Poly[3-ethyl-1-vinyl-imidazolium] diethyl phosphate/Pebax® 1657 Composite Membranes and Their Gas Separation Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Scanning Electron Microscopy (SEM)
2.4. X-ray Diffraction (XRD)
2.5. Fourier-Transformed Infrared Spectroscopy (FT–IR)
2.6. Time-Lag
2.7. Gravimetric Sorption
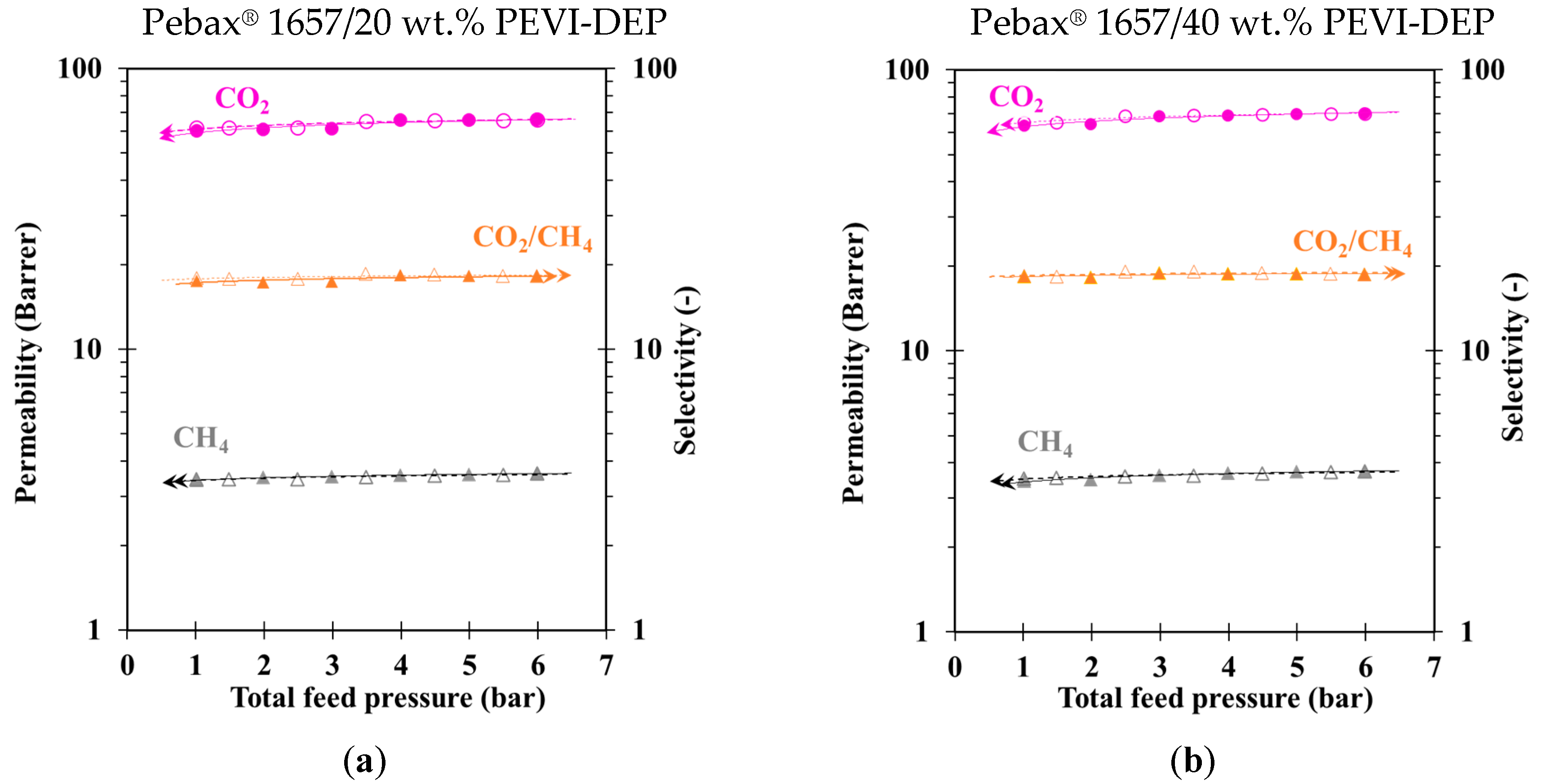
2.8. Mixed Gas Analysis
3. Results and Discussion
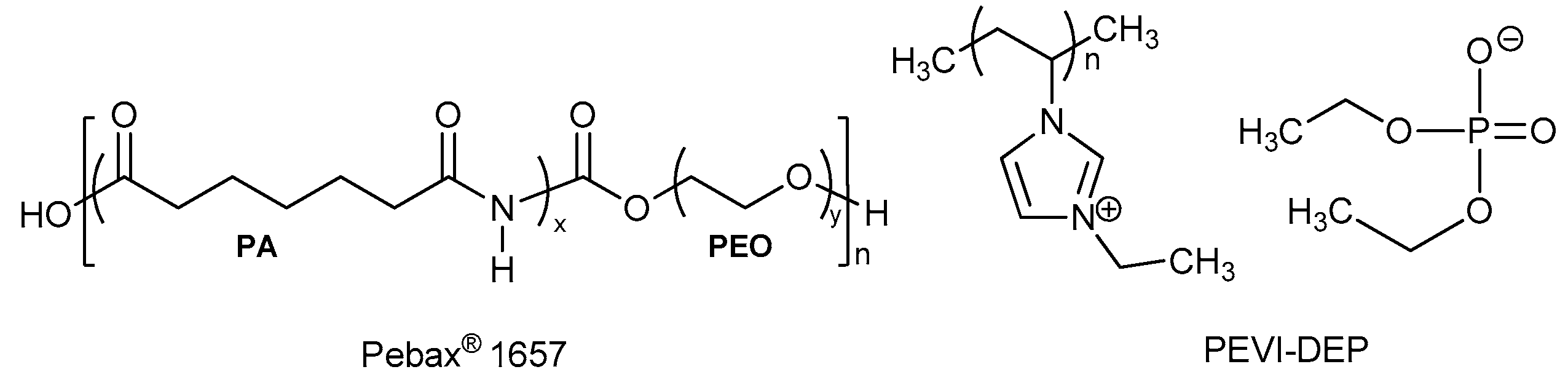
3.1. Morphology and Bulk Properties
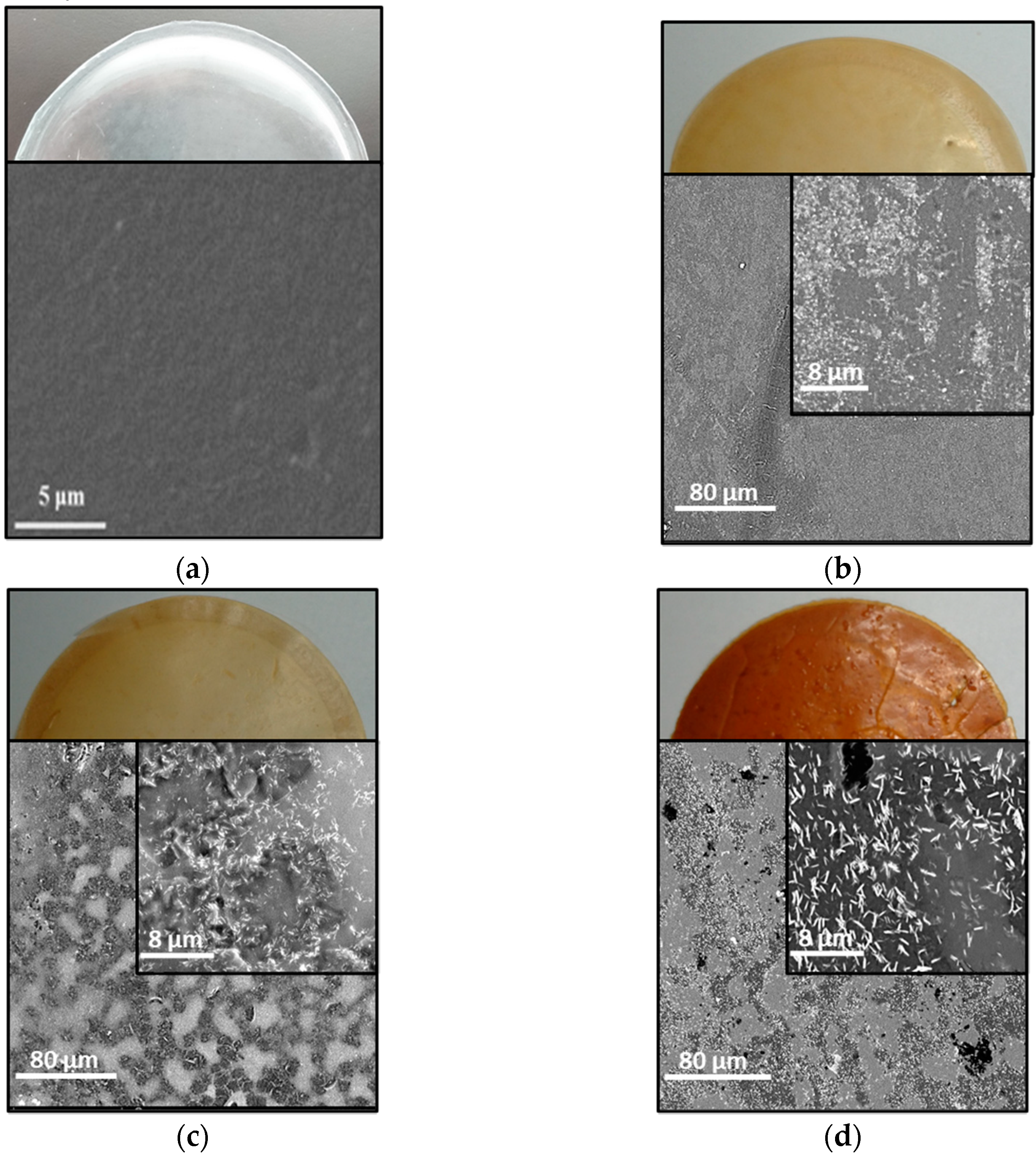
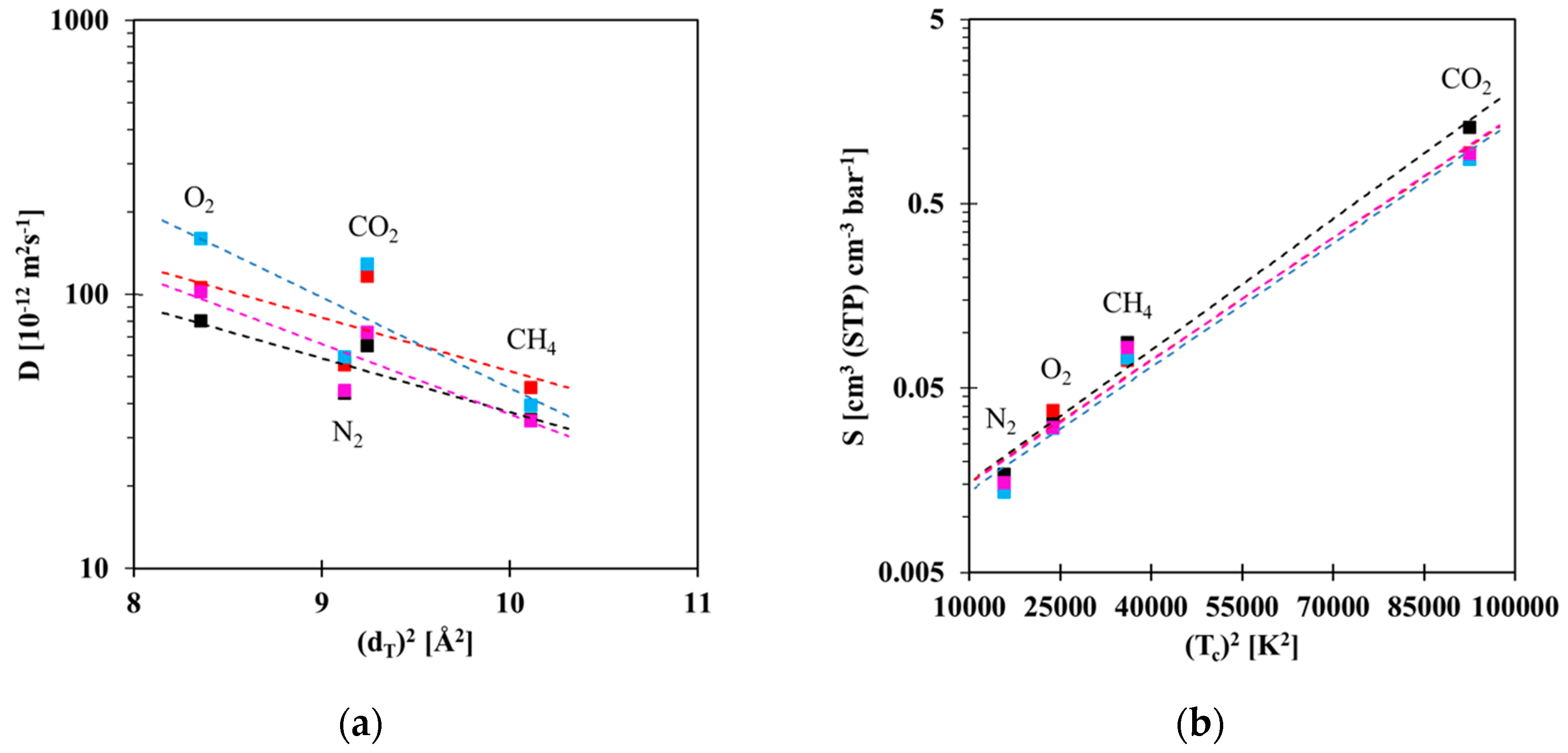
3.2. Gas Transport Properties
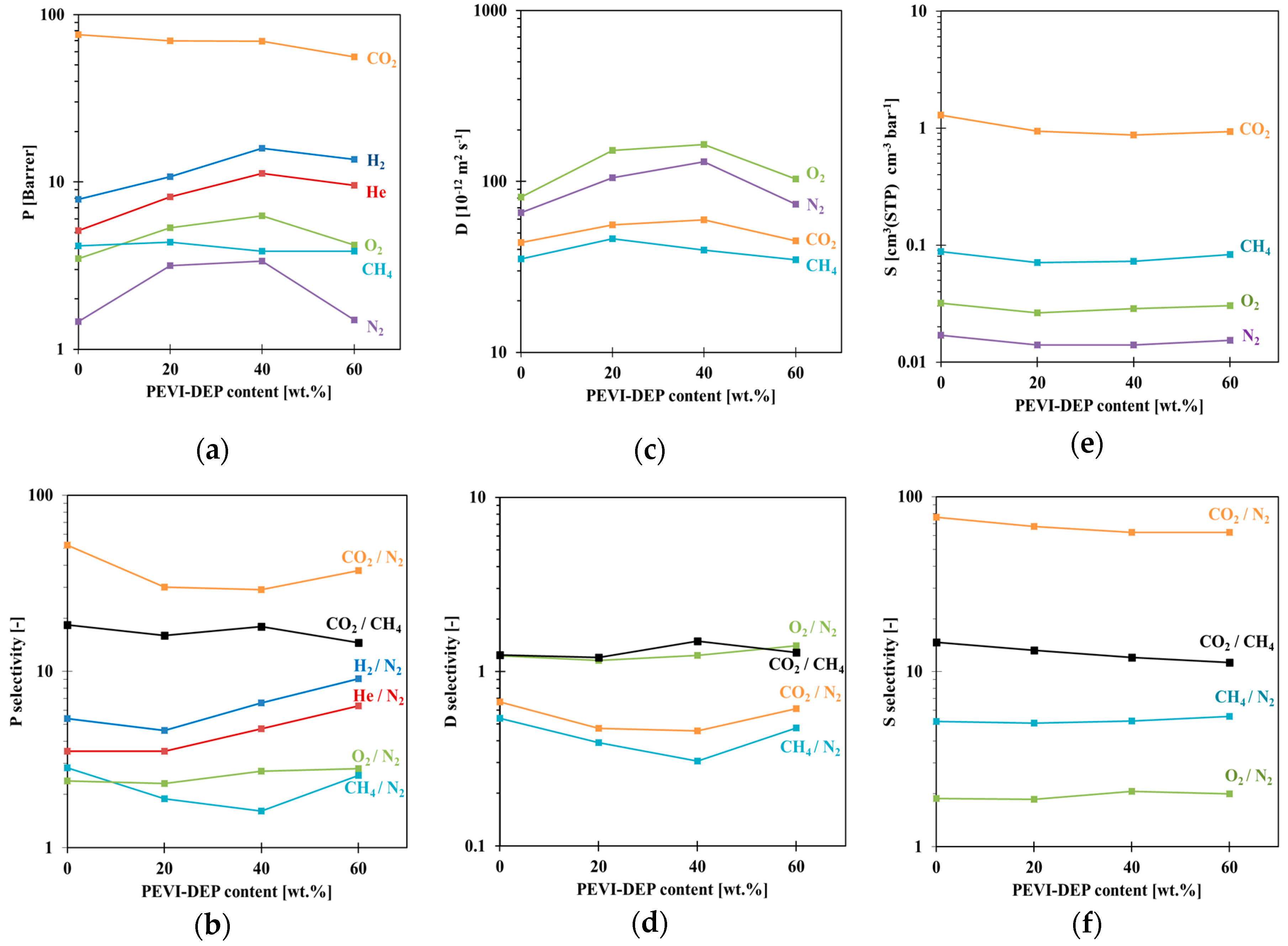
3.3. Gas Sorption Properties
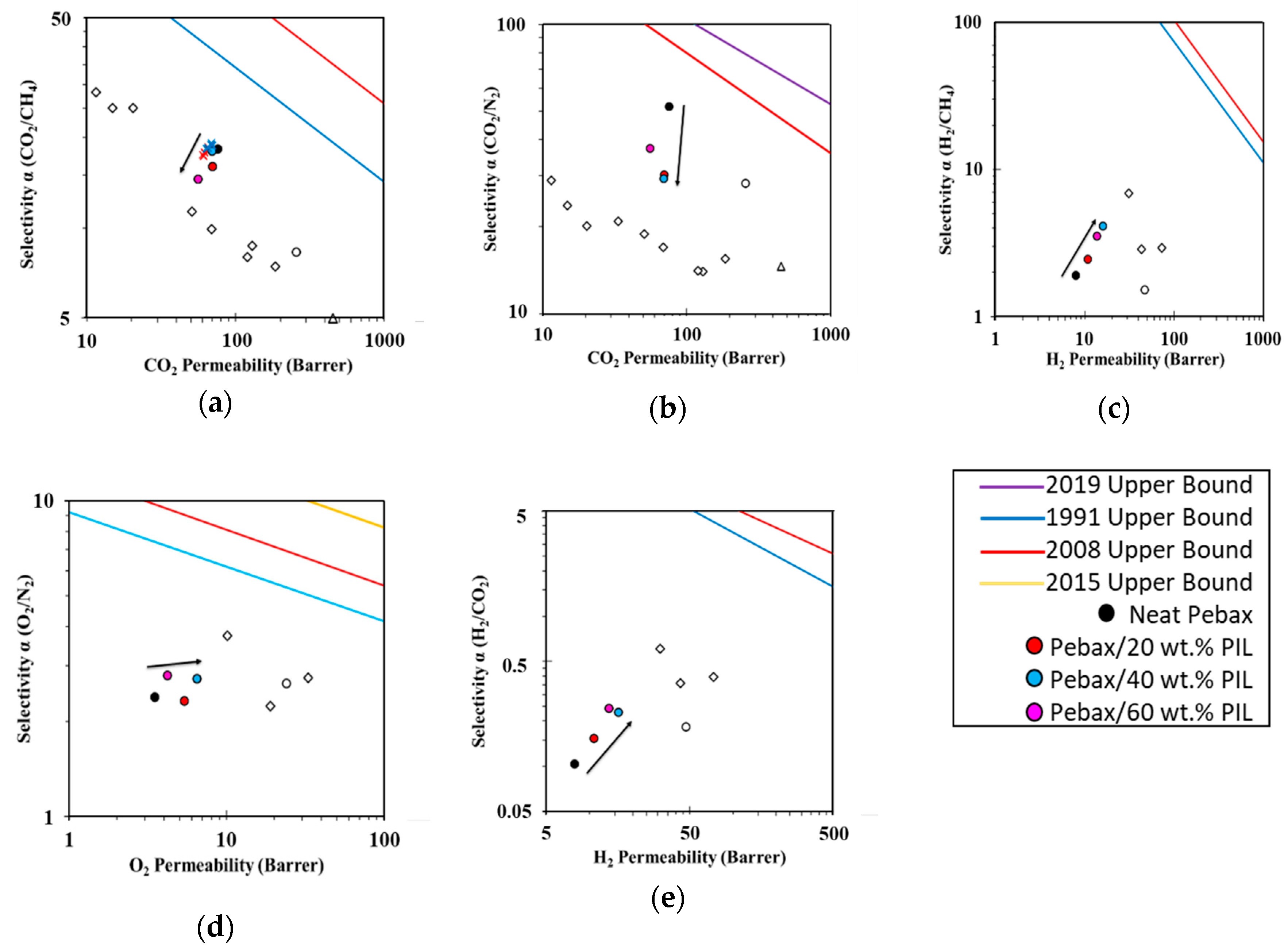
3.4. Robeson Plots and Correlations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Permeability (Barrer) | Selectivity (Px/PN2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PIL Content (wt.%) | H2 | He | O2 | N2 | CH4 | CO2 | H2/N2 | He/N2 | O2/N2 | CH4/N2 | CO2/N2 |
| 0 | 7.88 | 5.12 | 3.48 | 1.46 | 4.14 | 75.92 | 5.40 | 3.51 | 2.38 | 2.84 | 52.00 |
| 20 | 10.74 | 8.14 | 5.37 | 2.32 | 4.38 | 69.95 | 4.61 | 3.51 | 2.31 | 1.89 | 30.15 |
| 40 | 15.92 | 11.28 | 6.50 | 2.40 | 3.86 | 69.46 | 6.63 | 4.71 | 2.71 | 1.61 | 28.94 |
| 60 | 13.61 | 9.53 | 4.20 | 1.50 | 3.85 | 56.01 | 9.07 | 6.35 | 2.80 | 2.57 | 37.34 |
| Diffusivity (10−12·m2·s−1) | Selectivity (Dx/DN2) | ||||||||||
| 0 | - | - | 80.8 | 65.6 | 35.3 | 43.9 | - | - | 1.23 | 0.54 | 0.67 |
| 20 | - | - | 137 | 118 | 46.2 | 55.7 | - | - | 1.16 | 0.39 | 0.47 |
| 40 | - | - | 161 | 130 | 39.7 | 59.5 | - | - | 1.24 | 0.31 | 0.46 |
| 60 | - | - | 103 | 73.5 | 34.9 | 44.9 | - | - | 1.41 | 0.47 | 0.61 |
| Solubility (cm3(STP) cm−3·bar−1) | Selectivity (Sx/SN2) | ||||||||||
| 0 | - | - | 0.032 | 0.017 | 0.088 | 1.295 | - | - | 1.88 | 5.18 | 76.2 |
| 20 | - | - | 0.026 | 0.014 | 0.071 | 0.943 | - | - | 1.86 | 5.07 | 67.4 |
| 40 | - | - | 0.029 | 0.014 | 0.073 | 0.876 | - | - | 2.07 | 5.21 | 62.6 |
| 60 | - | - | 0.030 | 0.015 | 0.083 | 0.935 | - | - | 2.00 | 5.53 | 62.3 |
