Evaluation of a New Extracorporeal CO2 Removal Device in an Experimental Setting
Abstract
1. Introduction
2. Methods
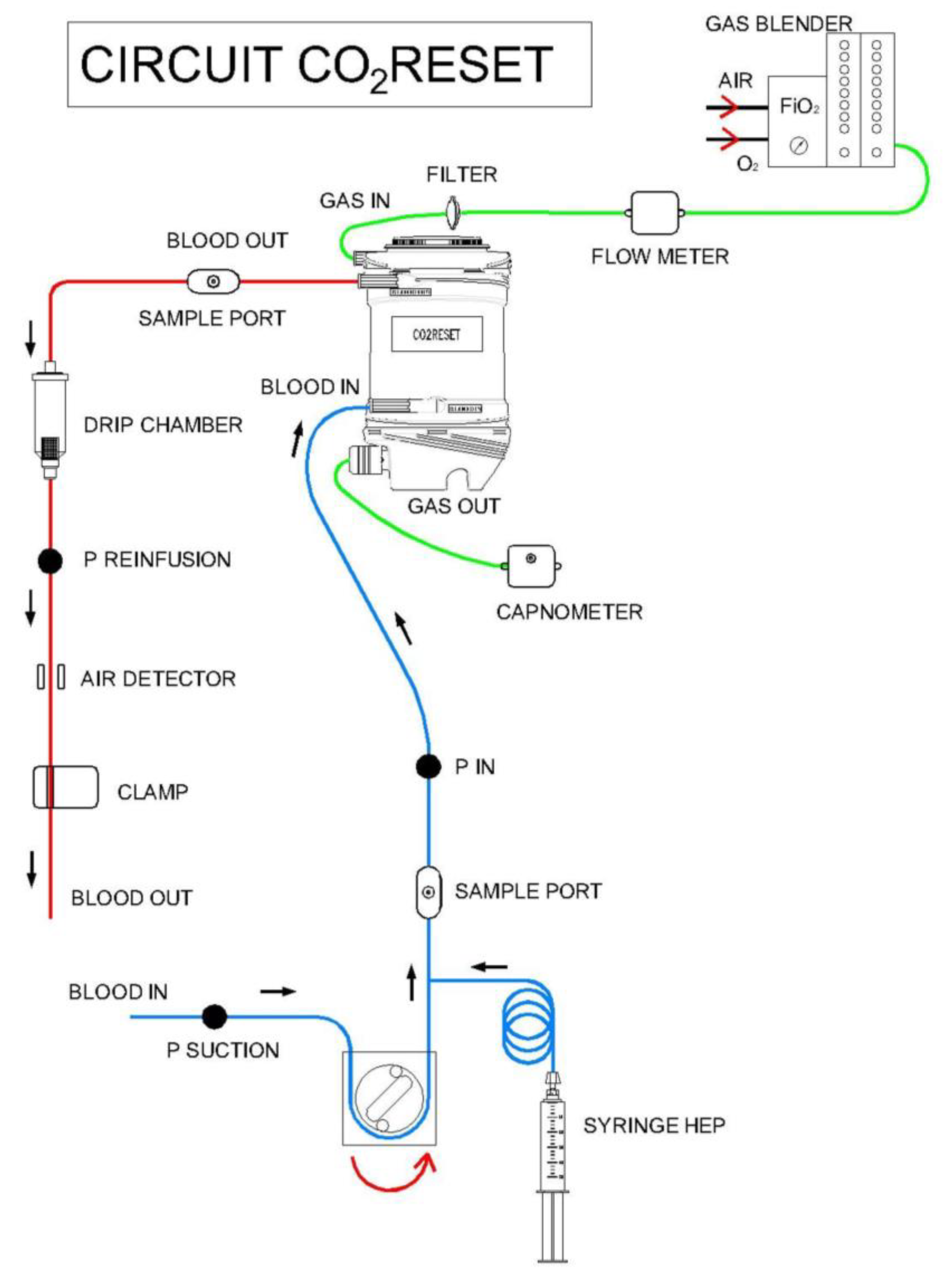
2.1. Extracorporeal CO2 Removal Technique
2.2. Animal Preparation
2.3. Study Design and Experiment Procedure
2.4. Statistical Analysis
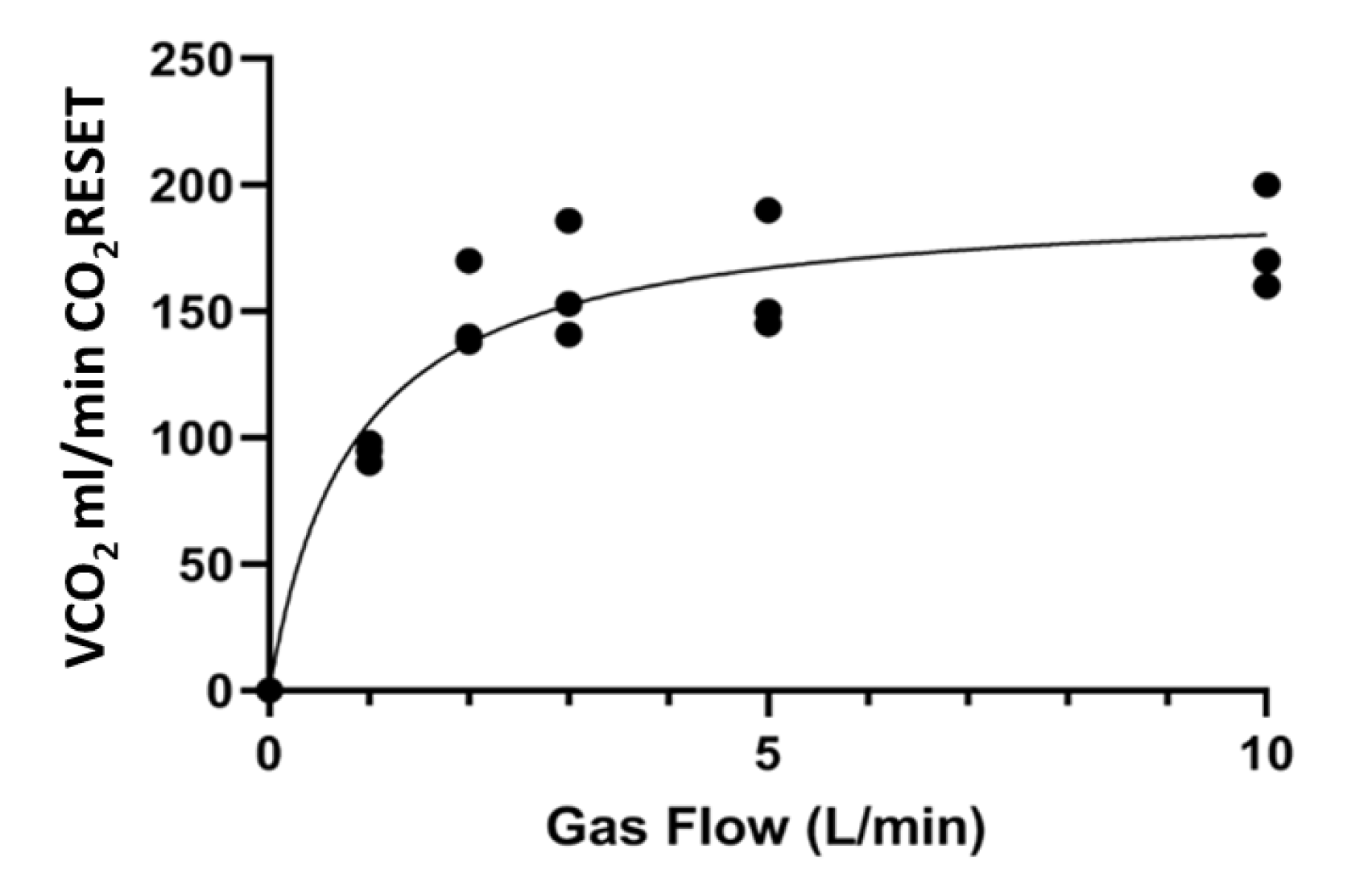
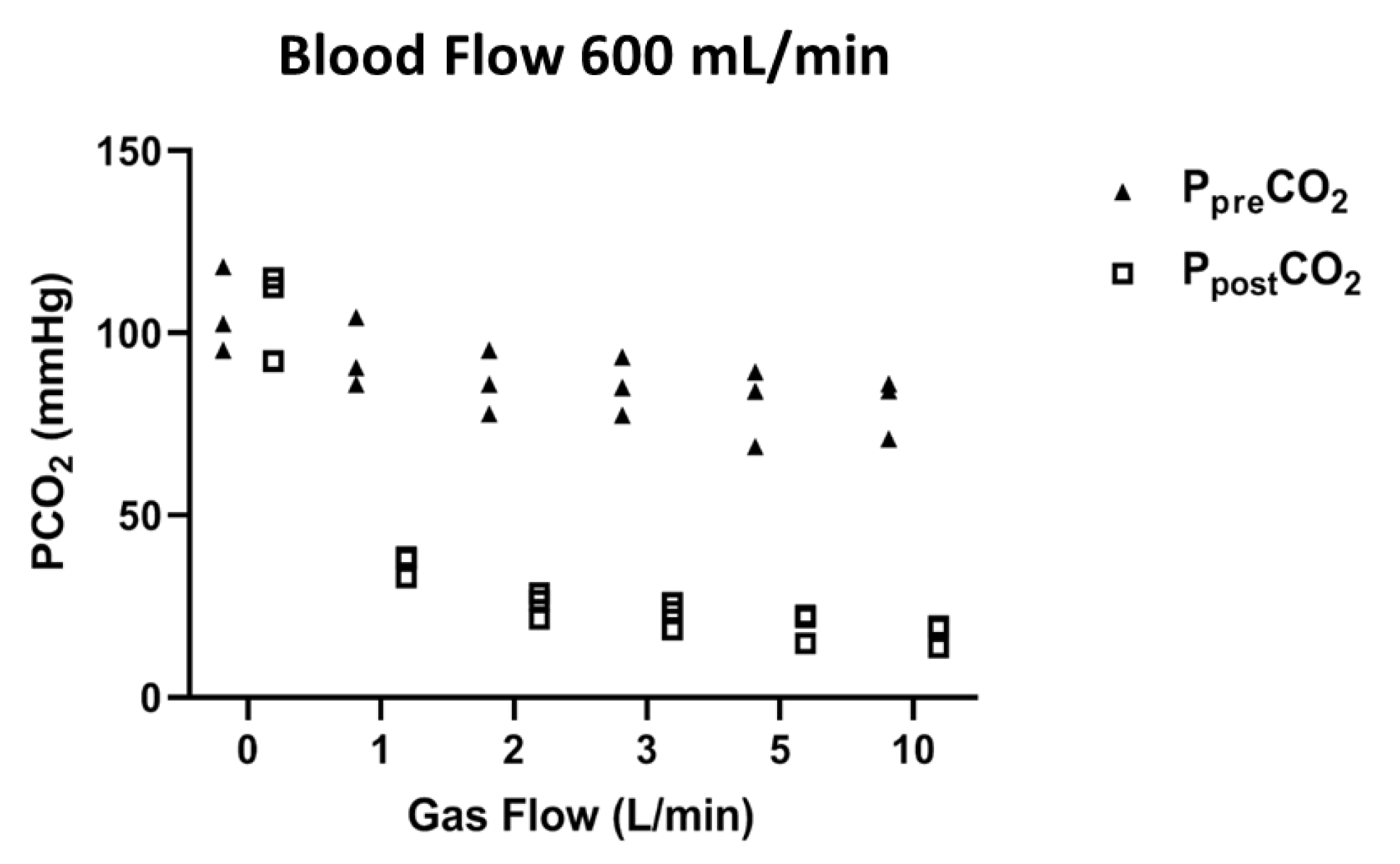
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Combes, A.; Fanelli, V.; Pham, T.; Ranieri, V.M.; On behalf of the European Society of Intensive Care Medicine Trials Group and the “Strategy of Ultra-Protective Lung Ventilation with Extracorporeal CO2 Removal for New-Onset Moderate to Severe ARDS” (SUPERNOVA) Investigators. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: The SUPERNOVA study. Intensiv. Care Med. 2019, 45, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Tonetti, T.; Fanelli, V.; Pham, T.; Pesenti, A.; Mancebo, J.; Brodie, D.; Ranieri, V.M. Efficacy and safety of lower versus higher CO2 extraction devices to allow ultraprotective ventilation: Secondary analysis of the SUPERNOVA study. Thorax 2019, 74, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, L.; Pisani, L.; Filippini, C.; Fanelli, V.; Fasano, L.; Terragni, P.; Dell’Amore, A.; Urbino, R.; Mascia, L.; Evangelista, A.; et al. Extracorporeal Co2 Removal in Hypercapnic Patients at Risk of Noninvasive Ventilation Failure. Crit. Care Med. 2015, 43, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Del Sorbo, L.; Pesenti, A.; Ranieri, V.M.; Fan, E. Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensiv. Care Med. 2017, 43, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Jaber, S.; Zogheib, E.; Godet, T.; Capellier, G.; Combes, A. Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit. Care 2018, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; Sieweke, A.; Brettner, F.; Staudinger, T.; Joannidis, M.; Verbrugge, S.; Frings, D.; Nierhaus, A.; Wegscheider, K.; Kluge, S. The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): Multicentre case–control study. Intensiv. Care Med. 2016, 42, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Polastri, M.; Pacilli, A.M.G.; Nava, S. Extracorporeal Lung Support for Hypercapnic Ventilatory Failure. Respir. Care 2018, 63, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Zanella, A.; Castagna, L.; Salerno, D.; Scaravilli, V.; Deab, S.A.E.A.E.S.; Magni, F.; Giani, M.; Mazzola, S.; Albertini, M.; Patroniti, N.; et al. Respiratory Electrodialysis. A Novel, Highly Efficient Extracorporeal CO2Removal Technique. Am. J. Respir. Crit. Care Med. 2015, 192, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Cove, M.E.; Vu, L.H.; Ring, T.; Federspiel, W.J.; Kellum, J.A. Respiratory Dialysis-A Novel Low Bicarbonate Dialysate to Provide Extracorporeal CO2 Removal. Crit. Care Med. 2020, 48, e592–e598. [Google Scholar] [CrossRef] [PubMed]
