Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture
Abstract
1. Introduction
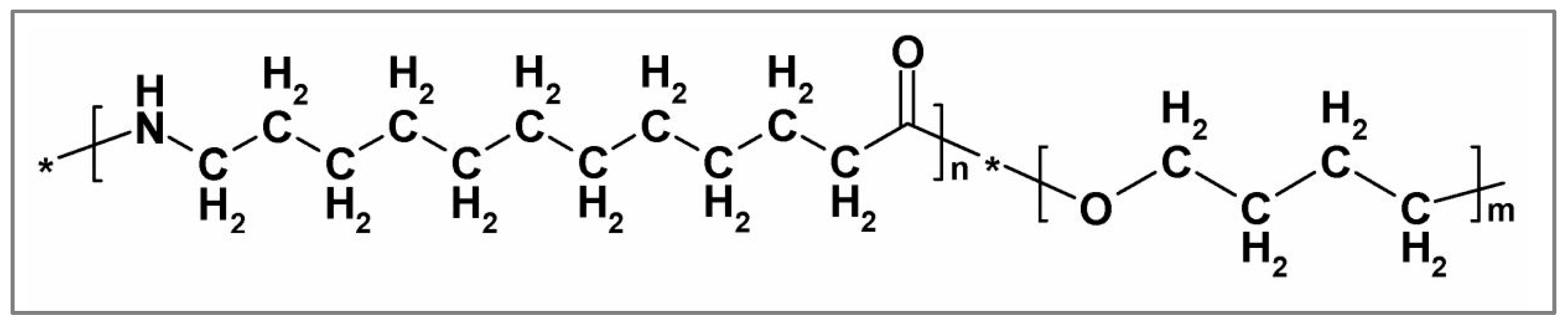
2. Materials and Methods
2.1. Nanofiller Preparation
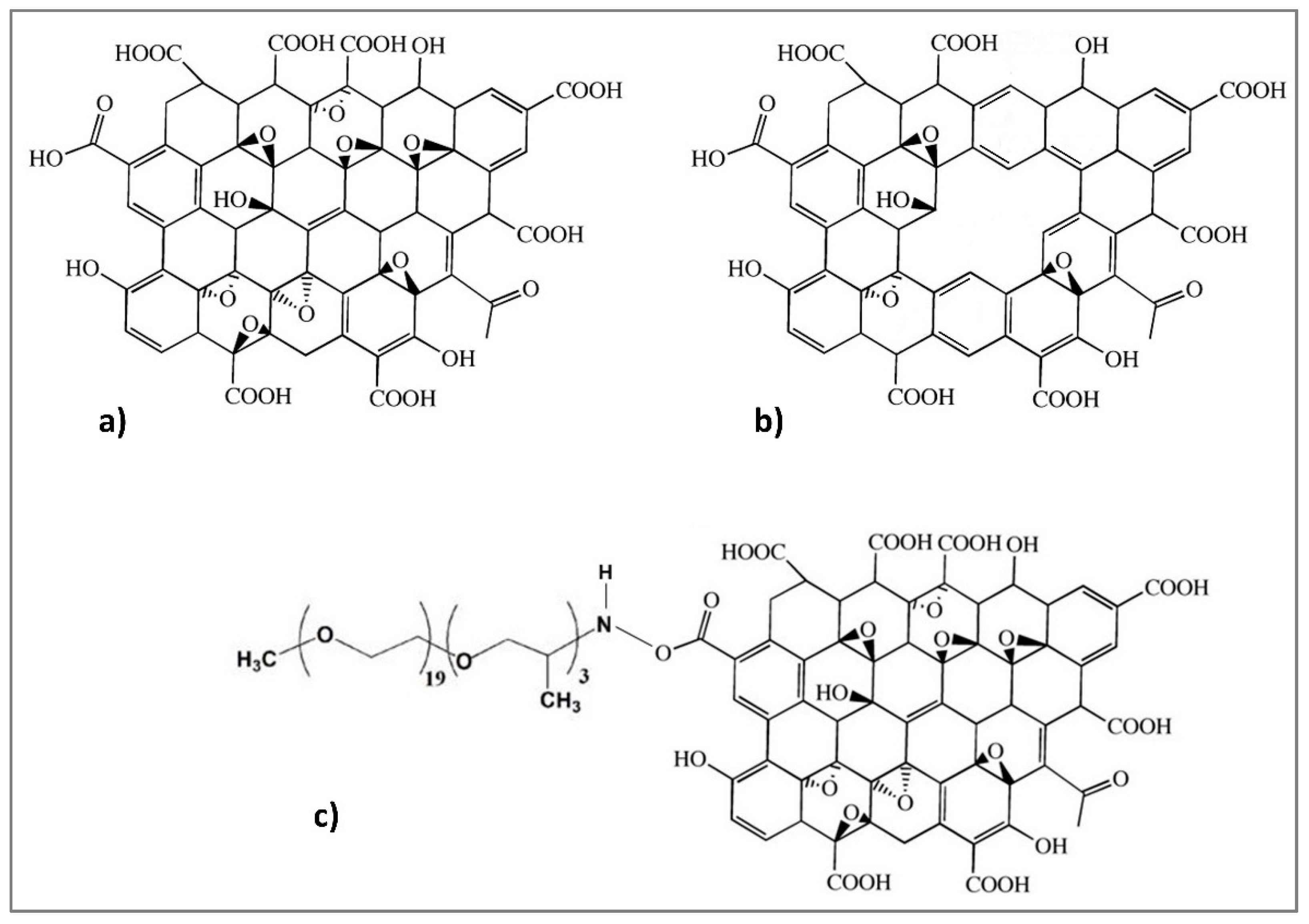
2.1.1. Graphene Oxide
2.1.2. Porous Graphene Oxide
