The Role of Lipid Environment in Ganglioside GM1-Induced Amyloid β Aggregation
Abstract
1. Introduction
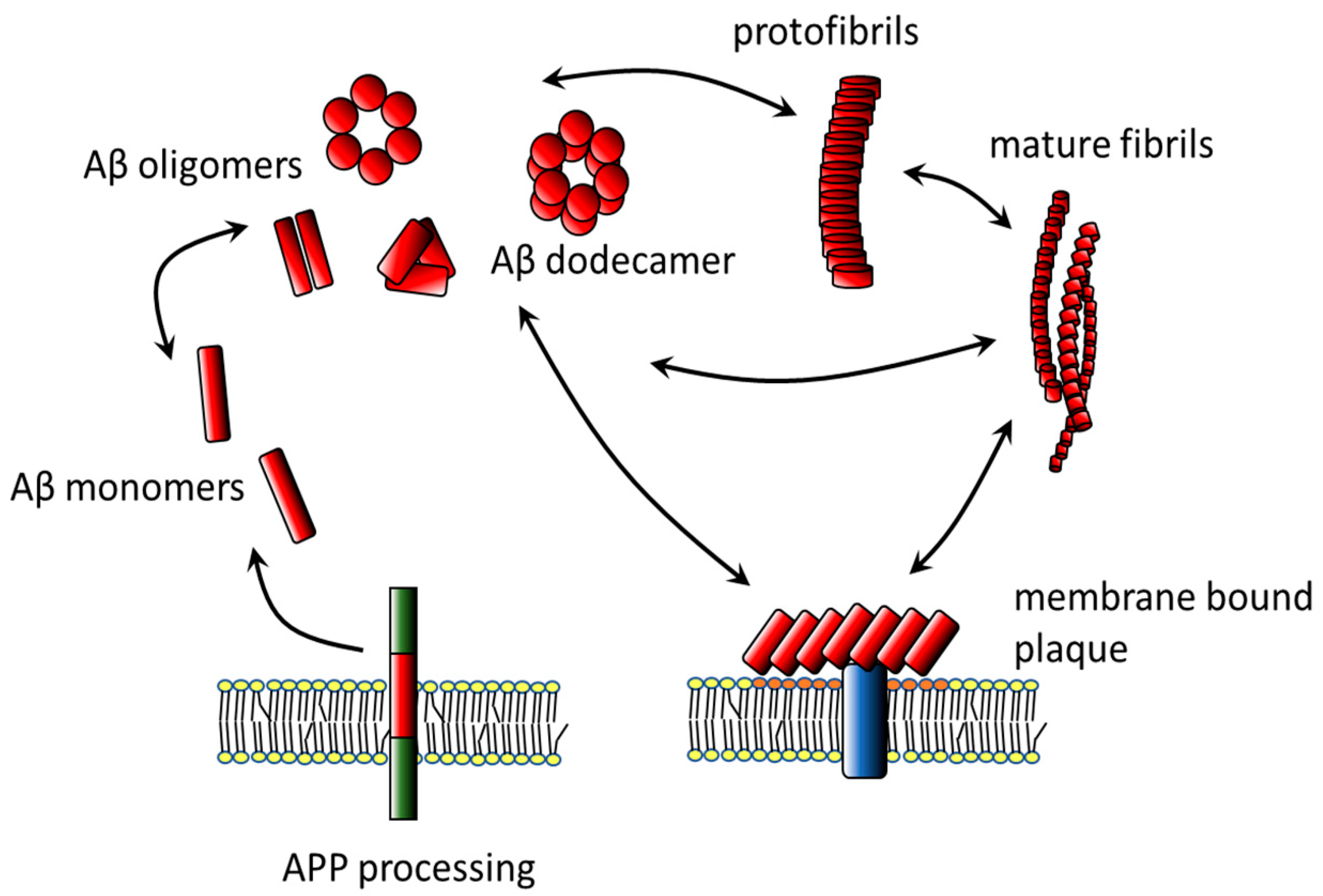
2. Amyloid β
2.1. Aggregates
2.2. Secondary and Tertiary Structure
2.3. Variability
2.4. Interactions of Aβ with the Membrane
2.4.1. Membrane Binding
2.4.2. Aβ–Lipid Interactions
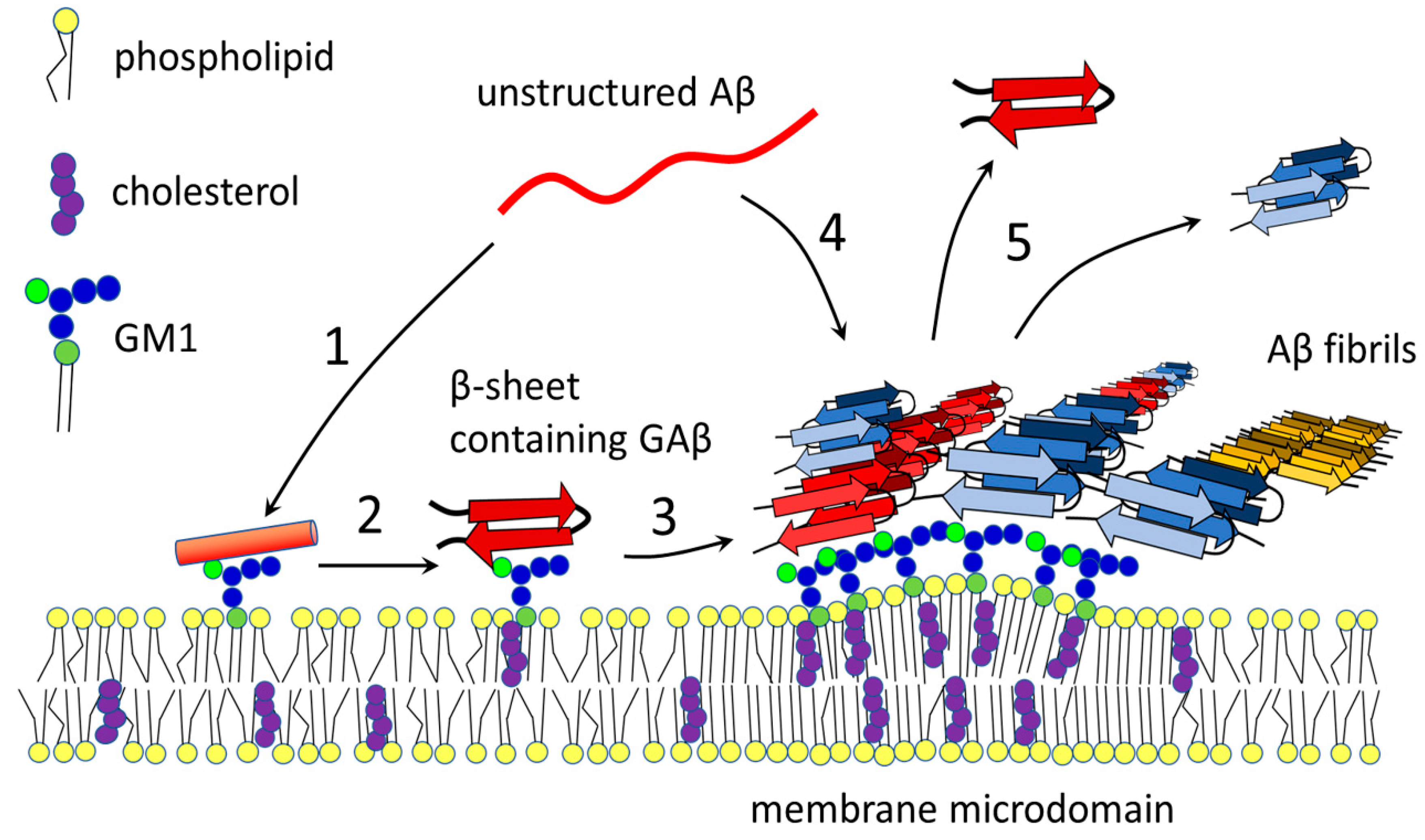
2.4.3. Aβ and Membrane Microdomains
3. Gangliosides and Aβ
3.1. Gangliosides
3.2. GM1
3.3. The Role of GM1 in Seeding and Accumulation of Aβ
3.4. The Effect of Other Sphingolipids and Cholesterol
3.5. Clustering of GM1
3.6. Neuroprotective Effect of GM1 in Neurodegeneration
4. Conclusions
Funding
Conflicts of Interest
References
- Apostolova, L.G. Alzheimer Disease. Continuum (Minneap. Minn.) 2016, 22, 419–434. [Google Scholar] [CrossRef]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Chen, X.Q.; Mobley, W.C. Exploring the Pathogenesis of Alzheimer Disease in Basal Forebrain Cholinergic Neurons: Converging Insights from Alternative Hypotheses. Front. Neurosci. 2019, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Adolfsson, O.; Allaman, I.; Buccarello, A.L.; Magistretti, P.J.; Pfeifer, A.; Muhs, A.; Lashuel, H.A. Abeta42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete Abeta42 species. J. Biol. Chem. 2011, 286, 8585–8596. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Palmqvist, S.; Scholl, M.; Strandberg, O.; Mattsson, N.; Stomrud, E.; Zetterberg, H.; Blennow, K.; Landau, S.; Jagust, W.; Hansson, O. Earliest accumulation of beta-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 2017, 8, 1214. [Google Scholar] [CrossRef]
- Wong, P.T.; Schauerte, J.A.; Wisser, K.C.; Ding, H.; Lee, E.L.; Steel, D.G.; Gafni, A. Amyloid-beta membrane binding and permeabilization are distinct processes influenced separately by membrane charge and fluidity. J. Mol. Biol. 2009, 386, 81–96. [Google Scholar] [CrossRef]
- Evangelisti, E.; Cascella, R.; Becatti, M.; Marrazza, G.; Dobson, C.M.; Chiti, F.; Stefani, M.; Cecchi, C. Binding affinity of amyloid oligomers to cellular membranes is a generic indicator of cellular dysfunction in protein misfolding diseases. Sci. Rep. 2016, 6, 32721. [Google Scholar] [CrossRef]
- Wakabayashi, M.; Matsuzaki, K. Formation of amyloids by Abeta-(1-42) on NGF-differentiated PC12 cells: Roles of gangliosides and cholesterol. J. Mol. Biol. 2007, 371, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Amar, F.; Sherman, M.A.; Rush, T.; Larson, M.; Boyle, G.; Chang, L.; Gotz, J.; Buisson, A.; Lesne, S.E. The amyloid-beta oligomer A beta*56 induces specific alterations in neuronal signaling that lead to tau phosphorylation and aggregation. Sci. Signal. 2017, 10, eaal2021. [Google Scholar] [CrossRef] [PubMed]
- Vahed, M.; Neya, S.; Matsuzaki, K.; Hoshino, T. Analysis of Physicochemical Interaction of Abeta40 with a GM1 Ganglioside-Containing Lipid Membrane. J. Phys. Chem. B 2018, 122, 3771–3781. [Google Scholar] [CrossRef] [PubMed]
- Micelli, S.; Meleleo, D.; Picciarelli, V.; Gallucci, E. Effect of sterols on beta-amyloid peptide (AbetaP 1-40) channel formation and their properties in planar lipid membranes. Biophys. J. 2004, 86, 2231–2237. [Google Scholar] [CrossRef][Green Version]
- Demuro, A.; Mina, E.; Kayed, R.; Milton, S.C.; Parker, I.; Glabe, C.G. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 2005, 280, 17294–17300. [Google Scholar] [CrossRef]
- Fantini, J.; Yahi, N. Molecular insights into amyloid regulation by membrane cholesterol and sphingolipids: Common mechanisms in neurodegenerative diseases. Expert Rev. Mol. Med. 2010, 12, e27. [Google Scholar] [CrossRef]
- Di Scala, C.; Chahinian, H.; Yahi, N.; Garmy, N.; Fantini, J. Interaction of Alzheimer’s beta-amyloid peptides with cholesterol: Mechanistic insights into amyloid pore formation. Biochemistry 2014, 53, 4489–4502. [Google Scholar] [CrossRef]
- Sepulveda, F.J.; Fierro, H.; Fernandez, E.; Castillo, C.; Peoples, R.W.; Opazo, C.; Aguayo, L.G. Nature of the neurotoxic membrane actions of amyloid-beta on hippocampal neurons in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 472–481. [Google Scholar] [CrossRef]
- Revett, T.J.; Baker, G.B.; Jhamandas, J.; Kar, S. Glutamate system, amyloid ss peptides and tau protein: Functional interrelationships and relevance to Alzheimer disease pathology. J. Psychiatry Neurosci. 2013, 38, 6–23. [Google Scholar] [CrossRef]
- Kocahan, S.; Dogan, Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-methyl-D-aspartate Receptors, Tau Protein and Other Risk Factors. Clin. Psychopharmacol. Neurosci. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Baker-Nigh, A.; Vahedi, S.; Davis, E.G.; Weintraub, S.; Bigio, E.H.; Klein, W.L.; Geula, C. Neuronal amyloid-beta accumulation within cholinergic basal forebrain in ageing and Alzheimer’s disease. Brain 2015, 138, 1722–1737. [Google Scholar] [CrossRef]
- Richter, N.; Beckers, N.; Onur, O.A.; Dietlein, M.; Tittgemeyer, M.; Kracht, L.; Neumaier, B.; Fink, G.R.; Kukolja, J. Effect of cholinergic treatment depends on cholinergic integrity in early Alzheimer’s disease. Brain 2018, 141, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Sultzer, D.L. Cognitive ageing and Alzheimer’s disease: The cholinergic system redux. Brain 2018, 141, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Machova, E.; Rudajev, V.; Smyckova, H.; Koivisto, H.; Tanila, H.; Dolezal, V. Functional cholinergic damage develops with amyloid accumulation in young adult APPswe/PS1dE9 transgenic mice. Neurobiol. Dis. 2010, 38, 27–35. [Google Scholar] [CrossRef]