- Duscio, E.; Cipulli, F.; Vasques, F.; Collino, F.; Rapetti, F.; Romitti, F.; Behnemann, T.; Niewenhuys, J.; Tonetti, T.; Pasticci, I.; et al. Extracorporeal CO2 Removal. Crit. Care Med. 2019, 47, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Strassmann, S.; Brodie, D.; Ritter, P.; Larsson, A.; Borchardt, R.; Windisch, W. Impact of membrane lung surface area and blood flow on extracorporeal CO2 removal during severe respiratory acidosis. Intensiv. Care Med. Exp. 2017, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Strassmann, S.; Merten, M.; Schäfer, S.; De Moll, J.; Brodie, D.; Larsson, A.; Windisch, W.; Karagiannidis, C. Impact of sweep gas flow on extracorporeal CO2 removal (ECCO2R). Intensiv. Care Med. Exp. 2019, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, T.; Vasques, F.; Rapetti, F.; Maiolo, G.; Collino, F.; Romitti, F.; Camporota, L.; Cressoni, M.; Cadringher, P.; Quintel, M.; et al. Driving pressure and mechanical power: New targets for VILI prevention. Ann. Transl. Med. 2017, 5, 286. [Google Scholar] [CrossRef] [PubMed]
- Van Milgen, J.; Noblet, J.; Dubois, S.; Bernier, J.-F. Dynamic aspects of oxygen consumption and carbon dioxide production in swine. Br. J. Nutr. 1997, 78, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Hospach, I.; Goldstein, J.; Harenski, K.; Laffey, J.G.; Pouchoulin, D.; Raible, M.; Votteler, S.; Storr, M. In vitro characterization of PrismaLung+: A novel ECCO2R device. Intensiv. Care Med. Exp. 2020, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Barrett, N.A.; Hart, N.; Camporota, L. In vivo carbon dioxide clearance of a low-flow