Liquid Membranes for Efficient Recovery of Phenolic Compounds Such as Vanillin and Catechol
Abstract
1. Introduction
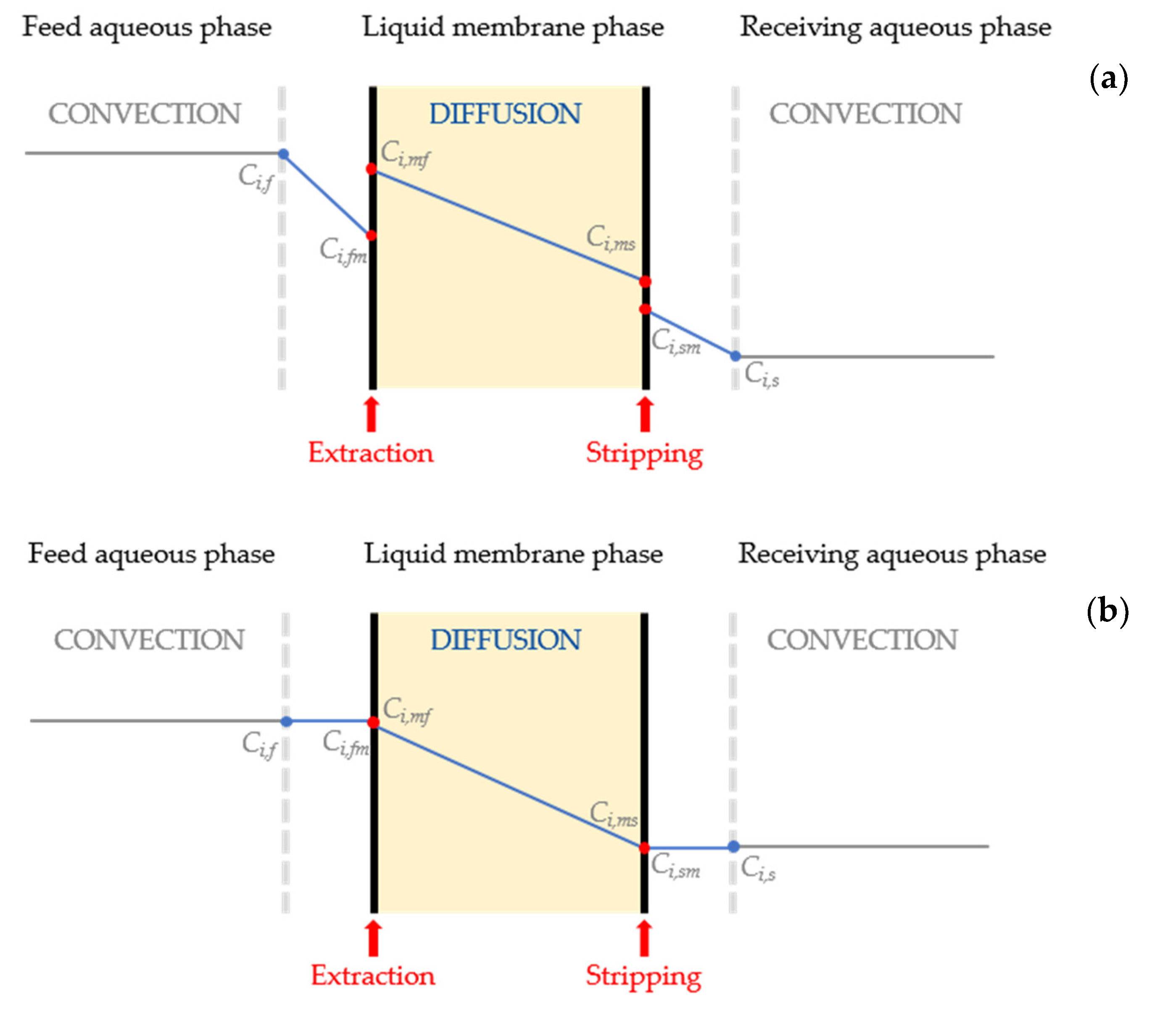
2. Theoretical Background
3. Materials and Methods
3.1. Materials
3.2. Experimental Procedure
3.2.1. Solvent Extraction
3.2.2. Transport
3.3. Analyses
4. Results and Discussion
4.1. Solvent Extraction
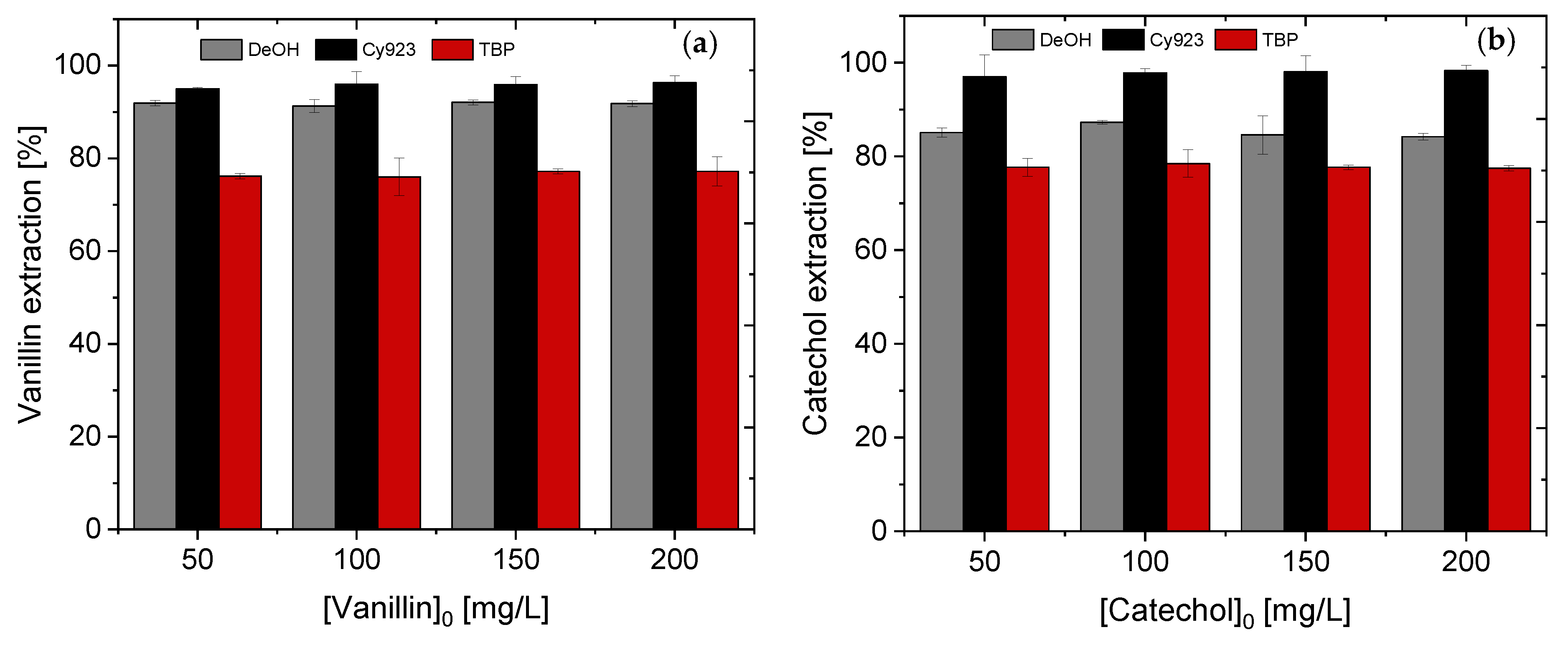
4.1.1. Effect of Different Extractants and Initial pH
4.1.2. Effect of Different Monomer Concentrations
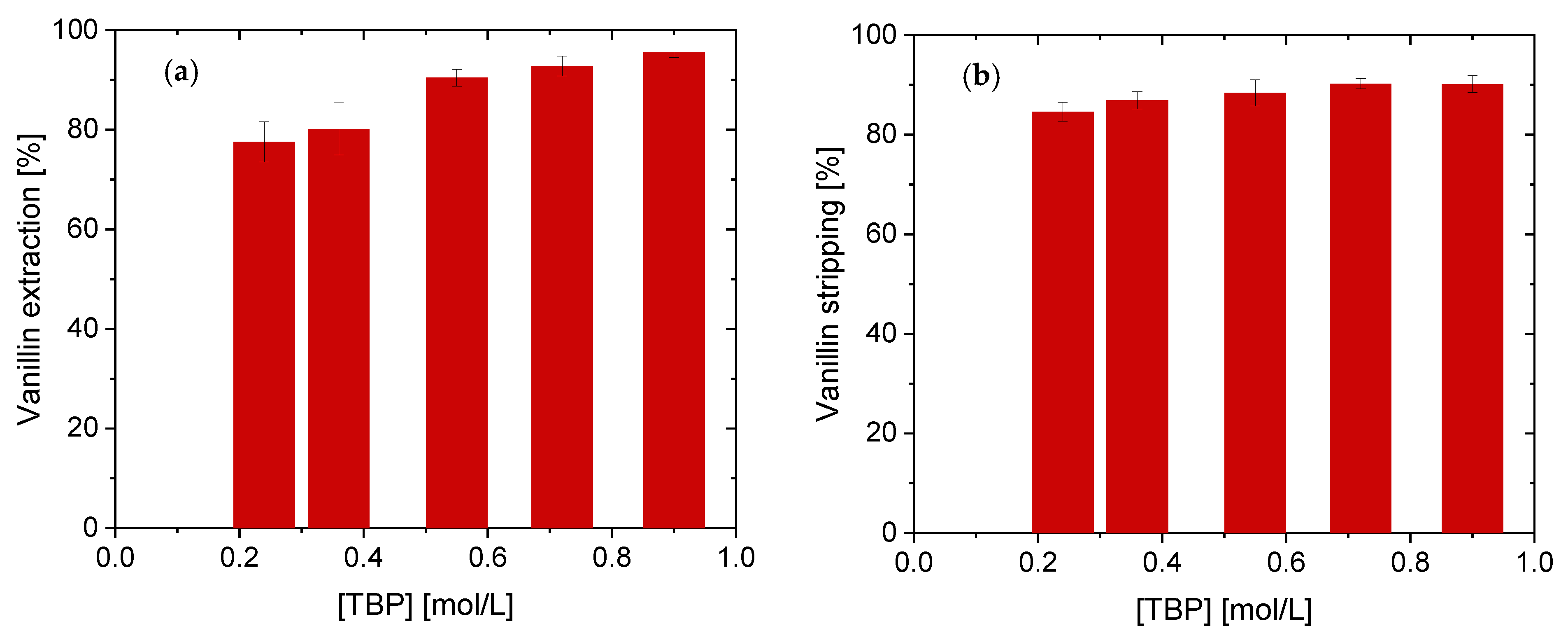
4.1.3. Effect of Different TBP Concentrations on Vanillin Extraction
4.2. SLM experiments
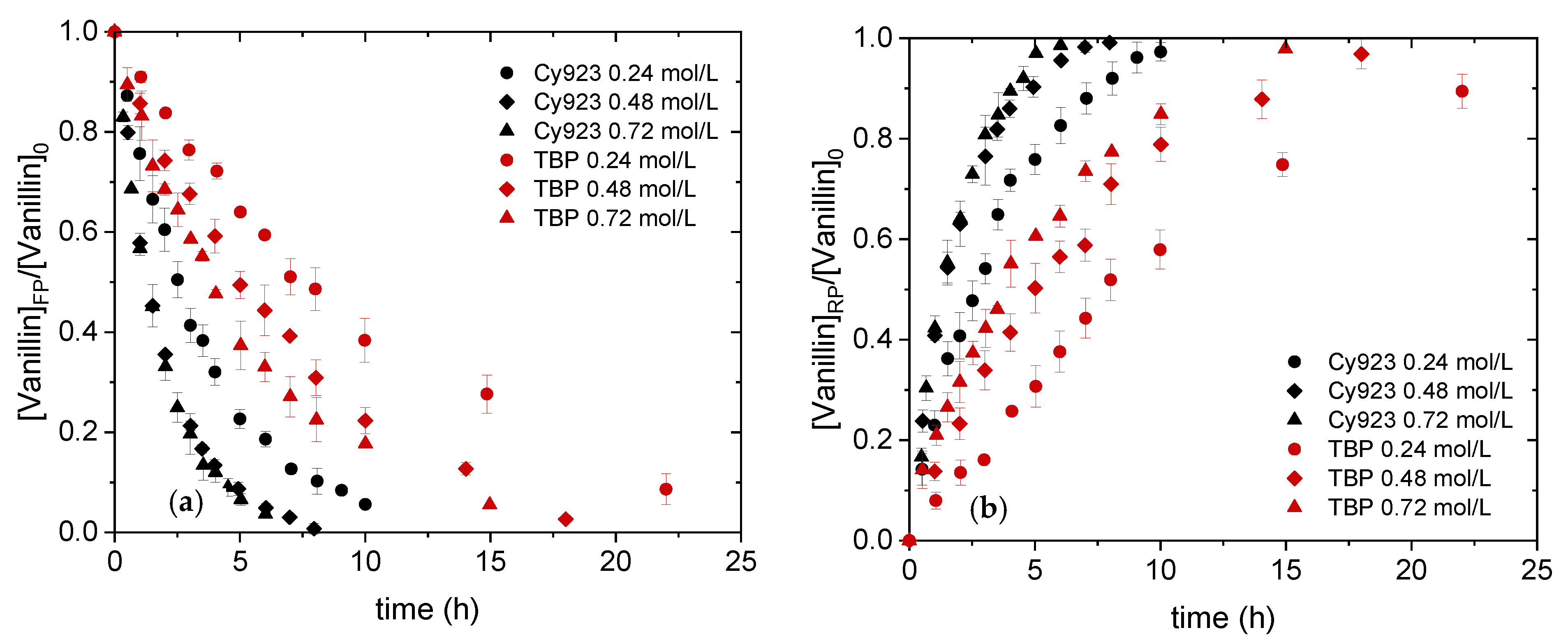
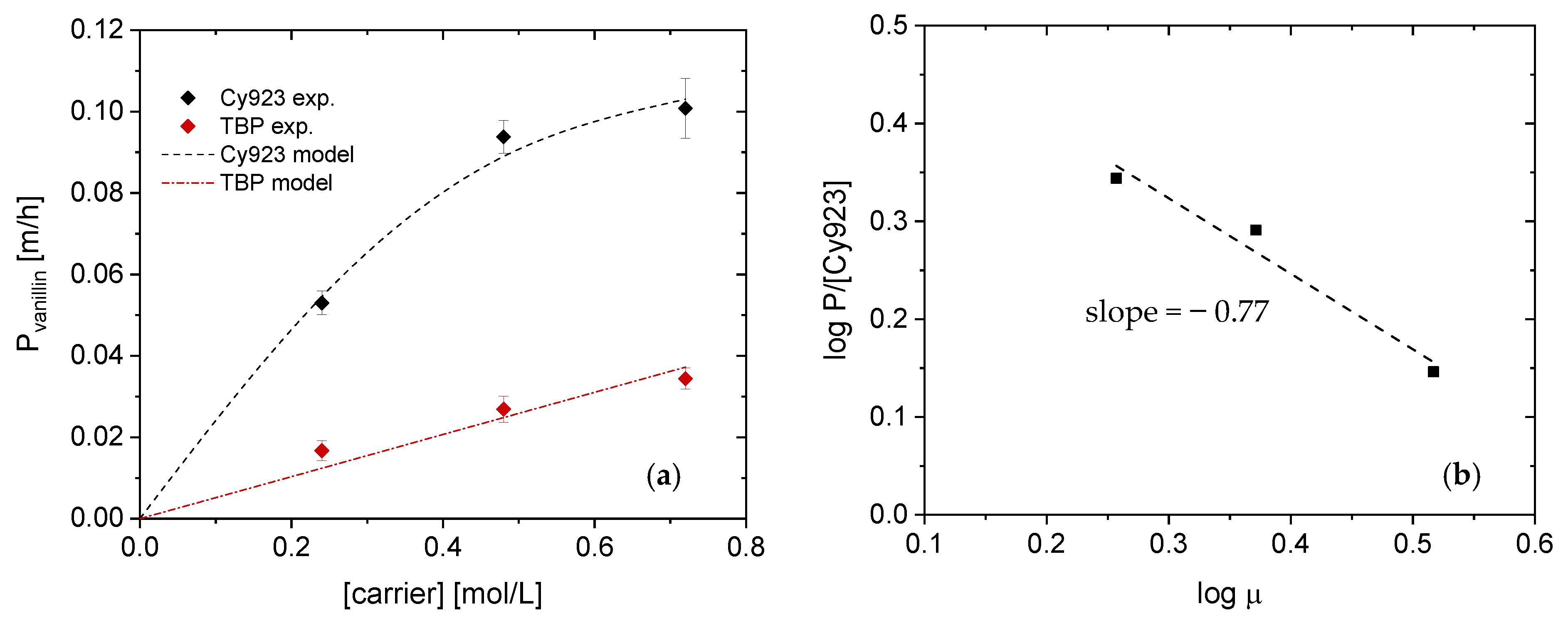
4.2.1. Effect of Carrier Concentration on Vanillin Transport
4.2.2. Permeability Coefficient Determination
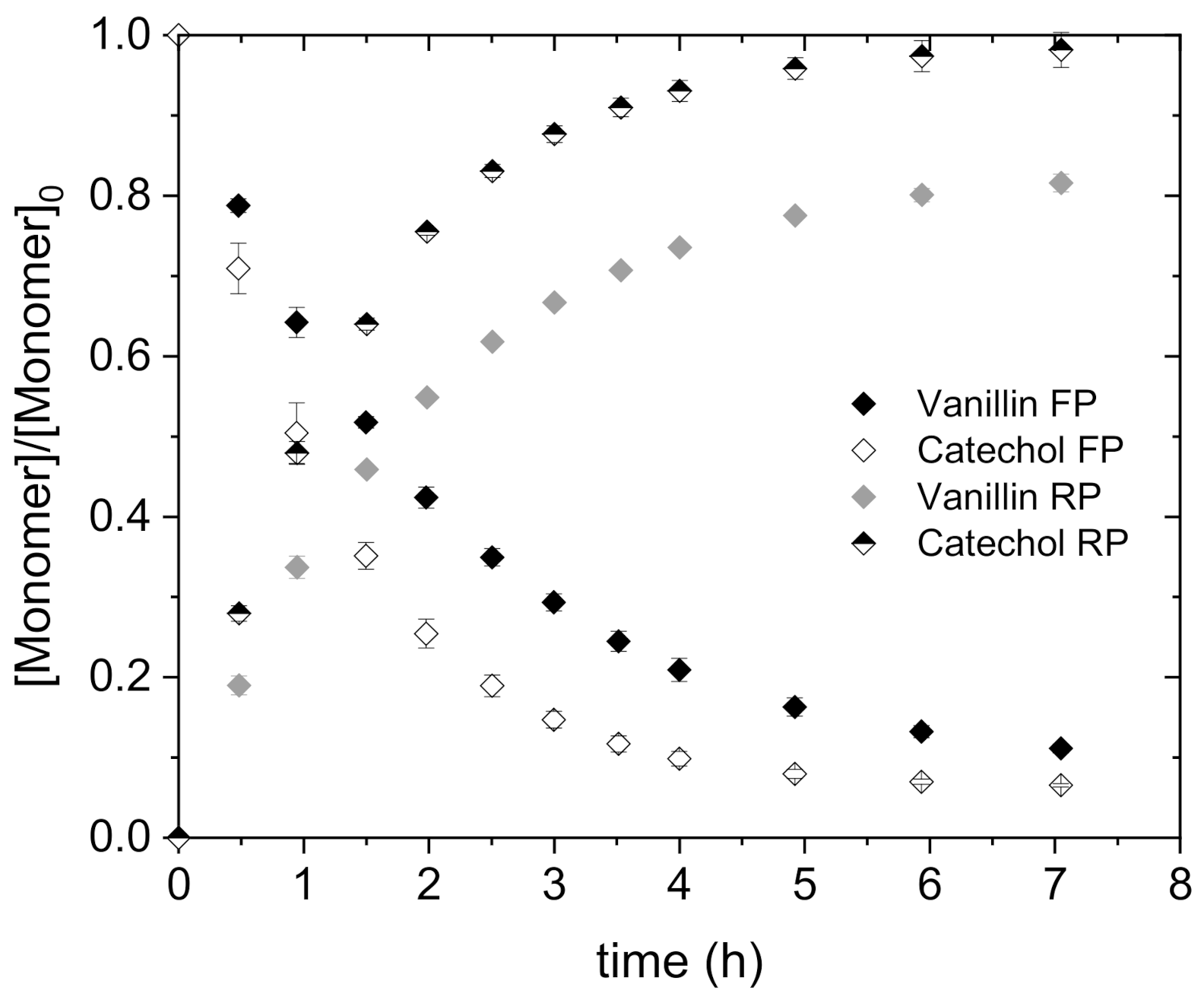
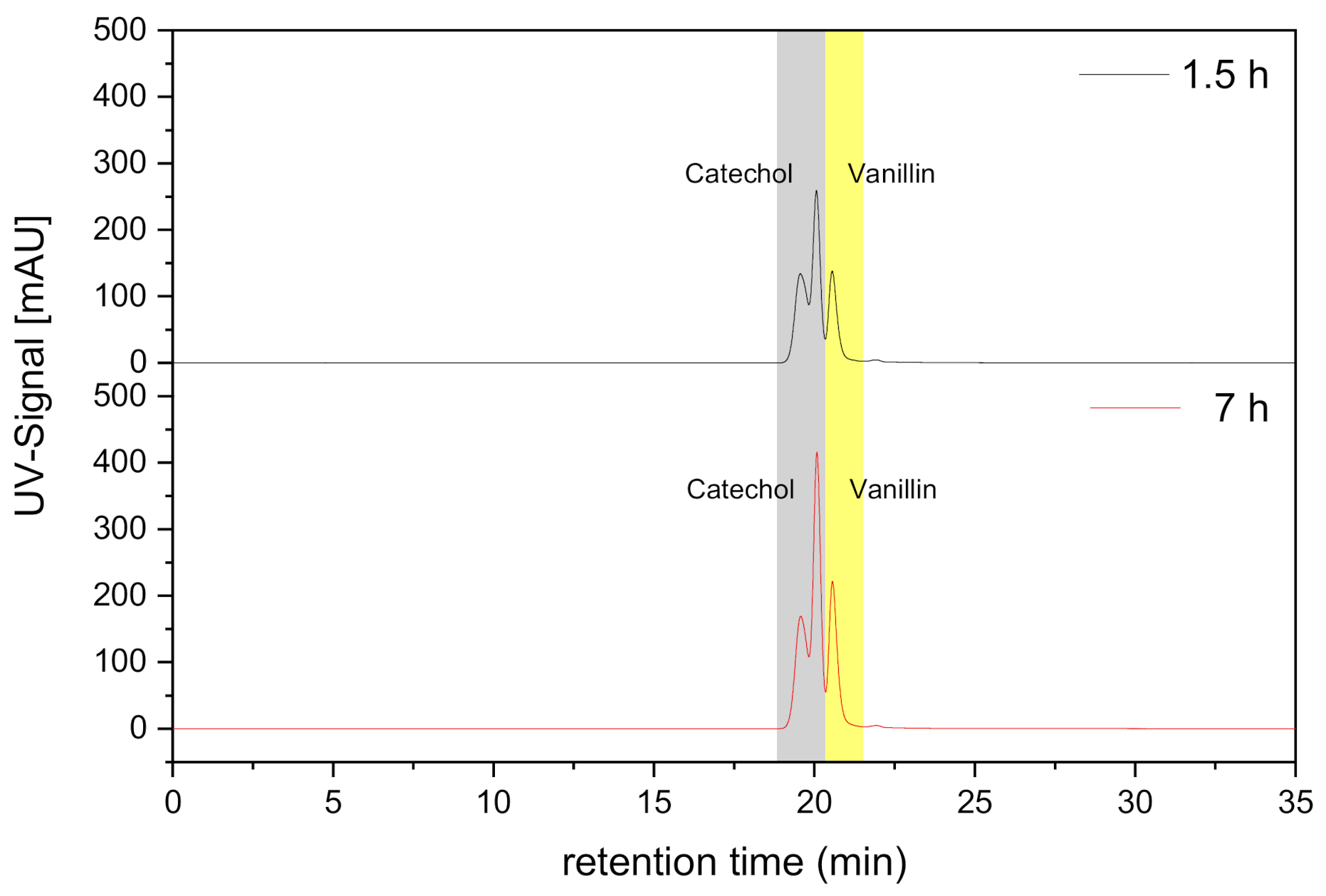
4.2.3. Transport and Permeability Coefficient of Vanillin and Catechol in Mixture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zidi, C.; Tayeb, R.; Boukhili, N.; Dhahbi, M. A supported liquid membrane system for efficient extraction of vanillin from aqueous solutions. Sep. Purif. Technol. 2011, 82, 36–42. [Google Scholar] [CrossRef]