| Volume Concentration (cm3 (STP) cm−3) | |||||
|---|---|---|---|---|---|
| Pressure (bar) | Neat Pebax® 1657 | Pebax® 1657/20 wt.%PEVI-DEP | Pebax® 1657/40 wt.%PEVI-DEP | Pebax® 1657/60 wt.%PEVI-DEP | Neat PEVI-DEP |
| 1.0 | 1.79 | 1.78 | 2.02 | 1.87 | 1.35 |
| 2.0 | 3.16 | 3.29 | 3.51 | 3.19 | 2.93 |
| 2.9 | 4.40 | 4.65 | 4.87 | 4.66 | 4.15 |
| 3.9 | 5.51 | 5.87 | 6.08 | 5.89 | 5.57 |
| 5.9 | 7.82 | 8.15 | 8.10 | 8.18 | 7.69 |
| 7.9 | 9.99 | 10.39 | 10.33 | 10.08 | 9.84 |
| 9.8 | 12.53 | 12.01 | 12.30 | 12.16 | 11.67 |
References
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Env. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Drieoli, E.; Giorno, L. (Eds.) Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783662443231. [Google Scholar]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Tomé, L.C.; Gouveia, A.S.L.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric ionic liquid-based membranes: Influence of polycation variation on gas transport and CO2 selectivity properties. J. Membr. Sci. 2015, 486, 40–48. [Google Scholar] [CrossRef]
- Noble, R.D.; Gin, D.L. Perspective on ionic liquids and ionic liquid membranes. J. Membr. Sci. 2011, 369, 1–4. [Google Scholar] [CrossRef]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- Kocherginsky, N.M.; Yang, Q.; Seelam, L. Recent advances in supported liquid membrane technology. Sep. Purif. Technol. 2007, 53, 171–177. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2018, 34, 341–363. [Google Scholar] [CrossRef]
- Bara, J.E.; Lessmann, S.; Gabriel, C.J.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. Synthesis and performance of polymerizable room-temperature ionic liquids as gas separation membranes. Ind. Eng. Chem. Res. 2007, 46, 5397–5404. [Google Scholar] [CrossRef]
- Bara, J.E.; Carlisle, T.K.; Gabriel, C.J.; Camper, D.; Finotello, A.; Gin, D.L.; Noble, R.D. Guide to CO2 separations in imidazolium-based room-temperature ionic liquids. Ind. Eng. Chem. Res. 2009, 48, 2739–2751. [Google Scholar] [CrossRef]