extracorporeal carbon dioxide removal circuit in patients with acute exacerbations of chronic obstructive pulmonary disease. Perfusion 2020, 35, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Gross-Hardt, S.; Hesselmann, F.; Arens, J.; Steinseifer, U.; Vercaemst, L.; Windisch, W.; Brodie, D.; Karagiannidis, C. Low-flow assessment of current ECMO/ECCO2R rotary blood pumps and the potential effect on hemocompatibility. Crit. Care 2019, 23, 348. [Google Scholar] [CrossRef] [PubMed]
- Giosa, L.; Busana, M.; Bonifazi, M.; Romitti, F.; Vassalli, F.; Pasticci, I.; Macrì, M.M.; D’Albo, R.; Collino, F.; Gatta, A.; et al. Mobilizing Carbon Dioxide Stores: An Experimental Study. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef] [PubMed]

| Time 1 | Time 2 | |
|---|---|---|
| Respiratory rate (breaths/min) | 18 (17–20) | 9 (8–10) |
| Tidal volume-pig (mL) | 430.00 (400.00–450.00) | 216.00 (200.00–224.00) |
| Minute ventilation (L/min) | 7.74 (6.80–9.00) | 1.80 (1.72–2.24) |
| Positive end-expiratory pressure (cmH2O) | 5.00 (5.00–5.00) | 5.00 (5.00–5.00) |
| Compliance respiratory system (cmH2O) | 34.00 (28.00–36.00) | 28.00 (20.00–30.00) |
| Respiratory system mechanical power (J/min) | 12.18 (10.00–13.25) | 1.91 (1.66–2.15) |
| Heart rate (beats/min) | 73 (65–85) | 77 (70–81) |
| Central venous pressure (mmHg) | 8.00 (6.00–10.00) | 9.00 (6.00–10.00) |
| Mean systemic arterial pressure (mmHg) | 92 (88–100) | 93 (87–99) |
| Arterial lactates (mmol/L) | 1.20 (0.90–1.60) | 1.35 (0.88–1.55) |
