Argon and Argon–Oxygen Plasma Surface Modification of Gelatin Nanofibers for Tissue Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
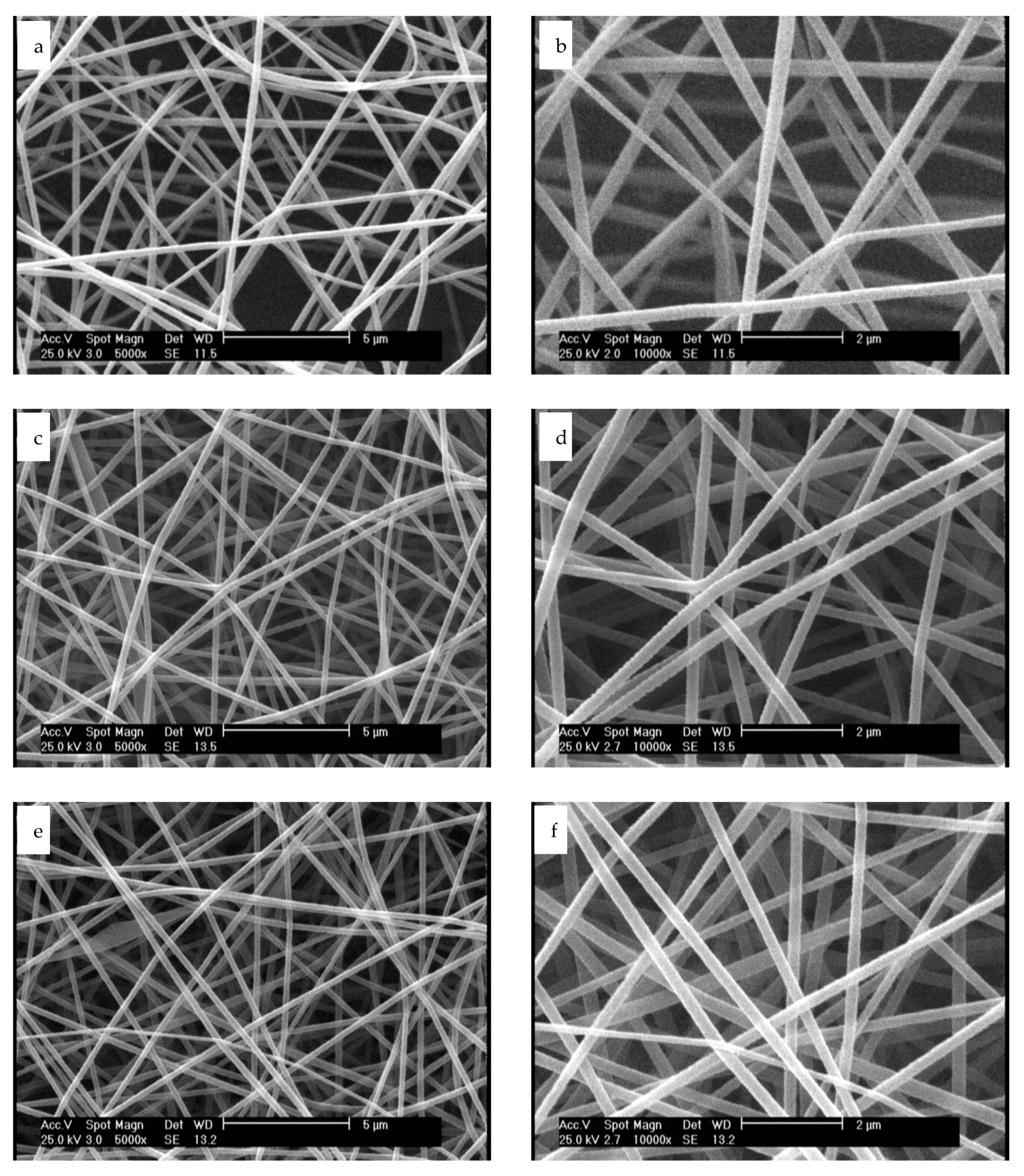
3.1. Microscopic Evaluation of Plasma Treated Nanofibers
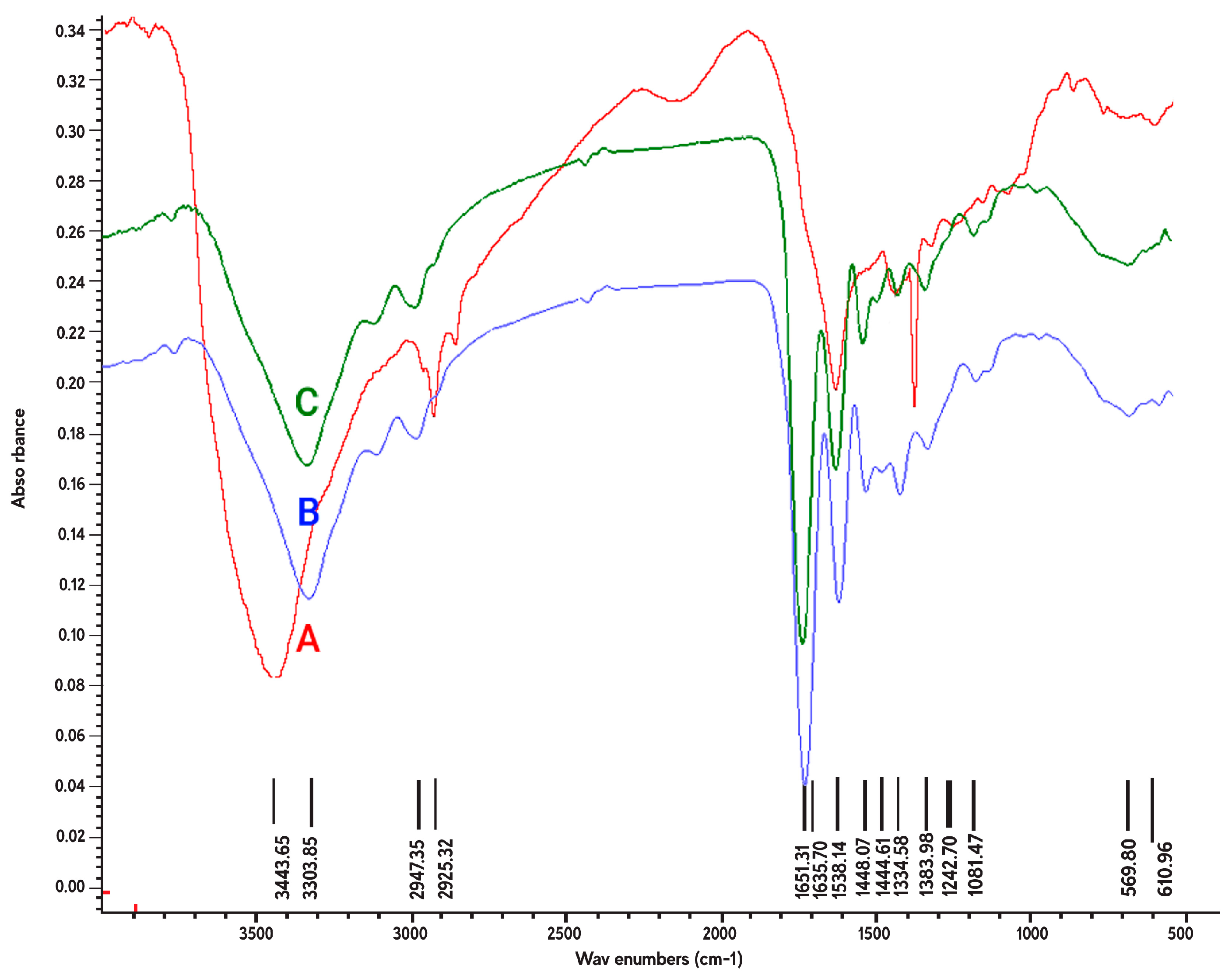
3.2. Chemical Characteristics of Plasma Treated Nanofibers
3.3. Water Contact Angle Properties of Nanofibers
3.4. XRD Analysis of Nanofibers
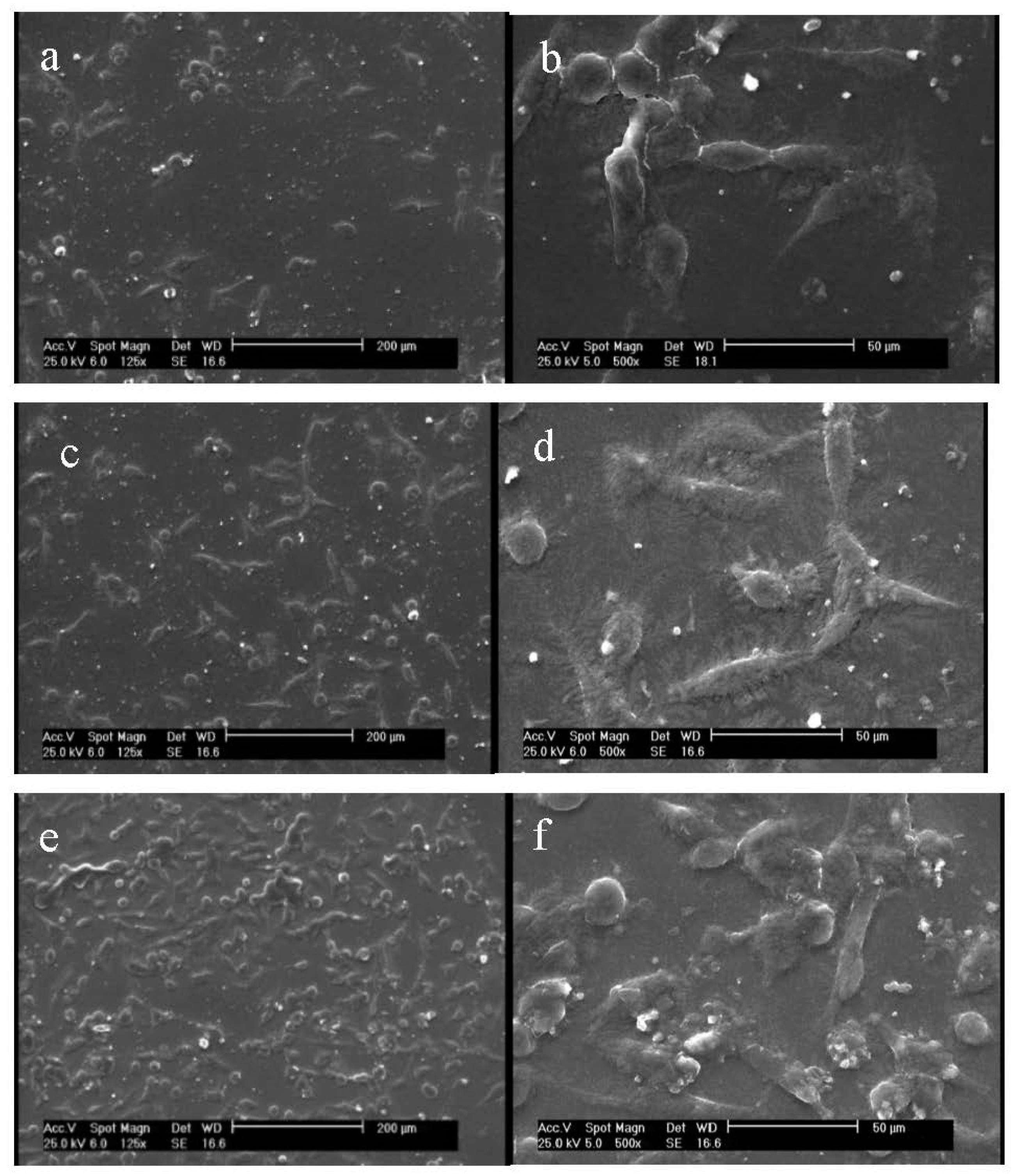
3.5. Morphological Characterization of Cells on Nanofiber Scaffolds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bodillard, J.; Pattappa, G.; Pilet, P.; Weiss, P.; Réthoré, G. Functionalisation of Polysaccharides for the Purposes of Electrospinning: A Case Study Using HPMC and Si-HPMC. Gels 2015, 1, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, P.K.; Ura, D.P.; Stachewicz, U. Humidity Controlled Mechanical Properties of Electrospun Polyvinylidene Fluoride (PVDF) Fibers. Fibers 2020, 8, 65. [Google Scholar] [CrossRef]
- Lubasova, D.; Netravali, A.N. A Novel Method for Electrospinning Nanofibrous 3-D Structures. Fibers 2020, 8, 27. [Google Scholar] [CrossRef]
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Ehrmann, A. Increased Mechanical Properties of Carbon Nanofiber Mats for Possible Medical Applications. Fibers 2019, 7, 98. [Google Scholar] [CrossRef]
- Chiu, C.-M.; Nootem, J.; Santiwat, T.; Srisuwannaket, C.; Pratumyot, K.; Lin, W.-C.; Mingvanish, W.; Niamnont, N. Enhanced Stability and Bioactivity of Curcuma comosa Roxb. Extract in Electrospun Gelatin Nanofibers. Fibers 2019, 7, 76. [Google Scholar] [CrossRef]
- Bezir, N.Ç.; Evcin, A.; Diker, R.; Özcan, B.; Kır, E.; Akarca, G.; Çetin, E.S.; Kayalı, R.; Özen, M.K. Investigation of Antibacterial Properties of Ag Doped TiO2 Nanofibers Prepared by Electrospinning Process. Open Chem. 2018, 16, 732–737. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Mirjalili, M.; Parvinzadeh Gashti, M.; Fard, G.C. Zwitter Ionic Modification of Cobalt-Ferrite Nanofiber for the Removal of Anionic and Cationic Dyes. J. Taiwan Inst. Chem. Eng. 2016, 67, 306–317. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Feng, F.; Zhang, H. Study on Wettability, Mechanical Property and Biocompatibility of Electrospun Gelatin/Zein Nanofibers Cross-linked by Glucose. Food Hydrocoll. 2019, 87, 1–10. [Google Scholar] [CrossRef]
- Feng, B.; Tu, H.; Yuan, H.; Peng, H.; Zhang, Y. Acetic-Acid-Mediated Miscibility toward Electrospinning Homogeneous Composite Nanofibers of GT/PCL. Biomacromolecules 2012, 13, 3917–3925. [Google Scholar] [CrossRef]
- Feng, B.; Duan, H.; Fu, W.; Cao, Y.; Zhang, W.J.; Zhang, Y. Effect of Inhomogeneity of the Electrospun Fibrous Scaffolds of Gelatin/Polycaprolactone Hybrid on Cell Proliferation. J. Biomed. Mater. Res. Part A 2015, 103, 431–438. [Google Scholar] [CrossRef]
- Duan, H.; Xue, J.; Liu, Y.; Feng, B.; Zhao, S.; Zhu, Y.; Liu, Y.; He, A.; Zhang, W.; Liu, W.; et al. The Influence of Gelatin/PCL Ratio and 3-D Construct Shape of Electrospun Membranes on Cartilage Regeneration. Biomaterials 2014, 35, 152–164. [Google Scholar]
- He, X.; Feng, B.; Huang, C.; Wang, H.; Ge, Y.; Hu, R.; Yin, M.; Xu, Z.; Wang, W.; Fu, W.; et al. Electrospun Gelatin/Polycaprolactone Nanofibrous Membranes Combined with a Coculture of Bone Marrow Stromal Cells and Chondrocytes for Cartilage Engineering. Int. J. Nanomed. 2015, 17, 2089–2099. [Google Scholar]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Fabrication and Characterization of Electrospun Gelatin Nanofibers Crosslinked with Oxidized Phenolic Compounds. Int. J. Biol. Macromol. 2017, 103, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-H.; Lee, C.-Y.; Huang, C.H.; Chen, Y.S.; Che, K.Y. Novel Bilayer Wound Dressing Based on Electrospun Gelatin/Keratin Nanofibrous Mats for Skin Wound Repair. Mater. Sci. Eng. C 2017, 79, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Sisson, K.; Zhang, C.; Farach-Carson, M.C.; Chase, D.B.; Rabolt, J.F. Evaluation of Cross-Linking Methods for Electrospun Gelatin on Cell Growth and Viability. Biomacromolecules 2009, 10, 1675–1680. [Google Scholar] [CrossRef]