2.1.3. Graphene Oxide Functionalized with Polyetheramine
2.2. Membranes Preparation: “Double-Solvent Compatibilization”
2.3. Materials Characterization
2.3.1. XPS
2.3.2. SEM Analysis
2.3.3. DSC
- (1)
- Cooling from room temperature to −80 °C
- (2)
- Heating from −80 to 250 °C
- (3)
- Cooling from 250 to −80 °C
- (4)
- Heating from −80 to 250 °C
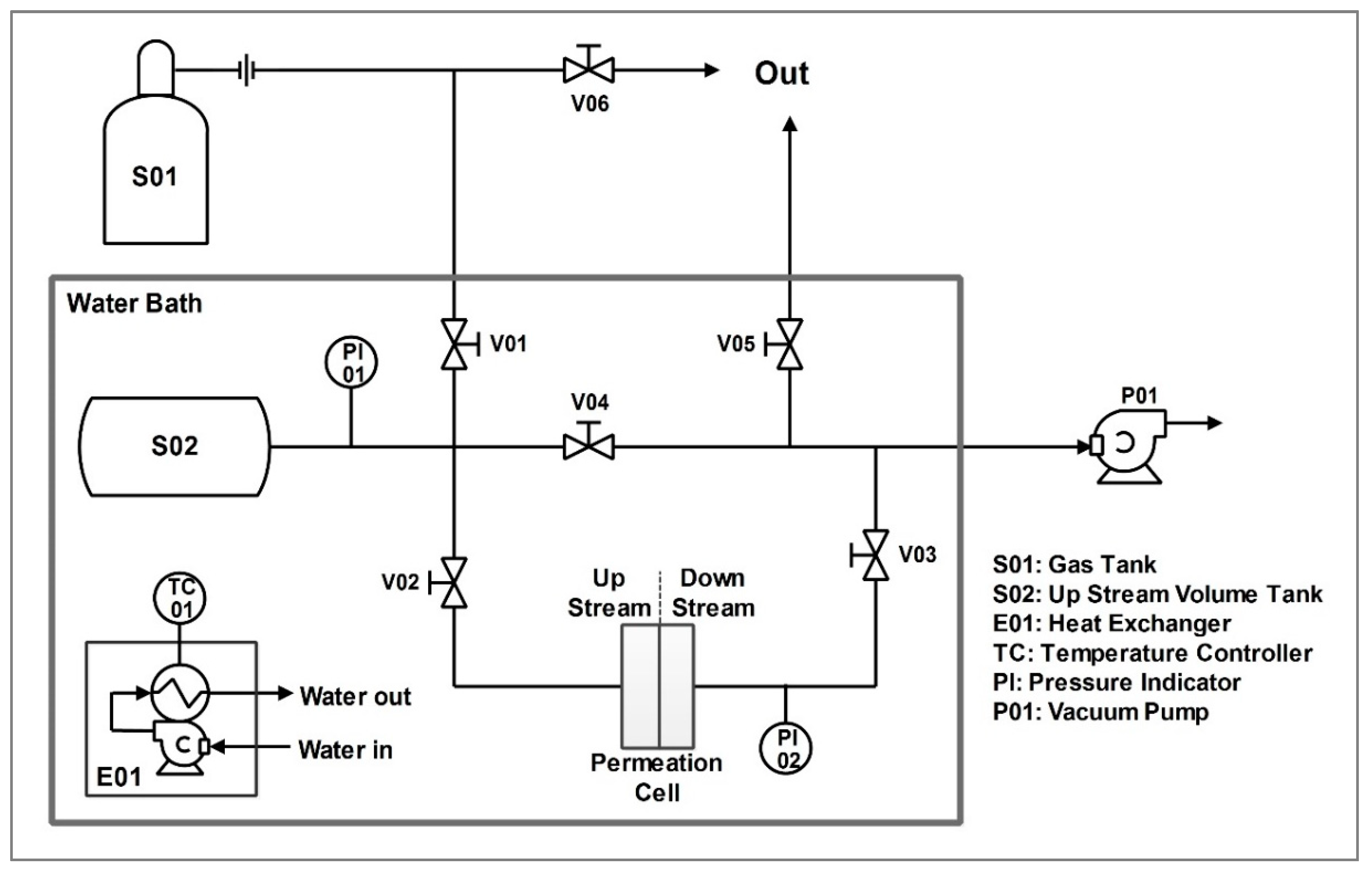
2.3.4. Permeation Test
3. Results and Discussion
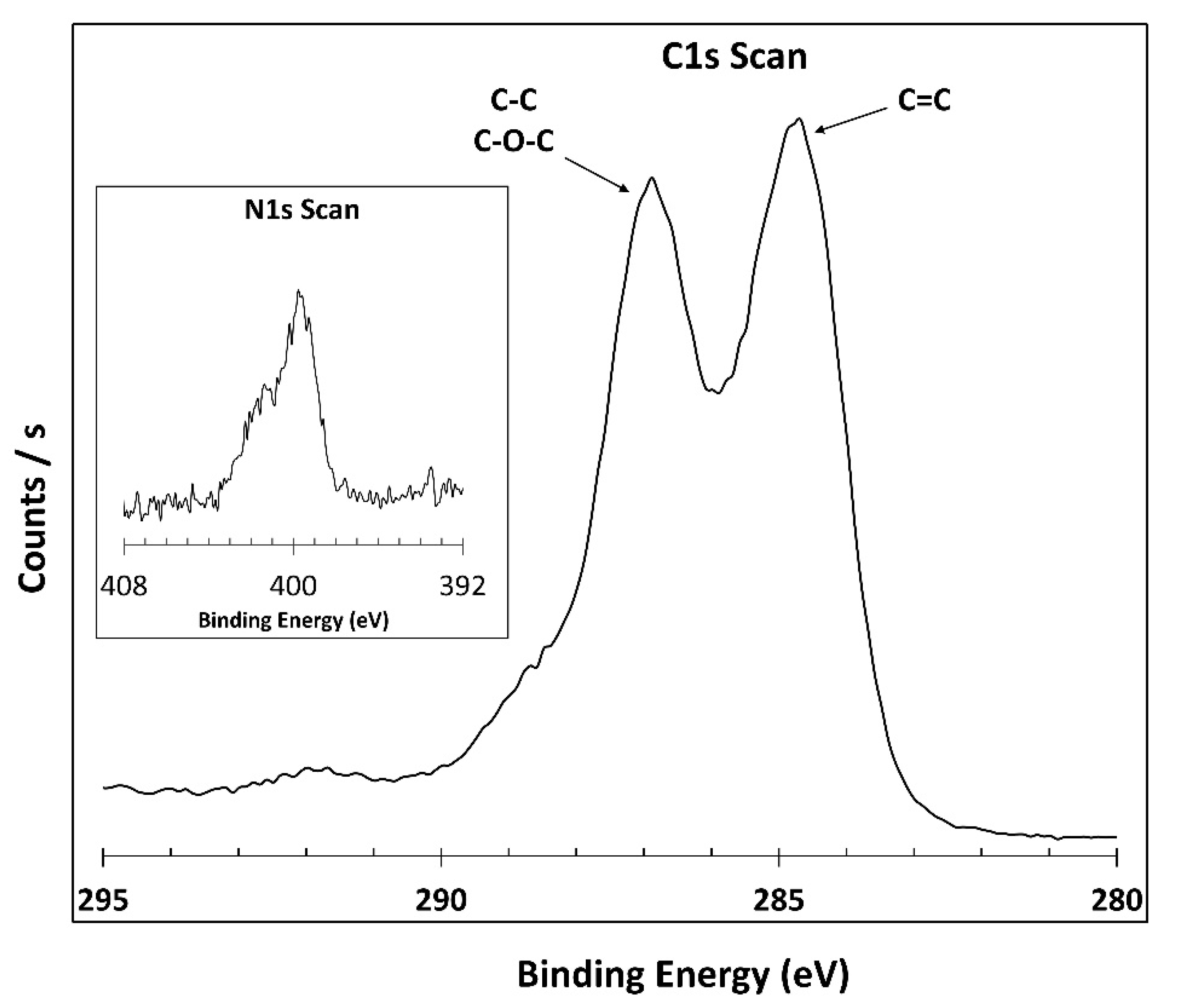
3.1. XPS
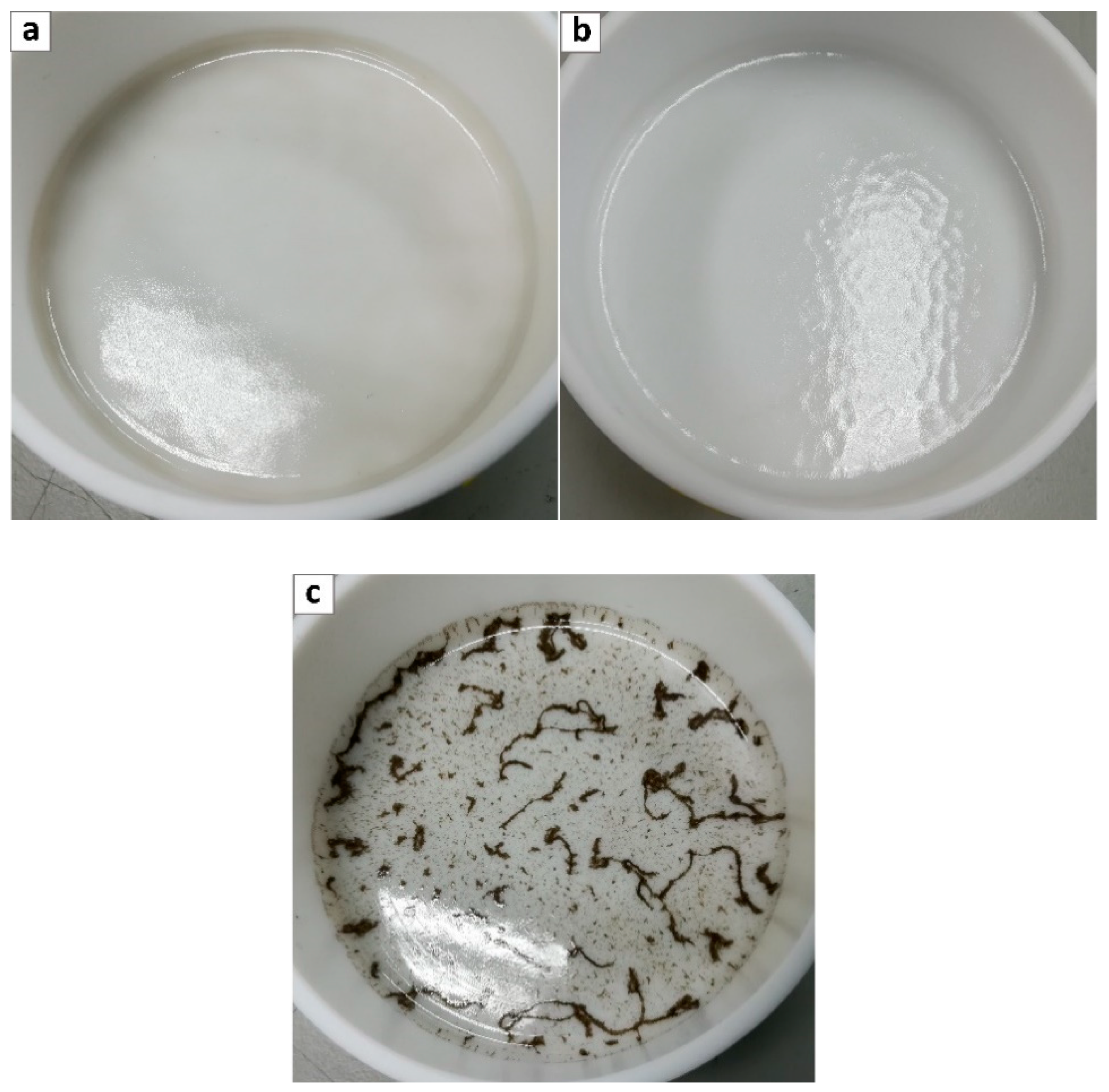
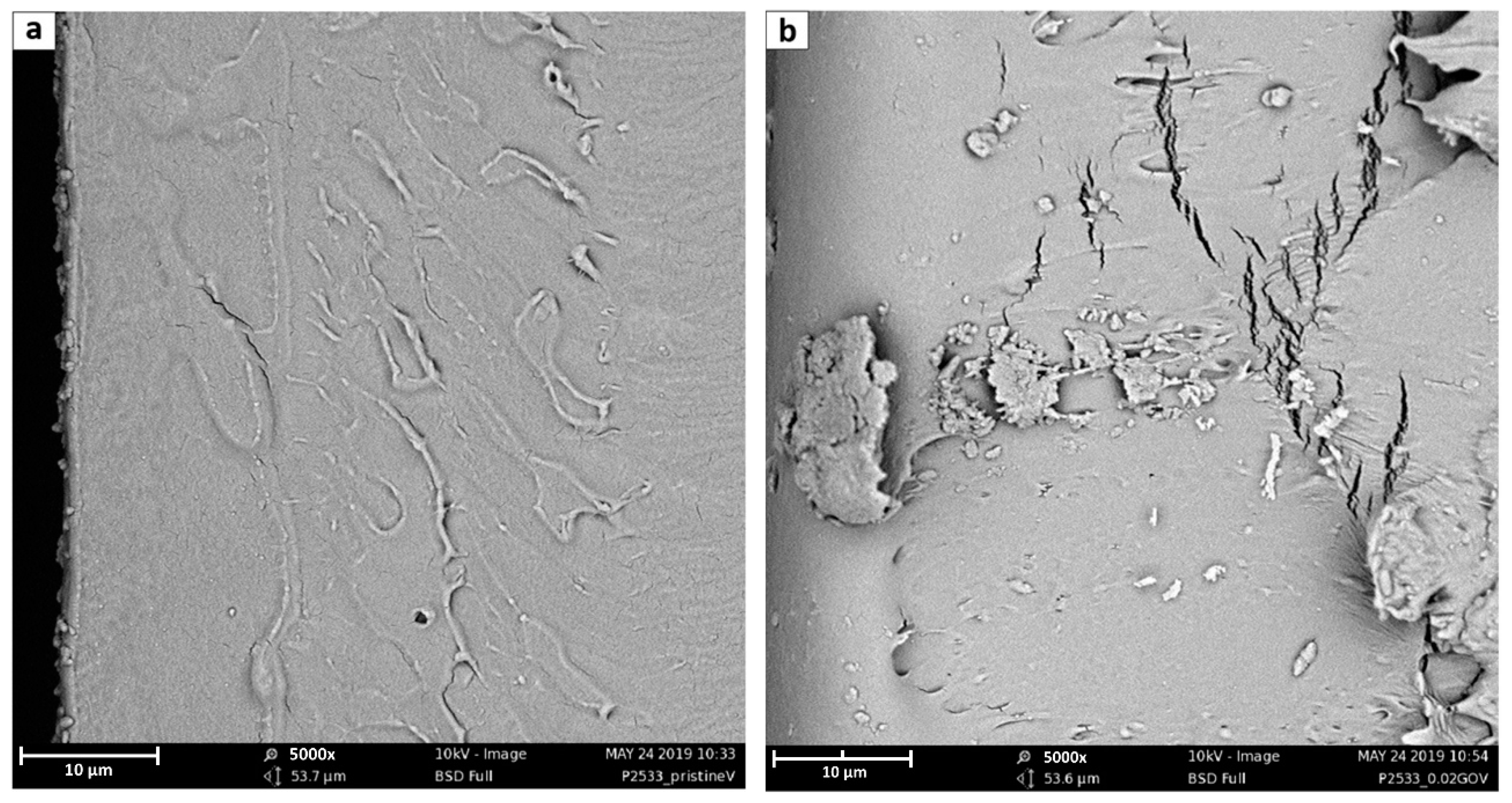