- Janickova, H.; Rudajev, V.; Zimcik, P.; Jakubik, J.; Tanila, H.; El-Fakahany, E.E.; Dolezal, V. Uncoupling of M1 muscarinic receptor/G-protein interaction by amyloid beta(1-42). Neuropharmacology 2013, 67, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Janickova, H.; Rudajev, V.; Dolejsi, E.; Koivisto, H.; Jakubik, J.; Tanila, H.; El-Fakahany, E.E.; Dolezal, V. Lipid-Based Diets Improve Muscarinic Neurotransmission in the Hippocampus of Transgenic APPswe/PS1dE9 Mice. Curr. Alzheimer Res. 2015, 12, 923–931. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Viola, K.L.; Klein, W.L. Amyloid beta oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015, 129, 183–206. [Google Scholar] [CrossRef]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Molander-Melin, M.; Blennow, K.; Bogdanovic, N.; Dellheden, B.; Mansson, J.E.; Fredman, P. Structural membrane alterations in Alzheimer brains found to be associated with regional disease development; increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane domains. J. Neurochem. 2005, 92, 171–182. [Google Scholar] [CrossRef]
- Pernber, Z.; Blennow, K.; Bogdanovic, N.; Mansson, J.E.; Blomqvist, M. Altered distribution of the gangliosides GM1 and GM2 in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2012, 33, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Arbor, S.C.; Lafontaine, M.; Cumbay, M. Amyloid-beta Alzheimer targets—Protein processing, lipid rafts, and amyloid-beta pores. Yale J. Biol. Med. 2016, 89, 5–21. [Google Scholar]
- Owen, M.C.; Kulig, W.; Poojari, C.; Rog, T.; Strodel, B. Physiologically-relevant levels of sphingomyelin, but not GM1, induces a beta-sheet-rich structure in the amyloid-beta(1-42) monomer. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K. GM1 ganglioside and Alzheimer’s disease. Glycoconj. J. 2015, 32, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Yuyama, K.; Yanagisawa, K. Sphingomyelin accumulation provides a favorable milieu for GM1 ganglioside-induced assembly of amyloid beta-protein. Neurosci. Lett. 2010, 481, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Kakio, A.; Nishimoto, S.; Yanagisawa, K.; Kozutsumi, Y.; Matsuzaki, K. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid beta-protein, an endogenous seed for Alzheimer amyloid. J. Biol. Chem. 2001, 276, 24985–24990. [Google Scholar] [CrossRef] [PubMed]
- Kamenetz, F.; Tomita, T.; Hsieh, H.; Seabrook, G.; Borchelt, D.; Iwatsubo, T.; Sisodia, S.; Malinow, R. APP processing and synaptic function. Neuron 2003, 37, 925–937. [Google Scholar] [CrossRef]
- Luhrs, T.; Ritter, C.; Adrian, M.; Riek-Loher, D.; Bohrmann, B.; Dobeli, H.; Schubert, D.; Riek, R. 3D structure of Alzheimer’s amyloid-beta(1-42) fibrils. Proc. Natl. Acad. Sci. USA 2005, 102, 17342–17347. [Google Scholar] [CrossRef]
- Ahmed, M.; Davis, J.; Aucoin, D.; Sato, T.; Ahuja, S.; Aimoto, S.; Elliott, J.I.; Van Nostrand, W.E.; Smith, S.O. Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat. Struct. Mol. Biol. 2010, 17, 561–567. [Google Scholar] [CrossRef]
- Gouras, G.K. Aging, Metabolism, Synaptic Activity, and Abeta in Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 185. [Google Scholar] [CrossRef]
- Nasica-Labouze, J.; Nguyen, P.H.; Sterpone, F.; Berthoumieu, O.; Buchete, N.V.; Cote, S.; De Simone, A.; Doig, A.J.; Faller, P.; Garcia, A.; et al. Amyloid beta Protein and Alzheimer’s Disease: When Computer Simulations Complement Experimental Studies. Chem. Rev. 2015, 115, 3518–3563. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Vats, A.; Taneja, V. Toxic species in amyloid disorders: Oligomers or mature fibrils. Ann. Indian Acad. Neurol. 2015, 18, 138–145. [Google Scholar] [PubMed]
- Tran, J.; Chang, D.; Hsu, F.; Wang, H.; Guo, Z. Cross-seeding between Abeta40 and Abeta42 in Alzheimer’s disease. FEBS Lett. 2017, 591, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Tran, J.; Wang, H.; Park, G.; Hsu, F.; Guo, Z. Abeta42 fibril formation from predominantly oligomeric samples suggests a link between oligomer heterogeneity and fibril polymorphism. R. Soc. Open Sci. 2019, 6, 190179. [Google Scholar] [CrossRef]
- Ono, K.; Tsuji, M. Protofibrils of Amyloid-beta are Important Targets of a Disease-Modifying Approach for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 952. [Google Scholar] [CrossRef]
- Roychaudhuri, R.; Yang, M.; Hoshi, M.M.; Teplow, D.B. Amyloid beta-protein assembly and Alzheimer disease. J. Biol. Chem. 2009, 284, 4749–4753. [Google Scholar] [CrossRef]
- Williams, T.L.; Johnson, B.R.; Urbanc, B.; Jenkins, A.T.; Connell, S.D.; Serpell, L.C. Abeta42 oligomers, but not fibrils, simultaneously bind to and cause damage to ganglioside-containing lipid membranes. Biochem. J. 2011, 439, 67–77. [Google Scholar] [CrossRef]
- Bobo, C.; Chaignepain, S.; Henry, S.; Vignaud, H.; Ameadan, A.; Marchal, C.; Prado, E.; Doutch, J.; Schmitter, J.M.; Nardin, C.; et al. Synthetic toxic Abeta1-42 oligomers can assemble in different morphologies. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1168–1176. [Google Scholar] [CrossRef]
