Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Enzyme Activity
2.3. Batch Conversion
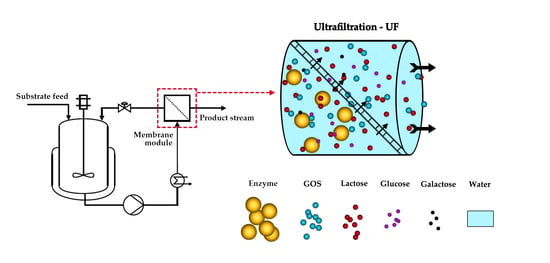
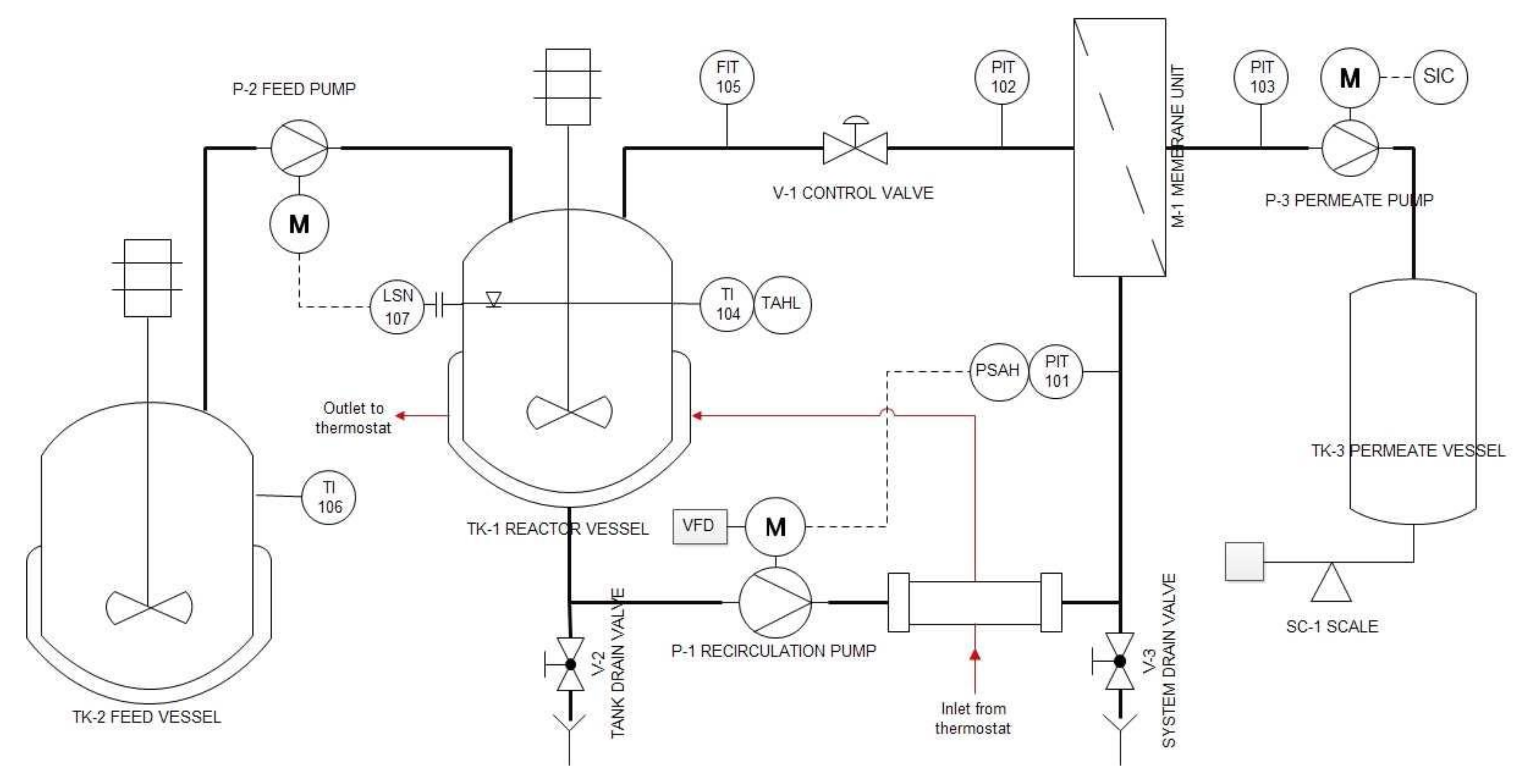
2.4. Enzyme Membrane Reactor (EMR)
2.5. Terminology
- Relative mass fraction () was calculated as the ratio of the mass of a saccharide fraction i () to the total mass of saccharides present in the solution:
- Relative mass percentage was the relative mass fraction ( ) expressed in percentage;
- Residence time (τ) was given as the weight of the reaction liquor in the reactor (mR) divided by the mass flow rate of the permeate (q):
- Yield (Y) was defined as the concentration of the generated DP3-6 fractions ( divided by the concentration of lactose in the feed ( ):
- Biocatalyst productivity (P) was the total quantity of DP3-6 formed by one unit of crude enzyme preparation per hour:
2.6. Preliminary Filtration Tests
2.6.1. Pressure-Scan
2.6.2. Determination of Limiting Flux
2.6.3. Membrane Cleaning
- The membrane plant was drained and flashed several times with deionized water.
- Membrane cleaning was carried out by circulating a NaOH solution (pH = 10–11) for 1–2 h at 40–50 °C under 0.5–1 bar pressure.
- The plant was drained and flushed several times with water to remove the cleaning agent.