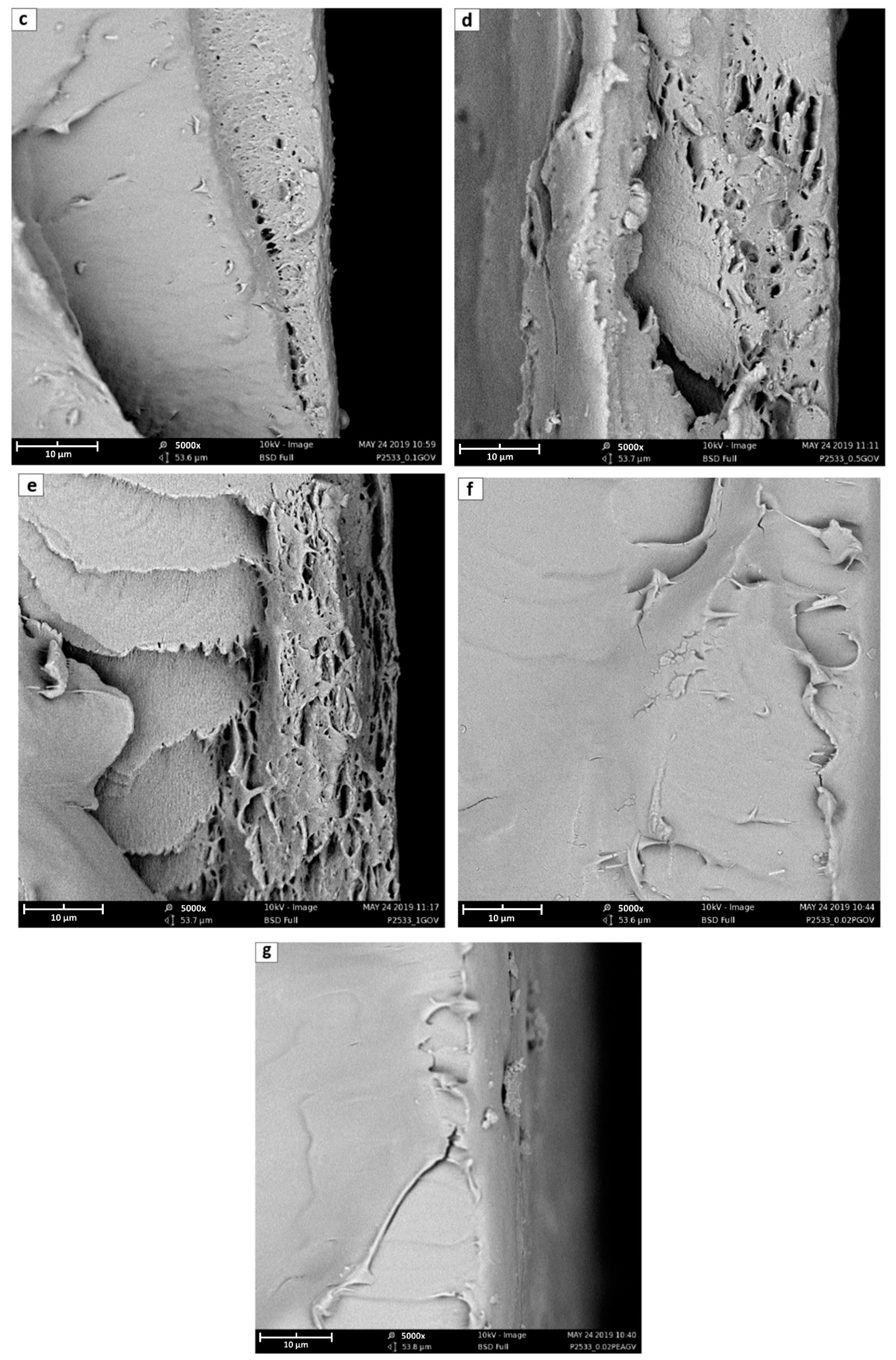
3.2. SEM
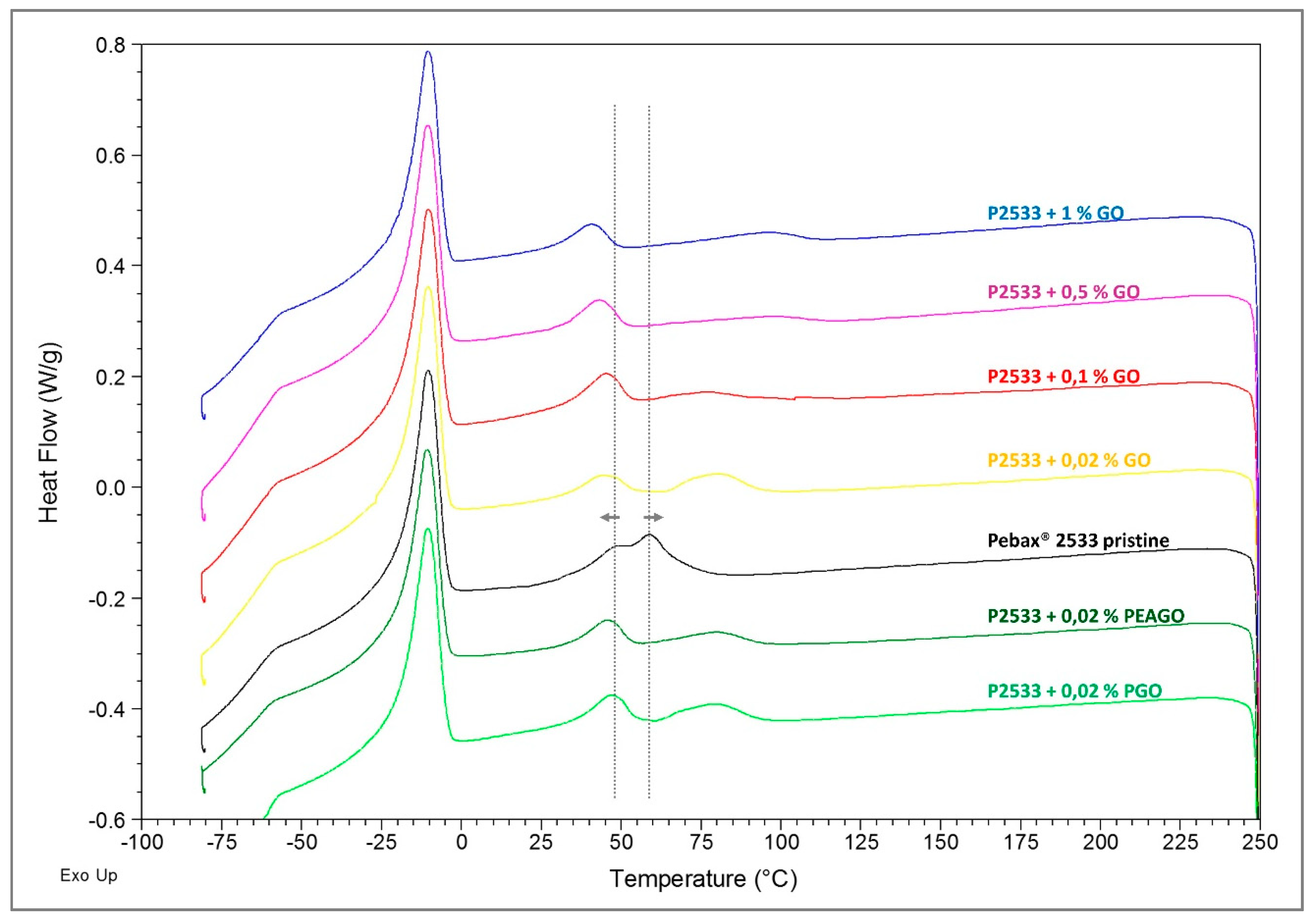
3.3. DSC
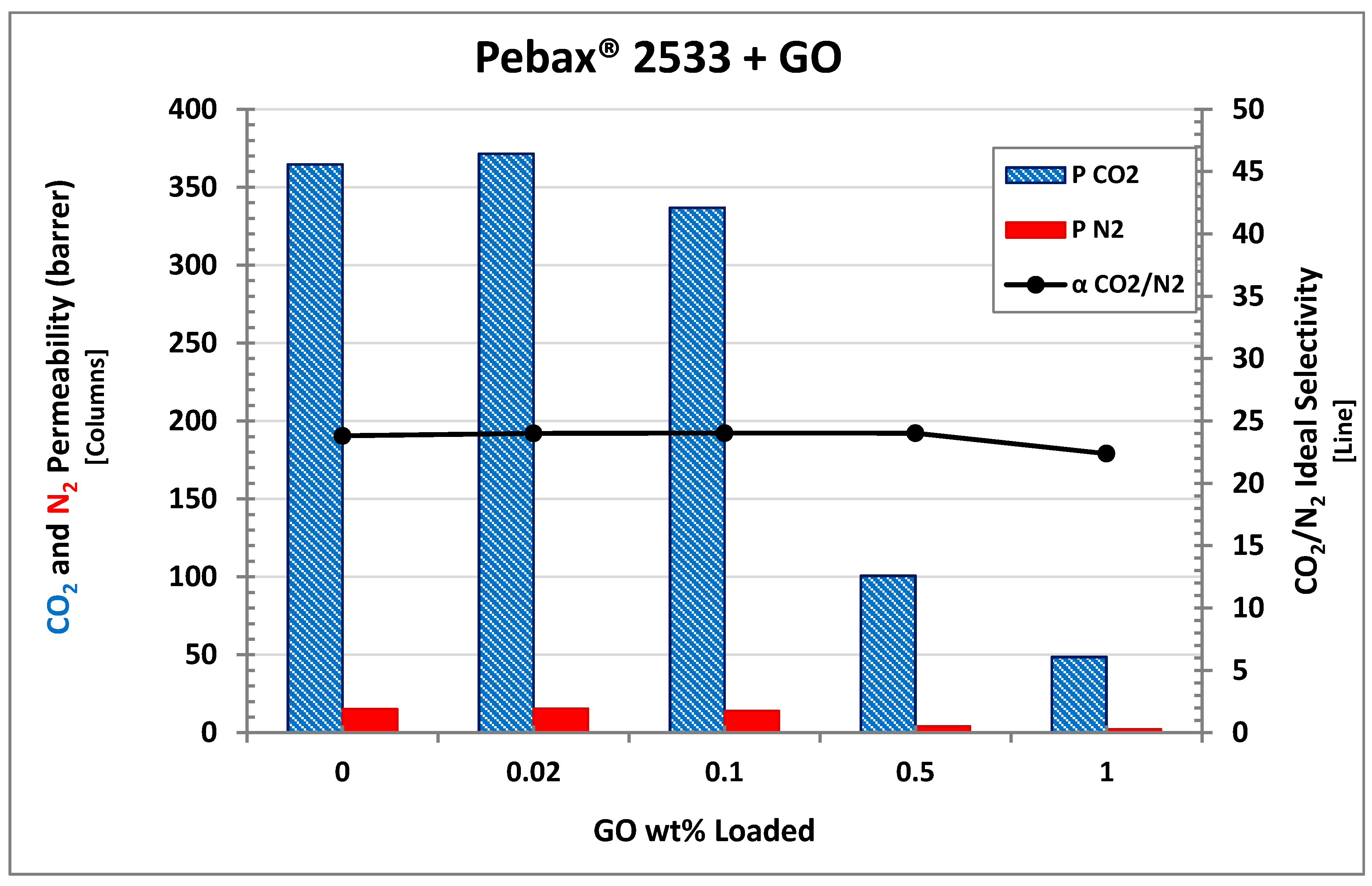
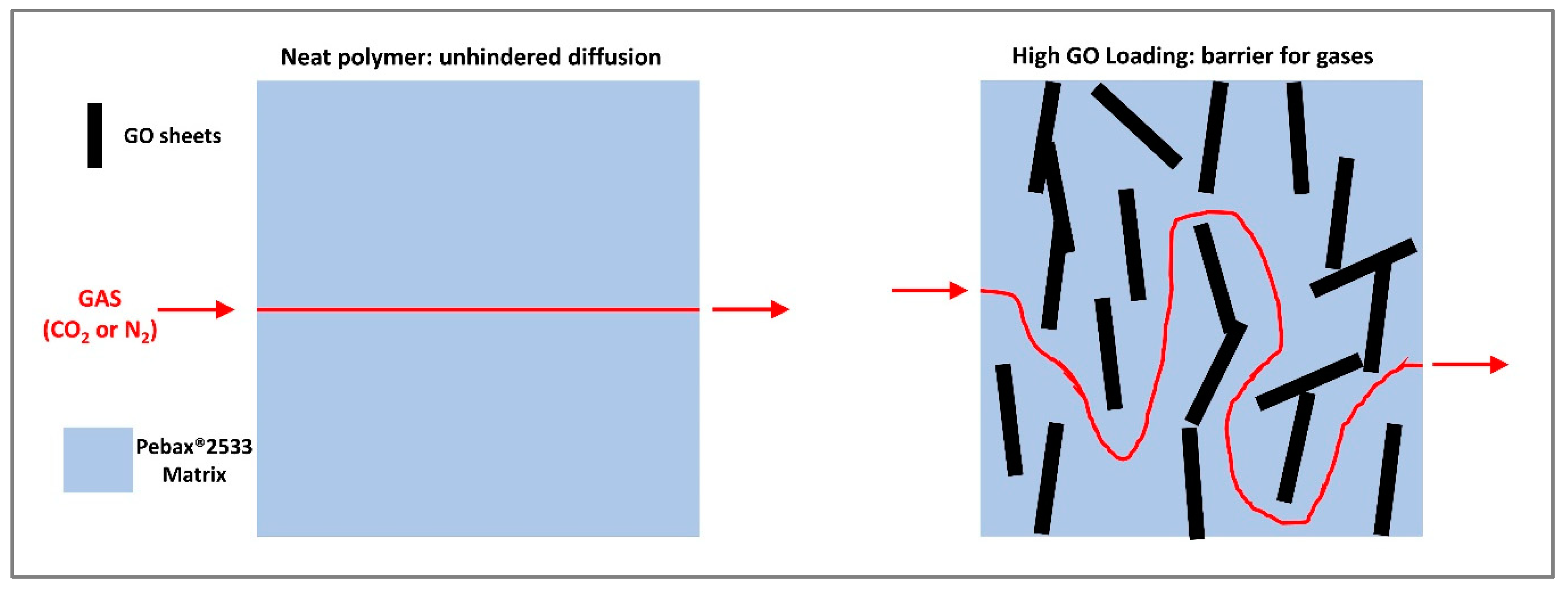
3.4. Permeation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IPCC—Intergovernmental Panel on Climate Change Global Warming Potential Values. Available online: https://www.ghgprotocol.org/sites/default/files/ghgp/Global-Warming-Potential-Values%20%28Feb%2016%202016%29_1.pdf (accessed on 10 September 2019).
- IPCC, Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: New York, NY, USA, 2014.