- Tengroth, C.; Gasslander, U.; Andersson, F.O.; Jacobsson, S.P. Cross-Linking of Gelatin Capsules with Formaldehyde and other Aldehydes: An FTIR Spectroscopy Study. Pharm. Dev. Technol. 2005, 10, 405–412. [Google Scholar] [CrossRef]
- Laha, A.; Yadav, S.; Majumdar, S.; Sharma, C.S. In-Vitro Release Study of Hydrophobic Drug Using Electrospun Cross-Linked Gelatin Nanofibers. Biochem. Eng. J. 2016, 105, 481–488. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, S.B.; Hong, S.R.; Lee, Y.M.; Song, K.W.; Park, M.H. Studies on Gelatin-Based Sponges. Part III: A Comparative Study of Cross-Linked Gelatin/Alginate, Gelatin/Hyaluronate and Chitosan/Hyaluronate Sponges and Their Application as a Wound Dressing in Full-Thickness Skin Defect of Rat. J. Mater. Sci. Mater. Med. 2001, 12, 67–73. [Google Scholar] [CrossRef]
- Zhang, X.; Do, M.D.; Casey, P.; Sulistio, A.; Qiao, G.G.; Lundin, L.; Lillford, P.; Kosaraju, S. Chemical Modification of Gelatin by a Natural Phenolic Cross-Linker, Tannic Acid. J. Agric. Food Chem. 2010, 58, 6809–6815. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Parvinzadeh Gashti, M.; Ebrahimi, I.; Pousti, M. New Insights into Corona Discharge Surface Ionization of Polyethylene Terephthalate via a Combined Computational and Experimental Assessment. Curr. Appl. Phys. 2015, 15, 1075–1083. [Google Scholar] [CrossRef]
- Ebrahimi, I.; Kiumarsi, A.; Parvinzadeh Gashti, M.; Rashidian, R.; Norouzi, M.H. Atmospheric-Air Plasma Enhances Coating of Different Lubricating Agents on Polyester Fiber. Eur. Phys. J. Appl. Phys. 2011, 56, 10801. [Google Scholar] [CrossRef]
- Parvinzadeh Gashti, M.; Hegemann, D.; Stir, M.; Hulliger, J. Thin Film Plasma Functionalization of Polyethylene Terephthalate to Induce Bone-Like Hydroxyapatite Nanocrystals. Plasma Process. Polym. 2014, 11, 37–43. [Google Scholar] [CrossRef]
