Dual-Functional Phosphorene Nanocomposite Membranes for the Treatment of Perfluorinated Water: An Investigation of Perfluorooctanoic Acid Removal via Filtration Combined with Ultraviolet Irradiation or Oxygenation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Phosphorene
2.3. Sulfonation of Poly Ether Ether Ketone
2.4. Water Flux Analysis
2.5. Determination of the Water Interaction Parameter of the Membranes
2.6. Treatment Processes
2.7. Membrane PFOA Adsorption Analysis
2.8. Measuring Ion Fluoride Concentration
2.9. Structural and Profile Studies with Scanning Electron Microscope (SEM) and X-ray Photoelectron Spectroscopy (XPS)
2.10. Surface Roughness Characterization
2.11. Data Analysis
3. Results and Discussion
3.1. PFOA Filtration Studies
3.2. Hydrophobicity
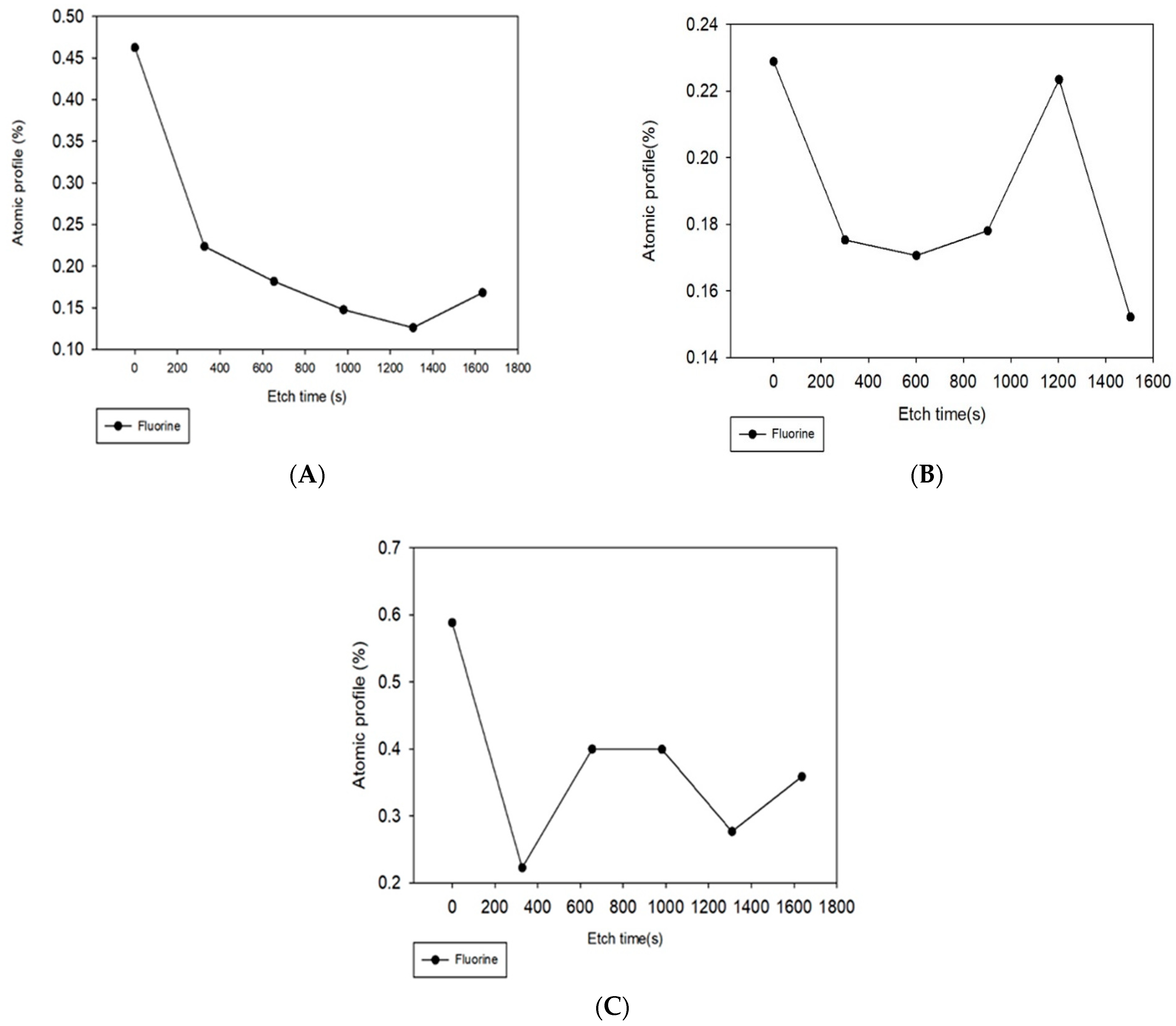
3.3. Adsorbed PFOA Removal
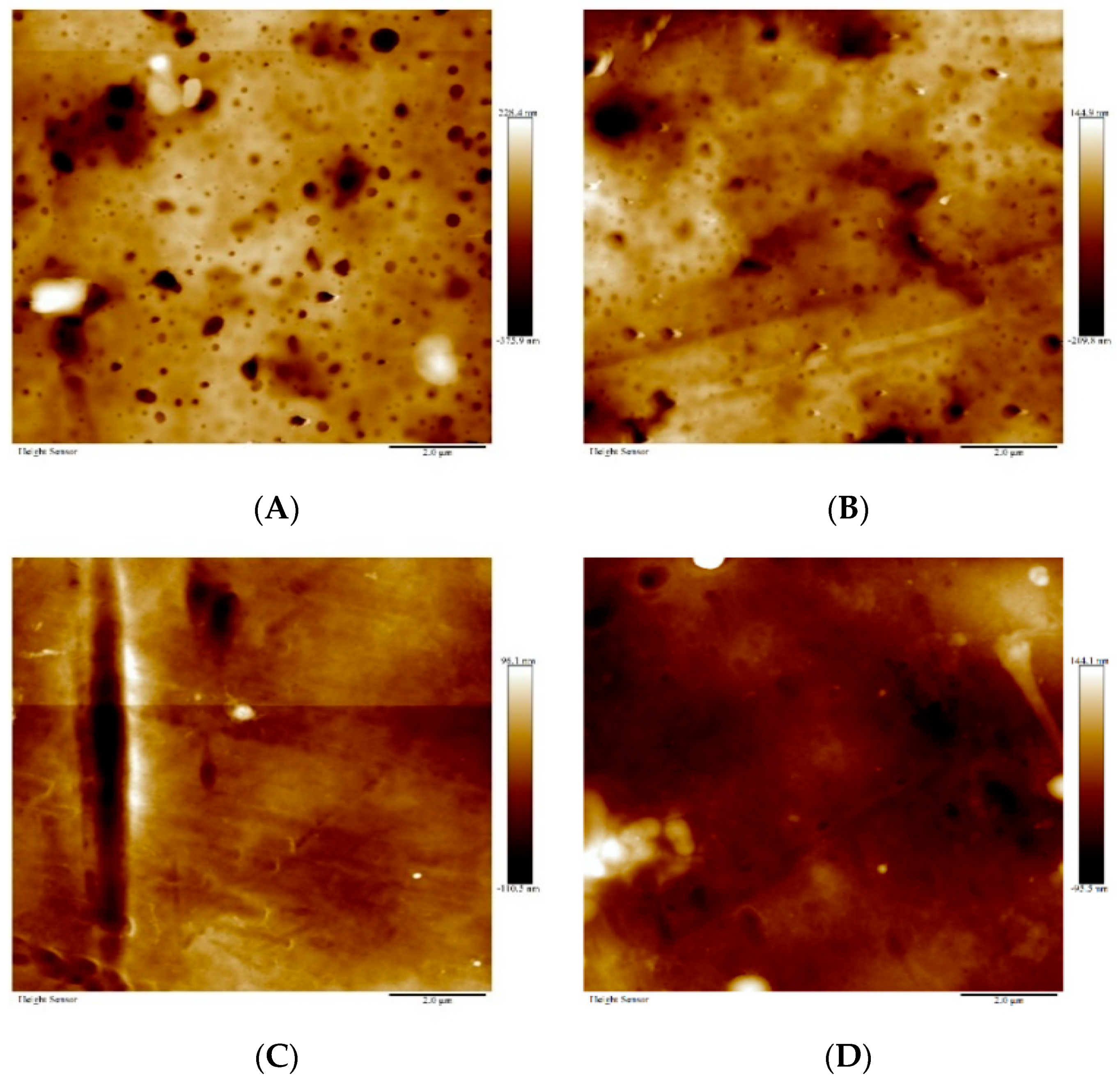
3.4. Membrane Morphology
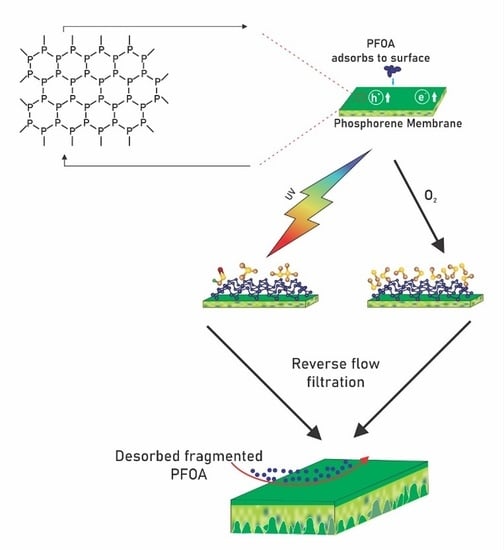
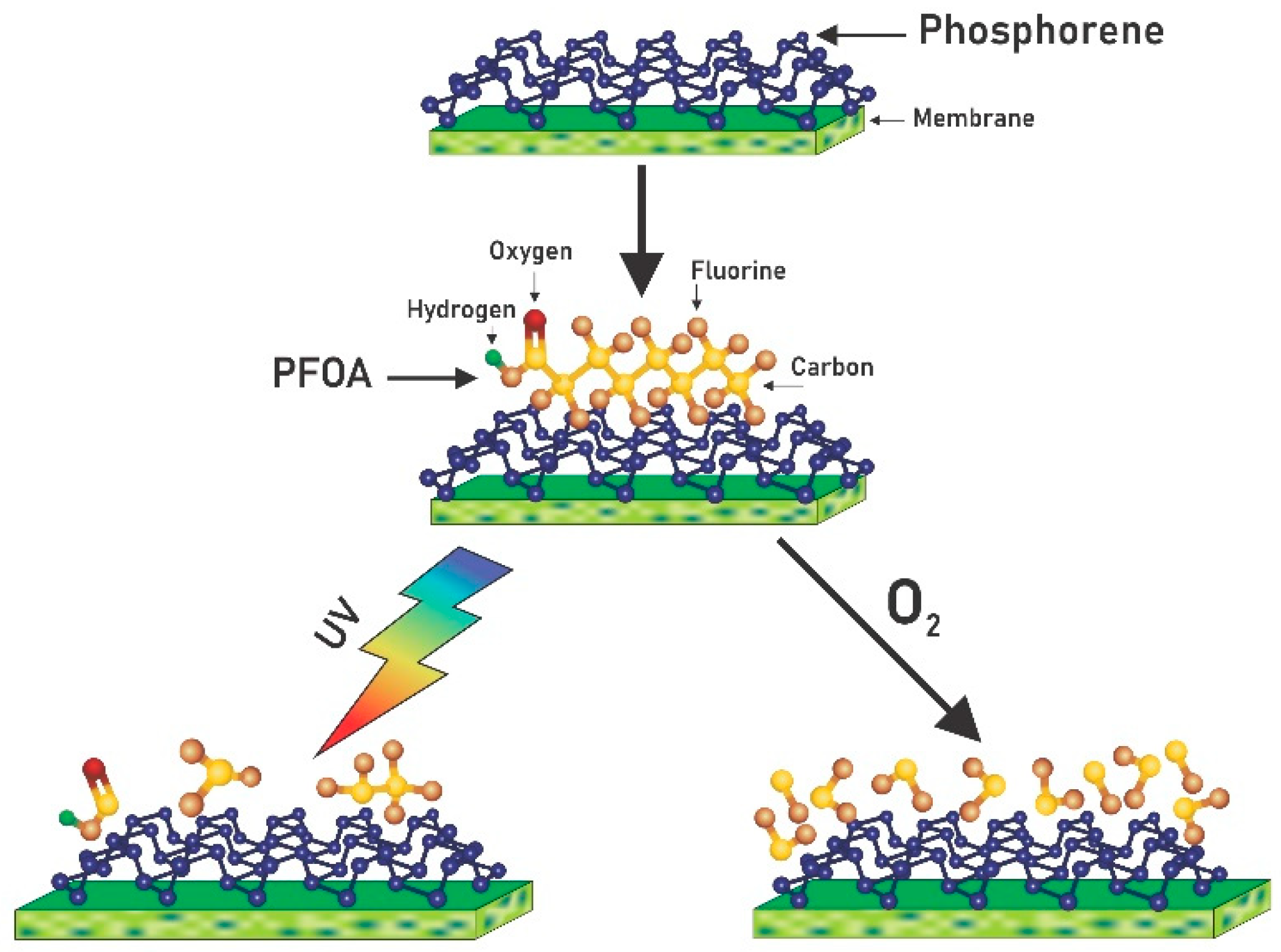
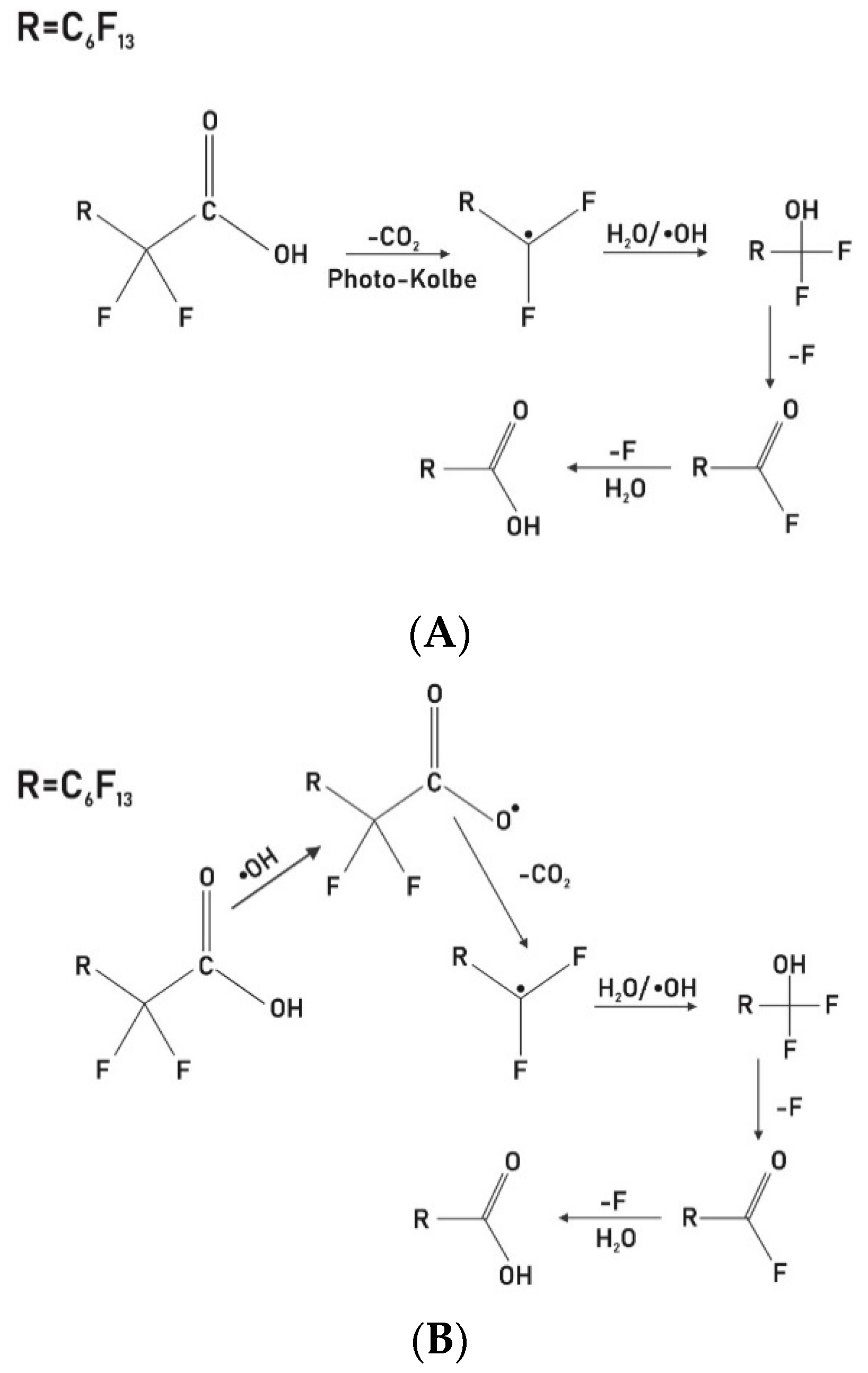
3.5. Proposed Reaction Pathway Based on Literature Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, K.; Yang, J.; Lu, Y. Phosphorene: An emerging 2d material. J. Mater. Res. 2017, 32, 2839–2847. [Google Scholar] [CrossRef]
- Aversa, R.; RPetrescu, V.; Apicella, A.; Petrescu, F.I. The basic elements of life’s. Am. J. Eng. Appl. Sci. 2016, 17, 1189–1197. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Deng, Y.; Ye, P.D. Semiconducting black phosphorus: Synthesis, transport properties and electronic applications. Chem. Soc. Rev. 2015, 44, 2732–2743. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, H.; Huang, S.; Xia, F.; Dresselhaus, M.S. The renaissance of black phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An unexplored 2d semiconductor with a high hole mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef]
- Koenig, S.P.; Doganov, R.A.; Schmidt, H.; Neto, A.H.C.; Özyilmaz, B. Electric field effect in ultrathin black phosphorus. Appl. Phys. Lett. 2014, 104, 103106. [Google Scholar] [CrossRef]