- Permeability of the cleaned membrane was measured with DI water. In certain cases, when the original permeability of the membrane (<25%) was not recovered by the alkaline cleaning procedure, then additional cleaning with citric acid and/or Ultrasil (Ecolab, Paul, MN, USA) was performed (1 w/w%, 40–50 °C, 0.5–1 bar, 0.5–1 h).
2.7. Short-Term Catalytic Runs
2.8. Long-Term Catalyst Runs
2.9. Statistical Test
2.10. High Performance Liquid Chromatography
3. Results and Discussion
3.1. Preliminary Filtration Experiments
3.1.1. Pressure-Scan
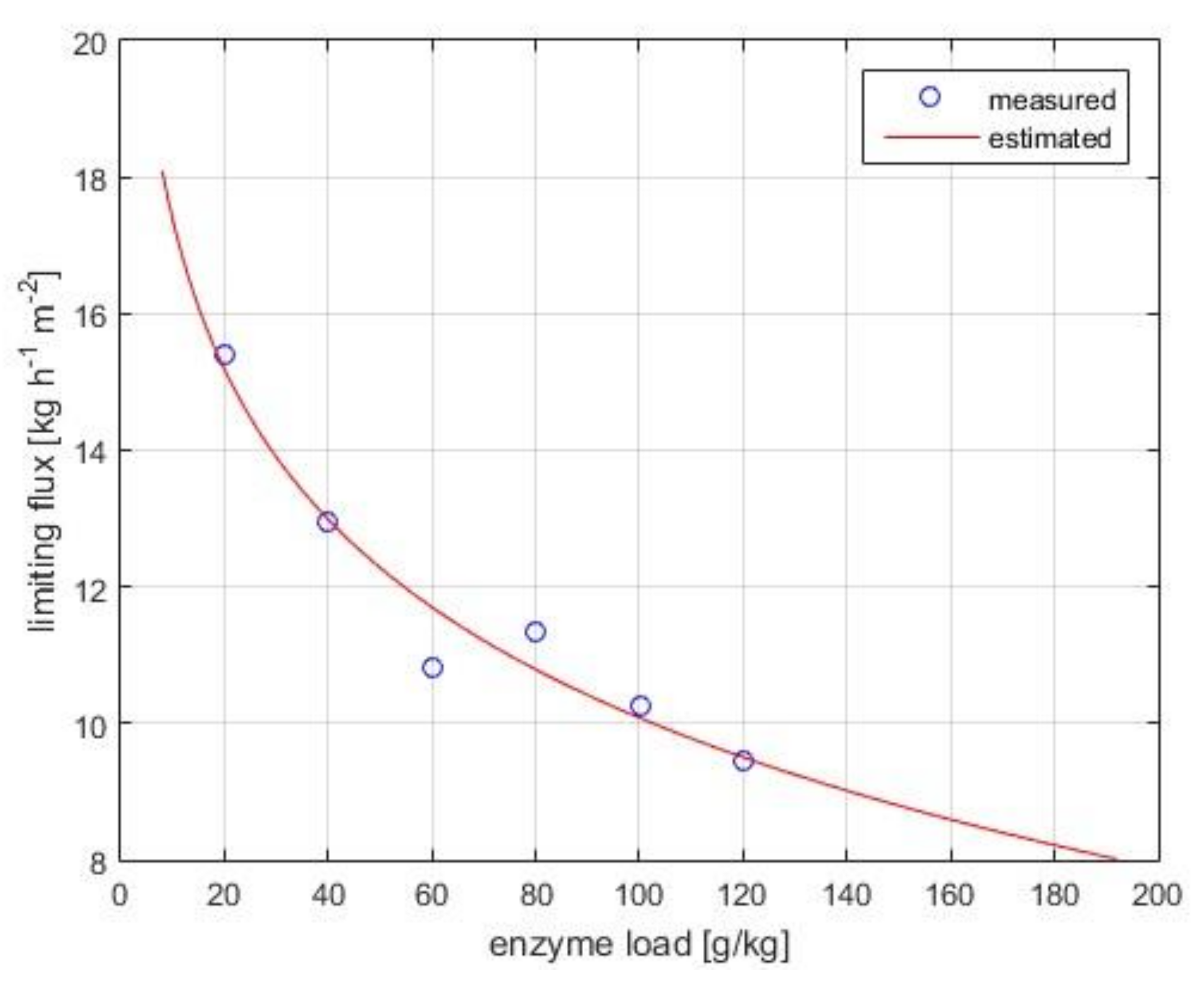
3.1.2. Limiting Flux
3.2. Catalytic Performance
3.2.1. Batch Conversion in STR
3.2.2. Short-Term Runs in EMR
3.2.3. Long-Term Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GRAS | generally recognized as safe |
| QPS | qualified presumption of safety |
| DP | degree of polymerization |
| DP2 | disaccharides (lactose and non-lactose) |
| DP3-6 | galacto-oligosaccharide fractions with a degree of polarization between 3 and 6 |
| EMR | enzymatic membrane reactor |
| GOS | galacto-oligosaccharides |
| RMSE | root mean squared error |
| SSE | sum of squares due to error |
| STR | stirred tank reactor |
| UF ONPG | ultrafiltration ortho-Nitrophenyl-β-galactoside |
| List of symbols | |
| cb | bulk concentration of retained compounds (g·kg−1) |
| cE | enzyme concentration in reaction liquid (U·g−1) |