| Characteristics | Value |
|---|---|
| Access pressure (mmHg) | −7.00 (−10.50–−3.50) |
| Pre-membrane pressure (mmHg) | 40.00 (46.75–69.50) |
| Post-membrane pressure (mmHg) | 17.00 (15.75–32.00) |
| Δ pressure (mmHg) | 32.00 (30.00–34.00) |
| Activate Clotting Time | 187.00 (184.00–191.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Nardo, M.; Annoni, F.; Su, F.; Belliato, M.; Lorusso, R.; Broman, L.M.; Malfertheiner, M.; Creteur, J.; Taccone, F.S. Evaluation of a New Extracorporeal CO2 Removal Device in an Experimental Setting. Membranes 2021, 11, 8. https://doi.org/10.3390/membranes11010008
Di Nardo M, Annoni F, Su F, Belliato M, Lorusso R, Broman LM, Malfertheiner M, Creteur J, Taccone FS. Evaluation of a New Extracorporeal CO2 Removal Device in an Experimental Setting. Membranes. 2021; 11(1):8. https://doi.org/10.3390/membranes11010008
Chicago/Turabian StyleDi Nardo, Matteo, Filippo Annoni, Fuhong Su, Mirko Belliato, Roberto Lorusso, Lars Mikael Broman, Maximilian Malfertheiner, Jacques Creteur, and Fabio Silvio Taccone. 2021. "Evaluation of a New Extracorporeal CO2 Removal Device in an Experimental Setting" Membranes 11, no. 1: 8. https://doi.org/10.3390/membranes11010008
APA StyleDi Nardo, M., Annoni, F., Su, F., Belliato, M., Lorusso, R., Broman, L. M., Malfertheiner, M., Creteur, J., & Taccone, F. S. (2021). Evaluation of a New Extracorporeal CO2 Removal Device in an Experimental Setting. Membranes, 11(1), 8. https://doi.org/10.3390/membranes11010008







