Diffusion Dialysis for Acid Recovery from Acidic Waste Solutions: Anion Exchange Membranes and Technology Integration
Abstract
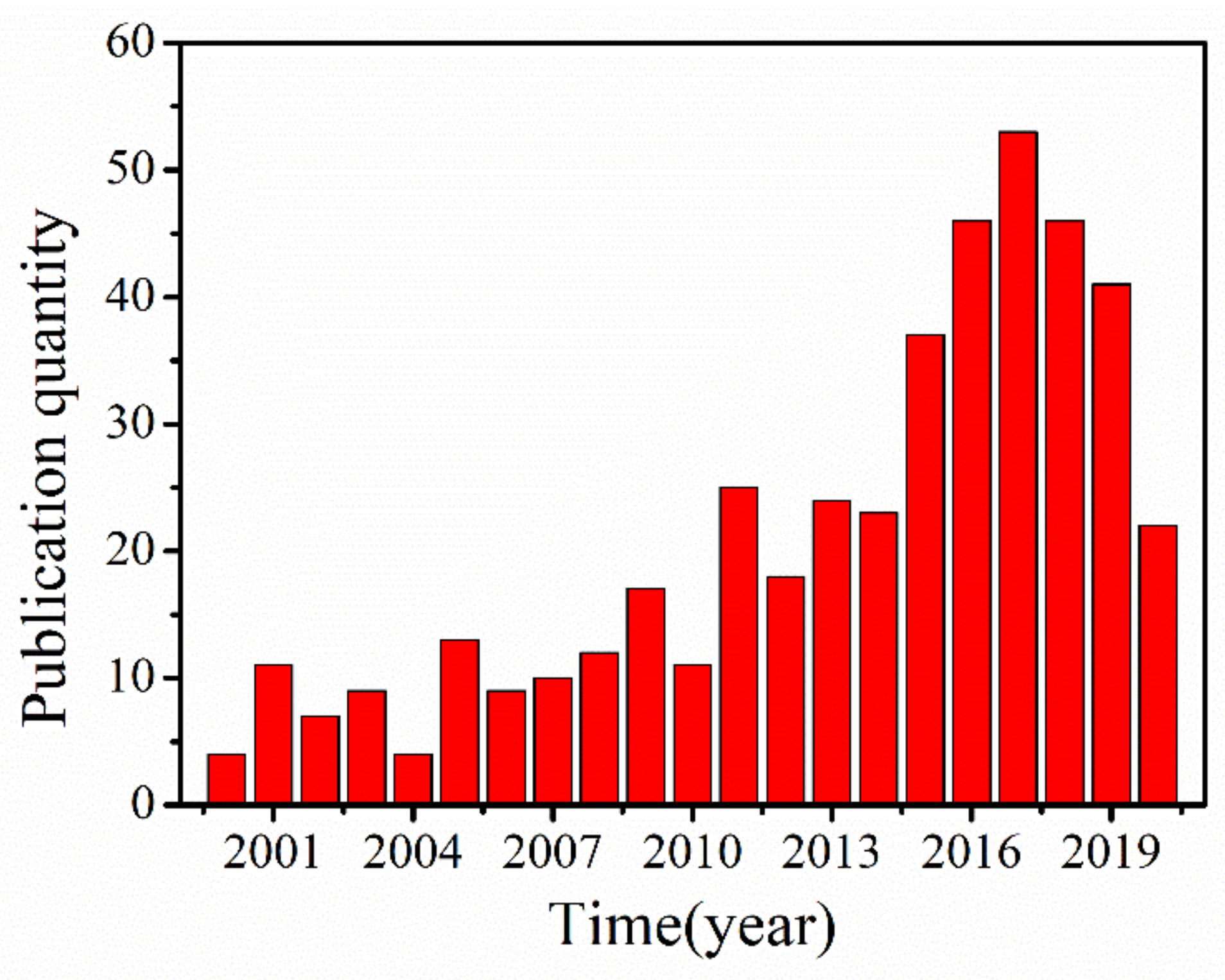
1. Introduction
- Low energy consumption owing to the spontaneity of the process driven by an activity gradient;
- Low installation costs, simple operation, and maintenance;
- High product quality due to the high selectivity of AEMs for acids;
- Environmentally friendliness because of no extra postprocessing and chemical agents.
