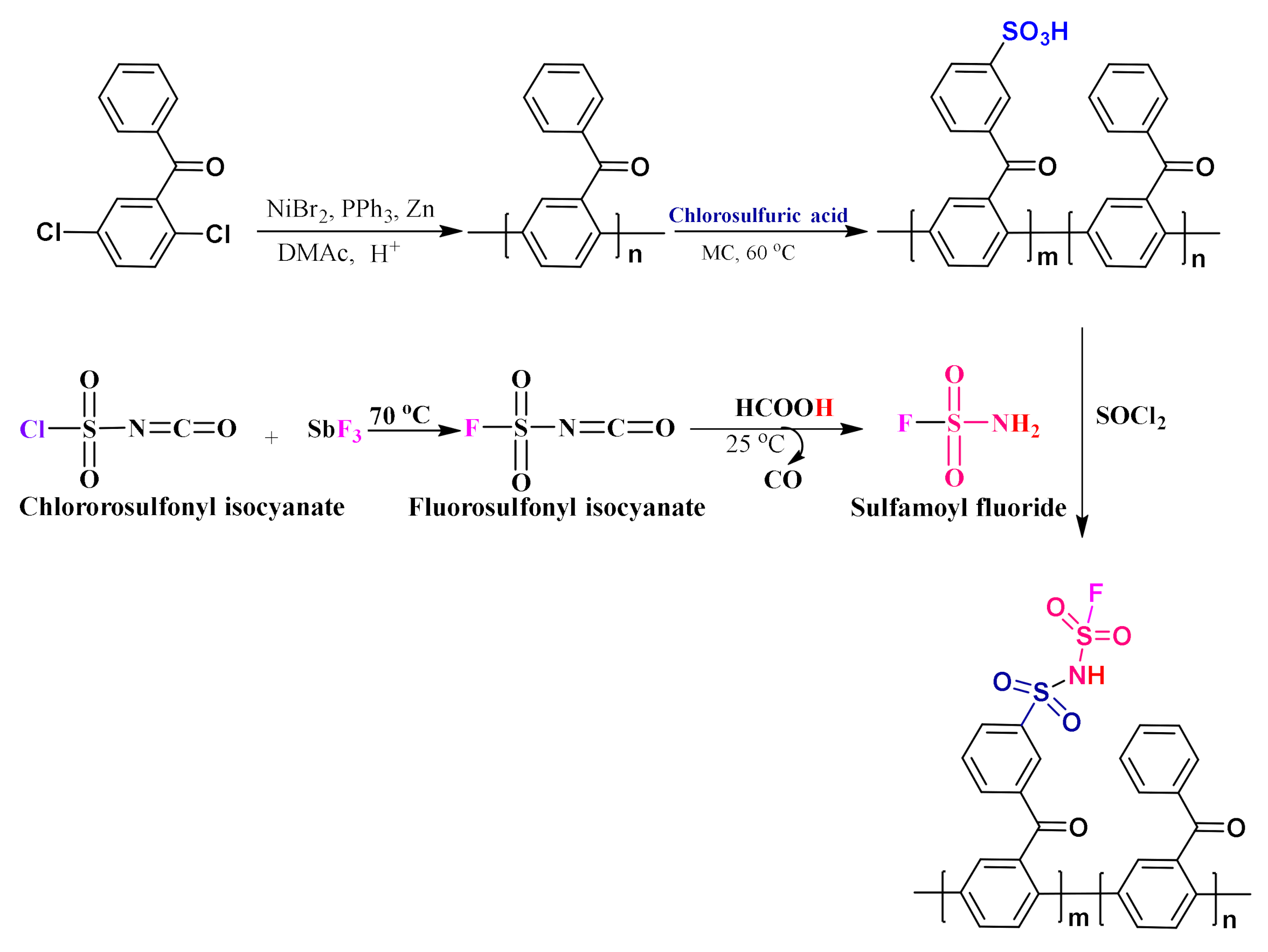
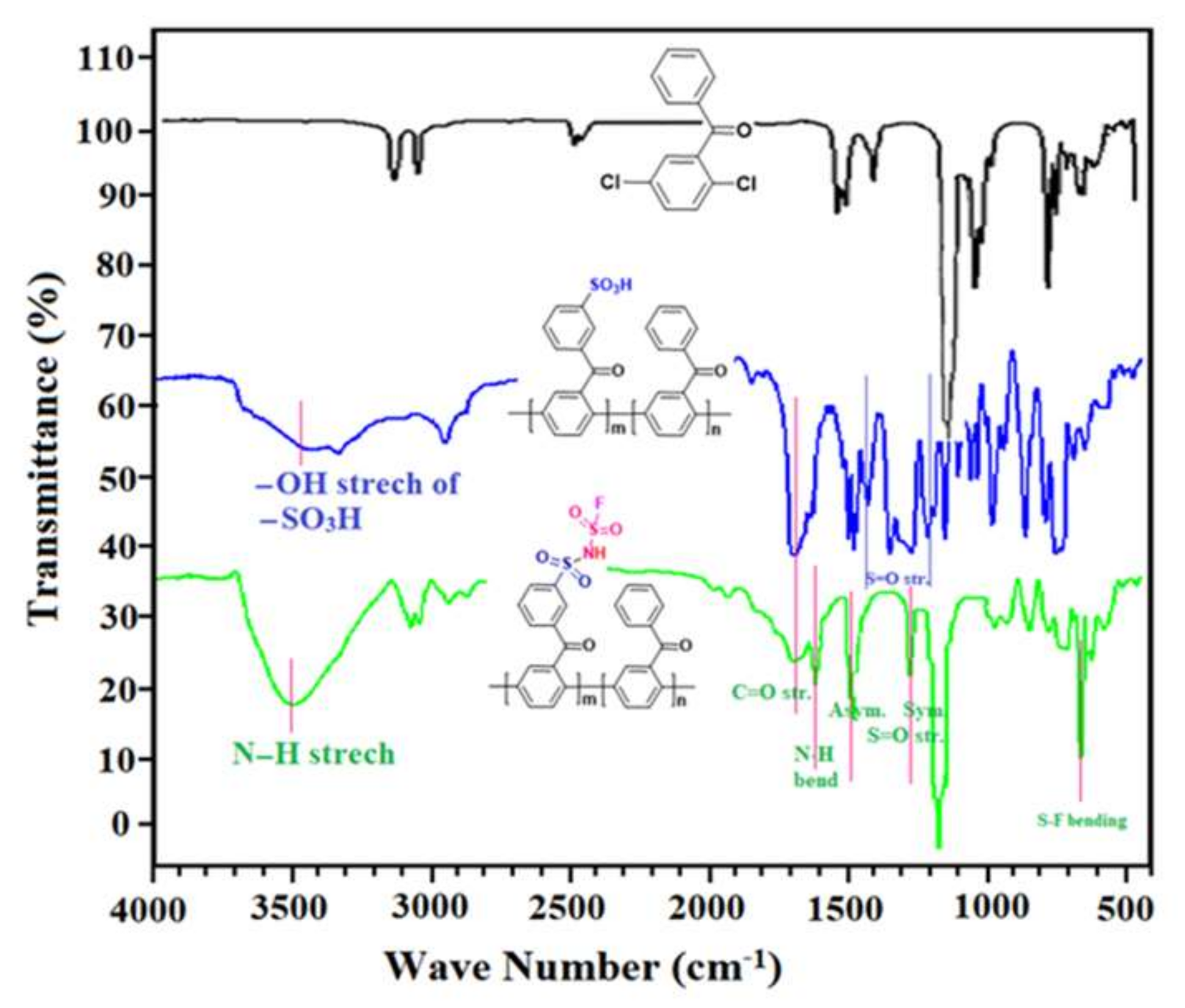
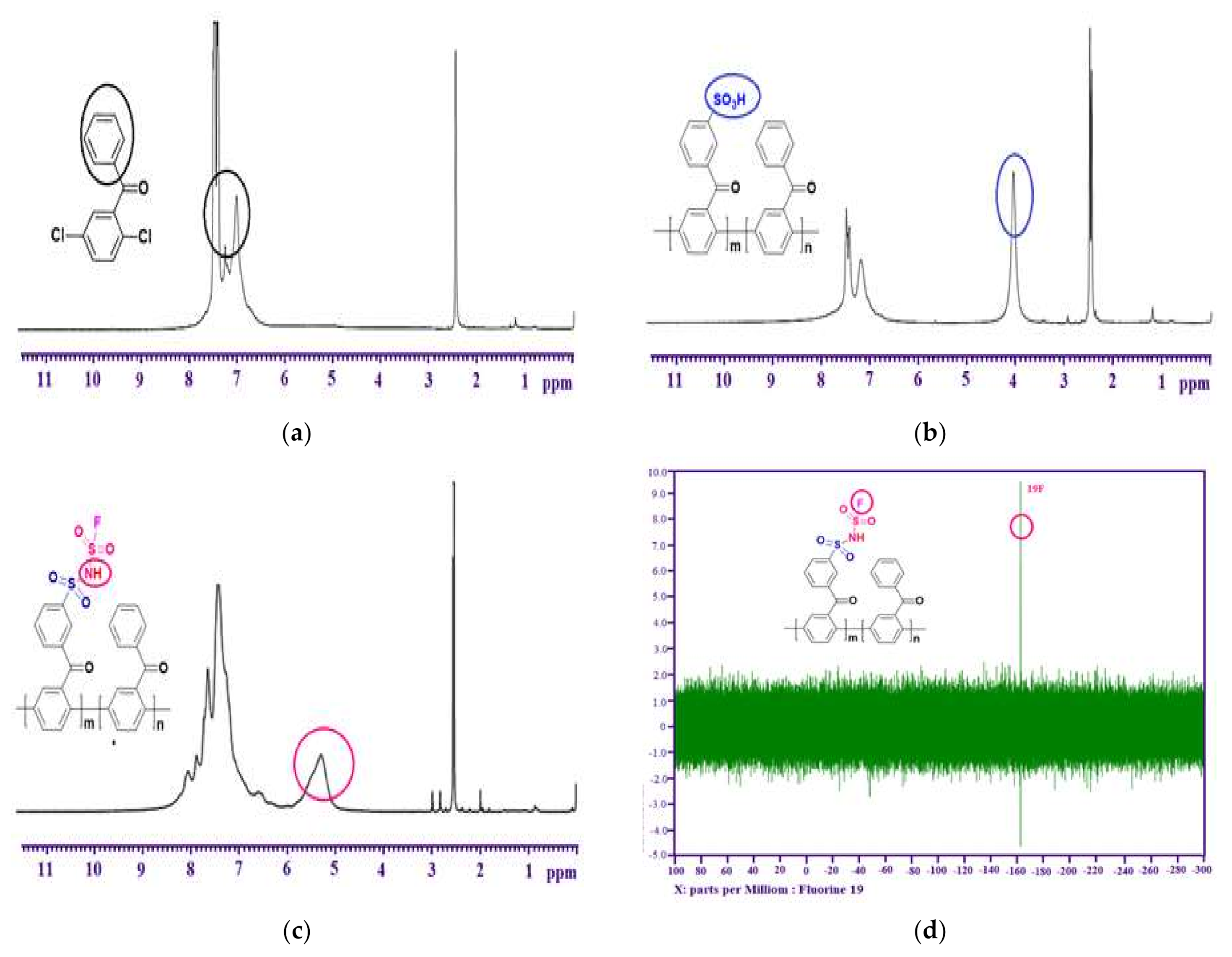
3.1. Preparation of Monomer (DCBP)
3.2. Preparation of Polymers (SI-PBP)
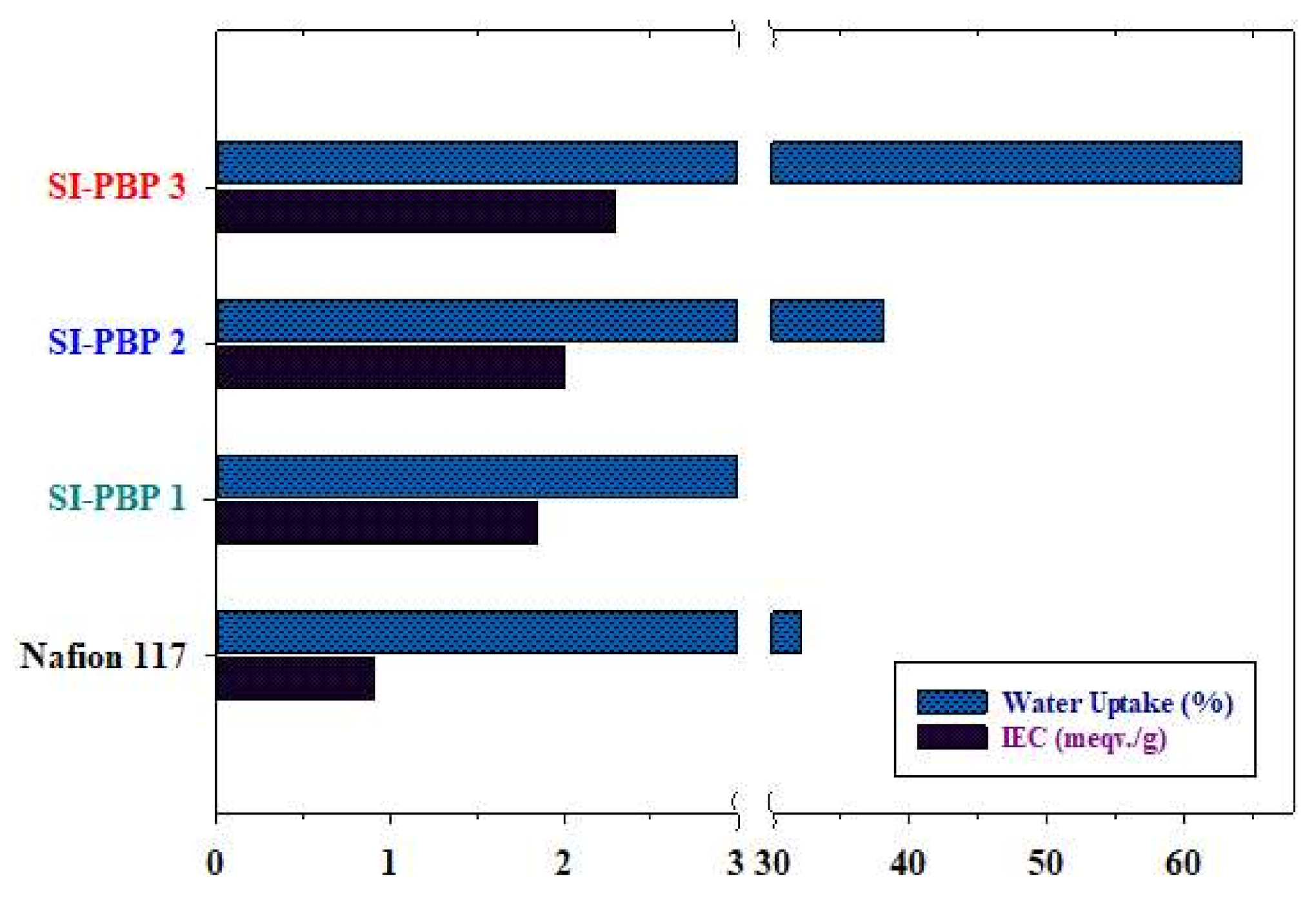
3.3. IEC, Water Uptake and Dimensional Stability of Membranes
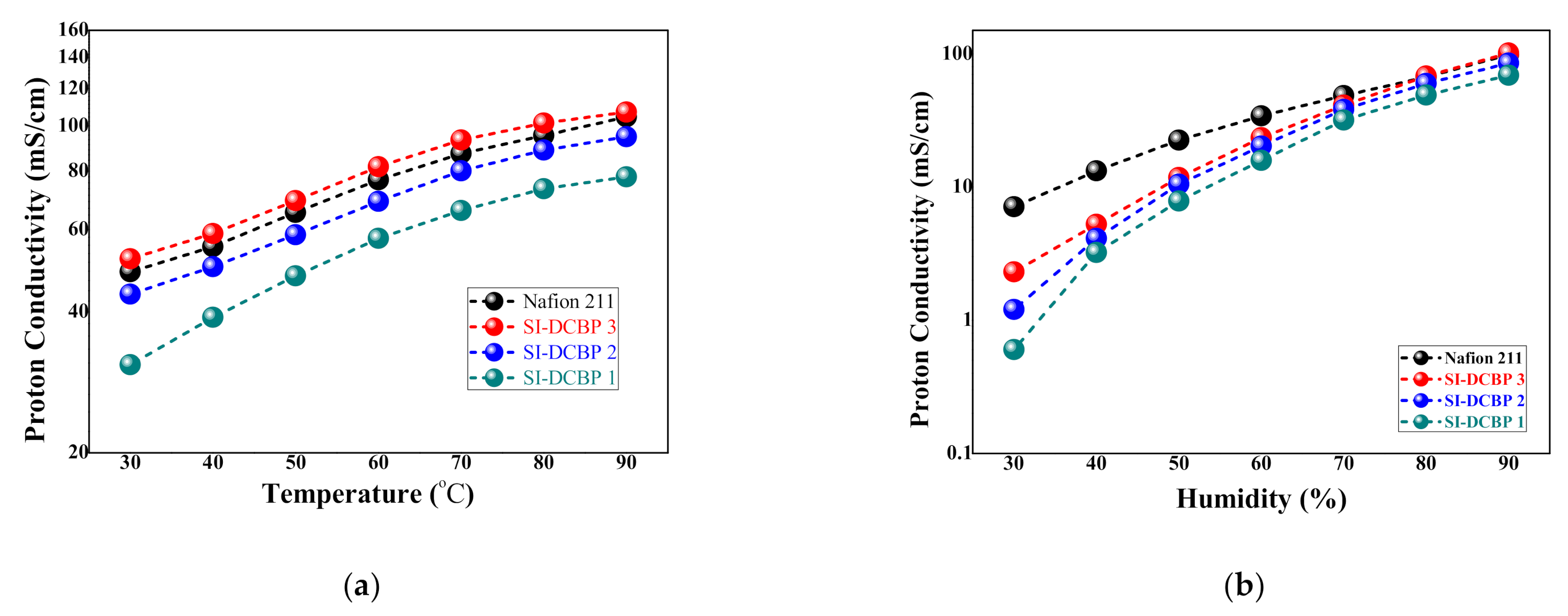
3.4. Proton Conductivity of the SI-PBP Membranes
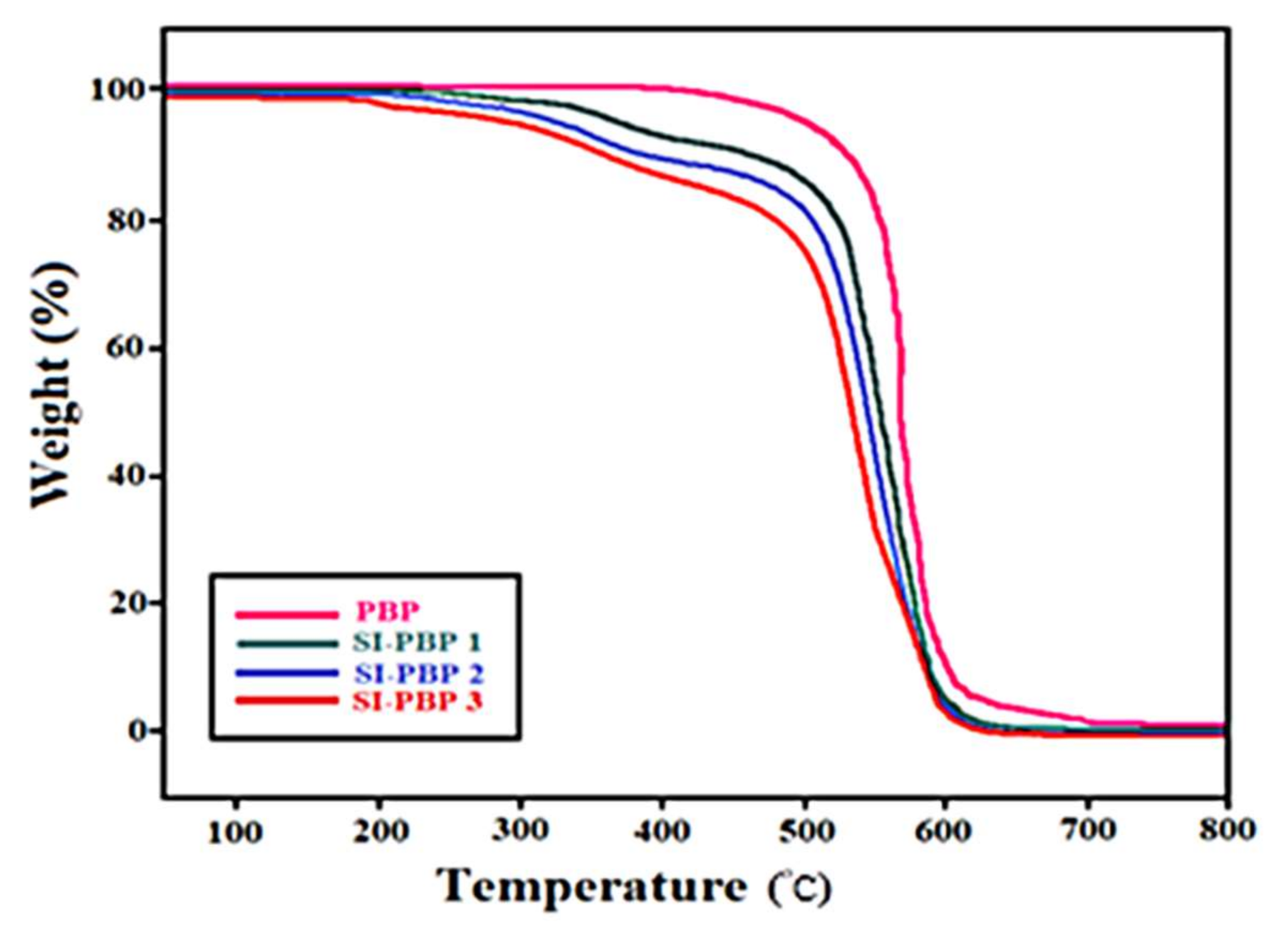
3.5. Thermo-Oxidative Stability of Membranes
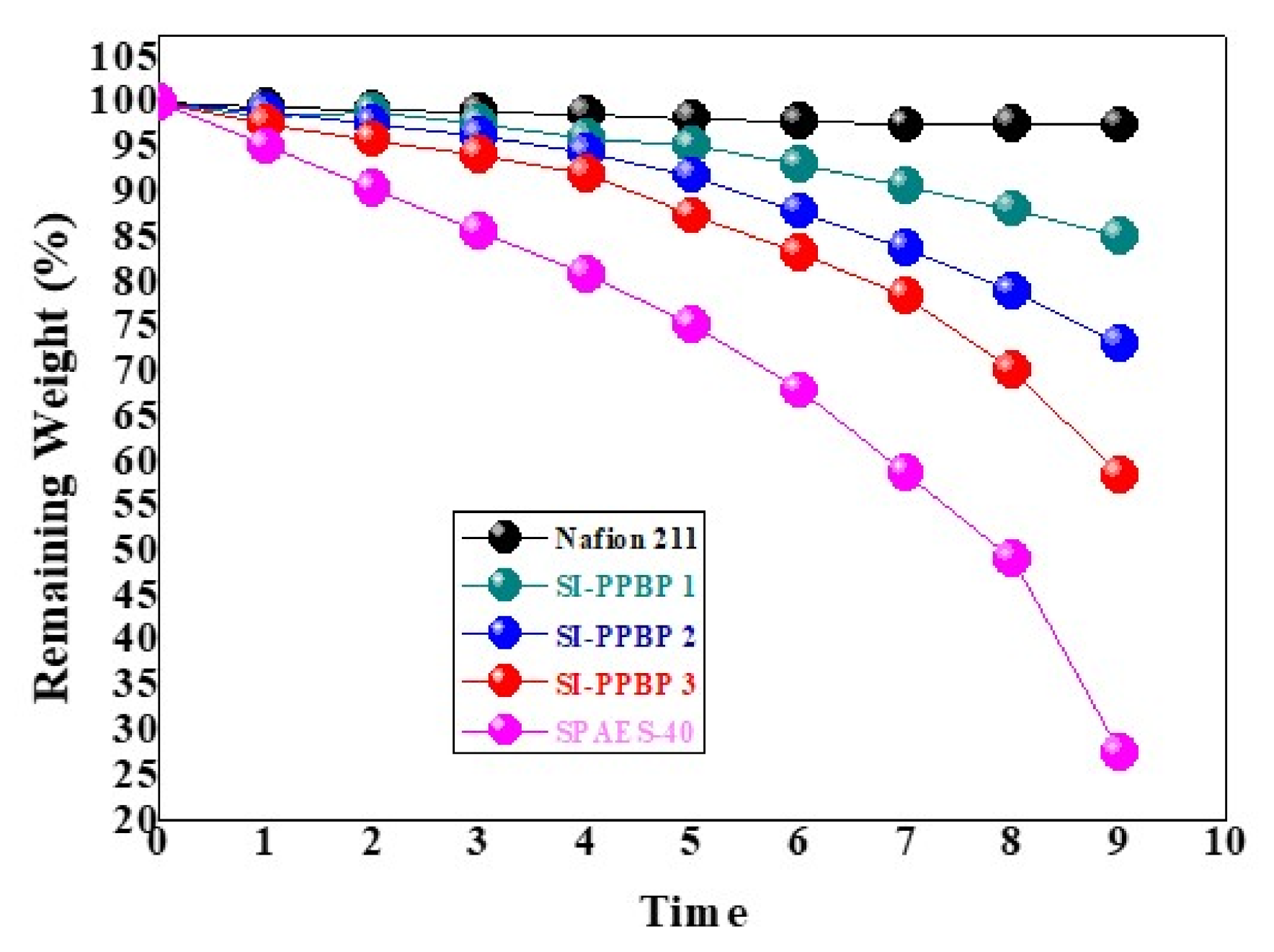
3.6. Chemical Stability of Membranes
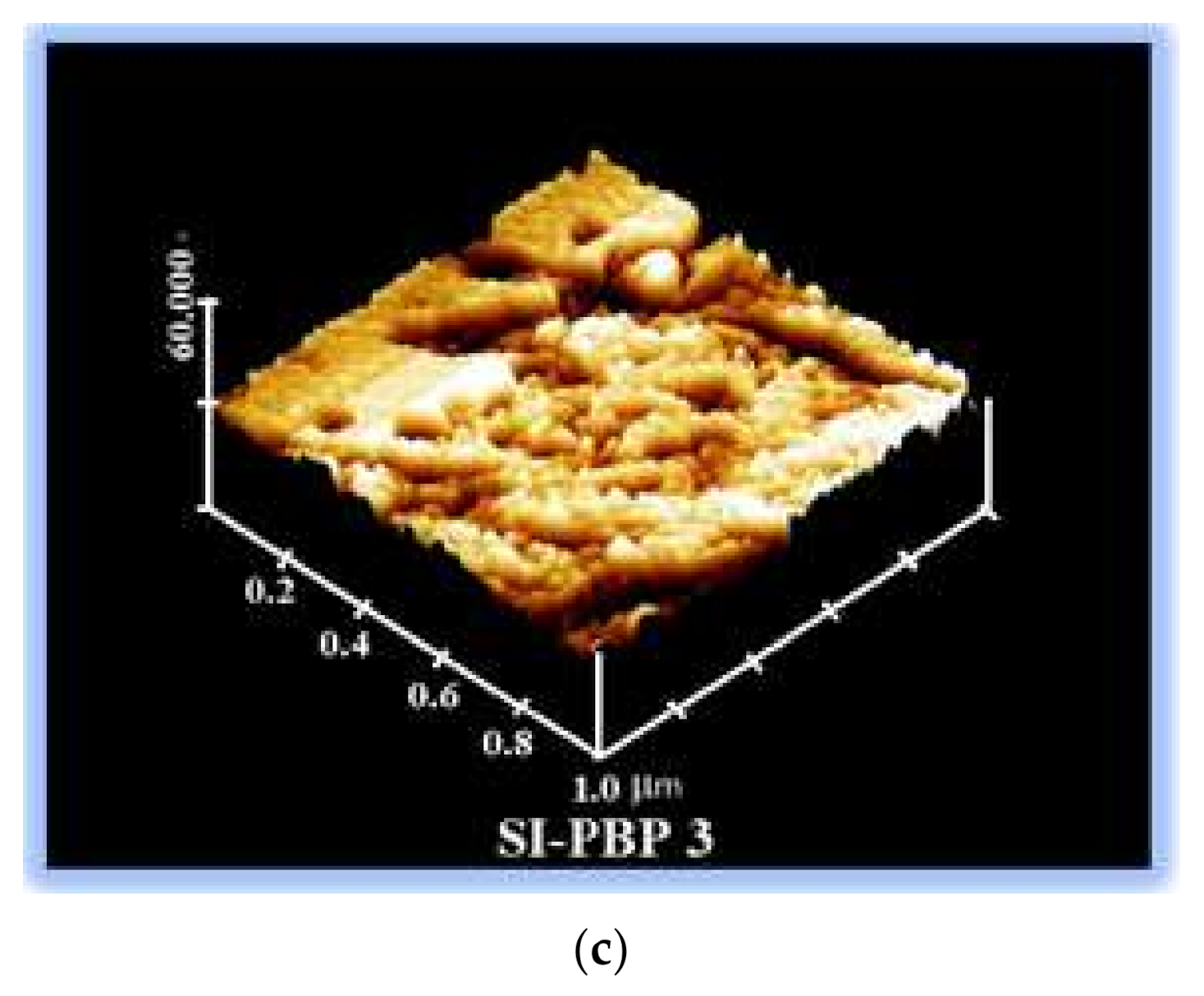
3.7. Morphology of the Membranes
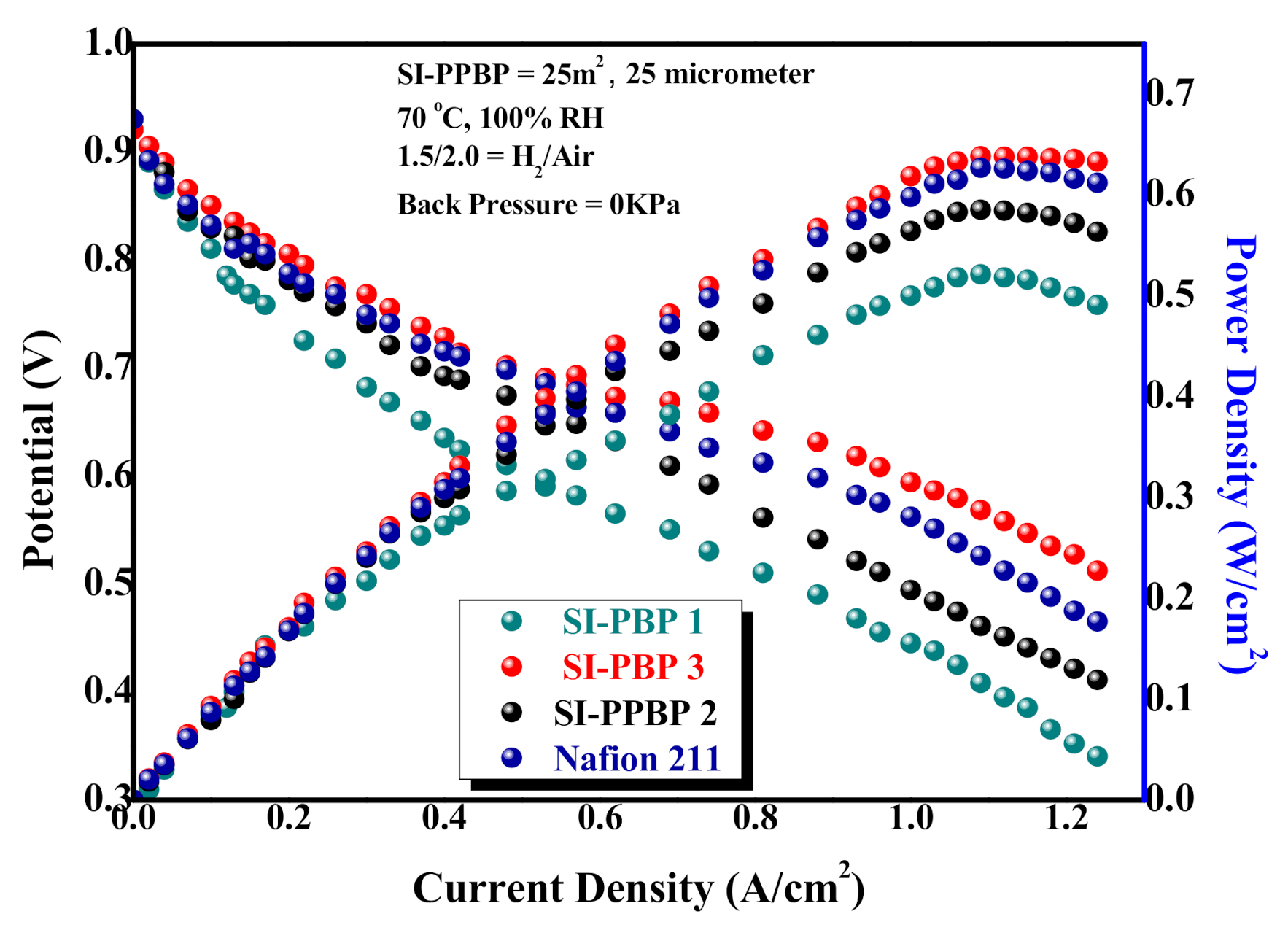
3.8. Cell Performance of the SI-PBP Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, H.; Hong, T.; Yoo, J.; Lee, S.; Ha, J.; Choi, K.; Lee, C.; Kim, W. Synthesis and characterization of sulfonated polyphenylene containing benzophenone moiety via nickel catalyzed polymerization. Electrochim. Acta 2015, 177, 161–167. [Google Scholar] [CrossRef]
- Marx, N.; Boulon, L.; Gustin, F.; Hissel, D.; Agbossou, K. A review of multi-stack and modular fuel cell systems: Interests, application areas and on-going research activities. Int. J. Hydrog. Energy 2014, 39, 12101–12111. [Google Scholar] [CrossRef]
