Mechanical Power during Veno-Venous Extracorporeal Membrane Oxygenation Initiation: A Pilot-Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. MV Parameters before and after ECMO Initiation
3.3. MV Parameters during Early Phases of ECMO Run and ICU Mortality
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rezoagli, E.; Fumagalli, R.; Bellani, G. Definition and epidemiology of acute respiratory distress syndrome. Ann. Transl. Med. 2017, 5, 282. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A. The concept of “baby lung”. Intensive Care Med. 2005, 31, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Network, A.R.D.S.; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.S.; Mercat, A.; et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef]
- Bugedo, G.; Retamal, J.; Bruhn, A. Driving pressure: A marker of severity, a safety limit, or a goal for mechanical ventilation? Crit. Care 2017, 21, 1–7. [Google Scholar] [CrossRef]
- Papazian, L.; Aubron, C.; Brochard, L.; Chiche, J.-D.; Combes, A.; Dreyfuss, D.; Forel, J.M.; Guérin, C.; Jaber, S.; Dessap, A.M.; et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 69. [Google Scholar] [CrossRef]
- Parekh, M.; Abrams, D.; Brodie, D. Extracorporeal techniques in acute respiratory distress syndrome. Ann. Transl. Med. 2017, 5, 296. [Google Scholar] [CrossRef]
- Gattinoni, L.; Vasques, F.; Quintel, M. Use of ECMO in ARDS: Does the EOLIA trial really help? Crit. Care 2018, 22, 171. [Google Scholar] [CrossRef]
- Rozencwajg, S.; Guihot, A.; Franchineau, G.; Lescroat, M.; Bréchot, N.; Hékimian, G.; Lebreton, G.; Autran, B.; Luyt, C.-E.; Combes, A.; et al. Ultra-Protective Ventilation Reduces Biotrauma in Patients on Venovenous Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. Crit. Care Med. 2019, 47, 1505–1512. [Google Scholar] [CrossRef]
- Gattinoni, L.; Tonetti, T.; Moerer, O. How best to set the ventilator on extracorporeal membrane lung oxygenation. Curr. Opin. Crit. Care 2017, 23, 66–72. [Google Scholar] [CrossRef]
- Gattinoni, L.; Kolobow, T.; Tomlinson, T.; Iapichino, G.; Samaja, M.; White, D.; Pierce, J. Low-Frequency Positive Pressure Ventilation with Extracorporeal Carbon Dioxide Removal (LFPPV-ECCO2R). Anesth. Analg. 1978, 57, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, L.; Goffi, A.; Tomlinson, G.; Pettenuzzo, T.; Facchin, F.; Vendramin, A.; Goligher, E.C.; Cypel, M.; Slutsky, A.S.; Keshavjee, S.; et al. Effect of Driving Pressure Change During Extracorporeal Membrane Oxygenation in Adults with Acute Respiratory Distress Syndrome: A Randomized Crossover Physiologic Study. Crit. Care Med. 2020, 48, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Hubmayr, R.D.; Kallet, R.H. Understanding Pulmonary Stress-Strain Relationships in Severe ARDS and Its Implications for Designing a Safer Approach to Setting the Ventilator. Respir. Care 2018, 63, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Tonetti, T.; Cressoni, M.; Cadringher, P.; Herrmann, P.; Moerer, O.; Protti, A.; Gotti, M.; Chiurazzi, C.; Carlesso, E.; et al. Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 2016, 42, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.L.; Ball, L.; Rocco, P.R.; Pelosi, P. Power to mechanical power to minimize ventilator-induced lung injury? Intensive Care Med. Exp. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Giosa, L.; Busana, M.; Pasticci, I.; Bonifazi, M.; Macrì, M.M.; Romitti, F.; Vassalli, F.; Chiumello, D.; Quintel, M.; Marini, J.J.; et al. Mechanical power at a glance: A simple surrogate for volume-controlled ventilation. Intensive Care Med. Exp. 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Cressoni, M.; Gotti, M.; Chiurazzi, C.; Massari, D.; Algieri, I.; Amini, M.; Cammaroto, A.; Brioni, M.; Montaruli, C.; Nikolla, K.; et al. Mechanical Power and Development of Ventilator-induced Lung Injury. Anesthesiology 2016, 124, 1100–1108. [Google Scholar] [CrossRef]
- Tonetti, T.; Vasques, F.; Rapetti, F.; Maiolo, G.; Collino, F.; Romitti, F.; Camporota, L.; Cressoni, M.; Cadringher, P.; Quintel, M.; et al. Driving pressure and mechanical power: New targets for VILI prevention. Ann. Transl. Med. 2017, 5, 286. [Google Scholar] [CrossRef]
- Marini, J.J.; Rocco, P.R.M.; Gattinoni, L. Static and Dynamic Contributors to Ventilator-induced Lung Injury in Clinical Practice. Pressure, Energy, and Power. Am. J. Respir. Crit. Care Med. 2020, 201, 767–774. [Google Scholar] [CrossRef]
- Neto, A.S.; for the PROVE Network Investigators; Deliberato, R.O.; Johnson, A.E.W.; Bos, L.D.; Amorim, P.; Pereira, S.M.; Cazati, D.C.; Cordioli, R.L.; Correa, T.D.; et al. Mechanical power of ventilation is associated with mortality in critically ill patients: An analysis of patients in two observational cohorts. Intensive Care Med. 2018, 44, 1914–1922. [Google Scholar] [CrossRef]
- World Medical Association. WMA Declaration of Lisbon on the Rights of the Patient. WMA Declar. 2015. Available online: https://www.wma.net/policies-post/wma-declaration-of-lisbon-on-the-rights-of-the-patient (accessed on 14 November 2020).
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization ELSO Guidelines for Adult Respiratory Failure, Version 1.4. August 2017, pp. 1–32. Available online: https://www.elso.org/Portals/0/ELSO%20Guidelines%20For%20Adult%20Respiratory%20Failure%201_4.pdf (accessed on 14 November 2020).
- Vincent, J.-L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Bailey, M.; Sheldrake, J.; Hodgson, C.; Aubron, C.; Rycus, P.T.; Scheinkestel, C.; Cooper, D.J.; Brodie, D.; Pellegrino, V.; et al. Predicting Survival after Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) Score. Am. J. Respir. Crit. Care Med. 2014, 189, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.; Zochios, V.; Parhar, K.K.S. Re-examining Permissive Hypercapnia in ARDS. Chest 2018, 154, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Quintel, M.; Tonetti, T.; Herrmann, P. Energy Calculator Vers. 1.2.6 by P. Herrman. Available online: http://www.ains.med.uni-goettingen.de/de/abteilung-anaesthesiologie/forschung/energy-calculator-software (accessed on 17 January 2019).