- Tang, J.; Sun, W.; Tang, H.; Radosz, M.; Shen, Y. Enhanced CO2 absorption of poly (ionic liquid)s. Macromolecules 2005, 38, 2037–2039. [Google Scholar] [CrossRef]
- Camper, D.; Bara, J.; Koval, C.; Noble, R. Bulk-fluid solubility and membrane feasibility of Rmim-based room-temperature ionic liquids. Ind. Eng. Chem. Res. 2006, 45, 6279–6283. [Google Scholar] [CrossRef]
- Blasig, A.; Tang, J.; Hu, X.; Tan, S.P.; Shen, Y.; Radosz, M. Carbon dioxide solubility in polymerized ionic liquids containing ammonium and imidazolium cations from magnetic suspension balance: P[VBTMA][BF4] and P[VBMI][BF4]. Ind. Eng. Chem. Res. 2007, 46, 5542–5547. [Google Scholar] [CrossRef]
- Tang, J.; Shen, Y.; Radosz, M.; Sun, W. Isothermal Carbon Dioxide Sorption in Poly (ionic liquid)s. Ind. Eng. Chem. Res. 2009, 48, 9113–9118. [Google Scholar] [CrossRef]
- Cserjési, P.; Nemestóthy, N.; Bélafi-Bakó, K. Gas separation properties of supported liquid membranes prepared with unconventional ionic liquids. J. Membr. Sci. 2010, 349, 6–11. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Sheridan, E.; Sandru, M.; Genua, A.; Tanczyk, M. Poly (vinylbenzyl chloride)-based poly (ionic liquids) as membranes for CO2 capture from flue gas. J. Mater. Chem. A Mater. Energy Sustain. 2017, 5, 19808–19818. [Google Scholar] [CrossRef]
- Bara, J.E.; Gin, D.L.; Noble, R.D. Effect of Anion on Gas Separation Performance of Polymer-Room-Temperature Ionic Liquid Composite Membranes. Ind. Eng. Chem. Res. 2008, 47, 9919–9924. [Google Scholar] [CrossRef]
- Tome, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6435–6451. [Google Scholar] [CrossRef]
- Uchytil, P.; Schauer, J.; Petrychkovych, R.; Setnickova, K.; Suen, S.Y. Ionic liquid membranes for carbon dioxide–methane separation. J. Membr. Sci. 2011, 383, 262–271. [Google Scholar] [CrossRef]
- Carlisle, T.K.; Bara, J.E.; Lafrate, A.L.; Gin, D.L.; Noble, R.D. Main-chain imidazolium polymer membranes for CO2 separations: An initial study of a new ionic liquid-inspired platform. J. Membr. Sci. 2010, 359, 37–43. [Google Scholar] [CrossRef]