- Zheng, J.; He, Q.; Gao, N.; Yuan, T.; Zhang, S.; Yang, H. Novel proton exchange membranes based on cardo poly(arylene ether sulfone/nitrile)s with perfluoroalkyl sulfonic acid moieties for passive direct methanol fuel cells. J. Power Sources 2014, 261, 38–45. [Google Scholar] [CrossRef]
- Lin, R.; Weng, Y.; Lin, X.; Xiong, F. Rapid cold start of proton exchange membrane fuel cells by the printed circuit board technology. Int. J. Hydrog. Energy 2014, 39, 18369–18378. [Google Scholar] [CrossRef]
- Ettihir, K.; Boulon, L.; Becherif, M.; Agbossou, K.; Ramadan, H.S. Online identification of semi-empirical model parameters for PEMFCs. Int. J. Hydrog. Energy 2014, 39, 21165–21176. [Google Scholar] [CrossRef]
- Petrone, R.; Zheng, Z.; Hissel, D.; Péra, M.; Pianese, C.; Sorrentino, M.C.; Becherif, M.; Yousfi-Steiner, N. A review on model-based diagnosis methodologies for PEMFCs. Int. J. Hydrog. Energy 2013, 38, 7077–7091. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific Aspects of Polymer Electrolyte Fuel Cell Durability and Degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Ying, Y.; Kamarudin, S.K.; Masdar, M.S. Silica-related membranes in fuel cell applications: An overview. Int. J. Hydrog. Energy 2018, 43, 16068–16084. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Graphene in electrocatalyst and proton conductiong membrane in fuel cell applications: An overview. Renew. Sustain. Energy Rev. 2017, 69, 862–870. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, M.J.; Nam, S.Y. A review of polymer–nanocomposite electrolyte membranes for fuel cell application. J. Ind. Eng. Chem. 2015, 21, 36–52. [Google Scholar] [CrossRef]
- Liu, B.; Robertson, G.P.; Kim, D.S.; Guiver, M.D.; Hu, W.; Jiang, Z. Aromatic Poly(ether ketone)s with Pendant Sulfonic Acid Phenyl Groups Prepared by a Mild Sulfonation Method for Proton Exchange Membranes. Macromolecules 2007, 40, 1934–1944. [Google Scholar] [CrossRef]
- Liu, B.; Robertson, G.P.; Kim, D.-S.; Sun, X.; Jiang, Z.; Guiver, M.D. Enhanced thermo-oxidative stability of sulfophenylated poly(ether sulfone)s. Polymer 2010, 51, 403–413. [Google Scholar] [CrossRef][Green Version]
- Alberti, G.; Costantino, U.; Casciola, M.; Ferroni, S.; Massinelli, L.; Staiti, P. Preparation, characterization and proton conductivity of titanium phosphate sulfophenylphosphonate. Solid State Ionics 2001, 145, 249–255. [Google Scholar] [CrossRef]