- Xue, W.F.; Hellewell, A.L.; Gosal, W.S.; Homans, S.W.; Hewitt, E.W.; Radford, S.E. Fibril fragmentation enhances amyloid cytotoxicity. J. Biol. Chem. 2009, 284, 34272–34282. [Google Scholar] [CrossRef]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from A beta(1-42) are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef]
- Lesne, S.; Koh, M.T.; Kotilinek, L.; Kayed, R.; Glabe, C.G.; Yang, A.; Gallagher, M.; Ashe, K.H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature 2006, 440, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Matsubara, E.; Maeda, S.; Minagawa, H.; Takashima, A.; Maruyama, W.; Michikawa, M.; Yanagisawa, K. A ganglioside-induced toxic soluble Abeta assembly. Its enhanced formation from Abeta bearing the Arctic mutation. J. Biol. Chem. 2007, 282, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Ewald, M.; Henry, S.; Lambert, E.; Feuillie, C.; Bobo, C.; Cullin, C.; Lecomte, S.; Molinari, M. High speed atomic force microscopy to investigate the interactions between toxic Abeta1-42 peptides and model membranes in real time: Impact of the membrane composition. Nanoscale 2019, 11, 7229–7238. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.L.; Dupuis, N.F.; Lazo, N.D.; Wyttenbach, T.; Condron, M.M.; Bitan, G.; Teplow, D.B.; Shea, J.E.; Ruotolo, B.T.; Robinson, C.V.; et al. Amyloid-beta protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat. Chem. 2009, 1, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Lesne, S.E.; Sherman, M.A.; Grant, M.; Kuskowski, M.; Schneider, J.A.; Bennett, D.A.; Ashe, K.H. Brain amyloid-beta oligomers in ageing and Alzheimer’s disease. Brain 2013, 136, 1383–1398. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.K.; Li, H.; Nowick, J.S. X-ray crystallographic structures of trimers and higher-order oligomeric assemblies of a peptide derived from Abeta(17-36). J. Am. Chem. Soc. 2014, 136, 5595–5598. [Google Scholar] [CrossRef]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid-beta Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef]
- Esparza, T.J.; Zhao, H.; Cirrito, J.R.; Cairns, N.J.; Bateman, R.J.; Holtzman, D.M.; Brody, D.L. Amyloid-beta oligomerization in Alzheimer dementia versus high-pathology controls. Ann. Neurol. 2013, 73, 104–119. [Google Scholar] [CrossRef]
- Hong, S.; Ostaszewski, B.L.; Yang, T.; O’malley, T.T.; Jin, M.; Yanagisawa, K.; Li, S.; Bartels, T.; Selkoe, D.J. Soluble Abeta oligomers are rapidly sequestered from brain ISF in vivo and bind GM1 ganglioside on cellular membranes. Neuron 2014, 82, 308–319. [Google Scholar] [CrossRef]
- Jeong, J.S.; Ansaloni, A.; Mezzenga, R.; Lashuel, H.A.; Dietler, G. Novel mechanistic insight into the molecular basis of amyloid polymorphism and secondary nucleation during amyloid formation. J. Mol. Biol. 2013, 425, 1765–1781. [Google Scholar] [CrossRef]
- Tycko, R. Amyloid polymorphism: Structural basis and neurobiological relevance. Neuron 2015, 86, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Yau, W.M.; Lu, J.X.; Collinge, J.; Tycko, R. Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature 2017, 541, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Petkova, A.T.; Leapman, R.D.; Guo, Z.; Yau, W.M.; Mattson, M.P.; Tycko, R. Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science 2005, 307, 262–265. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, Z.; Zhao, W.; Yang, J. Interactions between amyloid beta peptide and lipid membranes. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.; Mahler, J.; Beschorner, N.; Kaeser, S.A.; Hasler, L.M.; Baumann, F.; Nystrom, S.; Portelius, E.; Blennow, K.; Lashley, T.; et al. Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 13018–13023. [Google Scholar] [CrossRef]
- Larini, L.; Shea, J.E. Role of beta-Hairpin Formation in Aggregation: The Self-Assembly of the Amyloid-beta(25-35) Peptide. Biophys. J. 2012, 103, 576–586. [Google Scholar] [CrossRef]
- Nag, S.; Sarkar, B.; Chandrakesan, M.; Abhyanakar, R.; Bhowmik, D.; Kombrabail, M.; Dandekar, S.; Lerner, E.; Haas, E.; Maiti, S. A folding transition underlies the emergence of membrane affinity in amyloid-beta. Phys. Chem. Chem. Phys. 2013, 15, 19129–19133. [Google Scholar] [CrossRef]
- Wei, G.; Shea, J.E. Effects of solvent on the structure of the Alzheimer amyloid-beta(25-35) peptide. Biophys. J. 2006, 91, 1638–1647. [Google Scholar] [CrossRef]
- Choo, L.P.; Wetzel, D.L.; Halliday, W.C.; Jackson, M.; Levine, S.M.; Mantsch, H.H. In situ characterization of beta-amyloid in Alzheimer’s diseased tissue by synchrotron Fourier transform infrared microspectroscopy. Biophys. J. 1996, 71, 1672–1679. [Google Scholar] [CrossRef]
- Shao, H.; Jao, S.; Ma, K.; Zagorski, M.G. Solution structures of micelle-bound amyloid beta-(1-40) and beta-(1-42) peptides of Alzheimer’s disease. J. Mol. Biol. 1999, 285, 755–773. [Google Scholar] [CrossRef]
- Vivekanandan, S.; Brender, J.R.; Lee, S.Y.; Ramamoorthy, A. A partially folded structure of amyloid-beta(1-40) in an aqueous environment. Biochem. Biophys. Res. Commun. 2011, 411, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Maclaughlin, C.M.; Chandrakesan, M.; Ramesh, P.; Venkatramani, R.; Walker, G.C.; Maiti, S. pH changes the aggregation propensity of amyloid-beta without altering the monomer conformation. Phys. Chem. Chem. Phys. 2014, 16, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, C.; Klimov, D.K. Alzheimer’s Abeta10-40 peptide binds and penetrates DMPC bilayer: An isobaric-isothermal replica exchange molecular dynamics study. J. Phys. Chem. B 2014, 118, 2638–2648. [Google Scholar] [CrossRef]