- Parvinzadeh, M.; Ebrahimi, I. Influence of Atmospheric-Air Plasma on the Coating of a Nonionic Lubricating Agent on Polyester Fiber. Radiat. Eff. Def. Solid. 2011, 166, 408–416. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsumae, T.; Kurashima, Y.; Takagi, H.; Suga, T.; Itoh, T.; Higurashi, E. Comparison of Argon and Oxygen Plasma Treatments for Ambient Room-Temperature Wafer-Scale Au–Au Bonding Using Ultrathin Au Films. Micromachines 2019, 10, 119. [Google Scholar] [CrossRef]
- Demina, T.; Zotova, D.Z.; Yablokov, M.; Gilman, A.; Akopova, T.; Markvicheva, E.; Zelenetskii, A. DC Discharge Plasma Modification of Chitosan/Gelatin/PLLA Films: Surface Properties, Chemical Structure and Cell Affinity. Surf. Coat. Technol. 2012, 207, 508–516. [Google Scholar] [CrossRef]
- Ferreira, B.; Pinheiro, L.M.P.; Nascente, P.A.P.; Ferreira, M.J.; Duek, E.A.R. Plasma Surface Treatments of Poly (l-Lactic Acid)(PLLA) and Poly (Hydroxybutyrate-co-Hydroxyvalerate)(PHBV). Mat. Sci. Eng. C 2009, 29, 806–813. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-Surface Modification of Biomaterials. Mat. Sci. Eng. R Rep. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Cheng, Q.; Lee, B.L.P.; Komvopoulos, K.; Yan, Z.; Li, S. Plasma Surface Chemical Treatment of Electrospun Poly(l-Lactide) Microfibrous Scaffolds for Enhanced Cell Adhesion, Growth, and Infiltration. Tissue Eng. Part A 2013, 19, 1188–1198. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface Modification of Polymers by Plasma Treatments for the Enhancement of Biocompatibility and Controlled Drug Release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Ma, Z.; He, W.; Yong, T.; Ramakrishna, S. Grafting of Gelatin on Electrospun Poly(caprolactone) Nanofibers to Improve Endothelial Cell Spreading and Proliferation and to Control Cell Orientation. Tissue Eng. 2005, 11, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Hu, X.; Yang, F.; Bei, J.; Wang, S. Combining Oxygen Plasma Treatment with Anchorage of Cationized Gelatin for Enhancing Cell Affinity of Poly(Lactide-co-Glycolide). Biomaterials 2007, 28, 4219–4230. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Su, C.H. Surface Modification of Electrospun PLLA Nanofibers by Plasma Treatment and Cationized Gelatin Immobilization for Cartilage Tissue Engineering. Acta Biomater. 2011, 1, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Omrani, M.M.; Kumar, H.; Mohamed, M.G.A.; Golovin, K.; Milani, A.S.; Hadjizadeh, A.; Kim, K. Polyether Ether Ketone Surface Modification with Plasma and Gelatin for Enhancing Cell Attachment. J. Biomed. Mater. Res. Part B Appl. Biomat. 2020, 60. [Google Scholar] [CrossRef]
- Ghorbani, F.; Sahranavard, M.; Zamanian, A. Immobilization of Gelatin on the Oxygen Plasma-Modified Surface of Polycaprolactone Scaffolds with Tunable Pore Structure for Skin Tissue Engineering. J. Polym. Res. 2020, 27, 281. [Google Scholar] [CrossRef]
- Mirjalili, M.; Mozaffari, A.; Parvinzadeh Gashti, M.; Parsania, M. Effect of Tannic Acid on Properties of Electrospun Gelatin Nanofibers. Indian J. Fibre Text. Res. 2020, 45, 289–298. [Google Scholar]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun Poly(ε-Caprolactone)/Gelatin Nanofibrous Scaffolds for Nerve Tissue Engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Choktaweesap, N.; Arayanarakul, K.; Aht-ong, D.; Meechaisue, C.; Supaphol, P. Electrospun Gelatin Fibers: Effect of Solvent System on Morphology and Fiber Diameters. Polym. J. 2007, 39, 622–631. [Google Scholar] [CrossRef]
- Horuz, T.I.; Belibağlı, K.B. Production of Electrospun Gelatin Nanofibers: An Optimization Study by Using Taguchi’s Methodology. Mater. Res. Express 2017, 4, 015023. [Google Scholar] [CrossRef]
- Parvinzadeh Gashti, M.; Helali, M.; Karimi, S. Biomineralization-Inspired Green Synthesis of Zinc Phosphate-Based Nanosheets in Gelatin Hydrogel. Int. J. Appl. Ceram. Technol. 2016, 13, 1069–1073. [Google Scholar] [CrossRef]