- IPCC—Intergovernmental Panel on Climate Change. Climate Change 2014 Synthesis Report; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- IPCC—Intergovernmental Panel on Climate Change. Global warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018; ISBN 9789291691517. [Google Scholar]
- Raza, A.; Gholami, R.; Rezaee, R.; Rasouli, V.; Rabiei, M. Significant aspects of carbon capture and storage–A review. Petroleum 2019, 5, 335–340. [Google Scholar] [CrossRef]
- Wilberforce, T.; Baroutaji, A.; Soudan, B.; Al-alami, A.H.; Ghani, A. Science of the Total Environment Outlook of carbon capture technology and challenges. Sci. Total Environ. 2019, 657, 56–72. [Google Scholar] [CrossRef]
- Sreedhar, I.; Nahar, T.; Venugopal, A.; Srinivas, B. Carbon capture by absorption–Path covered and ahead. Renew. Sustain. Energy Rev. 2017, 76, 1080–1107. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Miller, D.C. Post-combustion CO2 capture technologies—A review of processes for solvent-based and sorbent-based CO2 capture. Curr. Opin. Chem. Eng. 2017, 17, 78–92. [Google Scholar] [CrossRef]
- Bernhardsen, I.M.; Knuutila, H.K. International Journal of Greenhouse Gas Control A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Naraharisetti, P.K.; Yeo, T.Y.; Bu, J. New classification of CO2 mineralization processes and economic evaluation. Renew. Sustain. Energy Rev. 2019, 99, 220–233. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Sreedhar, I.; Vaidhiswaran, R.; Kamani, B.M.; Venugopal, A. Process and engineering trends in membrane based carbon capture. Renew. Sustain. Energy Rev. 2017, 68, 659–684. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, Z.; Qiao, Z.; Zhao, S.; Wang, J. Chinese Journal of Chemical Engineering Recent advances on the membrane processes for CO2 separation. Chinese J. Chem. Eng. 2018, 26, 2280–2291. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S. Recent advances in polymeric membranes for CO2 capture. Chinese J. Chem. Eng. 2018, 26, 2238–2254. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhao, S.; Wang, J.; Wang, S. Recent advances on mixed matrix membranes for CO2 separation. Chinese J. Chem. Eng. 2017, 25, 1581–1597. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Memb. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Chung, T.; Ying, L.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Ebadi, A. Progress in Polymer Science State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Venturi, D.; Chrysanthou, A.; Dhuiège, B.; Missoum, K.; Baschetti, M.G. Arginine/Nanocellulose membranes for carbon capture applications. Nanomaterials 2019, 9, 877. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Enhancing CO2 separation performance of Pebax® MH-1657 with aromatic carboxylic acids. Sep. Purif. Technol. 2019, 212, 901–912. [Google Scholar] [CrossRef]
- Lee, S.; Chan, S.; Kim, T.; Wook, S.; Soo, Y. Direct molecular interaction of CO2 with KTFSI dissolved in Pebax 2533 and their use in facilitated CO2 transport membranes. J. Memb. Sci. 2018, 548, 358–362. [Google Scholar] [CrossRef]
- Eun, J.; Ki, S.; Hoon, Y.; Bum, H. Effect of PEG-MEA and graphene oxide additives on the performance of Pebax®1657 mixed matrix membranes for CO2 separation. J. Memb. Sci. 2019, 572, 300–308. [Google Scholar]
- Zheng, W.; Ding, R.; Yang, K.; Dai, Y.; Yan, X.; He, G. ZIF-8 nanoparticles with tunable size for enhanced CO2 capture of Pebax based MMMs. Sep. Purif. Technol. 2019, 214, 111–119. [Google Scholar] [CrossRef]
- Nafisi, V.; Hägg, M.B. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Memb. Sci. 2014, 459, 244–255. [Google Scholar] [CrossRef]
- Arkema ARKEMA Products Online Database. Available online: https://www.extremematerials-arkema.com/en/materials-database/products (accessed on 23 October 2019).
- Deluca, N. PEBA: TPE materials for high performance applications. In Proceedings of the Annual Technical Conference - ANTEC, Anaheim, CA, USA, 8–10 May 2017; pp. 2343–2393. [Google Scholar]
- Sheth, J.P.; Xu, J.; Wilkes, G.L. Solid state structure–property behavior of semicrystalline poly (ether-block-amide) PEBAX® thermoplastic elastomers. Polymer (Guildf.) 2003, 44, 743–756. [Google Scholar] [CrossRef]
- Arkema, Arkema Reference Document 2018 Including the Annual Financial Report. Labrador Information Design: Atlanta, GA, USA, 2019; pp. 1–364.
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Neumann, S.; Bolmer, S.; Khan, M.M.; Abetz, V. PEBAX® with PEG functionalized POSS as nanocomposite membranes for CO2 separation. J. Memb. Sci. 2013, 437, 286–297. [Google Scholar] [CrossRef]
- Dong, L.; Chen, M.; Li, J.; Shi, D.; Dong, W.; Li, X.; Bai, Y. Metal-organic framework-graphene oxide composites: A facile method to highly improve the CO2 separation performance of mixed matrix membranes. J. Memb. Sci. 2016, 520, 801–811. [Google Scholar] [CrossRef]