- Doganov, R.A.; O’Farrell, E.C.T.; Koenig, S.P.; Yeo, Y.; Ziletti, A.; Carvalho, A.; Campbell, D.K.; Coker, D.F.; Watanabe, K.; Taniguchi, T.; et al. Transport properties of pristine few-layer black phosphorus by van der waals passivation in an inert atmosphere. Nat. Commun. 2015, 6, 6647. [Google Scholar] [CrossRef]
- Wood, J.D.; Wells, S.A.; Jariwala, D.; Chen, K.-S.; Cho, E.; Sangwan, V.K.; Liu, X.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Effective passivation of exfoliated black phosphorus transistors against ambient degradation. Nano Lett. 2014, 14, 6964–6970. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Xia, F.; Wang, L.; Jiang, H.; Xia, Q.; Chin, M.L.; Dubey, M.; Han, S.-J. Black phosphorus radio-frequency transistors. Nano Lett. 2014, 14, 6424–6429. [Google Scholar] [CrossRef]
- Akhtar, M.; Anderson, G.; Zhao, R.; Alruqi, A.; Mroczkowska, J.E.; Sumanasekera, G.; Jasinski, J.B. Recent advances in synthesis, properties, and applications of phosphorene. npj 2D Mater. Appl. 2017, 1, 5. [Google Scholar] [CrossRef]
- Han, X.; Stewart, H.M.; Shevlin, S.A.; Catlow, C.R.A.; Guo, Z.X. Strain and orientation modulated bandgaps and effective masses of phosphorene nanoribbons. Nano Lett. 2014, 14, 4607–4614. [Google Scholar] [CrossRef] [PubMed]
- Rishabh, J.; Rekha, N.; Padmajan, S.S.; Eun, L.K.; Ju, J.H.; Ouk, K.S. Phosphorene for energy and catalytic application—Filling the gap between graphene and 2d metal chalcogenides. 2D Mater. 2017, 4, 042006. [Google Scholar]
- Akpan, U.; Hameed, B. Parameters affecting the photocatalytic degradation of dyes using tio2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Wang, H.; Xiao, D.; Dubey, M.; Ramasubramaniam, A. Two-dimensional material nanophotonics. Nat. Photonics 2014, 8, 899. [Google Scholar] [CrossRef]
- Low, J.; Cao, S.; Yu, J.; Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 2014, 50, 10768–10777. [Google Scholar] [CrossRef]
- Novoselov, K.; Jiang, D.; Schedin, F.; Booth, T.; Khotkevich, V.; Morozov, S.; Geim, A. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Kwong, C.W.; Davey, K.; Qiao, S.Z. 2d phosphorene as a water splitting photocatalyst: Fundamentals to applications. Energy Environ. Sci. 2016, 9, 709–728. [Google Scholar] [CrossRef]