- Scovazzo, P.; Kieft, J.; Finan, D.A.; Koval, C.; Dubois, D.; Noble, R. Gas separations using non-hexafluorophosphate [PF 6]—anion supported ionic liquid membranes. J. Membr. Sci. 2004, 238, 57–63. [Google Scholar] [CrossRef]
- Billard, I. Ionic Liquids: New hopes for efficient lanthanide/actinide extraction and separation. In Handbook on the Physics and Chemistry of Rare Earths, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 43, ISBN 9780444595362. [Google Scholar]
- Shi, G.; Zhao, H.; Chen, K.; Lin, W.; Li, H.; Wang, C. Efficient capture of CO2 from flue gas at high temperature by tunable polyamine-based hybrid ionic liquids. AIChE J. 2020, 66, 1–7. [Google Scholar] [CrossRef]
- Huang, K.; Chen, F.F.; Tao, D.J.; Dai, S. Ionic liquid–formulated hybrid solvents for CO2 capture. Curr. Opin. Green Sustain. Chem. 2017, 5, 67–73. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhuang, Q.; Zhang, M.; Wang, H.; Gao, Z.; Sun, J.-K.; Yuan, J. Poly (ionic liquid) composites. Chem. Soc. Rev. 2020, 49, 1726–1755. [Google Scholar] [CrossRef] [PubMed]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas Transport Properties of Poly (ether-b-amide) Segmented. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2051–2062. [Google Scholar] [CrossRef]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas Sorption and Characterization of Poly (ether-b-amide). J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2463–2475. [Google Scholar] [CrossRef]
- Esposito, E.; Bruno, R.; Monteleone, M.; Fuoco, A.; Pardo, E.; Armentano, D.; Jansen, J.C. Glassy PEEK-WC vs. Rubbery Pebax® 1657 Polymers: Effect on the Gas Transport in CuNi-MOF Based Mixed Matrix Membranes. Appl. Sci. 2020, 10, 1310. [Google Scholar] [CrossRef]
- Longo, M.; de Santo, M.P.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Jansen, J.C. Force spectroscopy determination of Young’s modulus in mixed matrix membranes. Polymer 2018, 156, 22–29. [Google Scholar] [CrossRef]