- Rao, S.R.; Ravishankar, G.A. Vanilla flavour: Production by conventional and biotechnological routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar] [CrossRef]
- Jahn, A.; Hoffmann, A.; Blaesing, L.; Kunde, F.; Bertau, M.; Bremer, M.; Fischer, S. Lignin from Annual Plants as Raw Material Source for Flavors and Basic Chemicals. Chem. Ing. Tech. 2020, 92, 1733–1740. [Google Scholar] [CrossRef]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2000; p. 275. [Google Scholar]
- Priefert, H.; Rabenhorst, J.; Steinbüchel, A. Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Sharma, U.K.; Sharma, N. A comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr. 2009, 59, 299–326. [Google Scholar] [CrossRef] [PubMed]
- Gallage, N.J.; Møller, B.L. Vanillin–Bioconversion and Bioengineering of the Most Popular Plant Flavor and Its De Novo Biosynthesis in the Vanilla Orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef]
- Žilnik, L.F.; Jazbinšek, A. Recovery of renewable phenolic fraction from pyrolysis oil. Sep. Purif. Technol. 2012, 86, 157–170. [Google Scholar] [CrossRef]
- Mante, O.D.; Thompson, S.J.; Mustapha, S.; Dayton, D.C.; Mustpha, S. A selective extraction method for recovery of monofunctional methoxyphenols from biomass pyrolysis liquids. Green Chem. 2019, 21, 2257–2265. [Google Scholar] [CrossRef]
- Wang, S.; Leng, F.; Chen, J.; Qiu, K.; Zhou, J. Separation and Enrichment of Catechol and Sugars from Bio-oil Aqueous Phase. BioResources 2016, 11, 1707–1720. [Google Scholar] [CrossRef][Green Version]
- Mantilla, S.V.; Manrique, A.M.; Gauthier-Maradei, P. Methodology for Extraction of Phenolic Compounds of Bio-oil from Agricultural Biomass Wastes. Waste Biomass Valorization 2015, 6, 371–383. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Freire, M.G.; Freire, C.S.; Silvestre, A.J.; Coutinho, J.A.P. Extraction of vanillin using ionic-liquid-based aqueous two-phase systems. Sep. Purif. Technol. 2010, 75, 39–47. [Google Scholar] [CrossRef]
- Smink, D.; Kersten, S.R.; Schuur, B. Recovery of lignin from deep eutectic solvents by liquid-liquid extraction. Sep. Purif. Technol. 2020, 235, 116127. [Google Scholar] [CrossRef]