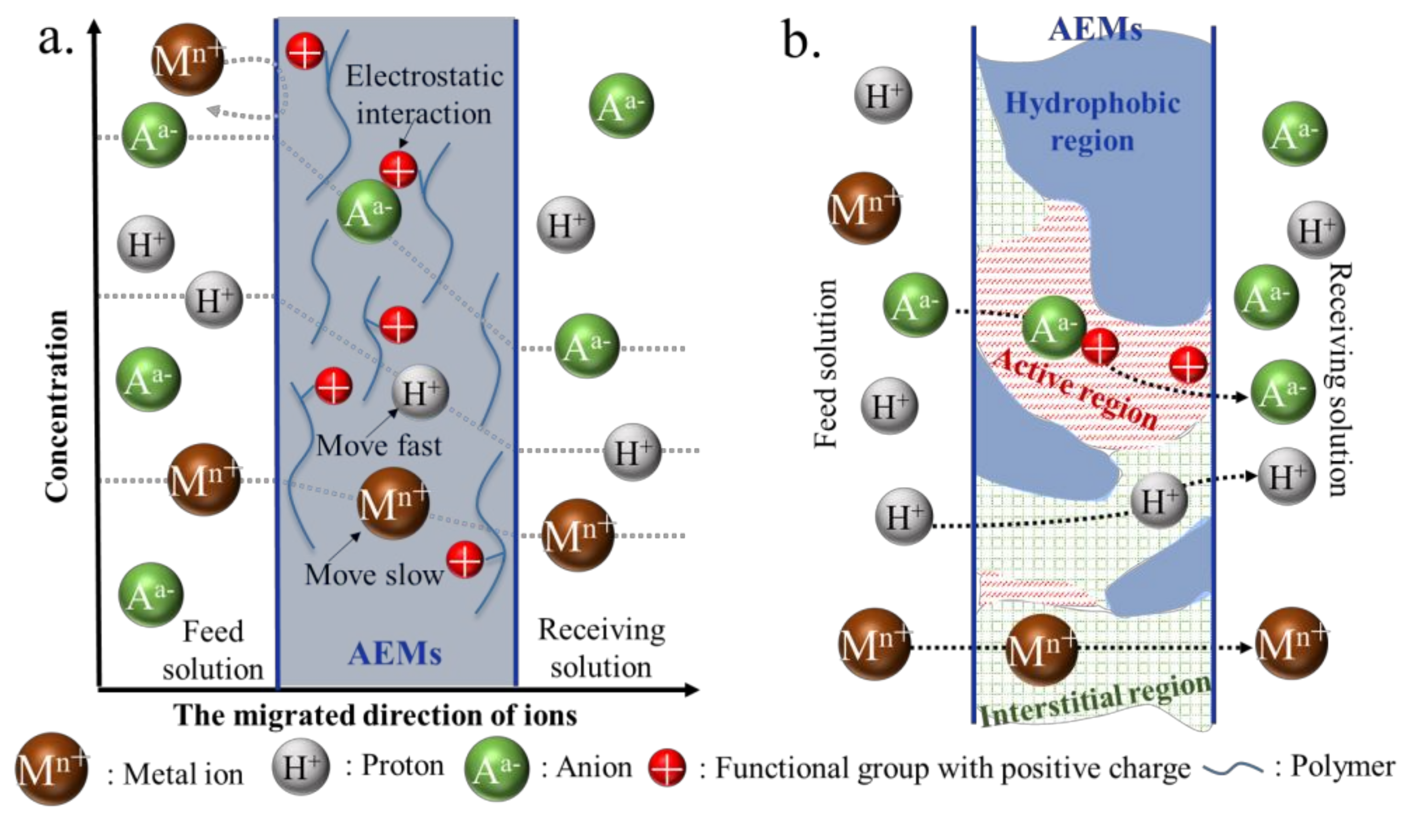
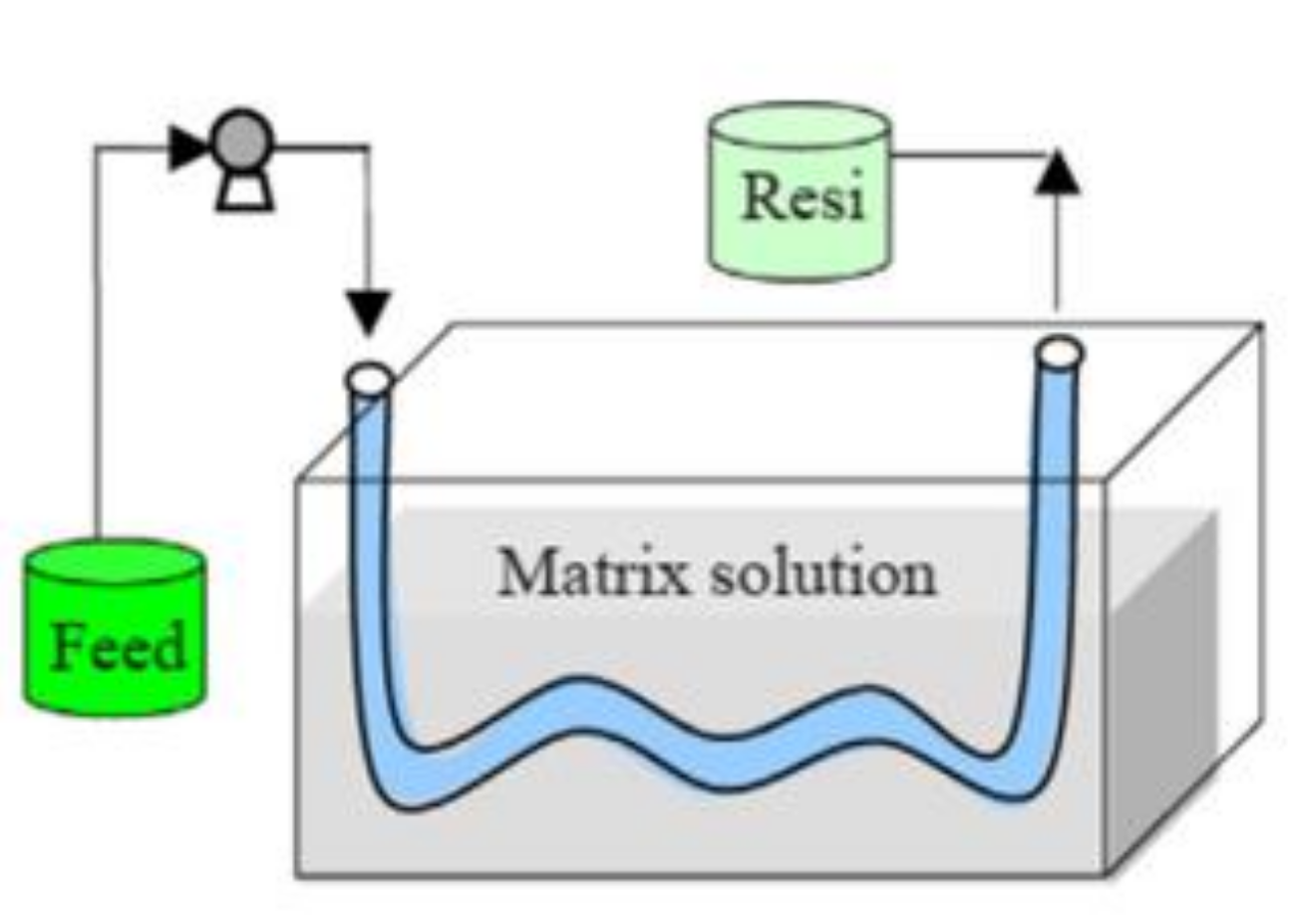
2. Description of Acid Recovery Using AEMs
3. Acid Permeability
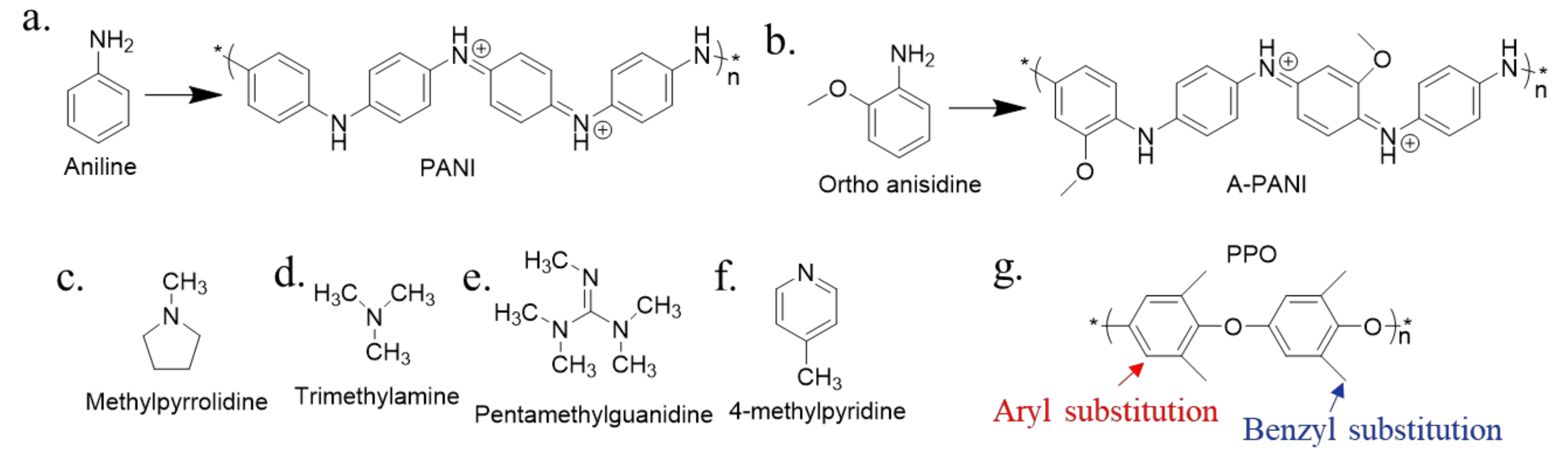
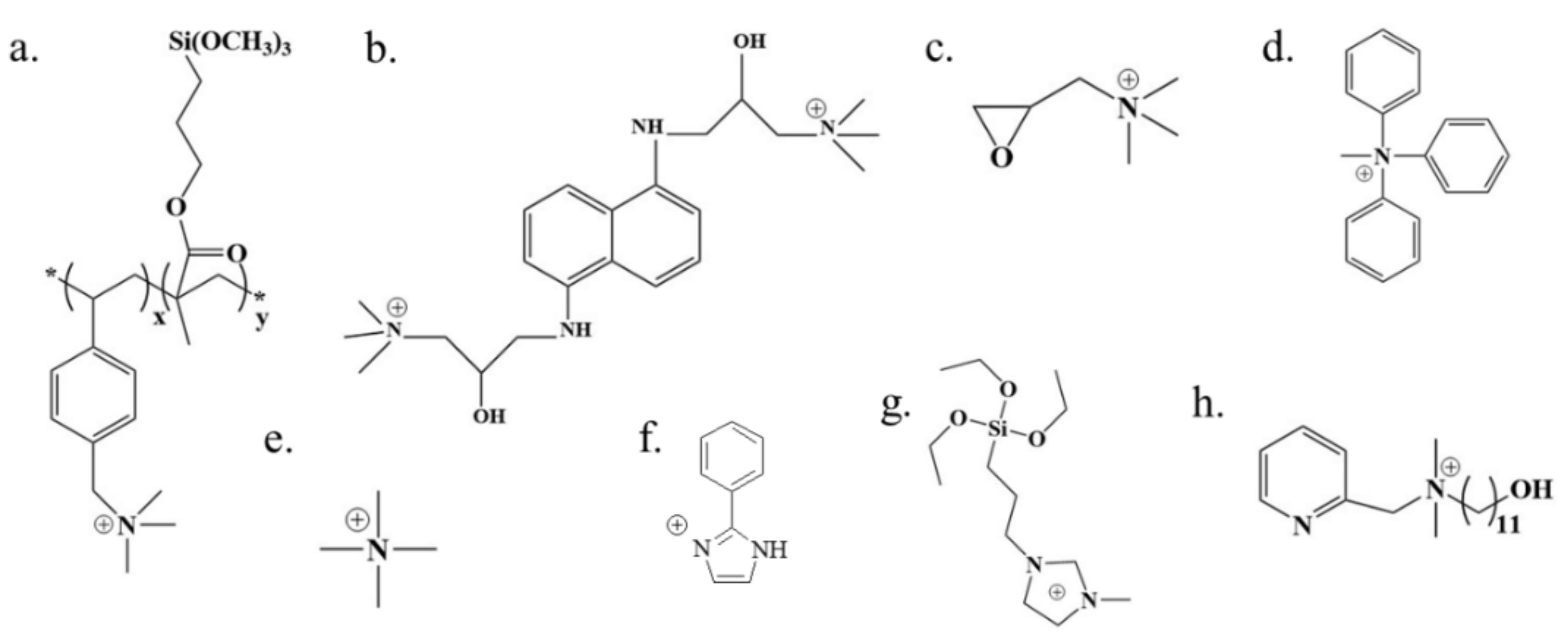
3.1. Alkaline Functional Groups for Permeability
3.2. Acid-Alkali Functional Groups for Permeability
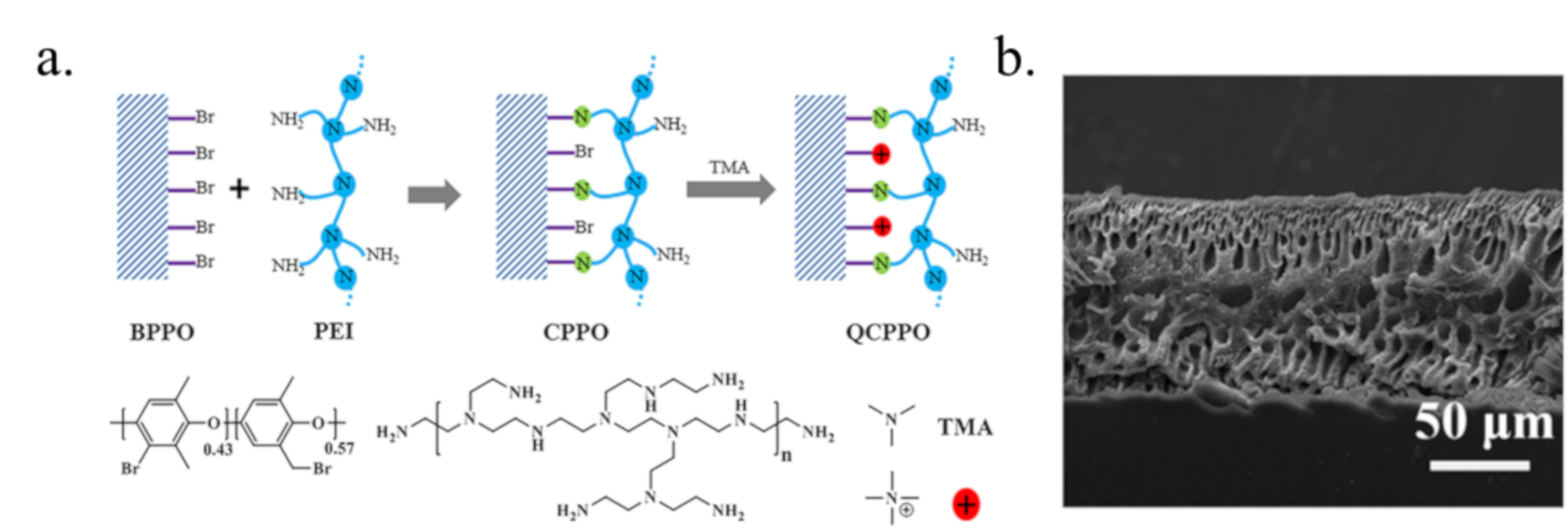
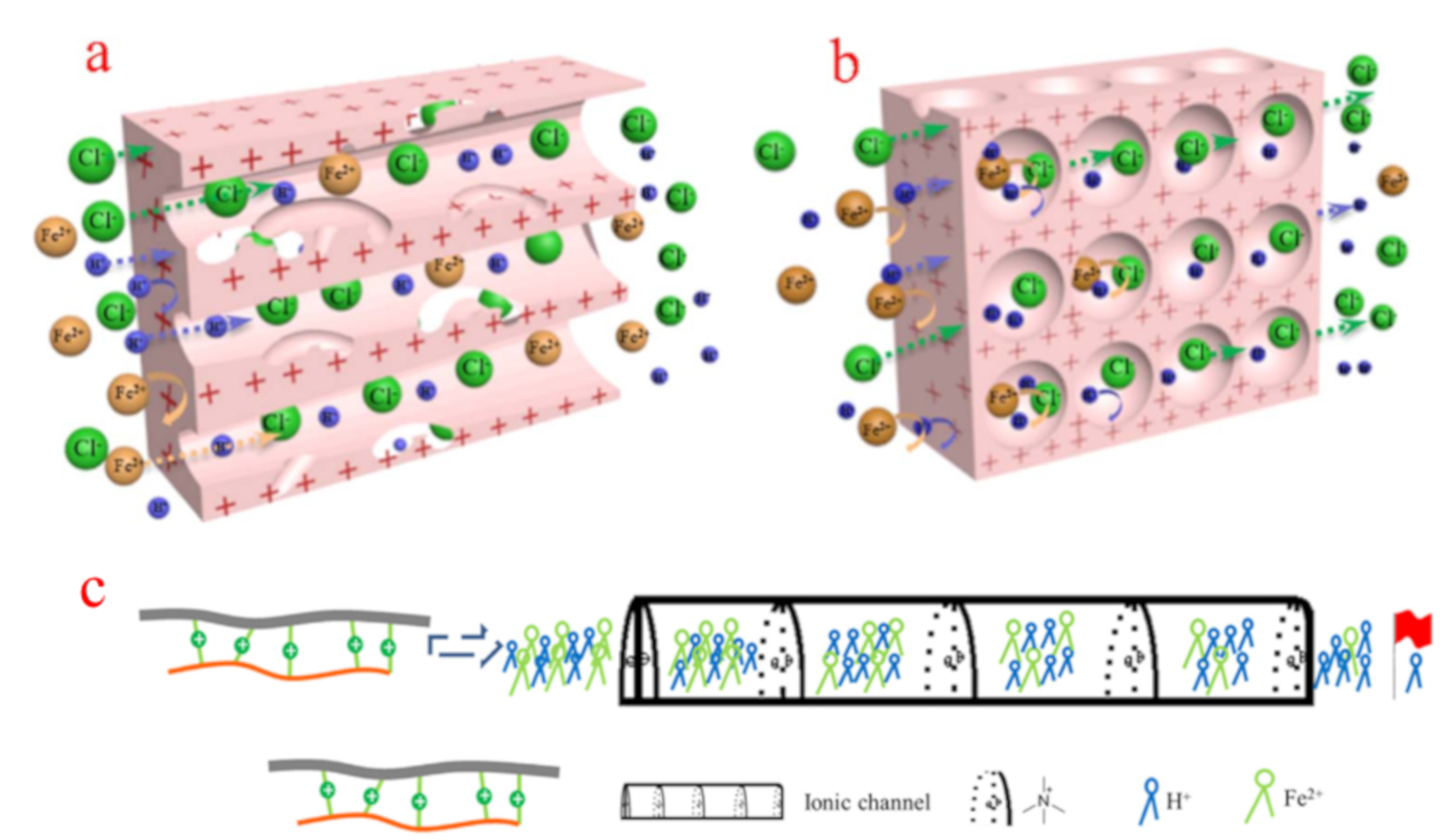
3.3. Membrane Structure
4. Acid Selectivity
4.1. Alkaline Functional Groups for Selectivity
4.2. Acid-Alkali Functional Groups for Selectivity
4.3. Size-Sieving Effect
5. Trade-Off Effects between Acid Permeability and Selectivity
6. Membrane Stability
6.1. Types of Functional Groups
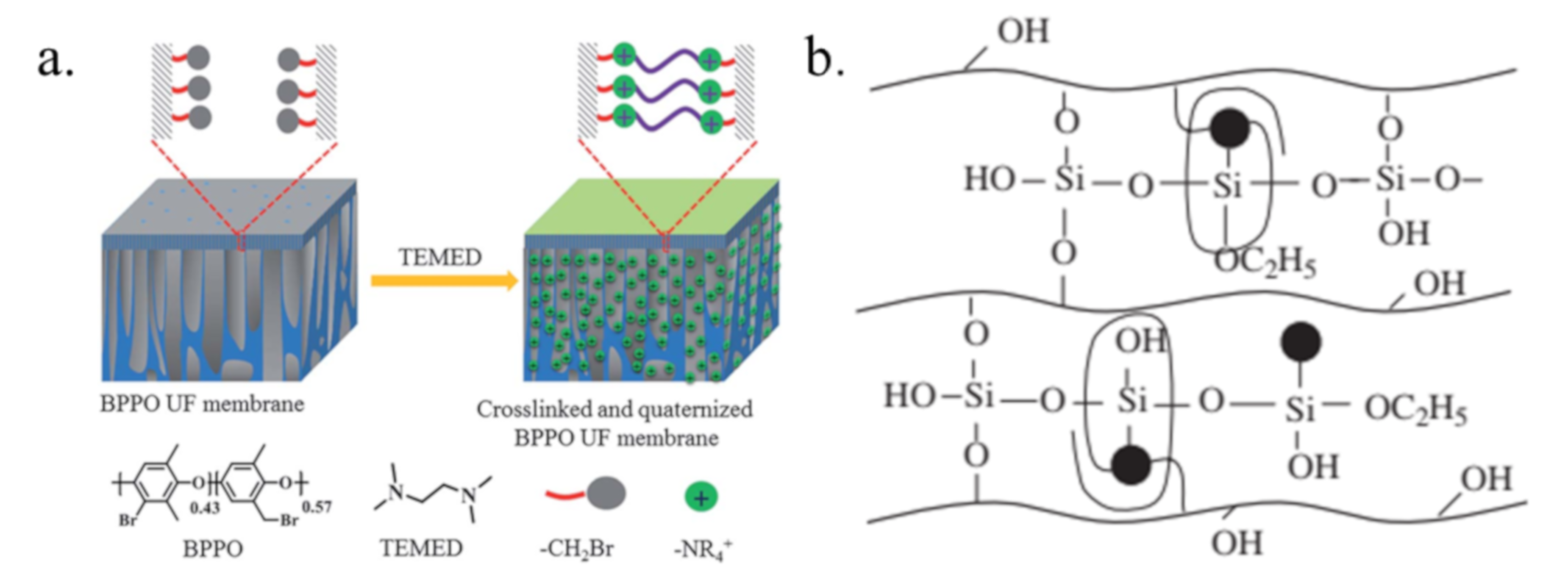
6.2. Crosslinking and Incorporating Inorganic Components
7. The Integration of Diffusion Dialysis with Other Technologies
7.1. The Integration of Diffusion Dialysis with Pressure
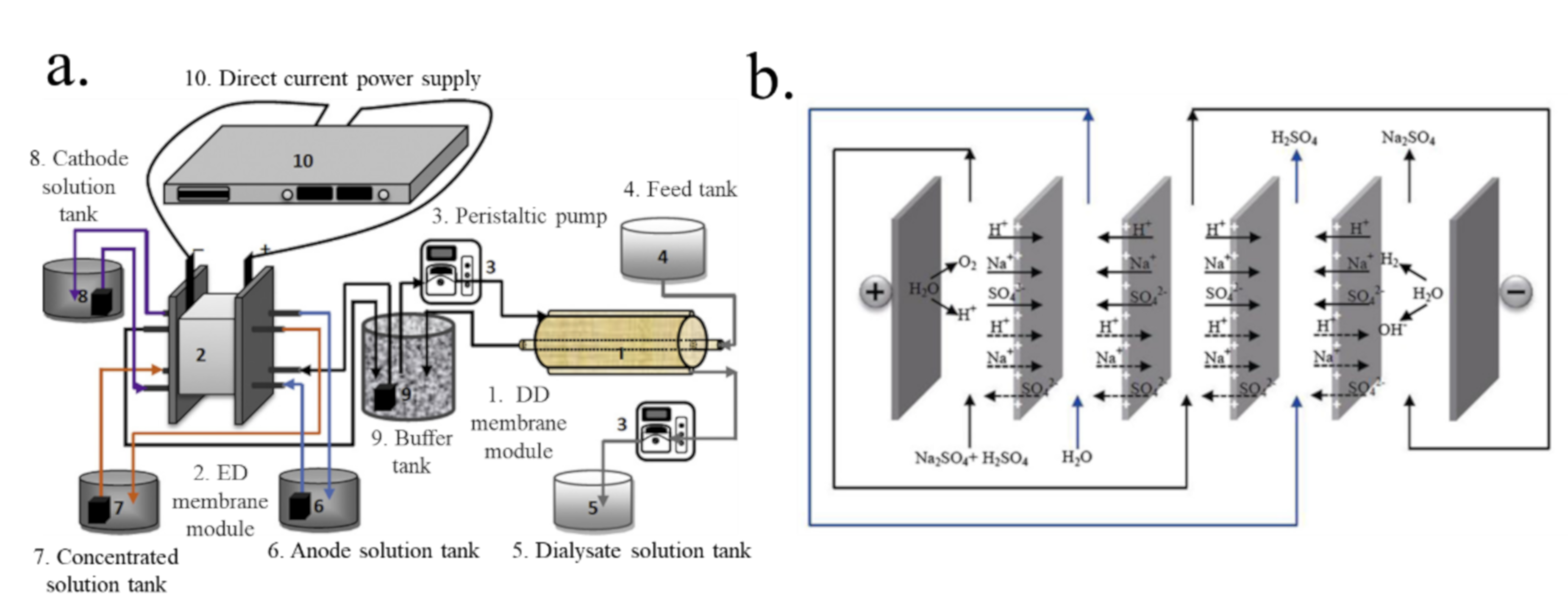
7.2. The Integration of Diffusion Dialysis with an Electric Field
7.3. The Integration of Diffusion Dialysis with a Continuous Process
8. Summary and Perspective
Author Contributions
Funding
Conflicts of Interest
Appendix A. List of Abbreviations
| A-PANI | Poly(o-anisidine) |
| b-PEI | Branched polyethyleneimine |
| BPPO | Brominated poly(2,6-dimethyl-1,4-phenylene oxide) |
| DABVO | 1,4-diazabicyclo[2,2,2]octane |
| DMAM | Dimethylaminoethyl methacrylate |
| DMEA | Dimethylethanolamine |
| DVB | Divinylbenzene |
| GMA | Glycidylmethacrylate |
| GO | Graphene oxide |
| NMP | 1-methyl-2-pyrrolidone |
| MPS | Methacryloxypropyl trimethoxy silane |
| PANI | Polyaniline |
| PE | Polyethylene |
| PEI | Polyethyleneimine |
| PES | Polyethersulfone |