- Eke, J.; Mills, P.A.; Page, J.R.; Wright, G.P.; Tsyusko, O.V.; Escobar, I.C. Nanohybrid membrane synthesis with phosphorene nanoparticles: A study of the addition, stability and toxicity. Polymers 2020, 12, 1555. [Google Scholar] [CrossRef]
- Clara, M.; Gans, O.; Weiss, S.; Sanz-Escribano, D.; Scharf, S.; Scheffknecht, C.J.W.R. Perfluorinated alkylated substances in the aquatic environment: An austrian case study. Water Res. 2009, 43, 4760–4768. [Google Scholar] [CrossRef]
- Lau, C.; Butenhoff, J.L.; Rogers, J.M. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol. Appl. Pharmacol. 2004, 198, 231–241. [Google Scholar] [CrossRef]
- Apelberg, B.J.; Witter, F.R.; Herbstman, J.B.; Calafat, A.M.; Halden, R.U.; Needham, L.L.; Goldman, L.R. Cord serum concentrations of perfluorooctane sulfonate (pfos) and perfluorooctanoate (pfoa) in relation to weight and size at birth. Environ. Health Perspect. 2007, 115, 1670–1676. [Google Scholar] [CrossRef] [PubMed]
- Rappazzo, K.M.; Coffman, E.; Hines, E.P. Exposure to perfluorinated alkyl substances and health outcomes in children: A systematic review of the epidemiologic literature. Int. J. Environ. Res. Public Health 2017, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Sáez, M.; de Voogt, P.; Parsons, J.R. Persistence of perfluoroalkylated substances in closed bottle tests with municipal sewage sludge. Environ. Sci. Pollut. Res. 2008, 15, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K. Perfluoroalkyl and polyfluoroalkyl substances: Current and future perspectives. Environ. Chem. 2011, 8, 333–338. [Google Scholar] [CrossRef]
- Kannan, K.; Koistinen, J.; Beckmen, K.; Evans, T.; Gorzelany, J.F.; Hansen, K.J.; Jones, P.D.; Helle, E.; Nyman, M.; Giesy, J.P. Accumulation of perfluorooctane sulfonate in marine mammals. Environ. Sci. Technol. 2001, 35, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Bundschuh, M. Fate and effects of poly-and perfluoroalkyl substances in the aquatic environment: A review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef]
- Pontius, F. Regulation of perfluorooctanoic acid (pfoa) and perfluorooctane sulfonic acid (pfos) in drinking water: A comprehensive review. Water 2019, 11, 2003. [Google Scholar] [CrossRef]