| cL | lactose concentration in feed (g·kg−1) |
| clim | limiting concentration of retained compounds (g·kg−1) |
| Jlim | permeate flux in Equation (5) (kg·h−1·m−2) |
| k | mass transfer coefficient (kg·h−1·m−2) |
| P | biocatalyst productivity (g·U−1·h−1) |
| q | permeate mass flow rate (kg·h−1) |
| t | operational time (h) |
| Y | yield of DP3-6 (w/w%) |
| Greek letters | |
| τ | residence time (h) |
References
- Carol, T.C.; Linda, T. Generally Recognized as Safe (GRAS) Determination for the Use of Galacto-Oligosaccharides as a Food Ingredient. In GRAS Notice 000489; FDA: Silver Spring, MD, USA, 2013. [Google Scholar]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.; Tomar, S.; Singh, R.; Singh, A.; Ali, B. Galactooligosaccharides: Novel components of designer foods. J. Food Sci. 2011, 76, R103–R111. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Forsythe, S.J.; El-Nezami, H. Probiotics interaction with foodborne pathogens: A potential alternative to antibiotics and future challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 3320–3333. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Global Galactooligosaccharibeds (GOS) Market Insights, Forecast to 2025. Available online: https://www.360marketupdates.com/global-galactooligosaccharides-gos-market-13729874 (accessed on 16 June 2020).