| PFDD | Plate-and-frame diffusion dialysis |
| PP | Polypropylene |
| PPO | Poly(2,6-dimethyl-1,4-phenylene oxide) |
| PSF | Polysulfone |
| PVA | Polyvinyl alcohol |
| PVC | Polyvinyl chloride |
| QBAPB | 4,4′-(1,1′-biphenyl-4,4′-diyldioxy)dianiline |
| Q-DAN | Quaternary 1,5-diaminonaphthalene |
| QUDAP | Quaternary 1-hydroxy-N,N-dimethyl-N-(pyridine-2-ylmethyl) methanaminium |
| SWDD | Spiral wound diffusion dialysis |
| TDA | Tris(2-(2-methoxyethoxy)ethyl)amine |
| TEMED | N,N,N′,N′-tetramethylethylenediamine |
| TMPDA | N,N,N′,N′-tetramethyl-1,3-propanediamine |
References
- Yang, C.-C.; Pan, J.; Zhu, D.-Q.; Guo, Z.-Q.; Li, X.-M. Pyrometallurgical recycling of stainless steel pickling sludge: A review. J. Iron Steel Res. Int. 2019, 26, 547–557. [Google Scholar] [CrossRef]
- Foureaux, A.F.S.; Moreira, V.R.; Lebron, Y.A.R.; Santos, L.V.S.; Amaral, M.C.S. Direct contact membrane distillation as an alternative to the conventional methods for value-added compounds recovery from acidic effluents: A review. Sep. Purif. Technol. 2020, 236, 116251. [Google Scholar] [CrossRef]
- Hogle, B.P.; Shekhawat, D.; Nagarajan, K.; Jackson, J.E.; Miller, D.J. Formation and Recovery of Itaconic Acid from Aqueous Solutions of Citraconic Acid and Succinic Acid. Ind. Eng. Chem. Res. 2002, 41, 2069–2073. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Du, C.; Blaga, A.-C.; Cămăruţ, M.; Webb, C.; Stevens, C.V.; Soetaert, W. Novel resin-based vacuum distillation-crystallisation method for recovery of succinic acid crystals from fermentation broths. Green Chem. 2010, 12, 666. [Google Scholar] [CrossRef]