- Avila, M.; Zougagh, M.; Escarpa, A.; Ríos, Á. Supported liquid membrane-modified piezoelectric flow sensor with molecularly imprinted polymer for the determination of vanillin in food samples. Talanta 2007, 72, 1362–1369. [Google Scholar] [CrossRef]
- Mahdavi, H.R.; Arzani, M.; Peydayesh, M.; Mohammadi, T. Pertraction of l-lysine by supported liquid membrane using D2EHPA/M2EHPA. Chem. Eng. Process. Process. Intensif. 2016, 106, 50–58. [Google Scholar] [CrossRef]
- Cichy, W.; Schlosser, S.W.; Szymanowski, J. Recovery of phenol with cyanex® 923 in membrane extraction-stripping systems. Solvent Extr. Ion Exch. 2001, 19, 905–923. [Google Scholar] [CrossRef]
- Rosly, M.B.; Jusoh, N.; Othman, N.; Rahman, H.A.; Sulaiman, R.N.R.; Noah, N.F.M. Stability of emulsion liquid membrane using bifunctional diluent and blended nonionic surfactant for phenol removal. Chem. Eng. Process. Process. Intensif. 2020, 148, 107790. [Google Scholar] [CrossRef]
- Zidi, C.; Tayeb, R.; Dhahbi, M. Extraction of phenol from aqueous solutions by means of supported liquid membrane (MLS) containing tri-n-octyl phosphine oxide (TOPO). J. Hazard. Mater. 2011, 194, 62–68. [Google Scholar] [CrossRef]
- Pavón, S.; Fortuny, A.; Coll, M.; Bertau, M.; Sastre, A. Permeability dependencies on the carrier concentration and membrane viscosity for Y(III) and Eu(III) transport by using liquid membranes. Sep. Purif. Technol. 2020, 239, 116573. [Google Scholar] [CrossRef]
- Mei, X.; Li, J.; Jing, C.; Fang, C.; Liu, Y.; Wang, Y.; Liu, J.; Bi, S.; Chen, Y.; Xiao, Y.; et al. Separation and recovery of phenols from an aqueous solution by a green membrane system. J. Clean. Prod. 2020, 251, 119675. [Google Scholar] [CrossRef]
- Othman, N.; Noah, N.F.M.; Shu, L.Y.; Ooi, Z.-Y.; Jusoh, N.; Idroas, M.; Goto, M. Easy removing of phenol from wastewater using vegetable oil-based organic solvent in emulsion liquid membrane process. Chin. J. Chem. Eng. 2017, 25, 45–52. [Google Scholar] [CrossRef]
- Venkateswaran, P.; Palanivelu, K. Recovery of phenol from aqueous solution by supported liquid membrane using vegetable oils as liquid membrane. J. Hazard. Mater. 