- Parvinzadeh Gashti, M.; Alimohammadi, F.; Hulliger, J.; Burgener, M.; Oulevey-Aboulfad, H.; Bowlin, G.L. Microscopic methods to study the structure of scaffolds in bone tissue engineering: A brief review. In Current Microscopy Contribution to Advances in Science and Technology; Méndez-Vilas, A., Ed.; Microscopy Book Series Number 5; Formatex Research Center: Badajoz, Spain, 2012; Volume 1, pp. 625–638. [Google Scholar]
- Gashti, M.P.; Dehghan, N. Gel Diffusion-Inspired Biomimetic Calcium Iodate/Gelatin Composite Particles: Structural Characterization and Antibacterial Activity. J. Solid State Chem. 2020, 285, 121262. [Google Scholar] [CrossRef]
- Parvinzadeh Gashti, M.; Shokri, A. Hydrogel-Assisted Low-Temperature Synthesis of Calcium Borate Nanoparticles. J. Aust. Ceram. Soc. 2018, 54, 601–607. [Google Scholar] [CrossRef]
- Parvinzadeh Gashti, M.; Burgener, M.; Stir, M.; Hulliger, J. Barium Hydrogen Phosphate/Gelatin Composites Versus Gelatin-Free Barium Hydrogen Phosphate: Synthesis and Characterization of Properties. J. Colloid Interf. Sci. 2014, 431, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Parvinzadeh Gashti, M.; Burgener, M.; Stir, M.; Hulliger, J. Growth of Strontium Hydrogen Phosphate/Gelatin Composites: A Biomimetic Approach. New J. Chem. 2016, 40, 5495–5500. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhang, M.; He, J.; Zheng, B.; Liu, F.; Zhao, Z.; Liu, Y. Use of Silver Nanoparticle–Gelatin/Alginate Scaffold to Repair Skull Defects. Coatings 2020, 10, 948. [Google Scholar] [CrossRef]
- Char, C.; Padilla, C.; Campos, V.; Pepczynska, M.; Díaz-Calderón, P.; Enrione, J. Characterization and Testing of a Novel Sprayable Crosslinked Edible Coating Based on Salmon Gelatin. Coatings 2019, 9, 595. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M.C. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef]