- Tycko, R. Molecular Structure of Aggregated Amyloid-beta: Insights from Solid-State Nuclear Magnetic Resonance. Cold Spring Harb. Perspect. Med. 2016, 6, a024083. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Arce, F.T.; Ramachandran, S.; Capone, R.; Lal, R.; Nussinov, R. beta-Barrel topology of Alzheimer’s beta-amyloid ion channels. J. Mol. Biol. 2010, 404, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Sachse, C.; Richter, W.; Xu, C.; Fandrich, M.; Grigorieff, N. Comparison of Alzheimer Abeta(1-40) and Abeta(1-42) amyloid fibrils reveals similar protofilament structures. Proc. Natl. Acad. Sci. USA 2009, 106, 19813–19818. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, B.; Mcelheny, D.; Parthasarathy, S.; Long, F.; Hoshi, M.; Nussinov, R.; Ishii, Y. Abeta(1-42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 2015, 22, 499–505. [Google Scholar] [CrossRef]
- Barz, B.; Olubiyi, O.O.; Strodel, B. Early amyloid beta-protein aggregation precedes conformational change. Chem. Commun. (Camb.) 2014, 50, 5373–5375. [Google Scholar] [CrossRef]
- Vignaud, H.; Bobo, C.; Lascu, I.; Sorgjerd, K.M.; Zako, T.; Maeda, M.; Salin, B.; Lecomte, S.; Cullin, C. A structure-toxicity study of Ass42 reveals a new anti-parallel aggregation pathway. PLoS ONE 2013, 8, e80262. [Google Scholar] [CrossRef]
- Bonhommeau, S.; Talaga, D.; Hunel, J.; Cullin, C.; Lecomte, S. Tip-Enhanced Raman Spectroscopy to Distinguish Toxic Oligomers from Abeta1-42 Fibrils at the Nanometer Scale. Angew. Chem. Int. Ed. Engl. 2017, 56, 1771–1774. [Google Scholar] [CrossRef]
- Davis, C.H.; Berkowitz, M.L. A molecular dynamics study of the early stages of amyloid-beta(1-42) oligomerization: The role of lipid membranes. Proteins 2010, 78, 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Perez, E.J.; Sepulveda, F.J.; Peoples, R.; Aguayo, L.G. Role of membrane GM1 on early neuronal membrane actions of Abeta during onset of Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Simakova, O.; Arispe, N.J. The cell-selective neurotoxicity of the Alzheimer’s Abeta peptide is determined by surface phosphatidylserine and cytosolic ATP levels. Membrane binding is required for Abeta toxicity. J. Neurosci. 2007, 27, 13719–13729. [Google Scholar] [CrossRef] [PubMed]
- Mclaurin, J.; Chakrabartty, A. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J. Biol. Chem. 1996, 271, 26482–26489. [Google Scholar] [CrossRef] [PubMed]
- Korshavn, K.J.; Bhunia, A.; Lim, M.H.; Ramamoorthy, A. Amyloid-beta adopts a conserved, partially folded structure upon binding to zwitterionic lipid bilayers prior to amyloid formation. Chem. Commun. (Camb.) 2016, 52, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Yates, E.A.; Owens, S.L.; Lynch, M.F.; Cucco, E.M.; Umbaugh, C.S.; Legleiter, J. Specific domains of Abeta facilitate aggregation on and association with lipid bilayers. J. Mol. Biol. 2013, 425, 1915–1933. [Google Scholar] [CrossRef]
- Sasahara, K.; Morigaki, K.; Shinya, K. Effects of membrane interaction and aggregation of amyloid beta-peptide on lipid mobility and membrane domain structure. Phys. Chem. Chem. Phys. 2013, 15, 8929–8939. [Google Scholar] [CrossRef]
- Ji, S.R.; Wu, Y.; Sui, S.F. Cholesterol is an important factor affecting the membrane insertion of beta-amyloid peptide (A beta 1-40), which may potentially inhibit the fibril formation. J. Biol. Chem. 2002, 277, 6273–6279. [Google Scholar] [CrossRef]
- Wakabayashi, M.; Okada, T.; Kozutsumi, Y.; Matsuzaki, K. GM1 ganglioside-mediated accumulation of amyloid beta-protein on cell membranes. Biochem. Biophys. Res. Commun. 2005, 328, 1019–1023. [Google Scholar] [CrossRef]
- Nicholson, A.M.; Ferreira, A. Increased membrane cholesterol might render mature hippocampal neurons more susceptible to beta-amyloid-induced calpain activation and tau toxicity. J. Neurosci. 2009, 29, 4640–4651. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Odaka, A.; Suzuki, N.; Ihara, Y. GM1 ganglioside-bound amyloid beta-protein (A beta): A possible form of preamyloid in Alzheimer’s disease. Nat. Med. 1995, 1, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.; Sachl, R.; Aydogan, G.; Mikhalyov, I.; Vacha, R.; Hof, M. GM1 Ganglioside Inhibits beta-Amyloid Oligomerization Induced by Sphingomyelin. Angew. Chem. Int. Ed. Engl. 2016, 55, 9411–9415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, J. Free cholesterol induces higher beta-sheet content in Abeta peptide oligomers by aromatic interaction with Phe19. PLoS ONE 2012, 7, e46245. [Google Scholar]
- Van Weering, J.R.T.; Scheper, W. Endolysosome and Autolysosome Dysfunction in Alzheimer’s Disease: Where Intracellular and Extracellular Meet. CNS Drugs 2019, 33, 639–648. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, J. Cholesterol promotes the interaction of Alzheimer beta-amyloid monomer with lipid bilayer. J. Mol. Biol. 2012, 421, 561–571. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Kato, K.; Yanagisawa, K. Abeta polymerization through interaction with membrane gangliosides. Biochim. Biophys. Acta 2010, 1801, 868–877. [Google Scholar] [CrossRef]