2006, 131, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Pavón, S.; Kutucu, M.; Coll, M.T.; Fortuny, A.; Sastre, A.M. Comparison of Cyanex 272 and Cyanex 572 for the separation of Neodymium from a Nd/Tb/Dy mixture by pertraction. J. Chem. Technol. Biotechnol. 2017, 93, 2152–2159. [Google Scholar] [CrossRef]
- Ehtash, M.; Fournier-Salaün, M.-C.; Dimitrov, K.; Salaün, P.; Saboni, A. Phenol removal from aqueous media by pertraction using vegetable oil as a liquid membrane. Chem. Eng. J. 2014, 250, 42–47. [Google Scholar] [CrossRef]
- Blaesing, L.; Jahn, A.; Bertau, M. Comparison of laccase and peroxidase to depolymerize lignin. In Proceedings of the 28th European Biomass Conference and Exhibition, Marseille, France, 6–9 July 2020. [Google Scholar]
- Heinzkill, M.; Bech, L.; Halkier, T.; Schneider, P.; Anke, T. Characterization of Laccases and Peroxidases from Wood-Rotting Fungi (Family Coprinaceae). Appl. Environ. Microbiol. 1998, 64, 1601–1606. [Google Scholar] [CrossRef]
- Tonin, F.; Melis, R.; Cordes, A.; Sanchez-Amat, A.; Pollegioni, L.; Rosini, E. Comparison of different microbial laccases as tools for industrial uses. New Biotechnol. 2016, 33, 387–398. [Google Scholar] [CrossRef]