- Sahu, A.K.; Pitchumani, S.; Sridhar, P.; Shukla, A.K. Nafion and modified-Nafion membranes for polymer electrolyte fuel cells: An overview. Bull. Mater. Sci. 2009, 32, 285–294. [Google Scholar] [CrossRef]
- Pourcelly, G. Membranes for low and medium temperature fuel cells. State-of-the-art and new trends. Pet. Chem. 2011, 51, 480–491. [Google Scholar] [CrossRef]
- Kang, K.; Kim, D. Pendant dual-sulfonated poly(arylene ether ketone) multi-block copolymer membranes for enhanced proton conductivity at reduced water swelling. J. Membr. Sci. 2019, 578, 103–110. [Google Scholar] [CrossRef]
- Liang, J.; Ge, J.; Wu, K.; Zhang, Q.; Wang, J.; Ye, Z. Sulfonated polyaryletherketone with pendant benzimidazole groups for proton exchange membranes. J. Membr. Sci. 2020, 597, 117626. [Google Scholar] [CrossRef]
- Rikukawa, M.; Sanui, K. Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog. Polym. Sci. 2000, 25, 1463–1502. [Google Scholar] [CrossRef]
- Yandrasits, M.; Lindell, M.; Komlev, A.; Fort, E.; Hamrock, S.; Peppin, D.M.; Kalstabakken, K. Stability of Perfluoro Bis(Sulfonyl)Imide-Based Ionomers in Fuel Cell Membranes and Electrodes. ECS Trans. 2018, 86, 381–394. [Google Scholar] [CrossRef]