- Marhong, J.D.; Telesnicki, T.; Munshi, L.; Del Sorbo, L.; Detsky, M.; Fan, E. Mechanical Ventilation during Extracorporeal Membrane Oxygenation. An International Survey. Ann. Am. Thorac. Soc. 2014, 11, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Moerer, O.; Busana, M.; Gattinoni, L. Breathing and Ventilation during Extracorporeal Membrane Oxygenation: How to Find the Balance between Rest and Load. Am. J. Respir. Crit. Care Med. 2019, 200, 954–956. [Google Scholar] [CrossRef]
- Jenks, C.L.; Tweed, J.; Gigli, K.H.; Venkataraman, R.; Raman, L. An International Survey on Ventilator Practices Among Extracorporeal Membrane Oxygenation Centers. ASAIO J. 2017, 63, 787–792. [Google Scholar] [CrossRef]
- Terragni, P.P.; Del Sorbo, L.; Mascia, L.; Urbino, R.; Martin, E.L.; Birocco, A.; Faggiano, C.; Quintel, M.; Gattinoni, L.; Ranieri, V.M. Tidal Volume Lower than 6 ml/kg Enhances Lung Protection. Anesthesiol. 2009, 111, 826–835. [Google Scholar] [CrossRef]
- Swol, J.; Fülling, Y.; Ull, C.; Bechtel, J.M.; Schildhauer, T.A. 48 h cessation of mechanical ventilation during venovenous extracorporeal membrane oxygenation in severe trauma: A case report. J. Artif. Organs 2017, 20, 280–284. [Google Scholar] [CrossRef]
- Neto, A.S.; The ReVA Research Network and the PROVE Network Investigators; Schmidt, M.; Azevedo, L.C.P.; Bein, T.; Brochard, L.; Beutel, G.; Combes, A.; Costa, E.L.V.; Hodgson, C.; et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: A pooled individual patient data analysis. Intensive Care Med. 2016, 42, 1672–1684. [Google Scholar] [CrossRef] [PubMed]
- Gupta, E.; Awsare, B.; Hiroshi, H.; Cavarocchi, N.; Baram, M. Don’t Drive Blind: Driving Pressure to Optimize Ventilator Management in ECMO. Lung 2020, 198, 1–8. [Google Scholar] [CrossRef]
- Magunia, H.; Haeberle, H.A.; Henn, P.; Mehrländer, M.; Vlatten, P.O.; Mirakaj, V.; Rosenberger, P.; Koeppen, M. Early Driving Pressure Changes Predict Outcomes during Venovenous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. Crit. Care Res. Pract. 2020, 2020, 6958152. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Pham, T.; Arcadipane, A.; Agerstrand, C.; Ohshimo, S.; Pellegrino, V.; Vuylsteke, A.; Guervilly, C.; McGuinness, S.; Pierard, S.; et al. Mechanical Ventilation Management during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. An International Multicenter Prospective Cohort. Am. J. Respir. Crit. Care Med. 2019, 200, 1002–1012. [Google Scholar] [CrossRef]