- Cordner, A.; de la Rosa, V.Y.; Schaider, L.A.; Rudel, R.A.; Richter, L.; Brown, P. Guideline levels for pfoa and pfos in drinking water: The role of scientific uncertainty, risk assessment decisions, and social factors. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 157–171. [Google Scholar] [CrossRef]
- Cui, J.; Gao, P.; Deng, Y. Destruction of per-and polyfluoroalkyl substances (pfas) with advanced reduction processes (arps): A critical review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef]
- Espana, V.A.A.; Mallavarapu, M.; Naidu, R. Treatment technologies for aqueous perfluorooctanesulfonate (pfos) and perfluorooctanoate (pfoa): A critical review with an emphasis on field testing. Environ. Technol. Innov. 2015, 4, 168–181. [Google Scholar] [CrossRef]
- Ross, I.; McDonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.T.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A review of emerging technologies for remediation of pfass. Remediat. J. 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, W.; Wang, C.; Liang, Y. Sonochemical degradation of poly-and perfluoroalkyl substances-a review. Ultrason. Sonochem. 2020, 69, 105245. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.P.; Qanbarzadeh, M.; Ateia, M.; Torkzadeh, H.; Maroli, A.S.; Cates, E.L. Rapid degradation and mineralization of perfluorooctanoic acid by a new petitjeanite bi3o (oh)(po4) 2 microparticle ultraviolet photocatalyst. Environ. Sci. Technol. Lett. 2018, 5, 533–538. [Google Scholar] [CrossRef]
- Merino, N.; Qu, Y.; Deeb, R.A.; Hawley, E.L.; Hoffmann, M.R.; Mahendra, S.J.E.E.S. Degradation and removal methods for perfluoroalkyl and polyfluoroalkyl substances in water. Environ. Eng. Sci. 2016, 33, 615–649. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Alam, M.M.; Zhou, J.L.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Rahman, M.S.; Hossen, J.; Hasan, A.K.; Moni, M.A. Advanced treatment technologies efficacies and mechanism of per-and poly-fluoroalkyl substances removal from water. Process Saf. Environ. Prot. 2020, 136, 1–14. [Google Scholar] [CrossRef]
- Hone, C.A.; Kappe, C.O. The use of molecular oxygen for liquid phase aerobic oxidations in continuous flow. In Accounts on Sustainable Flow Chemistry 2020; Springer: Cham, Switzerland, 2020; pp. 67–110. [Google Scholar]