- Rasrendra, C.B.; Girisuta, B.; Bovenkamp, H.V.D.; Winkelman, J.G.M.; Leijenhorst, E.; Venderbosch, R.; Windt, M.; Meier, D.; Heeres, H. Recovery of acetic acid from an aqueous pyrolysis oil phase by reactive extraction using tri-n-octylamine. Chem. Eng. J. 2011, 176, 244–252. [Google Scholar] [CrossRef]
- Ijmker, H.; Gramblicka, M.; Kersten, S.; Ham, A.V.D.; Schuur, B. Acetic acid extraction from aqueous solutions using fatty acids. Sep. Purif. Technol. 2014, 125, 256–263. [Google Scholar] [CrossRef]
- Shin, C.-H.; Kim, J.; Kim, H.-S.; Lee, H.-S.; Mohapatra, D.; Ahn, J.-W.; Ahn, J.-G.; Bae, W. Recovery of nitric acid from waste etching solution using solvent extraction. J. Hazard. Mater. 2009, 163, 729–734. [Google Scholar] [CrossRef]
- Lin, S.H.; Kiang, C.D. Chromic acid recovery from waste acid solution by an ion exchange process: Equilibrium and column ion exchange modeling. Chem. Eng. J. 2003, 92, 193–199. [Google Scholar] [CrossRef]
- Moldes, A.B.; Alonso, J.L.; Parajó, J.C. Resin selection and single-step production and recovery of lactic acid from pretreated wood. Appl. Biochem. Biotechnol. 2001, 95, 69–82. [Google Scholar] [CrossRef]
- Nenov, V.; Dimitrova, N.; Dobrevsky, I. Recovery of sulphuric acid from waste aqueous solutions containing arsenic by ion exchange. Hydrometallurgy 1997, 44, 43–52. [Google Scholar] [CrossRef]
- Agrawal, A.; Sahu, K.K. An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries. J. Hazard. Mater. 2009, 171, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, M. Recovery of hydrochloric acid from metal pickling solutions by membrane distillation. Sep. Purif. Technol. 2001, 22, 591–600. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Kim, B.; Kim, S.; Kim, W.; Lee, J. Recovery of H2SO4 from waste acid solution by a diffusion dialysis method. J. Hazard. Mater. 2005, 124, 230–235. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Stocks, C.; Wood, J.; Guy, S. Minimisation and recycling of spent acid wastes from galvanizing plants. Resour. Conserv. Recycl. 2005, 44, 153–166. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Kobuchi, Y.; Motomura, H.; Noma, Y.; Hanada, F. Application of ion exchange membranes to the recovery of acids by diffusion dialysis. J. Membr. Sci. 1986, 27, 173–179. [Google Scholar] [CrossRef]
- Zaman, N.K.; Rohani, R.; Mohammad, A.W.; Isloor, A.M.; Jahim, J.M. Investigation of succinic acid recovery from aqueous solution and fermentation broth using polyimide nanofiltration membrane. J. Environ. Chem. Eng. 2020, 8, 101895. [Google Scholar] [CrossRef]
- Sun, X.; Lu, H.; Wang, J. Recovery of citric acid from fermented liquid by bipolar membrane electrodialysis. J. Clean. Prod. 2017, 143, 250–256. [Google Scholar] [CrossRef]
- Palatý, Z.; Žáková, A. Separation of H2SO4 + ZnSO4 mixture by diffusion dialysis. Desalination 2004, 169, 277–285. [Google Scholar] [CrossRef]
- Palatý, Z.; Žáková, A. Separation of HCl + NiCl2 Mixture by Diffusion Dialysis. Sep. Sci. Technol. 2007, 42, 1965–1983. [Google Scholar] [CrossRef]
- Xu, T.; Yang, W. Industrial recovery of mixed acid (HF + HNO3) from the titanium spent leaching solutions by diffusion dialysis with a new series of anion exchange membranes. J. Membr. Sci. 2003, 220, 89–95. [Google Scholar] [CrossRef]
- Palatý, Z.; Bendová, H. Permeability of a Fumasep-FAD Membrane for Selected Inorganic Acids. Chem. Eng. Technol. 2018, 41, 385–391. [Google Scholar] [CrossRef]
- Lan, S.; Wen, X.; Zhu, Z.; Shao, F.; Zhu, C. Recycling of spent nitric acid solution from electrodialysis by diffusion dialysis. Desalination 2011, 278, 227–230. [Google Scholar] [CrossRef]