- Esposito, E.; Clarizia, G.; Bernardo, P.; Carolus, J.; Sedláková, Z.; Izák, P.; Curcio, S.; de Cindio, B.; Tasselli, F. Pebax/PAN hollow fi ber membranes for CO2/CH4 separation. Chem. Eng. Process. Process Intensif. 2015, 94, 53–61. [Google Scholar] [CrossRef]
- Jomekian, A.; Bazooyar, B.; Mosayebi, R.; Mohammadi, T. Ionic liquid-modified Pebax® 1657 membrane fi lled by ZIF-8 particles for. J. Membr. Sci. 2017, 524, 652–662. [Google Scholar] [CrossRef]
- Dai, Z.; Bai, L.; Hval, K.N.; Zhang, X.; Zhang, S.; Deng, L. Pebax®/TSIL blend thin film composite membranes for CO2 separation. Sci. China Chem. 2016, 59, 538–546. [Google Scholar] [CrossRef]
- Bernardo, P.; Jansen, J.C.; Bazzarelli, F.; Tasselli, F.; Fuoco, A.; Friess, K.; Izák, P.; Jarmarová, V.; Kačírková, M.; Clarizia, G. Gas transport properties of Pebax®/room temperature ionic liquid gel membranes. Sep. Purif. Technol. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Macchione, M.; Jansen, J.C.; de Luca, G.; Tocci, E.; Longeri, M.; Drioli, E. Experimental analysis and simulation of the gas transport in dense Hyflon® AD60X membranes: Influence of residual solvent. Polymer 2007, 48, 2619–2635. [Google Scholar] [CrossRef]
- Frisch, H.L. The time lag in diffusion. J. Phys. Chem. 1957, 61, 93–95. [Google Scholar] [CrossRef]
- Rutherford, S.W.; Do, D.D. Review of time lag permeation technique as a method for characterisation of porous media and membranes. Adsorption 1997, 3, 283–312. [Google Scholar] [CrossRef]
- Vopička, O.; Hynek, V.; Friess, K.; Šípek, M.; Sysel, P. Aparatura pro stanovení sorpce par v polymerech. Chem. List. 2009, 103, 310–314. [Google Scholar]
- Vopička, O.; Hynek, V.; Zgažar, M.; Friess, K.; Šípek, M. A new sorption model with a dynamic correction for the determination of diffusion coefficients. J. Membr. Sci. 2009, 330, 51–56. [Google Scholar] [CrossRef]
- Lanč, M.; Pilnáček, K.; Mason, C.R.; Budd, P.M.; Rogan, Y.; Malpass-Evans, R.; Carta, M.; Gándara, B.C.; McKeown, N.B.; Jansen, J.C.; et al. Gas sorption in polymers of intrinsic microporosity: The difference between solubility coefficients determined via time-lag and direct sorption experiments. J. Membr. Sci. 2019, 570–571, 522–536. [Google Scholar]