- Mori, K.; Mahmood, M.I.; Neya, S.; Matsuzaki, K.; Hoshino, T. Formation of GM1 ganglioside clusters on the lipid membrane containing sphingomyeline and cholesterol. J. Phys. Chem. B 2012, 116, 5111–5121. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Brown, D.A.; London, E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: Physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 1997, 36, 10944–10953. [Google Scholar] [CrossRef]
- Anderson, R.G.; Jacobson, K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 2002, 296, 1821–1825. [Google Scholar] [CrossRef]
- Pike, L.J. Lipid rafts: Heterogeneity on the high seas. Biochem. J. 2004, 378, 281–292. [Google Scholar] [CrossRef]
- Garner, A.E.; Smith, D.A.; Hooper, N.M. Visualization of detergent solubilization of membranes: Implications for the isolation of rafts. Biophys. J. 2008, 94, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Aureli, M.; Mauri, L.; Ciampa, M.G.; Prinetti, A. Membrane lipid domains in the nervous system. Front. Biosci. (Landmark Ed.) 2015, 20, 280–302. [Google Scholar] [CrossRef] [PubMed]
- Haughey, N.J.; Bandaru, V.V.; Bae, M.; Mattson, M.P. Roles for dysfunctional sphingolipid metabolism in Alzheimer’s disease neuropathogenesis. Biochim. Biophys. Acta 2010, 1801, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, M.P.; Scherer, P.E.; Tang, Z.; Sargiacomo, M. Caveolae, Caveolin and Caveolin-Rich Membrane Domains: A Signalling Hypothesis. Trends Cell Biol. 1994, 4, 231–235. [Google Scholar] [CrossRef]
- Eckert, G.P.; Igbavboa, U.; Muller, W.E.; Wood, W.G. Lipid rafts of purified mouse brain synaptosomes prepared with or without detergent reveal different lipid and protein domains. Brain Res. 2003, 962, 144–150. [Google Scholar] [CrossRef]
- Moravcova, Z.; Rudajev, V.; Stohr, J.; Novotny, J.; Cerny, J.; Parenti, M.; Milligan, G.; Svoboda, P. Long-term agonist stimulation of IP prostanoid receptor depletes the cognate G(s)alpha protein in membrane domains but does not change the receptor level. Biochim. Biophys. Acta 2004, 1691, 51–65. [Google Scholar] [CrossRef][Green Version]
- Matousek, P.; Novotny, J.; Rudajev, V.; Svoboda, P. Prolonged agonist stimulation does not alter the protein composition of membrane domains in spite of dramatic changes induced in a specific signaling cascade. Cell Biochem. Biophys. 2005, 42, 21–40. [Google Scholar] [CrossRef]
- Rudajev, V.; Novotny, J.; Hejnova, L.; Milligan, G.; Svoboda, P. Dominant portion of thyrotropin-releasing hormone receptor is excluded from lipid domains. Detergent-resistant and detergent-sensitive pools of TRH receptor and Gqalpha/G11alpha protein. J. Biochem. 2005, 138, 111–125. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chang, A.; Gintzler, A.R. Subcellular localization of mu-opioid receptor G(s) signaling. J. Pharmacol. Exp. Ther. 2010, 333, 193–200. [Google Scholar] [CrossRef]
- Lauren, J.; Gimbel, D.A.; Nygaard, H.B.; Gilbert, J.W.; Strittmatter, S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 2009, 457, 1128–U1184. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, J.V.; Hooper, N.M. Lipid Rafts: Linking Alzheimer’s Amyloid-beta Production, Aggregation, and Toxicity at Neuronal Membranes. Int. J. Alzheimers Dis. 2010, 2011, 603052. [Google Scholar] [CrossRef] [PubMed]
- Staneva, G.; Puff, N.; Stanimirov, S.; Tochev, T.; Angelova, M.I.; Seigneuret, M. The Alzheimer’s disease amyloid-beta peptide affects the size-dynamics of raft-mimicking Lo domains in GM1-containing lipid bilayers. Soft Matter 2018, 14, 9609–9618. [Google Scholar] [CrossRef] [PubMed]
- Azouz, M.; Cullin, C.; Lecomte, S.; Lafleur, M. Membrane domain modulation of Abeta1-42 oligomer interactions with supported lipid bilayers: An atomic force microscopy investigation. Nanoscale 2019, 11, 20857–20867. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, M.S.; Lin, Y.; Kinoshita, M.; Kanemura, S.; Itoh, D.; Sugiki, T.; Okumura, M.; Ramamoorthy, A.; Lee, Y.H. Impact of membrane curvature on amyloid aggregation. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1741–1764. [Google Scholar] [CrossRef] [PubMed]
- Drolle, E.; Negoda, A.; Hammond, K.; Pavlov, E.; Leonenko, Z. Changes in lipid membranes may trigger amyloid toxicity in Alzheimer’s disease. PLoS ONE 2017, 12, e0182194. [Google Scholar] [CrossRef]
- Fabelo, N.; Martin, V.; Marin, R.; Santpere, G.; Aso, E.; Ferrer, I.; Diaz, M. Evidence for premature lipid raft aging in APP/PS1 double-transgenic mice, a model of familial Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012, 71, 868–881. [Google Scholar] [CrossRef]
- Fabelo, N.; Martin, V.; Marin, R.; Moreno, D.; Ferrer, I.; Diaz, M. Altered lipid composition in cortical lipid rafts occurs at early stages of sporadic Alzheimer’s disease and facilitates APP/BACE1 interactions. Neurobiol. Aging 2014, 35, 1801–1812. [Google Scholar] [CrossRef]
- Malchiodi-Albedi, F.; Contrusciere, V.; Raggi, C.; Fecchi, K.; Rainaldi, G.; Paradisi, S.; Matteucci, A.; Santini, M.T.; Sargiacomo, M.; Frank, C.; et al. Lipid raft disruption protects mature neurons against amyloid oligomer toxicity. Biochim. Biophys. Acta 2010, 1802, 406–415. [Google Scholar] [CrossRef]