- Sun, S.; Ling, L.; Xiong, Y.; Zhang, Y.; Li, Z. Trifluoromethanesulfonimide-based hygroscopic semi-interpenetrating polymer network for enhanced proton conductivity of nafion-based proton exchange membranes at low humidity. J. Membr. Sci. 2020, 612, 118339. [Google Scholar] [CrossRef]
- Eikerling, M.; Paddison, S.J.; Zawodzinski, T.A. Molecular orbital calculations of proton dissociation and hydration of various acidic moieties for fuel cell polymers. J. New Mat. Electrochem. Syst. 2002, 5, 15–23. [Google Scholar]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Phan, T.N.T.; Gigmes, D.; Drockenmuller, E. Enhancing Properties of Anionic Poly(ionic liquid)s with 1,2,3-Triazolium Counter Cations. ACS Macro Lett. 2014, 3, 658–662. [Google Scholar] [CrossRef]
- Morozova, S.M.; Shaplov, A.S.; Lozinskaya, E.I.; Mecerreyes, D.; Sardon, H.; Zulfiqar, S.; Suárez-García, F.; Vygodskii, Y.S. Ionic Polyurethanes as a New Family of Poly(ionic liquid)s for Efficient CO2 Capture. Macromolecules 2017, 50, 2814–2824. [Google Scholar] [CrossRef]