- Haverlock, T.J.; Bonnesen, P.V.; Moyer, B.A. Separation of NaOH by Solvent Extraction Using Weak Hydroxy Acids. Solvent Extr. Ion Exch. 2003, 21, 483–504. [Google Scholar] [CrossRef]
- Yang, X.; Zou, A.; Qiu, J.; Wang, S.; Guo, H. Phenol Removal from Aqueous System by Bis(2-ethylhexyl) Sulfoxide Extraction. Sep. Sci. Technol. 2014, 49, 2495–2501. [Google Scholar] [CrossRef]
- Luque, M.; Luque-Pérez, E.; Ríos, A.; Valcárcel, M. Supported liquid membranes for the determination of vanillin in food samples with amperometric detection. Anal. Chim. Acta 2000, 410, 127–134. [Google Scholar] [CrossRef]
- Sciubba, L.; Di Gioia, D.; Fava, F.; Gostoli, C. Membrane-based solvent extraction of vanillin in hollow fiber contactors. Desalination 2009, 241, 357–364. [Google Scholar] [CrossRef]
- IFA Institut für Arbeitsschutz der Deutschen Gesetzlichen Unfallversicherung. GESTIS-Stoffdatenbank: Solubility of Butanol in Water. Available online: http://gestis.itrust.de/nxt/gateway.dll/gestis_de/000000.xml?f=templates&fn=default.htm&vid=gestisdeu:sdbdeu (accessed on 2 December 2020).