- Collino, F.; Rapetti, F.; Vasques, F.; Maiolo, G.; Tonetti, T.; Romitti, F.; Niewenhuys, J.; Behnemann, T.; Camporota, L.; Hahn, G.; et al. Positive End-expiratory Pressure and Mechanical Power. Anesthesiol. 2019, 130, 119–130. [Google Scholar] [CrossRef]
- Huhle, R.; Neto, A.S.; Schultz, M.J.; De Abreu, M.G. Is mechanical power the final word on ventilator-induced lung injury?—No. Ann. Transl. Med. 2018, 6, 394. [Google Scholar] [CrossRef]
- Lachmann, B. (Burkhard) Open up the lung and keep the lung open. Intensive Care Med. 1992, 18, 319–321. [Google Scholar] [CrossRef]
- Van Der Zee, P.; Gommers, D. Recruitment Maneuvers and Higher PEEP, the So-Called Open Lung Concept, in Patients with ARDS. Crit. Care 2019, 23, 73. [Google Scholar] [CrossRef]
- Protti, A.; Andreis, D.T.; Monti, M.; Santini, A.; Sparacino, C.C.; Langer, T.; Votta, E.; Gatti, S.; Lombardi, L.; Leopardi, O.; et al. Lung Stress and Strain During Mechanical Ventilation. Crit. Care Med. 2013, 41, 1046–1055. [Google Scholar] [CrossRef]
- Van Der Zee, P.; Miranda, D.R.; Meeder, H.; Endeman, H.; Gommers, D. vvECMO can be avoided by a transpulmonary pressure guided open lung concept in patients with severe ARDS. Crit. Care 2019, 23, 133. [Google Scholar] [CrossRef]
- Camporota, L.; Caricola, E.V.; Bartolomeo, N.; Di Mussi, R.; Wyncoll, D.L.A.; Meadows, C.I.S.; Amado-Rodriguez, L.; Vasques, F.; Sanderson, B.; Glover, G.W.; et al. Lung Recruitability in Severe Acute Respiratory Distress Syndrome Requiring Extracorporeal Membrane Oxygenation. Crit. Care Med. 2019, 47, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Lee, H.S.; Kim, H.S.; Ha, S.O.; Lee, W.Y.; Park, S.J.; Lee, S.H.; Lee, T.H.; Seo, J.Y.; Choi, H.H.; et al. The pre-ECMO simplified acute physiology score II as a predictor for mortality in patients with initiation ECMO support at the emergency department for acute circulatory and/or respiratory failure: A retrospective study. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Ha, S.O.; Kim, H.S.; Park, S.; Han, S.J.; Lee, S.H. The Simplified Acute Physiology Score II as a Predictor of Mortality in Patients Who Underwent Extracorporeal Membrane Oxygenation for Septic Shock. Ann. Thorac. Surg. 2017, 103, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019, 5, 1–22. [Google Scholar] [CrossRef]
- Grant, A.A.; Hart, V.J.; Lineen, E.B.; Badiye, A.; Byers, P.M.; Patel, A.; Vianna, R.; Koerner, M.M.; El Banayosy, A.; Loebe, M.; et al. A Weaning Protocol for Venovenous Extracorporeal Membrane Oxygenation with a Review of the Literature. Artif. Organs 2018, 42, 605–610. [Google Scholar] [CrossRef]