- Santos, G.; Diaz, M.; Torres, N.V. Lipid Raft Size and Lipid Mobility in Non-raft Domains Increase during Aging and Are Exacerbated in APP/PS1 Mice Model of Alzheimer’s Disease. Predictions from an Agent-Based Mathematical Model. Front. Physiol. 2016, 7, 90. [Google Scholar] [CrossRef][Green Version]
- Chan, R.B.; Oliveira, T.G.; Cortes, E.P.; Honig, L.S.; Duff, K.E.; Small, S.A.; Wenk, M.R.; Shui, G.; Di Paolo, G. Comparative lipidomic analysis of mouse and human brain with Alzheimer disease. J. Biol. Chem. 2012, 287, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Nakatani, Y.; Yanagisawa, M. The role of glycosphingolipid metabolism in the developing brain. J. Lipid Res. 2009, 50, S440–S445. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T.; Mcdonald, M.P.; Yu, R.K. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease—A review. J. Lipid Res. 2008, 49, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Yagi-Utsumi, M.; Kato, K. Structural and dynamic views of GM1 ganglioside. Glycoconj. J. 2015, 32, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Geisler, F.H.; Schneider, J.S.; Li, P.A.; Fiumelli, H.; Sipione, S. Gangliosides: Treatment Avenues in Neurodegenerative Disease. Front. Neurol. 2019, 10, 859. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Grey, C.; Sparr, E. Self-Assembly in Ganglioside-Phospholipid Systems: The Co-Existence of Vesicles, Micelles, and Discs. Int. J. Mol. Sci. 2019, 21, 56. [Google Scholar] [CrossRef]
- Sonnino, S.; Mauri, L.; Chigorno, V.; Prinetti, A. Gangliosides as components of lipid membrane domains. Glycobiology 2007, 17, 1R–13R. [Google Scholar] [CrossRef]
- Ohmi, Y.; Tajima, O.; Ohkawa, Y.; Yamauchi, Y.; Sugiura, Y.; Furukawa, K.; Furukawa, K. Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: Elucidation by a series of ganglioside-deficient mutant mice. J. Neurochem. 2011, 116, 926–935. [Google Scholar] [CrossRef]
- Herzer, S.; Meldner, S.; Rehder, K.; Grone, H.J.; Nordstrom, V. Lipid microdomain modification sustains neuronal viability in models of Alzheimer’s disease. Acta Neuropathol. Commun. 2016, 4, 103. [Google Scholar] [CrossRef]
- Herzer, S.; Hagan, C.; Von Gerichten, J.; Dieterle, V.; Munteanu, B.; Sandhoff, R.; Hopf, C.; Nordstrom, V. Deletion of Specific Sphingolipids in Distinct Neurons Improves Spatial Memory in a Mouse Model of Alzheimer’s Disease. Front. Mol. Neurosci. 2018, 11, 206. [Google Scholar] [CrossRef]
- Vajn, K.; Viljetic, B.; Degmecic, I.V.; Schnaar, R.L.; Heffer, M. Differential distribution of major brain gangliosides in the adult mouse central nervous system. PLoS ONE 2013, 8, e75720. [Google Scholar] [CrossRef] [PubMed]
- Fukami, Y.; Ariga, T.; Yamada, M.; Yuki, N. Brain Gangliosides in Alzheimer’s Disease: Increased Expression of Cholinergic Neuron-Specific Gangliosides. Curr. Alzheimer Res. 2017, 14, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Caughlin, S.; Maheshwari, S.; Agca, Y.; Agca, C.; Harris, A.J.; Jurcic, K.; Yeung, K.K.; Cechetto, D.F.; Whitehead, S.N. Membrane-lipid homeostasis in a prodromal rat model of Alzheimer’s disease: Characteristic profiles in ganglioside distributions during aging detected using MALDI imaging mass spectrometry. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Hicks, D.A.; Nalivaeva, N.N.; Turner, A.J. Lipid rafts and Alzheimer’s disease: Protein-lipid interactions and perturbation of signaling. Front. Physiol. 2012, 3, 189. [Google Scholar] [CrossRef] [PubMed]
- Chiricozzi, E.; Lunghi, G.; Di Biase, E.; Fazzari, M.; Sonnino, S.; Mauri, L. GM1 Ganglioside Is A Key Factor in Maintaining the Mammalian Neuronal Functions Avoiding Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 868. [Google Scholar] [CrossRef]
- Kim, S.I.; Yi, J.S.; Ko, Y.G. Amyloid beta oligomerization is induced by brain lipid rafts. J. Cell. Biochem. 2006, 99, 878–889. [Google Scholar] [CrossRef]
- Marconi, S.; De Toni, L.; Lovato, L.; Tedeschi, E.; Gaetti, L.; Acler, M.; Bonetti, B. Expression of gangliosides on glial and neuronal cells in normal and pathological adult human brain. J. Neuroimmunol. 2005, 170, 115–121. [Google Scholar] [CrossRef]
- Matsuzaki, K. How do membranes initiate Alzheimer’s Disease? Formation of toxic amyloid fibrils by the amyloid beta-protein on ganglioside clusters. Acc. Chem. Res. 2014, 47, 2397–2404. [Google Scholar] [CrossRef]
- Yamamoto, N.; Igbabvoa, U.; Shimada, Y.; Ohno-Iwashita, Y.; Kobayashi, M.; Wood, W.G.; Fujita, S.C.; Yanagisawa, K. Accelerated Abeta aggregation in the presence of GM1-ganglioside-accumulated synaptosomes of aged apoE4-knock-in mouse brain. FEBS Lett. 2004, 569, 135–139. [Google Scholar] [CrossRef]
- Gylys, K.H.; Fein, J.A.; Yang, F.; Miller, C.A.; Cole, G.M. Increased cholesterol in Abeta-positive nerve terminals from Alzheimer’s disease cortex. Neurobiol. Aging 2007, 28, 8–17. [Google Scholar] [CrossRef]
- Kaya, I.; Jennische, E.; Dunevall, J.; Lange, S.; Ewing, A.G.; Malmberg, P.; Baykal, A.T.; Fletcher, J.S. Spatial Lipidomics Reveals Region and Long Chain Base Specific Accumulations of Monosialogangliosides in Amyloid Plaques in Familial Alzheimer’s Disease Mice (5xFAD) Brain. ACS Chem. Neurosci. 2020, 11, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Choo-Smith, L.P.; Surewicz, W.K. The interaction between Alzheimer amyloid beta(1-40) peptide and ganglioside GM1-containing membranes. FEBS Lett. 1997, 402, 95–98. [Google Scholar] [CrossRef]