- Narębska, A.; Staniszewski, M.; Narȩbska, A. Separation of Carboxylic Acids from Carboxylates by Diffusion Dialysis. Sep. Sci. Technol. 2008, 43, 490–501. [Google Scholar] [CrossRef]
- Palatý, Z.; Stoček, P.; Bendová, H.; Prchal, P. Continuous dialysis of carboxylic acids: Solubility and diffusivity in Neosepta-AMH membranes. Desalination 2009, 243, 65–73. [Google Scholar] [CrossRef]
- Khan, M.I.; Luque, R.; Akhtar, S.; Shaheen, A.; Mehmood, A.; Idress, S.; Buzdar, S.A.; Rehman, A.U. Design of Anion Exchange Membranes and Electrodialysis Studies for Water Desalination. Materials 2016, 9, 365. [Google Scholar] [CrossRef]
- Kim, J.H.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Anion dxchange membranes obtained from poly(arylene ether sulfone) block copolymers comprising hydrophilic and hydrophobic segments. Polymers 2020, 12, 325. [Google Scholar] [CrossRef]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Improved electrochemical performance of composite anion exchange membranes for fuel cells through cross linking of the polymer chain with functionalized graphene oxide. J. Membr. Sci. 2020, 611, 118385. [Google Scholar] [CrossRef]
- Chu, J.Y.; Lee, K.H.; Kim, A.R.; Yoo, D.J. Study on the Chemical Stabilities of Poly(arylene ether) Random Copolymers for Alkaline Fuel Cells: Effect of Main Chain Structures with Different Monomer Units. ACS Sustain. Chem. Eng. 2019, 7, 20077–20087. [Google Scholar] [CrossRef]
- Khan, M.I.; Wu, L.; Hossain, M.; Pan, J.; Ran, J.; Mondal, A.N.; Xu, T. Preparation of diffusion dialysis membrane for acid recovery via a phase-inversion method. Membr. Water Treat. 2015, 6. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Yao, Z.; Pan, J.; Hossain, M.; Khan, M.I.; Yang, Z.; Wu, L.; Xu, T. Novel quaternized aromatic amine based hybrid PVA membranes for acid recovery. J. Membr. Sci. 2015, 490, 29–37. [Google Scholar] [CrossRef]