- Klepić, M.; Setničková, K.; Lanč, M.; Žák, M.; Izák, P.; Dendisová, M.; Fuoco, A.; Jansen, J.C.; Friess, K. Permeation and sorption properties of CO2-selective blend membranes based on polyvinyl alcohol (PVA) and 1-ethyl-3-methylimidazolium dicyanamide ([EMIM][DCA]) ionic liquid for effective CO2/H2 separation. J. Membr. Sci. 2020, 597, 117623. [Google Scholar] [CrossRef]
- Barrer, R.M. Diffusivities in glassy polymers for the dual mode sorption model. J. Membr. Sci. 1984, 18, 25–35. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Gas Sorption in Polymers, Molecular Sieves, and Mixed Matrix Membranes. J. Appl. Polym. Sci. 2007, 104, 4053–4059. [Google Scholar] [CrossRef]
- Barrer, R.M.; Barrie, J.A.; Slater, J. Sorption and diffusion in ethyl cellulose. Part I. History-dependence of sorption isotherms and permeation rates. J. Polym. Sci. 1957, 23, 315–329. [Google Scholar] [CrossRef]
- Barrer, R.M.; Barrie, J.A.; Slater, J. Sorption and diffusion in ethyl cellulose. Part III. Comparison between ethyl cellulose and rubber. J. Polym. Sci. 1958, 27, 177–197. [Google Scholar] [CrossRef]
- Fraga, S.C.; Monteleone, M.; Lanč, M.; Esposito, E.; Fuoco, A.; Giorno, L.; Pilnáček, K.; Friess, K.; Carta, M.; McKeown, N.B.; et al. A novel time lag method for the analysis of mixed gas diffusion in polymeric membranes by on-line mass spectrometry: Method development and validation. J. Membr. Sci. 2018, 561, 39–58. [Google Scholar] [CrossRef]
- Fuoco, A.; Monteleone, M.; Esposito, E.; Bruno, R.; Ferrando-Soria, J.; Pardo, E.; Armentano, D.; Jansen, C.J. Gas Transport in Mixed Matrix Membranes: Two Methods for Time Lag Determination. Computation 2020, 8, 28. [Google Scholar] [CrossRef]
- Peng, D.; Wang, S.; Tian, Z.; Wu, X.; Wu, Y.; Wu, H.; Xin, Q.; Chen, J.; Cao, X.; Jiang, Z. Facilitated transport membranes by incorporating graphene nanosheets with high zinc ion loading for enhanced CO2 separation. J. Membr. Sci. 2017, 522, 351–362. [Google Scholar] [CrossRef]