- Chen, X.Y.; Gänzle, M.G. Lactose and lactose-derived oligosaccharides: More than prebiotics? Int. Dairy J. 2017, 67, 61–72. [Google Scholar] [CrossRef]
- Park, A.-R.; Oh, D.-K. Galacto-oligosaccharide production using microbial β-galactosidase: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Warmerdam, A.; Zisopoulos, F.K.; Boom, R.M.; Janssen, A.E. Kinetic characterization of galacto-oligosaccharide (GOS) synthesis by three commercially important β-galactosidases. Biotechnol. Prog. 2014, 30, 38–47. [Google Scholar] [CrossRef]
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.; Gomez-Zavaglia, A. Technological aspects of the production of fructo and galacto-oligosaccharides. Enzymatic synthesis and hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar] [CrossRef]
- Su, Z.; Luo, J.; Li, X.; Pinelo, M. Enzyme membrane reactors for production of oligosaccharides: A review on the interdependence between enzyme reaction and membrane separation. Sep. Purif. Technol. 2020, 243. [Google Scholar] [CrossRef]
- Tymczyszyn, E.; Santos, M.; Costa, M.d.C.; Illanes, A.; Gómez-Zavaglia, A. History, synthesis, properties, applications and regulatory issues of prebiotic oligosaccharides. In Carbohydrates Applications in Medicine; Research Signpost: Kerala, India, 2014. [Google Scholar]
- Kovács, Z.; Benjamins, E.; Grau, K.; Rehman, A.U.; Ebrahimi, M.; Czermak, P. Recent developments in manufacturing oligosaccharides with prebiotic functions. In Biotechnology of Food and Feed Additives; Springer: Berlin, Heidelberg, Germany, 2013; pp. 257–295. [Google Scholar] [CrossRef]
- Illanes, A.; Guerrero, C.; Vera, C.; Wilson, L.; Conejeros, R.; Scott, F. Enzymatic production of galacto-oligosaccharides. In Lactose-Derived Prebiotics, 1st ed.; Academic Press: Valparaíso, Chile, 2016. [Google Scholar]
- Satyawali, Y.; Vanbroekhoven, K.; Dejonghe, W. Process intensification: The future for enzymatic processes? Biochem. Eng. J. 2017, 121, 196–223. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Athanasopoulos, V.I.; Niranjan, K.; Rastall, R.A. Synthesis of galacto-oligosaccharide from lactose using β-galactosidase from Kluyveromyces lactis: Studies on batch and continuous UF membrane-fitted bioreactors. Biotechnol. Bioeng. 2005, 89, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Czermak, P.; Ebrahimi, M.; Grau, K.; Netz, S.; Sawatzki, G.; Pfromm, P.H. Membrane-assisted enzymatic production of galactosyl-oligosaccharides from lactose in a continuous process. J. Membr. Sci. 2004, 232, 85–91. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Placido, L.; Engel, L.; Shams-Ashaghi, K.; Czermak, P. Two-Stage Integrated Ceramic Membrane Reactor System For The Continuous Enzymatic Synthesis Of Oligosaccharides. In Proceedings of the WFC10: Discover the Future of Filtration & Separation, Leipzig, Germany, 14–18 April 2008; pp. II-492–II-496. [Google Scholar]
- Foda, M.I.; Lopez-Leiva, M. Continuous production of oligosaccharides from whey using a membrane reactor. Process. Biochem. 2000, 35, 581–587. [Google Scholar] [CrossRef]