- Scofield, J.M.P.; Gurr, P.A.; Kim, J.; Fu, Q.; Kentish, S.E.; Qiao, G.G. Development of novel fluorinated additives for high performance CO2 separation thin-film composite membranes. J. Memb. Sci. 2016, 499, 191–200. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, C.Y.; Lin, G.S.; Chen, C.H.; Wu, K.C.W.; Lin, C.H.; Tung, K.L. Characterization and molecular simulation of Pebax-1657-based mixed matrix membranes incorporating MoS2 nanosheets for carbon dioxide capture enhancement. J. Memb. Sci. 2019, 582, 358–366. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ahmadi, R.; Ebadi Amooghin, A.; Ghanbari, D. A novel ternary mixed matrix membrane containing glycerol-modified poly(ether-block-amide) (Pebax 1657)/copper nanoparticles for CO2 separation. J. Memb. Sci. 2019, 573, 234–246. [Google Scholar] [CrossRef]
- Wang, J.; Fang, W.; Luo, J.; Gao, M.; Wan, Y.; Zhang, S.; Zhang, X.; Park, A.H.A. Selective separation of CO2 using novel mixed matrix membranes based on Pebax and liquid-like nanoparticle organic hybrid materials. J. Memb. Sci. 2019, 584, 79–88. [Google Scholar] [CrossRef]
- Nafisi, V.; Hägg, M.B. Development of nanocomposite membranes containing modified Si nanoparticles in PEBAX-2533 as a block copolymer and 6FDADurene diamine as a glassy polymer. ACS Appl. Mater. Interfaces 2014, 6, 15643–15652. [Google Scholar] [CrossRef] [PubMed]
- Koolivand, H.; Razzaghi-kashani, M.; Karimi, M.; Koolivand, M. Functionalized graphene oxide/polyimide nanocomposites as highly CO2-selective membranes. J. Polym. Res. 2014, 21, 1–12. [Google Scholar] [CrossRef]
- Chen, M.; Soyekwo, F.; Zhang, Q.; Hu, C.; Zhu, A.; Liu, Q. Graphene oxide nanosheets to improve permeability and selectivity of PIM-1 membrane for carbon dioxide separation. J. Ind. Eng. Chem. 2018, 63, 296–302. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Li, S.; Yu, M. Ultrathin graphene oxide-based hollow fiber membranes with brush-like CO2-philic agent for highly efficient CO2 capture. Nat. Commun. 2017, 8, 1–8. [Google Scholar]
- Dong, G.; Hou, J.; Wang, J.; Zhang, Y.; Chen, V.; Liu, J. Enhanced CO2/N2 separation by porous reduced graphene oxide / Pebax mixed matrix membranes. J. Memb. Sci. 2016, 520, 860–868. [Google Scholar] [CrossRef]
- Wang, D.; Yao, D.; Wang, Y.; Wang, F.; Xin, Y.; Song, S.; Zhang, Z.; Su, F.; Zheng, Y. Carbon nanotubes and graphene oxide-based solvent-free hybrid nanofluids functionalized mixed-matrix membranes for efficient CO2/N2separation. Sep. Purif. Technol. 2019, 221, 421–432. [Google Scholar] [CrossRef]
- Anastasiou, S.; Bhoria, N.; Pokhrel, J.; Reddy, K.S.K.; Srinivasakannan, C.; Wang, K.; Karanikolos, G.N. Metal-organic framework / graphene oxide composite fillers in mixed-matrix membranes for CO2 separation. Mater. Chem. Phys. 2018, 212, 513–522. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Nasir, A.M.; Aziz, F.; Kumar, G.; Sallehhudin, W.; Jaafar, J.; Lau, W.J.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F. CO2/N2 selectivity enhancement of PEBAX MH 1657/Aminated Partially Reduced Graphene Oxide Mixed Matrix Composite Membrane. Sep. Purif. Technol. 2019. [Google Scholar] [CrossRef]
- Janakiram, S.; Ahmadi, M.; Dai, Z.; Ansaloni, L.; Deng, L. Performance of nanocomposite membranes containing 0D to 2D nanofillers for CO2 separation: A review. Membranes 2018, 8, 24. [Google Scholar] [CrossRef]
- Yoo, B.M.; Shin, J.E.; Lee, H.D.; Park, H.B. Graphene and graphene oxide membranes for gas separation applications. Curr. Opin. Chem. Eng. 2017, 16, 39–47. [Google Scholar] [CrossRef]
- Sun, M.; Li, J. Graphene oxide membranes: Functional structures, preparation and environmental applications. Nano Today 2018, 20, 121–137. [Google Scholar] [CrossRef]
- Karunakaran, M.; Shevate, R.; Kumar, M.; Peinemann, K.V. CO2-selective PEO-PBT (PolyActiveTM)/graphene oxide composite membranes. Chem. Commun. 2015, 51, 14187–14190. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, G.; Huang, K.; Jin, W.; Lee, K.R.; Xu, N. Membranes with fast and selective gas-transport channels of laminar graphene oxide for efficient CO2 capture. Angew. Chemie Int. Ed. 2015, 54, 578–582. [Google Scholar]
- Roussanaly, S.; Anantharaman, R.; Lindqvist, K.; Zhai, H.; Rubin, E. Membrane properties required for post-combustion CO2 capture at coal-fired power plants. J. Memb. Sci. 2016, 511, 250–264. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, B.; Yan, F.; Zhao, J.; Li, J.; Li, L. Engineering nano-porous graphene oxide by hydroxyl radicals. Carbon N. Y. 2019, 105, 291–296. [Google Scholar] [CrossRef]
- Yoo, M.J.; Kim, H.W.; Yoo, B.M.; Park, H.B. Highly soluble polyetheramine-functionalized graphene oxide and reduced graphene oxide both in aqueous and non-aqueous solvents. Carbon N. Y. 2014, 75, 149–160. [Google Scholar] [CrossRef]
- Lara-estévez, J.C.I.; Antônio, L.; de Almeida, S.; Schulte, K.; Bucio, E. PEBAX TM-Silanized Al2O3 Composite, Synthesis and Characterization. Open J. Polym. Chem. 2012, 2, 63–69. [Google Scholar]
- Armstrong, S.; Freeman, B.; Hiltner, A.; Baer, E. Gas permeability of melt-processed poly (ether block amide) copolymers and the effects of orientation. Polymer (Guildf.) 2012, 53, 1383–1392. [Google Scholar] [CrossRef]