- Hayashi, H.; Kimura, N.; Yamaguchi, H.; Hasegawa, K.; Yokoseki, T.; Shibata, M.; Yamamoto, N.; Michikawa, M.; Yoshikawa, Y.; Terao, K.; et al. A seed for Alzheimer amyloid in the brain. J. Neurosci. 2004, 24, 4894–4902. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Matsubara, T.; Sato, T.; Yanagisawa, K. Age-dependent high-density clustering of GM1 ganglioside at presynaptic neuritic terminals promotes amyloid beta-protein fibrillogenesis. Biochim. Biophys. Acta 2008, 1778, 2717–2726. [Google Scholar] [CrossRef]
- Kakio, A.; Nishimoto, S.; Yanagisawa, K.; Kozutsumi, Y.; Matsuzaki, K. Interactions of amyloid beta-protein with various gangliosides in raft-like membranes: Importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry 2002, 41, 7385–7390. [Google Scholar] [CrossRef]
- Matsubara, T.; Nishihara, M.; Yasumori, H.; Nakai, M.; Yanagisawa, K.; Sato, T. Size and Shape of Amyloid Fibrils Induced by Ganglioside Nanoclusters: Role of Sialyl Oligosaccharide in Fibril Formation. Langmuir 2017, 33, 13874–13881. [Google Scholar] [CrossRef]
- Chi, E.Y.; Frey, S.L.; Lee, K.Y. Ganglioside G(M1)-mediated amyloid-beta fibrillogenesis and membrane disruption. Biochemistry 2007, 46, 1913–1924. [Google Scholar] [CrossRef]
- Tachi, Y.; Okamoto, Y.; Okumura, H. Conformational Change of Amyloid-beta 40 in Association with Binding to GM1-Glycan Cluster. Sci. Rep. 2019, 9, 6853. [Google Scholar] [CrossRef]
- Ariga, T.; Kobayashi, K.; Hasegawa, A.; Kiso, M.; Ishida, H.; Miyatake, T. Characterization of high-affinity binding between gangliosides and amyloid beta-protein. Arch. Biochem. Biophys. 2001, 388, 225–230. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Tsuda, L.; Suzuki, A.; Yanagisawa, K. Induction of ganglioside synthesis in Drosophila brain accelerates assembly of amyloid beta protein. Sci. Rep. 2018, 8, 8345. [Google Scholar] [CrossRef]
- Bera, S.; Korshavn, K.J.; Kar, R.K.; Lim, M.H.; Ramamoorthy, A.; Bhunia, A. Biophysical insights into the membrane interaction of the core amyloid-forming Abeta40 fragment K16-K28 and its role in the pathogenesis of Alzheimer’s disease. Phys. Chem. Chem. Phys. 2016, 18, 16890–16901. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Mukhopadhyay, C. Binding, conformational transition and dimerization of amyloid-beta peptide on GM1-containing ternary membrane: Insights from molecular dynamics simulation. PLoS ONE 2013, 8, e71308. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Abeta-ganglioside interactions in the pathogenesis of Alzheimer’s disease. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183233. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, M.C.; Spigolon, D.; Librizzi, F.; Moran, O.; Ortore, M.G.; Bulone, D.; Biagio, P.L.; Carrotta, R. Amyloid beta-peptide insertion in liposomes containing GM1-cholesterol domains. Biophys. Chem. 2016, 208, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Iijima, K.; Yamamoto, N.; Yanagisawa, K.; Sato, T. Density of GM1 in nanoclusters is a critical factor in the formation of a spherical assembly of amyloid beta-protein on synaptic plasma membranes. Langmuir 2013, 29, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Thomaier, M.; Gremer, L.; Dammers, C.; Fabig, J.; Neudecker, P.; Willbold, D. High-Affinity Binding of Monomeric but Not Oligomeric Amyloid-beta to Ganglioside GM1 Containing Nanodiscs. Biochemistry 2016, 55, 6662–6672. [Google Scholar] [CrossRef] [PubMed]
- Dukhinova, M.; Veremeyko, T.; Yung, A.W.Y.; Kuznetsova, I.S.; Lau, T.Y.B.; Kopeikina, E.; Chan, A.M.L.; Ponomarev, E.D. Fresh evidence for major brain gangliosides as a target for the treatment of Alzheimer’s disease. Neurobiol. Aging 2019, 77, 128–143. [Google Scholar] [CrossRef]
- Mikhalyov, I.; Olofsson, A.; Grobner, G.; Johansson, L.B. Designed fluorescent probes reveal interactions between amyloid-beta(1-40) peptides and GM1 gangliosides in micelles and lipid vesicles. Biophys. J. 2010, 99, 1510–1519. [Google Scholar] [CrossRef]
- Michno, W.; Wehrli, P.M.; Zetterberg, H.; Blennow, K.; Hanrieder, J. GM1 locates to mature amyloid structures implicating a prominent role for glycolipid-protein interactions in Alzheimer pathology. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 458–467. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamaguchi, T.; Fukunaga, S.; Hoshino, M.; Matsuzaki, K. Mechanism of amyloid beta-protein aggregation mediated by GM1 ganglioside clusters. Biochemistry 2011, 50, 6433–6440. [Google Scholar] [CrossRef]
- Ahyayauch, H.; De La Arada, I.; Masserini, M.E.; Arrondo, J.L.R.; Goni, F.M.; Alonso, A. The Binding of Abeta42 Peptide Monomers to Sphingomyelin/Cholesterol/Ganglioside Bilayers Assayed by Density Gradient Ultracentrifugation. Int. J. Mol. Sci. 2020, 21, 1674. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Okubo, K.; Ikeda, K.; Yano, Y.; Hoshino, M.; Hayashi, Y.; Kiso, Y.; Itoh-Watanabe, H.; Naito, A.; Matsuzaki, K. Toxic Amyloid Tape: A Novel Mixed Antiparallel/Parallel beta-Sheet Structure Formed by Amyloid beta-Protein on GM1 Clusters. ACS Chem. Neurosci. 2019, 10, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, S.; Ueno, H.; Yamaguchi, T.; Yano, Y.; Hoshino, M.; Matsuzaki, K. GM1 cluster mediates formation of toxic Abeta fibrils by providing hydrophobic environments. Biochemistry 2012, 51, 8125–8131. [Google Scholar] [CrossRef]