- Anthony, J.L.; Anderson, J.L.; Maginn, E.J.; Brennecke, J.F. Anion Effects on Gas Solubility in Ionic Liquids. J. Phys. Chem. B 2005, 109, 6366–6374. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Zeng, S.; Bai, L.; Gao, H.; Deng, J.; Yang, Q.; Zhang, S. Pebax-based composite membranes with high gas transport properties enhanced by ionic liquids for CO2 separation. RSC Adv. 2017, 7, 6422–6431. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Kumbharkar, S.C.; Kharul, U.K. Polymeric ionic liquids (PILs): Effect of anion variation on their CO2 sorption. J. Membr. Sci. 2012, 389, 305–315. [Google Scholar] [CrossRef]
- Vollas, A.; Chouliaras, T.; Deimede, V.; Ioannides, T. New Pyridinium Type Poly (Ionic Liquids) as Membranes for CO2 Separation. Polymers 2018, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, T.K.; Wiesenauer, E.F.; Nicodemus, G.D.; Gin, D.L.; Noble, R.D. Ideal CO2/Light Gas Separation Performance of Poly (vinylimidazolium) Membranes and Poly (vinylimidazolium)-Ionic Liquid Composite Films. Ind. Eng. Chem. Res. 2013, 52, 1023–1032. [Google Scholar] [CrossRef]
- Cowan, M.G.; Masuda, M.; Mcdanel, W.M.; Kohno, Y.; Gin, D.L.; Noble, R.D. Phosphonium-based poly (Ionic liquid) membranes: The effect of cation alkyl chain length on light gas separation properties and Ionic conductivity. J. Membr. Sci. 2016, 498, 408–413. [Google Scholar] [CrossRef]
- Li, P.; Paul, D.R.; Chung, T. High performance membranes based on ionic liquidpolymers for CO2 separation from the flue gas. Green Chem. 2012, 14, 1052–1063. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-Tuned Intrinsically Ultramicroporous Polymers Redefine the Permeability/Selectivity Upper Bounds of Membrane-Based Air and Hydrogen Separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef]
- Ferguson, L.; Scovazzo, P. Solubility, diffusivity, and permeability of gases in phosphonium-based room temperature ionic liquids: Data and correlations. Ind. Eng. Chem. Res. 2007, 46, 1369–1374. [Google Scholar] [CrossRef]