- Burghoff, B.; De Haan, A.B. Liquid-Liquid Equilibrium Study of Phenol Extraction with Cyanex 923. Sep. Sci. Technol. 2009, 44, 1753–1771. [Google Scholar] [CrossRef]
- MacGlashan, J.D.; Bixby, J.L.; King, C.J. Separation of phenols from dilute aqueous solution by use of tri-o-octyl phosphine oxide as extractant. Solvent Extr. Ion Exch. 1985, 3, 1–25. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Luo, H.; Yan, S.; Dai, J.; Bai, Z. Efficient recovery of phenol from coal tar processing wastewater with tributylphosphane/diethyl carbonate/cyclohexane: Extraction cycle and mechanism study. Chem. Eng. Res. Des. 2020, 157, 104–113. [Google Scholar] [CrossRef]
- Zidi, C.; Tayeb, R.; Dhahbi, M. Comparison entre le transport facilité à traves une Membrane à Liquide Sup-porté (MLS) du phénol et de la vanilline extraits de milieux aqueux. J. Mater. Environ. Sci. 2014, 5, 779–782. [Google Scholar]
- Wongsawa, T.; Koonsang, T.; Kunthakudee, N.; Prapasawat, T.; Maneeintr, K.; Pancharoen, U. The experimental investigations on viscosity, surface tension, interfacial tension and solubility of the binary and ternary systems for tributyl phosphate (TBP) extractant in various organic solvents with water: Thermodynamic NRTL model and molecular interaction approach. J. Mol. Liq. 2018, 251, 229–237. [Google Scholar] [CrossRef]
- Reis, M.T.A.; Freitas, O.M.; Agarwal, S.; Ferreira, L.M.; Ismael, M.R.C.; Machado, R.M.; De Carvalho, J.M.R. Removal of phenols from aqueous solutions by emulsion liquid membranes. J. Hazard. Mater. 2011, 192, 986–994. [Google Scholar] [CrossRef]
- Tayeb, R.; Fontàs, C.; Dhahbi, M.; Tingry, S.; Seta, P. Cd(II) transport across supported liquid membranes (SLM) and polymeric plasticized membranes (PPM) mediated by Lasalocid A. Sep. Purif. Technol. 2005, 42, 189–193. [Google Scholar] [CrossRef]
- Bird, R.B. Transport phenomena. Appl. Mech. Rev. 2002, 55, R1–R4. [Google Scholar] [CrossRef]
- Hiss, T.G.; Cussler, E.L. Diffusion in high viscosity liquids. AIChE J. 1973, 19, 698–703. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Diameter (cm) | 4.7 |
| Pore diameter (µm) | 0.45 |
| Thickness (µm) | 150 * |
| Effective area (m2) | 11.4 |
| Porosity (%) | 85 |
| Monomers | Wavelength in cb pH 4 [nm] | Wavelength in NaOH pH 14 [nm] |
|---|---|---|
| Vanillin | 280 | 345 |
| Catechol | 276 | 318 |
| Stripping Efficiency [%] | ||||||||
|---|---|---|---|---|---|---|---|---|
| Initial pH | BuOH | DeOH | Cy923 | TBP | ||||
| 3 | 64.9 ± 4.45 | 99.8 ± 0.98 | 99.2 ± 2.14 | 85.2 ± 1.33 | ||||
| 4 | 64.9 ± 4.98 | 83.1 ± 4.15 | 99.9 ± 3.38 | 99.9 ± 3.11 | 98.7 ± 2.14 | 94.6 ± 2.24 | 84.6 ± 1.88 | 84.0 ± 1.93 |
| 5 | 61.6 ± 4.84 | 99.7 ± 3.43 | 99.2 ± 4.01 | 85.0 ± 1.42 | ||||
| Stripping Efficiency [%] | ||||||
|---|---|---|---|---|---|---|
| Monomer Concentration [mg/L] | DeOH | Cy923 | TBP | |||
| 50 | 97.9 ± 1.13 | 89.9 ± 1.80 | 96.3 ± 2.86 | 87.9 ± 1.01 | 99.9 ± 0.53 | 96.6 ± 3.57 |
| 100 | 99.9 ± 2.08 | 99.9 ± 2.61 | 99.9 ± 2.14 | 92.7 ± 2.28 | 91.3 ± 1.88 | 85.2 ± 3.94 |
| 150 | 96.9 ± 2.21 | 80.7 ± 1.60 | 99.9 ± 1.90 | 82.6 ± 2.00 | 97.6 ± 2.65 | 95.7 ± 2.18 |
| 200 | 99.9 ± 2.52 | 91.0 ± 1.30 | 96.8 ± 1.93 | 80.6 ± 1.50 | 94.4 ± 1.35 | 92.9 ± 3.20 |
| Carrier | Concentration [mol/L] | Density * [kg/m3] | ν * [10−6·m2/s] | µ [10−2·kg/m·s] |
|---|---|---|---|---|
| Cy923 | 0.24 | 803 | 2.25 | 1.81 |
| 0.48 | 814 | 2.89 | 2.35 | |
| 0.72 | 828 | 3.98 | 3.29 | |
| TBP | 0.24 | 806 | 1.90 | 1.53 |
| 0.48 | 812 | 1.92 | 1.56 | |
| 0.72 | 838 | 1.99 | 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavón, S.; Blaesing, L.; Jahn, A.; Aubel, I.; Bertau, M. Liquid Membranes for Efficient Recovery of Phenolic Compounds Such as Vanillin and Catechol. Membranes 2021, 11, 20. https://doi.org/10.3390/membranes11010020
Pavón S, Blaesing L, Jahn A, Aubel I, Bertau M. Liquid Membranes for Efficient Recovery of Phenolic Compounds Such as Vanillin and Catechol. Membranes. 2021; 11(1):20. https://doi.org/10.3390/membranes11010020
Chicago/Turabian StylePavón, Sandra, Luisa Blaesing, Annika Jahn, Ines Aubel, and Martin Bertau. 2021. "Liquid Membranes for Efficient Recovery of Phenolic Compounds Such as Vanillin and Catechol" Membranes 11, no. 1: 20. https://doi.org/10.3390/membranes11010020
APA StylePavón, S., Blaesing, L., Jahn, A., Aubel, I., & Bertau, M. (2021). Liquid Membranes for Efficient Recovery of Phenolic Compounds Such as Vanillin and Catechol. Membranes, 11(1), 20. https://doi.org/10.3390/membranes11010020