- Porcarelli, L.; Shaplov, A.S.; Salsamendi, M.; Nair, J.R.; Vygodskii, Y.S.; Mecerreyes, D.; Gerbaldi, C. Single-Ion Block Copoly(ionic liquid)s as Electrolytes for All-Solid State Lithium Batteries. ACS Appl. Mater. Interfaces 2016, 8, 10350–10359. [Google Scholar] [CrossRef]
- Jangu, C.; Savage, A.M.; Zhang, Z.; Schultz, A.R.; Madsen, L.A.; Beyer, F.L.; Long, T.E. Sulfonimide-Containing Triblock Copolymers for Improved Conductivity and Mechanical Performance. Macromolecules 2015, 48, 4520–4528. [Google Scholar] [CrossRef]
- Ma, Q.; Xia, Y.; Feng, W.; Nie, J.; Hu, Y.S.; Li, H.; Huang, X.; Chen, L.; Armand, M.; Zhou, Z. Impact of the functional group in the polyanion of single lithium-ion conducting polymer electrolytes on the stability of lithium metal electrodes. RSC Adv. 2016, 6, 32454–32461. [Google Scholar] [CrossRef]
- Feng, S.; Shi, D.; Liu, F.; Zheng, L.; Nie, J.; Feng, W.; Huang, X.; Armand, M.; Zhou, Z. Single lithium-ion conducting polymer electrolytes based on poly[(4-styrenesulfonyl)(trifluoromethanesulfonyl)imide] anions. Electrochim. Acta 2013, 93, 254–263. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Meziane, R.; Bonnet, J.P.; Courty, M.; Djellab, K.; Armand, M. Single-ion polymer electrolytes based on a delocalized polyanion for lithium batteries. Electrochim. Acta 2011, 57, 14–19. [Google Scholar] [CrossRef]