- Parvinzadeh, M.; Ebrahimi, I. Atmospheric Air-Plasma Treatment of Polyester Fiber to Improve the Performance of Nanoemulsion Silicone. Appl. Surf. Sci. 2011, 257, 4062–4068. [Google Scholar] [CrossRef]
- Peña, C.; de la Caba, K.; Eceiza, A.; Ruseckaite, R.; Mondragon, I. Enhancing Water Repellence and Mechanical Properties of Gelatin Films by Tannin Addition. Bioresour Technol. 2010, 101, 6836. [Google Scholar] [CrossRef]
- Al-Balakocy, N.G.; El-Ola, A. Effect of Surface Activation of Nylon-6 Fabrics by Plasma and Grafting with Vinyl Monomers on Its Functional Finishing with TiO2 Nanoparticles. J. Appl. Sci. Res. 2013, 9, 1743–1753. [Google Scholar]
- Gasi, F.; Petraconi, G.; Bittencourt, E.; Lourenço, S.R.; Castro, A.H.R.; Miranda, F.d.S.; Essiptchouk, A.M.; Nascimento, L.; Petraconi, A.; Fraga, M.A.; et al. Plasma Treatment of Polyamide Fabric Surface by Hybrid Corona-Dielectric Barrier Discharge: Material Characterization and Dyeing/Washing Processes. Mater. Res. 2020, 23, e20190255. [Google Scholar] [CrossRef]
- Chandrasekaran, A.R.; Venugopal, J.; Sundarrajan, S.; Ramakrishna, S. Fabrication of a Nanofibrous Scaffold with Improved Bioactivity for Culture of Human Dermal Fibroblasts for Skin Regeneration. Biomed. Mater. 2011, 6, 015001. [Google Scholar] [CrossRef] [PubMed]


| Samples | Plasma Treatment Time (s) | Maximum Peak Height, Ra (nm) | Maximum Valley Depth, Rz (nm) | Average Peak-to-Valley Height Rq (nm) | RMS (nm) |
|---|---|---|---|---|---|
| Untreated nanofibers | 0 | 6.585 | 276.8 | 47.48 | 5.1 |
| Argon plasma treated nanofibers | 90 | 1.635 | 22.95 | 11.79 | 31.54 |
| Argon–oxygen plasma treated nanofibers | 90 | 1.056 | 20.73 | 10.63 | 27.76 |
| Crosslinked Gelatin Nanofibers before Plasma Treatment | Crosslinked Gelatin Nanofibers after Argon Plasma Treatment | Crosslinked Gelatin Nanofibers after Argon/Oxygen Plasma Treatment | |||
|---|---|---|---|---|---|
| Peak Position (cm−1) | Band Assignment | Peak Position (cm−1) | Band Assignment | Peak Position (cm−1) | Band Assignment |