- Vasques, F.; Romitti, F.; Gattinoni, L.; Camporota, L. How I wean patients from veno-venous extra-corporeal membrane oxygenation. Crit. Care 2019, 23, 1–3. [Google Scholar] [CrossRef]
- Costa, E.L.V.; Amato, M.B.P. Ultra-protective tidal volume: How low should we go? Crit. Care 2013, 17, 1–2. [Google Scholar] [CrossRef]
- Umbrello, M.; Marino, A.; Chiumello, D. Tidal volume in acute respiratory distress syndrome: How best to select it. Ann. Transl. Med. 2017, 5, 287. [Google Scholar] [CrossRef]
- Santos, R.S.; Maia, L.D.A.; Oliveira, M.V.; Santos, C.L.; Moraes, L.; Pinto, E.F.; Samary, C.D.S.; Machado, J.A.; Carvalho, A.C.; Fernandes, M.V.D.S.; et al. Biologic Impact of Mechanical Power at High and Low Tidal Volumes in Experimental Mild Acute Respiratory Distress Syndrome. Anesthesiol. 2018, 128, 1193–1206. [Google Scholar] [CrossRef]
- Araos, J.; Alegria, L.; Garcia, P.; Cruces, P.; Soto, D.; Erranz, B.; Amthauer, M.; Salomon, T.; Medina, T.; Rodriguez, F.; et al. Near-Apneic Ventilation Decreases Lung Injury and Fibroproliferation in an Acute Respiratory Distress Syndrome Model with Extracorporeal Membrane Oxygenation. Am. J. Respir. Crit. Care Med. 2019, 199, 603–612. [Google Scholar] [CrossRef]
- Peek, G.J.; Clemens, F.; Elbourne, D.; Firmin, R.; Hardy, P.; Hibbert, C.; Killer, H.; Mugford, M.; Thalanany, M.; Tiruvoipati, R.; et al. CESAR: Conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Heal. Serv. Res. 2006, 6, 163. [Google Scholar] [CrossRef] [PubMed]
- Patroniti, N.; Bonatti, G.; Senussi, T.; Robba, C. Mechanical ventilation and respiratory monitoring during extracorporeal membrane oxygenation for respiratory support. Ann. Transl. Med. 2018, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.J.; Rocco, P.R. Which component of mechanical power is most important in causing VILI? Crit. Care 2020, 24, 1–3. [Google Scholar] [CrossRef] [PubMed]
| Study Population (n = 35) | ICU Non-Survivors (n = 11) | ICU Survivors (n = 24) | p-Value | |
|---|---|---|---|---|
| Age [years; median value (25p–75p)] | 53 (40–64) | 53 (39–67) | 53 (42–62) | 0.902 |
| Male sex [n; %] | 24 (68) | 6 (54) | 18 (75) | 0.233 |
| Weight [kg; median value (25p–75p)] | 84 (70–110) | 75 (60–85) | 85 (77–118) | 0.057 |
| Height [cm; median value (25p–75p)] | 175 (169–180) | 171 (167–177) | 177 (169–180) | 0.182 |