- Gavriilidis, A.; Constantinou, A.; Hellgardt, K.; Hii, K.K.M.; Hutchings, G.J.; Brett, G.L.; Kuhn, S.; Marsden, S.P. Aerobic oxidations in flow: Opportunities for the fine chemicals and pharmaceuticals industries. React. Chem. Eng. 2016, 1, 595–612. [Google Scholar] [CrossRef]
- Ye, X.; Johnson, M.D.; Diao, T.; Yates, M.H.; Stahl, S.S. Development of safe and scalable continuous-flow methods for palladium-catalyzed aerobic oxidation reactions. Green Chem. 2010, 12, 1180–1186. [Google Scholar] [CrossRef]
- Saad, A.; Mills, R.; Wan, H.Y.; Mottaleb, M.A.; Ormsbee, L.; Bhattacharyya, D. Thermo-responsive adsorption-desorption of perfluoroorganics from water using pnipam hydrogels and pore functionalized membranes. J. Membr. Sci. 2020, 599, 117821. [Google Scholar] [CrossRef]
- Thompson, B.C.; Fréchet, J.M.J. Polymer–fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Talapin, D.V.; Murray, C.B. Pbse nanocrystal solids for n- and p-channel thin film field-effect transistors. Science 2005, 310, 86–89. [Google Scholar] [CrossRef]
- Wu, J.; Agrawal, M.; Becerril, H.A.; Bao, Z.; Liu, Z.; Chen, Y.; Peumans, P. Organic light-emitting diodes on solution-processed graphene transparent electrodes. ACS Nano 2010, 4, 43–48. [Google Scholar] [CrossRef]
- Woomer, A.H.; Farnsworth, T.W.; Hu, J.; Wells, R.A.; Donley, C.L.; Warren, S.C. Phosphorene: Synthesis, scale-up, and quantitative optical spectroscopy. ACS Nano 2015, 9, 8869–8884. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lu, N.; Dai, J.; Wu, X.; Zeng, X.C. Phosphorene nanoribbons, phosphorus nanotubes, and van der waals multilayers. J. Phys. Chem. C 2014, 118, 14051–14059. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, H.; Lu, S.; Wang, Z.; Tang, S.; Shao, J.; Sun, Z.; Xie, H.; Wang, H.; Yu, X.F. From black phosphorus to phosphorene: Basic solvent exfoliation, evolution of raman scattering, and applications to ultrafast photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Eke, J.; Elder, K.; Escobar, I.C. Self-cleaning nanocomposite membranes with phosphorene-based pore fillers for water treatment. Membranes 2018, 8, 79. [Google Scholar] [CrossRef]