| 610–669 | –CH bending | 610–669 | –CH bending | 610–669 | –CH bending |
| 1242 | C–O stretching/Amide III | 1242 | Amide III | 1242 | Amide III |
| 1300 | C–O stretching | - | - | - | - |
| 1334 | C–N stretching/Amide III | 1334 | Amide III | 1334 | Amide III |
| - | - | 1444 | Amide II | 1444 | Amide II |
| - | - | 1448 | Amide II | 1448 | Amide II |
| 1538 | N–O stretching/Amide II | 1538 | Amide II | 1538 | Amide II |
| - | - | 1651 | C=O stretching | 1651 | C=O stretching |
| 2925 | –CH stretching | - | - | - | - |
| - | - | 2947 | –CH stretching | 2947 | –CH stretching |
| - | - | 3303 | Amide A | 3303 | Amide A |
| 3443 | O–H Stretching/Amide A | - | - | - | - |
| Samples | Mean Contact Angle, CA (°) | Camera Image |
|---|---|---|
| Untreated nanofibers | 20.65 |  |
| Argon plasma treated nanofibers | 14.6 |  |
| Argon–oxygen plasma treated nanofibers | 0 | Completely hydrophilic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozaffari, A.; Parvinzadeh Gashti, M.; Mirjalili, M.; Parsania, M. Argon and Argon–Oxygen Plasma Surface Modification of Gelatin Nanofibers for Tissue Engineering Applications. Membranes 2021, 11, 31. https://doi.org/10.3390/membranes11010031
Mozaffari A, Parvinzadeh Gashti M, Mirjalili M, Parsania M. Argon and Argon–Oxygen Plasma Surface Modification of Gelatin Nanofibers for Tissue Engineering Applications. Membranes. 2021; 11(1):31. https://doi.org/10.3390/membranes11010031
Chicago/Turabian StyleMozaffari, Abolfazl, Mazeyar Parvinzadeh Gashti, Mohammad Mirjalili, and Masoud Parsania. 2021. "Argon and Argon–Oxygen Plasma Surface Modification of Gelatin Nanofibers for Tissue Engineering Applications" Membranes 11, no. 1: 31. https://doi.org/10.3390/membranes11010031
APA StyleMozaffari, A., Parvinzadeh Gashti, M., Mirjalili, M., & Parsania, M. (2021). Argon and Argon–Oxygen Plasma Surface Modification of Gelatin Nanofibers for Tissue Engineering Applications. Membranes, 11(1), 31. https://doi.org/10.3390/membranes11010031