| BMI [kg/m2; median value (25p–75p)] | 27 (24–35) | 24 (21–29) | 28 (26–37) | 0.145 |
| SOFA score [n; median value (25p–75p)] | 12 (9–17) | 14 (11–18) | 12 (8–17) | 0.228 |
| SAPS II score [n; median value (25p–75p)] | 53 (42–68) | 68 (51–80) | 49 (37–60) | 0.005 |
| RESP score [n; median value (25p–75p)] | −4 (−7–0) | −6 (−9–−1) | −3 (−7–0) | 0.398 |
| pre-ECMO MP [J/min; median value (25p–75p)] | 32.4 (29.3–36.6) | 31.1 (29.4–35.8) | 32.6 (23.6–38.2) | 0.918 |
| pre-ECMO PEEP [cmH2O; median value (25p–75p)] | 14 (10–15) | 10 (8–12) | 15 (12–16) | 0.032 |
| pre-ECMO Pplat [cmH2O; median value (25p–75p)] | 27 (21–33) | 30 (19–38) | 27 (21–31) | 0.506 |
| pre-ECMO Ppeak [cmH2O; median value (25p–75p)] | 33 (29–37) | 34 (30–38) | 33 (28–35) | 0.604 |
| pre-ECMO ΔP [cmH2O; median value (25p–75p)] | 11 (7–23) | 22 (7–29) | 13 (7–13) | 0.237 |
| pre-ECMO RR [breaths/min; median value (25p–75p)] | 22 (20–30) | 25 (23–40) | 21 (18–27) | 0.059 |
| pre-ECMO VT/IBW [mL/kg; median value (25p–75p)] | 5.5 (4.3–7.4) | 5.1 (4.1–6.9) | 5.9 (4.8–7.4) | 0.441 |
| pre-ECMO FiO2 [ratio; median value (25p–75p)] | 1.00 (0.80–1.00) | 1.00 (0.80–1.00) | 1.00 (0.80–1.00) | 0.929 |
| ICU stay length [days; median value (25p–75p)] | 20 (11–33) | 11 (5–15) | 28 (16–38) | 0.009 |
| VV ECMO run length [days; median value (25p–75p)] | 10 (4–15) | 4 (2–11) | 10 (5–16) | 0.031 |
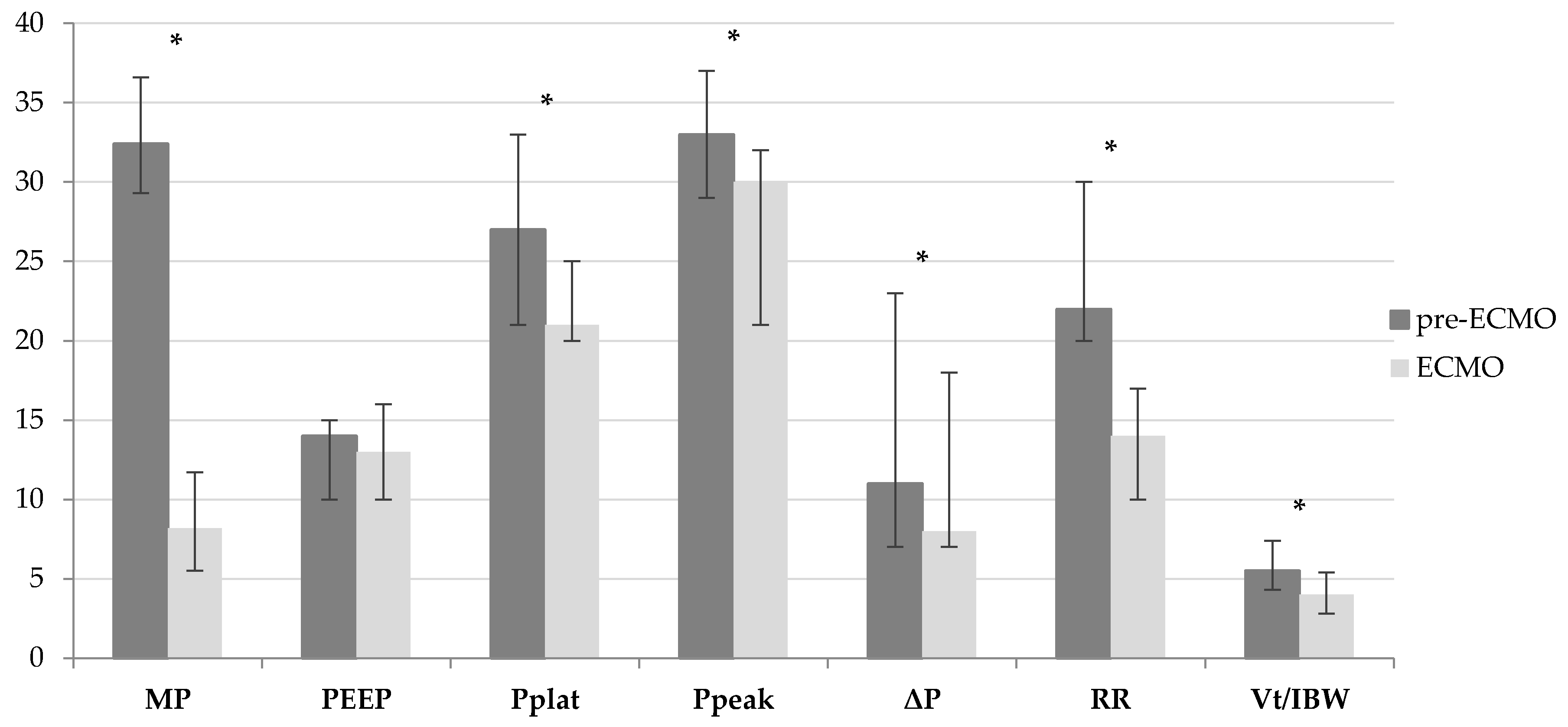
| pre-ECMO (n = 35) | ECMO (n = 35) | Δ% | p-Value | |
|---|---|---|---|---|
| MP [J/min; median value (25p–75p)] | 32.4 (29.3–36.6) | 8.2 (5.5–11.7) | −74.7% | <0.001 |
| PEEP [cmH2O; median value (25p–75p)] | 14 (10–15) | 13 (10–16) | −7.1% | 0.390 |
| Pplat [cmH2O; median value (25p–75p)] | 27 (21–33) | 21 (20–25) | −22.2% | 0.012 |
| Ppeak [cmH2O; median value (25p–75p)] | 33 (29–37) | 30 (21–32) | −9.1% | <0.001 |
| ΔP [cmH2O; median value (25p–75p)] | 11 (7–23) | 8 (7–10) | −27.3% | 0.014 |
| RR [breaths/min; median value (25p–75p)] | 22 (20–30) | 14 (10–17) | −36.4% | <0.001 |
| VT/IBW [mL/kg; median value (25p–75p)] | 5.5 (4.3–7.4) | 4.0 (2.8–5.4) | −27.3% | 0.001 |
| FiO2 [ratio; median value (25p–75p)] | 1.00 (0.80–1.00) | 0.60 (0.40–0.80) | −40.0% | <0.001 |
| Study Population (n = 35) | ICU Non-Survivors (n = 11) | ICU Survivors (n = 24) | p-Value | |
|---|---|---|---|---|
| MP [J/min; median value (25p–75p)] | 8.0 (5.0–14.0) | 8.0 (5.0–20.0) | 8.0 (6.0–13.0) | 0.530 |
| PEEP [cmH2O; median value (25p–75p)] | 12 (10–16) | 10 (8–12) | 14 (11–16) | 0.048 |