- Wei, C.; Li, X.; Deng, Z.; Fan, G.; Li, M.; Li, C. Recovery of H2SO4 from an acid leach solution by diffusion dialysis. J. Hazard. Mater. 2010, 176, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Lo, M.C. Recovery of sulfuric acid from waste aluminum surface processing solution by diffusion dialysis. J. Hazard. Mater. 1998, 60, 247–257. [Google Scholar] [CrossRef]
- Web of Science. 2020. Available online: http://apps.webofknowledge.com (accessed on 23 July 2020).
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, B.; Anderson, A. Sustainable resolutions for environmental threat of the acid mine drainage. Sci. Total. Environ. 2020, 717, 137211. [Google Scholar] [CrossRef]
- Aktij, S.A.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.; Tiraferri, A.; Rahimpour, A. Feasibility of membrane processes for the recovery and purification of bio-based volatile fatty acids: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar] [CrossRef]
- Handojo, L.; Wardani, A.K.; Regina, D.; Bella, C.; Kresnowati, M.T.A.P.; Wenten, I.G. Electro-membrane processes for organic acid recovery. RSC Adv. 2019, 9, 7854–7869. [Google Scholar] [CrossRef]
- Talnikar, V.D.; Mahajan, Y.S. Recovery of acids from dilute streams: A review of process technologies. Korean J. Chem. Eng. 2014, 31, 1720–1731. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Xu, T.; Yang, W. Tuning the diffusion dialysis performance by surface cross-linking of PPO anion exchange membranes-simultaneous recovery of sulfuric acid and nickel from electrolysis spent liquor of relatively low acid concentration. J. Hazard. Mater. 2004, 109, 157–164. [Google Scholar]