- Schaberg, M.S.; Abulu, J.E.; Haugen, G.M.; Emery, M.A.; O’Conner, S.J.; Xiong, P.N.; Hamrock, S. New Multi Acid Side-Chain Ionomers for Proton Exchange Membrane Fuel Cells. ECS Trans. 2019, 33, 627–633. [Google Scholar] [CrossRef]
- Savett, S.C.; Atkins, J.R.; Sides, C.R.; Harris, J.L.; Thomas, B.H.; Creager, S.E.; Pennington, W.T.; Desmarteau, D.D. A Comparison of Bis[(perfluoroalkyl)sulfonyl]imide Ionomers and Perfluorosulfonic Acid Ionomers for Applications in PEM Fuel-Cell Technology. J. Electrochem. Soc. 2002, 149, A1527–A1532. [Google Scholar] [CrossRef]
- Kusoglu, A.; Vezzu, K.; Hegde, G.A.; Nawn, G.; Motz, A.R.; Sarode, H.N.; Haugen, G.M.; Yang, Y.; Seifert, S.; Yandrasits, M.A.; et al. Transport and Morphology of a Proton Exchange Membrane Based on a Doubly Functionalized Perfluorosulfonic Imide Side Chain Perflourinated Polymer. Chem. Mater. 2020, 32, 38–59. [Google Scholar] [CrossRef]
- Handbook of Fluoropolymer Science and Technology—Google Books. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781118850220.ch8 (accessed on 2 May 2014).