- Ismail, M.F.; Khorshidi, B.; Sadrzadeh, M. New insights into the impact of nanoscale surface heterogeneity on the wettability of polymeric membranes. J. Membr. Sci. 2019, 590, 117270. [Google Scholar] [CrossRef]
- Rattanaoudom, R.; Visvanathan, C.; Boontanon, S.K. Removal of concentrated pfos and pfoa in synthetic industrial wastewater by powder activated carbon and hydrotalcite. J. Water Sustain. 2012, 2, 245–258. [Google Scholar]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Zhao, X.; Su, Y.; Chen, W.; Peng, J.; Jiang, Z. Grafting perfluoroalkyl groups onto polyacrylonitrile membrane surface for improved fouling release property. J. Membr. Sci. 2012, 415–416, 824–834. [Google Scholar] [CrossRef]
- Mottaleb, M.A.; Petriello, M.C.; Morris, A.J. High-throughput uhplc-ms/ms measurement of per-and poly-fluorinated alkyl substances in human serum. J. Anal. Toxicol. 2020, 44, 339–347. [Google Scholar] [CrossRef]
- Bonyadinejad, G.; Khosravi, M.; Ebrahimi, A.; Nateghi, R.; Taghavi-Shahri, S.M.; Mohammadi, H. Sonoelectrochemical mineralization of perfluorooctanoic acid using ti/pbo2anode assessed by response surface methodology. J. Environ. Health Sci. Eng. 2015, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Method 300.1: Determination of Inorganic Anions in Drinking Water by Ion Chromatography; US EPA: Cincinnati, OH, USA, 1997.
- Hobbs, C.; Taylor, J.; Hong, S. Effect of surface roughness on fouling of ro and nf membranes during filtration of a high organic surficial groundwater. J. Water Supply Res. Technol. AQUA 2006, 55, 559–570. [Google Scholar] [CrossRef]
- Chede, S.; Griffiths, P.; Escobar, I.C.; Harris, T.A.L. Does casting method matter in filtration membranes? A comparison in performance between doctor blade and slot-die extruded polymeric membranes. J. Appl. Polym. Sci. 2018, 20, 45563. [Google Scholar] [CrossRef]
- Thompson, J.; Eaglesham, G.; Reungoat, J.; Poussade, Y.; Bartkow, M.; Lawrence, M.; Mueller, J.F. Removal of pfos, pfoa and other perfluoroalkyl acids at water reclamation plants in south east queensland australia. Chemosphere 2011, 82, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, A.; Kirchgeorg, T.; Weinberg, I.; Matthias, V. Particle-size distribution of airborne poly- and perfluorinated alkyl substances. Chemosphere 2015, 129, 142–149. [Google Scholar] [CrossRef]