- Liu, K.; Fang, C.; Li, Z.; Young, M. Separation of thiophene/n-heptane mixtures using PEBAX/PVDF-composited membranes via pervaporation. J. Memb. Sci. 2014, 451, 24–31. [Google Scholar] [CrossRef]
- Clarizia, G.; Bernardo, P.; Gorrasi, G.; Zampino, D.; Carroccio, S.C. Influence of the Preparation Method and Mhoto-Oxidation Treatment on the Thermal and Gas Transport Properties of Dense Films Based on a Poly(ether-block-amide) Copolymer. Materials 2018, 11, 1326. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.S.A.; Sunarti, A.R. Development of PEBAX Based Membrane for Gas Separation: A Review. Int. J. Membr. Sci. Technol. 2015, 2, 78–84. [Google Scholar]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, H.W.; Lee, H.D.; Shin, J.E.; Yoo, B.M.; Park, H.B. Water and ion sorption, diffusion, and transport in graphene oxide membranes revisited. J. Memb. Sci. 2017, 544, 425–435. [Google Scholar] [CrossRef]
- Moghadam, F.; Lee, T.H.; Park, I.; Park, H.B. Thermally annealed polyimide-based mixed matrix membrane containing ZIF-67 decorated porous graphene oxide nanosheets with enhanced propylene/propane selectivity. J. Memb. Sci. 2020, 603, 118019. [Google Scholar] [CrossRef]
- Su, Y.; University of Manchester; Wilkinson, J.; AzoNano. Interview: Developing Graphene Oxide Membranes for the Purification of Water and Green Fuels. Available online: https://www.azonano.com/article.aspx?ArticleID=4275 (accessed on 14 May 2020).
- Ehsani, A.; Pakizeh, M. Journal of the Taiwan Institute of Chemical Engineers Synthesis, characterization and gas permeation study of ZIF-11 / Pebax ® 2533 mixed matrix membranes. J. Taiwan Inst. Chem. Eng. 2016, 66, 414–423. [Google Scholar] [CrossRef]
- Cho, Y.H.; Jeong, S.M.; Kim, S.; Kim, Y.; Lee, H.J.; Lee, T.H.; Park, H.B.; Park, H.; Nam, S.; Park, Y. Sacrificial graphene oxide interlayer for highly permeable ceramic thin film composite membranes. J. Memb. Sci. 2020, 118442. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.C.; Roh, J.S.; Moon, G.H.; Shin, J.E.; Kang, Y.S.; Park, H.B. Metal-organic frameworks grown on a porous planar template with an exceptionally high surface area: Promising nanofiller platforms for CO2 separation. J. Mater. Chem. A 2017, 5, 22500–22505. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Li, N.; Liao, J.; Zhao, S.; Wang, J.; Wang, S. Relationship between polymer-filler interfaces in separation layers and gas transport properties of mixed matrix composite membranes. J. Memb. Sci. 2015, 495, 252–268. [Google Scholar] [CrossRef]
- Takahashi, S.; Paul, D.R. Gas permeation in poly(ether imide) nanocomposite membranes based on surface-treated silica. Part 1: Without chemical coupling to matrix. Polymer (Guildf.) 2006, 47, 7519–7534. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic-inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive models for mixed-matrix membrane performance: A review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef] [PubMed]
- Baig, Z.; Mamat, O.; Mustapha, M.; Mumtaz, A.; Munir, K.S.; Sarfraz, M. Investigation of tip sonication effects on structural quality of graphene nanoplatelets (GNPs) for superior solvent dispersion. Ultrason. Sonochem. 2018, 45, 133–149. [Google Scholar] [CrossRef]


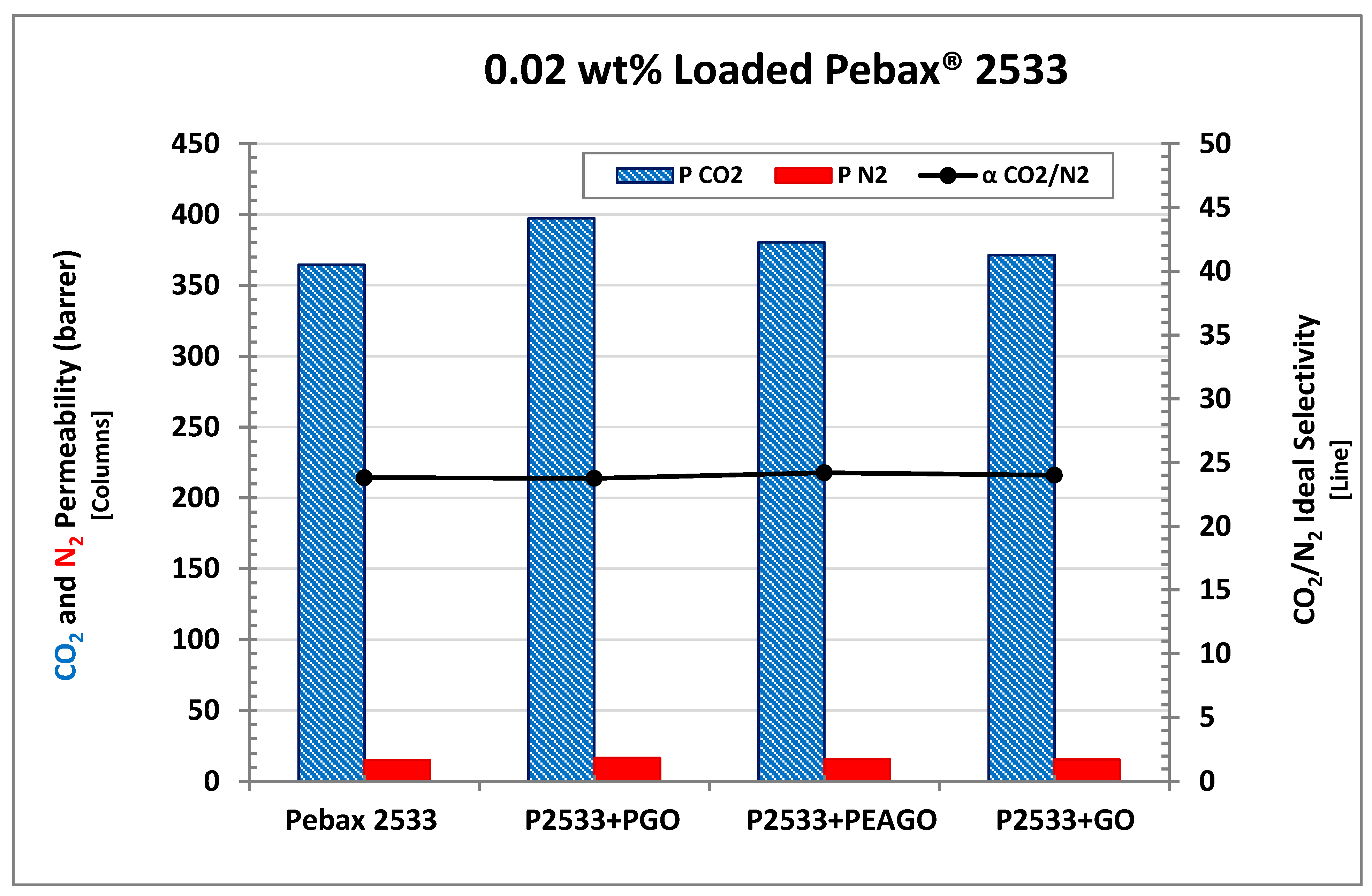
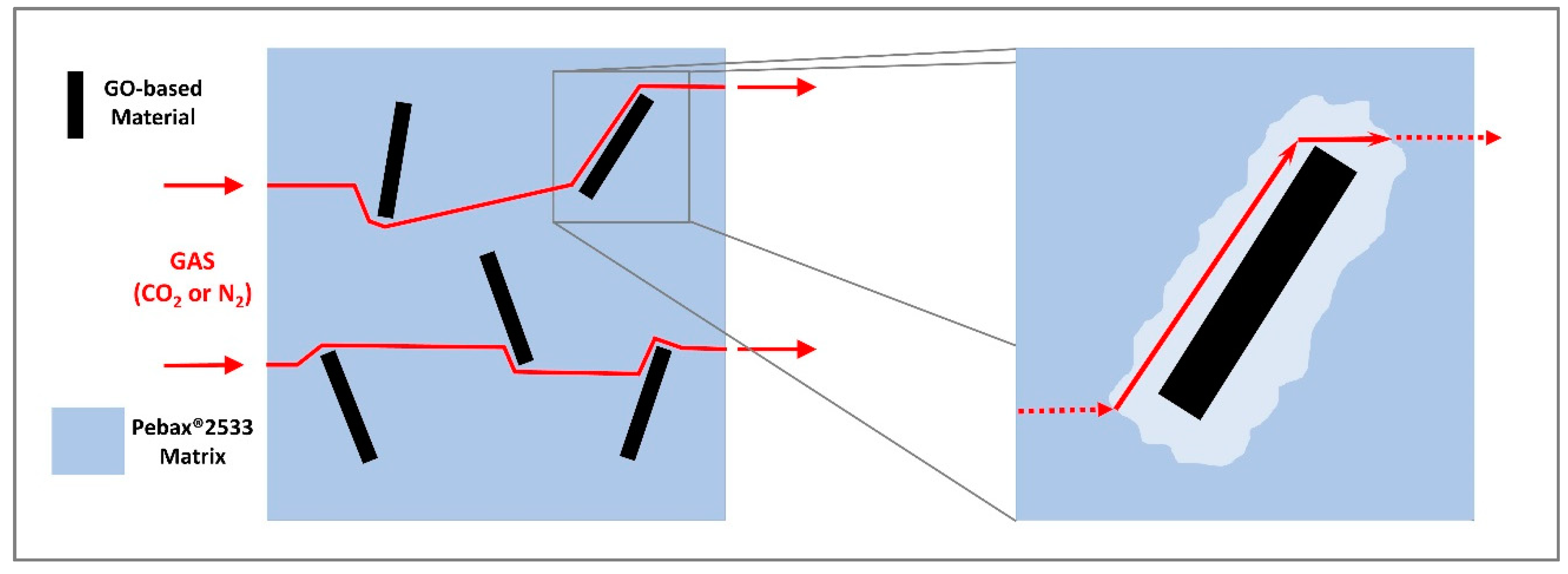
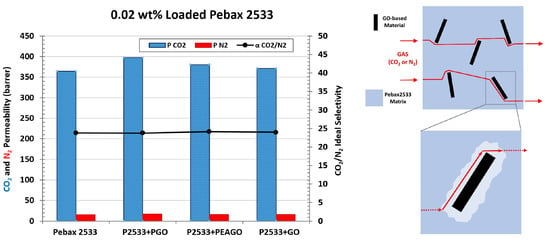
| Membrane | CO2 Permeability (Barrer) | CO2 Permeability Variation% | N2 Permeability (Barrer) | N2 Permeability Variation% | CO2/N2 Selectivity | CO2/N2 Variation% |
|---|---|---|---|---|---|---|
| Pebax 2533 | 364.61 | \ | 15.32 | \ | 23.80 | \ |
| +0.02% GO | 371.39 | 1.86 | 15.47 | 1.01 | 24.00 | 0.84 |
| +0.1% GO | 336.80 | −7.63 | 14.02 | −8.47 | 24.02 | 0.92 |
| +0.5% GO | 100.61 | −72.41 | 4.19 | −72.65 | 24.01 | 0.88 |
| +1% GO | 48.58 | −86.68 | 2.17 | −85.83 | 22.38 | −5.97 |
| +0.02% PGO | 397.35 | 8.98 | 16.73 | 9.19 | 23.75 | −0.19 |
| +0.02% PEAGO | 380.44 | 4.34 | 15.73 | 2.65 | 24.19 | 1.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casadei, R.; Giacinti Baschetti, M.; Yoo, M.J.; Park, H.B.; Giorgini, L. Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture. Membranes 2020, 10, 188. https://doi.org/10.3390/membranes10080188
Casadei R, Giacinti Baschetti M, Yoo MJ, Park HB, Giorgini L. Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture. Membranes. 2020; 10(8):188. https://doi.org/10.3390/membranes10080188
Chicago/Turabian StyleCasadei, Riccardo, Marco Giacinti Baschetti, Myung Jin Yoo, Ho Bum Park, and Loris Giorgini. 2020. "Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture" Membranes 10, no. 8: 188. https://doi.org/10.3390/membranes10080188
APA StyleCasadei, R., Giacinti Baschetti, M., Yoo, M. J., Park, H. B., & Giorgini, L. (2020). Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture. Membranes, 10(8), 188. https://doi.org/10.3390/membranes10080188