- Matsubara, T.; Yasumori, H.; Ito, K.; Shimoaka, T.; Hasegawa, T.; Sato, T. Amyloid-beta fibrils assembled on ganglioside-enriched membranes contain both parallel beta-sheets and turns. J. Biol. Chem. 2018, 293, 14146–14154. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, M.; Shi, X.; Wang, K.; Gao, G.; Shen, L.; Sun, T. Kinetic study of Abeta(1-42) amyloidosis in the presence of ganglioside-containing vesicles. Colloids Surf. B Biointerfaces 2020, 185, 110615. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.G.; Yagi-Utsumi, M.; Kato, K.; Okumura, H. Effects of a Hydrophilic/Hydrophobic Interface on Amyloid-beta Peptides Studied by Molecular Dynamics Simulations and NMR Experiments. J. Phys. Chem. B 2019, 123, 160–169. [Google Scholar] [CrossRef]
- Hirai, M.; Ajito, S.; Sato, S.; Ohta, N.; Igarashi, N.; Shimizu, N. Preferential Intercalation of Human Amyloid-beta Peptide into Interbilayer Region of Lipid-Raft Membrane in Macromolecular Crowding Environment. J. Phys. Chem. B 2018, 122, 9482–9489. [Google Scholar] [CrossRef]
- Yuyama, K.; Yanagisawa, K. Late endocytic dysfunction as a putative cause of amyloid fibril formation in Alzheimer’s disease. J. Neurochem. 2009, 109, 1250–1260. [Google Scholar] [CrossRef]
- Fantini, J.; Yahi, N.; Garmy, N. Cholesterol accelerates the binding of Alzheimer’s beta-amyloid peptide to ganglioside GM1 through a universal hydrogen-bond-dependent sterol tuning of glycolipid conformation. Front. Physiol. 2013, 4, 120. [Google Scholar] [CrossRef]
- Mao, Y.; Shang, Z.; Imai, Y.; Hoshino, T.; Tero, R.; Tanaka, M.; Yamamoto, N.; Yanagisawa, K.; Urisu, T. Surface-induced phase separation of a sphingomyelin/cholesterol/ganglioside GM1-planar bilayer on mica surfaces and microdomain molecular conformation that accelerates Abeta oligomerization. Biochim. Biophys. Acta 2010, 1798, 1090–1099. [Google Scholar] [CrossRef]
- Yanagisawa, K. Pathological significance of ganglioside clusters in Alzheimer’s disease. J. Neurochem. 2011, 116, 806–812. [Google Scholar] [CrossRef]
- Cebecauer, M.; Hof, M.; Amaro, M. Impact of GM1 on Membrane-Mediated Aggregation/Oligomerization of beta-Amyloid: Unifying View. Biophys. J. 2017, 113, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L. Gangliosides--a new therapeutic agent against stroke and Alzheimer’s disease. Life Sci. 1994, 55, 2125–2134. [Google Scholar] [CrossRef]
- Calamai, M.; Pavone, F.S. Partitioning and confinement of GM1 ganglioside induced by amyloid aggregates. FEBS Lett. 2013, 587, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, L.; Brane, G.; Karlsson, I.; Lekman, A.; Ramstrom, I.; Wikkelso, C. Alzheimer disease—Effect of continuous intracerebroventricular treatment with GM1 ganglioside and a systematic activation programme. Dement. Geriatr. Cogn. Disord. 2002, 14, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, T.V.; Zakharova, I.O.; Furaev, V.V.; Rychkova, M.P.; Avrova, N.F. Neuroprotective effect of ganglioside GM1 on the cytotoxic action of hydrogen peroxide and amyloid beta-peptide in PC12 cells. Neurochem. Res. 2007, 32, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Q.; Min, L.; Sui, R.; Li, J.; Liu, X. Monosialoanglioside improves memory deficits and relieves oxidative stress in the hippocampus of rat model of Alzheimer’s disease. Neurol. Sci. 2013, 34, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, F.; Scherer, E.B.; Ferreira, A.G.K.; Petry, F.D.; Pereira, C.L.; Santana, F.; Wyse, A.T.D.; Salbego, C.G.; Trindade, V.M.T. Alterations on Na+,K+-ATPase and Acetylcholinesterase Activities Induced by Amyloid-beta Peptide in Rat Brain and GM1 Ganglioside Neuroprotective Action. Neurochem. Res. 2013, 38, 2342–2350. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Saito, M.; Lafrancois, J.; Saito, M.; Gaynor, K.; Olm, V.; Wang, L.; Casey, E.; Lu, Y.; Shiratori, C.; et al. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J. Neurosci. 2003, 23, 29–33. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Yang, D.J.; Ngo, T.H.; Shih, C.H.; Wu, Y.F.; Lee, C.K.; Phraekanjanavichid, V.; Yen, S.F.; Kao, S.H.; Lee, H.M.; et al. Ganglioside Hp-s1 Analogue Inhibits Amyloidogenic Toxicity in Alzheimer’s Disease Model Cells. ACS Chem. Neurosci. 2019, 10, 528–536. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudajev, V.; Novotny, J. The Role of Lipid Environment in Ganglioside GM1-Induced Amyloid β Aggregation. Membranes 2020, 10, 226. https://doi.org/10.3390/membranes10090226
Rudajev V, Novotny J. The Role of Lipid Environment in Ganglioside GM1-Induced Amyloid β Aggregation. Membranes. 2020; 10(9):226. https://doi.org/10.3390/membranes10090226
Chicago/Turabian StyleRudajev, Vladimir, and Jiri Novotny. 2020. "The Role of Lipid Environment in Ganglioside GM1-Induced Amyloid β Aggregation" Membranes 10, no. 9: 226. https://doi.org/10.3390/membranes10090226
APA StyleRudajev, V., & Novotny, J. (2020). The Role of Lipid Environment in Ganglioside GM1-Induced Amyloid β Aggregation. Membranes, 10(9), 226. https://doi.org/10.3390/membranes10090226