- Dixit, F.; Barbeau, B.; Mostafavi, S.G.; Mohseni, M. Pfoa and pfos removal by ion exchange for water reuse and drinking applications: Role of organic matter characteristics. Environ. Sci. Water Res. Technol. 2019, 5, 1782–1795. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E. The role of membrane surface charge and solute physico-chemical properties in the rejection of organic acids by nf membranes. J. Membr. Sci. 2005, 249, 227–234. [Google Scholar] [CrossRef]
- Fujii, S.; Polprasert, C.; Tanaka, S.; Lien, N.P.H.; Qiu, Y. New pops in the water environment: Distribution, bioaccumulation and treatment of perfluorinated compounds–a review paper. J. Water Supply Res. Technol. AQUA 2007, 56, 313–326. [Google Scholar] [CrossRef]
- Ma, Z.; Shu, G.; Lu, X. Preparation of an antifouling and easy cleaning membrane based on amphiphobic fluorine island structure and chemical cleaning responsiveness. J. Membr. Sci. 2020, 611, 118403. [Google Scholar] [CrossRef]
- Giri, R.; Ozaki, H.; Morigaki, T.; Taniguchi, S.; Takanami, R. Uv photolysis of perfluorooctanoic acid (pfoa) in dilute aqueous solution. Water Sci. Technol. 2011, 63, 276–282. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Synergistic effects in combined fouling of a loose nanofiltration membrane by colloidal materials and natural organic matter. J. Membr. Sci. 2006, 278, 72–82. [Google Scholar] [CrossRef]
- Song, W.; Ravindran, V.; Koel, B.E.; Pirbazari, M. Nanofiltration of natural organic matter with h2o2/uv pretreatment: Fouling mitigation and membrane surface characterization. J. Membr. Sci. 2004, 241, 143–160. [Google Scholar] [CrossRef]
- Gomez-Ruiz, B.; Ribao, P.; Diban, N.; Rivero, M.J.; Ortiz, I.; Urtiaga, A. Photocatalytic degradation and mineralization of perfluorooctanoic acid (pfoa) using a composite tio2− rgo catalyst. J. Hazard. Mater. 2018, 344, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Lyu, C.; Sun, R.; Zhang, D.; Alvarez, P.J. Discerning the inefficacy of hydroxyl radicals during perfluorooctanoic acid degradation. Chemosphere 2020, 247, 125883. [Google Scholar] [CrossRef]