- Teplyakov, V.; Meares, P. Correlation aspects of the selective gas permeabilities of polymeric materials and membranes. Gas Sep. Purif. 1990, 4, 66–74. [Google Scholar] [CrossRef]
- Fuoco, A.; Rizzuto, C.; Tocci, E.; Monteleone, M.; Esposito, E.; Budd, P.M.; Carta, M.; Comesaña-Gándara, B.; McKeown, N.B.; Jansen, J.C. The origin of size-selective gas transport through polymers of intrinsic microporosity. J. Mater. Chem. A 2019, 7, 20121–20126. [Google Scholar] [CrossRef]
- Freeman, B.; Yampolskii, Y.; Pinnau, I. (Eds.) Materials Science of Membranes for Gas. and Vapor Separation; John Wiley & Sons: Hoboken, NY, USA, 2006. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzei, I.R.; Nikolaeva, D.; Fuoco, A.; Loïs, S.; Fantini, S.; Monteleone, M.; Esposito, E.; Ashtiani, S.J.; Lanč, M.; Vopička, O.; et al. Poly[3-ethyl-1-vinyl-imidazolium] diethyl phosphate/Pebax® 1657 Composite Membranes and Their Gas Separation Performance. Membranes 2020, 10, 224. https://doi.org/10.3390/membranes10090224
Mazzei IR, Nikolaeva D, Fuoco A, Loïs S, Fantini S, Monteleone M, Esposito E, Ashtiani SJ, Lanč M, Vopička O, et al. Poly[3-ethyl-1-vinyl-imidazolium] diethyl phosphate/Pebax® 1657 Composite Membranes and Their Gas Separation Performance. Membranes. 2020; 10(9):224. https://doi.org/10.3390/membranes10090224
Chicago/Turabian StyleMazzei, Irene R., Daria Nikolaeva, Alessio Fuoco, Sandrine Loïs, Sébastien Fantini, Marcello Monteleone, Elisa Esposito, Saeed Jamali Ashtiani, Marek Lanč, Ondřej Vopička, and et al. 2020. "Poly[3-ethyl-1-vinyl-imidazolium] diethyl phosphate/Pebax® 1657 Composite Membranes and Their Gas Separation Performance" Membranes 10, no. 9: 224. https://doi.org/10.3390/membranes10090224
APA StyleMazzei, I. R., Nikolaeva, D., Fuoco, A., Loïs, S., Fantini, S., Monteleone, M., Esposito, E., Ashtiani, S. J., Lanč, M., Vopička, O., Friess, K., Vankelecom, I. F. J., & Jansen, J. C. (2020). Poly[3-ethyl-1-vinyl-imidazolium] diethyl phosphate/Pebax® 1657 Composite Membranes and Their Gas Separation Performance. Membranes, 10(9), 224. https://doi.org/10.3390/membranes10090224