- Kutt, A.; Rodima, T.; Saame, J.; Raamat, E.; Maemets, V.; Kaljurand, I.; Koppel, I.A.; Garlyauskayte, R.Y.; Yagupolskii, Y.L.; Yagupolskii, L.M.; et al. Equilibrium Acidities of Superacids. J. Org. Chem. 2011, 76, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sun, J. Triflimide (HNTf2) in Organic Synthesis. Chem. Rev. 2018, 118, 10349–10392. [Google Scholar] [CrossRef] [PubMed]
- Appleby, A.J.; Velev, O.A.; LeHelloco, J.-G.; Parthasarthy, A.; Srinivasan, S.; DesMarteau, D.D.; Gillette, M.S.; Ghosh, J.K. Polymeric Perfluoro Bis-Sulfonimides as Possible Fuel Cell Electrolytes. J. Electrochem. Soc. 1993, 140, 109. [Google Scholar] [CrossRef]
- Razaq, M.; Razaq, A.; Yeager, E.; Desmarteau, D.D.; Singh, S. Perfluorosulfonimide as an Additive in Phosphoric Acid Fuel Cell. J. Electrochem. Soc. 1989, 136, 385–390. [Google Scholar] [CrossRef]
- Razaq, M.; Razaq, A.; Yeager, E.; Desmarteau, D.D.; Singh, S. Oxygen electroreduction in perfluorinated sulphonyl imides. J. Appl. Electrochem. 1987, 17, 1057–1064. [Google Scholar] [CrossRef]
- Porcarelli, L.; Manojkumar, K.; Sardon, H.; Llorente, O.; Shaplov, A.S.; Vijayakrishna, K.; Gerbaldi, C.; Mecerreyes, D. Single Ion Conducting Polymer Electrolytes Based On Versatile Polyurethanes. Electrochim. Acta 2017, 241, 526–534. [Google Scholar] [CrossRef]
- Rehahn, M.; Schlüter, A.-D.; Wegner, G. Soluble poly(para-phenylene)s, 3. Variation of the length and the density of the solubilizing side chains. Die Makromol. Chem. Macromol. Chem. Phys. 1990, 191, 1991–2003. [Google Scholar] [CrossRef]
- Sutradhar, S.C.; Ahmed, F.; Ryu, T.; Yoon, S.; Lee, S.; Rahman, M.; Kim, J.; Lee, Y.; Kim, W.; Jin, Y. A novel synthesis approach to partially fluorinated sulfonimide based poly (arylene ether sulfone) s for proton exchange membrane. Int. J. Hydrog. Energy 2019, 44, 11321–11331. [Google Scholar] [CrossRef]
- Rohan, R.; Pareek, K.; Chen, Z.; Cai, W.; Zhang, Y.; Xu, G.; Gao, Z.; Cheng, H. A high performance polysiloxane-based single ion conducting polymeric electrolyte membrane for application in lithium ion batteries. J. Mater. Chem. A 2015, 3, 20267–20276. [Google Scholar] [CrossRef]
- Rehahn, M.; Schlüter, A.-D.; Wegner, G.; Feast, W.J. Soluble poly(para-phenylene)s. 2. Improved synthesis of poly(para-2,5-di-n-hexylphenylene) via Pd-catalysed coupling of 4-bromo-2,5-di-n-hexylbenzeneboronic acid. Polymer 1989, 30, 1060–1062. [Google Scholar] [CrossRef]
- Wallow, T.I.; Novak, B.M. In aqua synthesis of water-soluble poly(p-phenylene) derivatives. J. Am. Chem. Soc. 1991, 113, 7411–7412. [Google Scholar] [CrossRef]
- Kuo, A.-T.; Shinoda, W.; Okazaki, S. Molecular Dynamics Study of the Morphology of Hydrated Perfluorosulfonic Acid Polymer Membranes. J. Phys. Chem. C 2016, 120, 25832–25842. [Google Scholar] [CrossRef]
- Hagberg, E.C.; Olson, D.A.; Sheares, V.V. Advances in Ni(0)-Catalyzed Coupling for the Synthesis of Polythiophenes and Polyphenylenes. Macromolecules 2004, 37, 4748–4754. [Google Scholar] [CrossRef]
- Liang, X.; Pan, G.; Xu, L.; Wang, J. A modified decal method for preparing the membrane electrode assembly of proton exchange membrane fuel cells. Fuel 2015, 139, 393–400. [Google Scholar] [CrossRef]

| Polymers | Viscosity a ηint (dl/g) | IEC b, (meq/g) | Water Content c, (%) | Dimensional Changes c | Hydration Number, Per Sulfonimide Group c, λ | Conductivity Measurement d, (mS/cm) | Young’s Modulus e (MPa) | ||
|---|---|---|---|---|---|---|---|---|---|
| Δt (%) | Δl (%) | Membrans Thickness, L | σ | ||||||
| SI-PBP 1 | 2.30 | 1.85 | 22.72 | 7.5 | 4.8 | 7.01 | 25 μm | 72.75 | 1005 |
| SI-PBP 2 | 2.12 | 2.00 | 38.09 | 12.5 | 8.0 | 10.58 | 25 μm | 94.80 | 1014 |
| SI-PBP 3 | 2.00 | 2.30 | 64.29 | 34.0 | 16.2 | 15.53 | 25 μm | 107.07 | 1019 |
| Nafion-211® | - | 0.91 | 32.1 | 31.98 | 14.2 | 19.95 | 25 μm | 104.5 | 208 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, S.C.; Yoon, S.; Ryu, T.; Jin, L.; Zhang, W.; Jang, H.; Kim, W. Sulfonyl Imide Acid-Functionalized Membranes via Ni (0) Catalyzed Carbon-Carbon Coupling Polymerization for Fuel Cells. Membranes 2021, 11, 49. https://doi.org/10.3390/membranes11010049
Sutradhar SC, Yoon S, Ryu T, Jin L, Zhang W, Jang H, Kim W. Sulfonyl Imide Acid-Functionalized Membranes via Ni (0) Catalyzed Carbon-Carbon Coupling Polymerization for Fuel Cells. Membranes. 2021; 11(1):49. https://doi.org/10.3390/membranes11010049
Chicago/Turabian StyleSutradhar, Sabuj Chandra, Sujin Yoon, Taewook Ryu, Lei Jin, Wei Zhang, Hohyoun Jang, and Whangi Kim. 2021. "Sulfonyl Imide Acid-Functionalized Membranes via Ni (0) Catalyzed Carbon-Carbon Coupling Polymerization for Fuel Cells" Membranes 11, no. 1: 49. https://doi.org/10.3390/membranes11010049
APA StyleSutradhar, S. C., Yoon, S., Ryu, T., Jin, L., Zhang, W., Jang, H., & Kim, W. (2021). Sulfonyl Imide Acid-Functionalized Membranes via Ni (0) Catalyzed Carbon-Carbon Coupling Polymerization for Fuel Cells. Membranes, 11(1), 49. https://doi.org/10.3390/membranes11010049