- Hori, H.; Yamamoto, A.; Hayakawa, E.; Taniyasu, S.; Yamashita, N.; Kutsuna, S.; Kiatagawa, H.; Arakawa, R. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Pan, Z.; Li, X.; Ling, Y.; Li, L. Efficient degradation of perfluorooctanoic acid (pfoa) by photocatalytic ozonation. Chem. Eng. J. 2016, 296, 329–334. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Pan, G.; Chen, H. Ferric ion mediated photochemical decomposition of perfluorooctanoic acid (pfoa) by 254 nm UV light. J. Hazard. Mater. 2008, 160, 181–186. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eke, J.; Banks, L.; Mottaleb, M.A.; Morris, A.J.; Tsyusko, O.V.; Escobar, I.C. Dual-Functional Phosphorene Nanocomposite Membranes for the Treatment of Perfluorinated Water: An Investigation of Perfluorooctanoic Acid Removal via Filtration Combined with Ultraviolet Irradiation or Oxygenation. Membranes 2021, 11, 18. https://doi.org/10.3390/membranes11010018
Eke J, Banks L, Mottaleb MA, Morris AJ, Tsyusko OV, Escobar IC. Dual-Functional Phosphorene Nanocomposite Membranes for the Treatment of Perfluorinated Water: An Investigation of Perfluorooctanoic Acid Removal via Filtration Combined with Ultraviolet Irradiation or Oxygenation. Membranes. 2021; 11(1):18. https://doi.org/10.3390/membranes11010018
Chicago/Turabian StyleEke, Joyner, Lillian Banks, M. Abdul Mottaleb, Andrew J. Morris, Olga V. Tsyusko, and Isabel C. Escobar. 2021. "Dual-Functional Phosphorene Nanocomposite Membranes for the Treatment of Perfluorinated Water: An Investigation of Perfluorooctanoic Acid Removal via Filtration Combined with Ultraviolet Irradiation or Oxygenation" Membranes 11, no. 1: 18. https://doi.org/10.3390/membranes11010018
APA StyleEke, J., Banks, L., Mottaleb, M. A., Morris, A. J., Tsyusko, O. V., & Escobar, I. C. (2021). Dual-Functional Phosphorene Nanocomposite Membranes for the Treatment of Perfluorinated Water: An Investigation of Perfluorooctanoic Acid Removal via Filtration Combined with Ultraviolet Irradiation or Oxygenation. Membranes, 11(1), 18. https://doi.org/10.3390/membranes11010018