- Gonzalez, R.; Ebrahimi, M.; Czermak, P. Experimental and modeling study of Galactosyl-Oligosaccharides formation in continuous recycle membrane reactors (CRMR). Open Food Sci. J. 2009, 3, 1–9. [Google Scholar] [CrossRef]
- Pocedičová, K.; Čurda, L.; Mišún, D.; Dryáková, A.; Diblíková, L. Preparation of galacto-oligosaccharides using membrane reactor. J. Food Eng. 2010, 99, 479–484. [Google Scholar] [CrossRef]
- Ren, H.; Fei, J.; Shi, X.; Zhao, T.; Cheng, H.; Zhao, N.; Chen, Y.; Ying, H. Continuous ultrafiltration membrane reactor coupled with nanofiltration for the enzymatic synthesis and purification of galactosyl-oligosaccharides. Sep. Purif. Technol. 2015, 144, 70–79. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Guerrero, C.; Vera, C.; Illanes, A. Assessment of the fouling mechanisms of an ultrafiltration membrane bioreactor during synthesis of galacto-oligosaccharides: Effect of the operational variables. Desalination 2016, 393, 79–89. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo, C.; Vera, C.; Guerrero, C.; Illanes, A. Performance of an ultrafiltration membrane bioreactor (UF-MBR) as a processing strategy for the synthesis of galacto-oligosaccharides at high substrate concentrations. J. Biotechnol. 2016, 223, 26–35. [Google Scholar] [CrossRef]
- Matella, N.; Dolan, K.; Lee, Y.S. Comparison of galactooligosaccharide production in free-enzyme ultrafiltration and in immobilized-enzyme systems. J. Food Sci. 2006, 71, C363–C368. [Google Scholar] [CrossRef]
- Das, R.; Sen, D.; Sarkar, A.; Bhattacharyya, S.; Bhattacharjee, C. A comparative study on the production of galacto-oligosaccharide from whey permeate in recycle membrane reactor and in enzymatic batch reactor. Ind. Eng. Chem. Res. 2011, 50, 806–816. [Google Scholar] [CrossRef]
- Splechtna, B.; Nguyen, T.-H.; Haltrich, D. Comparison between discontinuous and continuous lactose conversion processes for the production of prebiotic galacto-oligosaccharides using β-galactosidase from Lactobacillus reuteri. J. Agric. Food Chem. 2007, 55, 6772–6777. [Google Scholar] [CrossRef] [PubMed]
- Petzelbauer, I.; Splechtna, B.; Nidetzky, B. Development of an ultrahigh-temperature process for the enzymatic hydrolysis of lactose. III. Utilization of two thermostable β-glycosidases in a continuous ultrafiltration membrane reactor and galacto-oligosaccharide formation under steady-state conditions. Biotechnol. Bioeng. 2002, 77, 394–404. [Google Scholar] [CrossRef]
- Warmerdam, A.; Boom, R.M.; Janssen, A.E. β-galactosidase stability at high substrate concentrations. SpringerPlus 2013, 2, 402. [Google Scholar] [CrossRef]
- Pázmándi, M.; Maráz, A.; Ladányi, M.; Kovács, Z. The impact of membrane pretreatment on the enzymatic production of whey-derived galacto-oligosaccharides. J. Food Process. Eng. 2018, 41, e12649. [Google Scholar] [CrossRef]
- Paulen, R.; Foley, G.; Fikar, M.; Kovács, Z.; Czermak, P. Minimizing the process time for ultrafiltration/diafiltration under gel polarization conditions. J. Membr. Sci. 2011, 380, 148–154. [Google Scholar] [CrossRef]