| Pplat [cmH2O; median value (25p–75p)] | 22 (20–25) | 22 (19–29) | 22 (20–25) | 0.880 |
| Ppeak [cmH2O; median value (25p–75p)] | 26 (22–31) | 30 (24–34) | 26 (21–30) | 0.174 |
| ΔP [cmH2O; median value (25p–75p)] | 10 (7–12) | 12 (7–15) | 9 (6–12) | 0.229 |
| RR [breaths/min; median value (25p–75p)] | 14 (10–17) | 17 (15–25) | 13 (10–16) | 0.003 |
| VT/IBW [mL/kg; median value (25p–75p)] | 4.0 (3.0–5.0) | 3.0 (2.0–4.0) | 4.0 (3.5–6.0) | 0.028 |
| FiO2 [ratio; median value (25p–75p)] | 0.55 (0.43–0.73) | 0.43 (0.40–0.73) | 0.57 (0.50–0.75) | 0.345 |
| Variable Categorization | Pearson’s Chi-Square Test | p-Value | |
|---|---|---|---|
| MP (J/min) | ≤12; >12 | 0.475 | 0.491 |
| PEEP (cmH2O) | <12; ≥12 | 2.828 | 0.093 |
| Pplat (cmH2O) | ≤23; >23 | 2.198 | 0.138 |
| Ppeak (cmH2O) | ≤27; >27 | 0.957 | 0.328 |
| ΔP (cmH2O) | <10; ≥10 | 1.247 | 0.264 |
| RR (breaths/min) | <15; ≥15 | 7.098 | 0.008 |
| VT/IBW (mL/kg) | <4.5; ≥4.5 | 1.847 | 0.174 |
| FiO2 [ratio] | ≤0.6; >0.6 | 0.088 | 0.766 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belliato, M.; Epis, F.; Cremascoli, L.; Ferrari, F.; Quattrone, M.G.; Fisser, C.; Malfertheiner, M.V.; Taccone, F.S.; Di Nardo, M.; Broman, L.M.; et al. Mechanical Power during Veno-Venous Extracorporeal Membrane Oxygenation Initiation: A Pilot-Study. Membranes 2021, 11, 30. https://doi.org/10.3390/membranes11010030
Belliato M, Epis F, Cremascoli L, Ferrari F, Quattrone MG, Fisser C, Malfertheiner MV, Taccone FS, Di Nardo M, Broman LM, et al. Mechanical Power during Veno-Venous Extracorporeal Membrane Oxygenation Initiation: A Pilot-Study. Membranes. 2021; 11(1):30. https://doi.org/10.3390/membranes11010030
Chicago/Turabian StyleBelliato, Mirko, Francesco Epis, Luca Cremascoli, Fiorenza Ferrari, Maria Giovanna Quattrone, Christoph Fisser, Maximilian Valentin Malfertheiner, Fabio Silvio Taccone, Matteo Di Nardo, Lars Mikael Broman, and et al. 2021. "Mechanical Power during Veno-Venous Extracorporeal Membrane Oxygenation Initiation: A Pilot-Study" Membranes 11, no. 1: 30. https://doi.org/10.3390/membranes11010030
APA StyleBelliato, M., Epis, F., Cremascoli, L., Ferrari, F., Quattrone, M. G., Fisser, C., Malfertheiner, M. V., Taccone, F. S., Di Nardo, M., Broman, L. M., & Lorusso, R. (2021). Mechanical Power during Veno-Venous Extracorporeal Membrane Oxygenation Initiation: A Pilot-Study. Membranes, 11(1), 30. https://doi.org/10.3390/membranes11010030