- Élysée-Collen, B.; Lencki, R.W. Protein ultrafiltration concentration polarization layer flux resistance I. Importance of protein layer morphology on flux decline with gelatin. J. Membr. Sci. 1997, 129, 101–113. [Google Scholar] [CrossRef]
- Yazdanshenas, M.; Tabatabaeenezhad, A.; Roostaazad, R.; Khoshfetrat, A. Full scale analysis of apple juice ultrafiltration and optimization of diafiltration. Sep. Purif. Technol. 2005, 47, 52–57. [Google Scholar] [CrossRef]
- Ma, S.; Kassinos, S.C.; Kassinos, D. Direct simulation of the limiting flux: I. Interpretation of the experimental results. J. Membr. Sci. 2009, 337, 81–91. [Google Scholar] [CrossRef]
- Palai, T.; Mitra, S.; Bhattacharya, P.K. Kinetics and design relation for enzymatic conversion of lactose into galacto-oligosaccharides using commercial grade β-galactosidase. J. Biosci. Bioeng. 2012, 114, 418–423. [Google Scholar] [CrossRef] [PubMed]





| Component | No3 | No5 | No2 | No7 | No4 | No6 | No1 | No8 | Batch |
|---|---|---|---|---|---|---|---|---|---|
| τ [h] | 1.1 | 2.1 | 2.2 | 2.6 | 1.1 | 2.1 | 2.2 | 2.8 | 6.0 |
| cE [U·g−1] | 19.1 | 17.3 | 19.1 | 19.1 | 190.6 | 173.4 | 190.6 | 190.6 | 5.7 |
| τ × cE [U·h·g−1] | 21.5 | 36.1 | 41.9 | 49.8 | 215.4 | 360.8 | 423.6 | 537.5 | 34.3 |
| P [g·h−1·U−1] × 10−3 | 3.42 | 2.28 | 2.32 | 1.87 | 0.46 | 0.28 | 0.23 | 0.18 | 3.28 |
| DP2 | 63.8 | 61.7 | 50.5 | 53.8 | 45.0 | 41.9 | 40.2 | 41.7 | 44.2 |
| Glu | 11.7 | 10.5 | 17.1 | 14.3 | 18.6 | 20.8 | 20.8 | 22.2 | 17.2 |
| Gal | 0.0 | 0.4 | 0.0 | 0.9 | 3.3 | 3.8 | 6.2 | 4.2 | 1.0 |
| DP3 | 19.7 | 22.0 | 22.9 | 22.9 | 21.6 | 20.6 | 20.6 | 20.7 | 25.0 |
| DP4 | 4.4 | 5.4 | 7.5 | 6.8 | 7.9 | 8.8 | 8.3 | 8.6 | 10.5 |
| DP5 | 0.6 | 0.0 | 1.9 | 1.3 | 2.7 | 4.0 | 3.7 | 2.6 | 2.0 |
| DP6 | 0.0 | 0.0 | 0.2 | 0.0 | 1.0 | 0.0 | 0.1 | 0.0 | 0.1 |
| DP3-6 | 24.6 | 27.4 | 32.4 | 31.0 | 33.2 | 33.4 | 32.8 | 31.9 | 37.6 |
| Response Variable | Model Parameters | Goodness of Fit | ||||
|---|---|---|---|---|---|---|
| b1 | b2 | SSE | R2 | Adjusted-R2 | RMSE | |
| DP2 | 3.932 | 0.06594 | 65.39 | 0.9779 | 0.9754 | 2.695 |
| DP3-6 | 5.224 | 0.1547 | 22.26 | 0.9769 | 0.9744 | 1.573 |
| Glu | 0.951 | 0.04417 | 28.26 | 0.9307 | 0.923 | 1.772 |
| Gal | 0.01835 | 0.002243 | 28.26 | 0.9319 | 0.9244 | 0.5721 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, T.; Pázmándi, M.; Galambos, I.; Kovács, Z. Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes. Membranes 2020, 10, 203. https://doi.org/10.3390/membranes10090203
Cao T, Pázmándi M, Galambos I, Kovács Z. Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes. Membranes. 2020; 10(9):203. https://doi.org/10.3390/membranes10090203
Chicago/Turabian StyleCao, Teng, Melinda Pázmándi, Ildikó Galambos, and Zoltán Kovács. 2020. "Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes" Membranes 10, no. 9: 203. https://doi.org/10.3390/membranes10090203
APA StyleCao, T., Pázmándi, M., Galambos, I., & Kovács, Z. (2020). Continuous Production of Galacto-Oligosaccharides by an Enzyme Membrane Reactor Utilizing Free Enzymes. Membranes, 10(9), 203. https://doi.org/10.3390/membranes10090203