- Luo, J.; Wu, C.; Wu, Y.; Xu, T. Diffusion dialysis of hydrochloride acid at different temperatures using PPO–SiO2 hybrid anion exchange membranes. J. Membr. Sci. 2010, 347, 240–249. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Wu, Y.; Gu, J.; Xu, T. Polyelectrolyte complex/PVA membranes for diffusion dialysis. J. Hazard. Mater. 2013, 261, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Tugas, I.; Pourcelly, G.; Gavach, C. Electrotransport of protons and chloride ions in anion exchange membranes for the recovery of acids. Part I. Equilibrium properties. J. Membr. Sci. 1993, 85, 183–194. [Google Scholar] [CrossRef]
- Ersoz, M.; Gugul, I.; Şahin, A. Transport of Acids through Polyether–Sulfone Anion-Exchange Membrane. J. Colloid Interface Sci. 2001, 237, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kim, S.; Zhu, D.M.; Shamsaei, E.; Xu, T.; Fang, X.; Wang, H. Preparation of porous diffusion dialysis membranes by functionalization of polysulfone for acid recovery. J. Membr. Sci. 2017, 524, 557–564. [Google Scholar] [CrossRef]
- Mondal, P.; Samanta, N.S.; Kumar, A.; Purkait, M.K. Recovery of H2SO4 from wastewater in the presence of NaCl and KHCO3 through pH responsive polysulfone membrane: Optimization approach. Polym. Test. 2020, 86, 106463. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Cheng, C.; Pan, J.; Emmanuel, K.; Wu, L.; Xu, T. Porous BPPO-based membranes modified by aromatic amine for acid recovery. Sep. Purif. Technol. 2016, 157, 27–34. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Emmanuel, K.; Hossain, M.; Afsar, N.U.; Wu, L.; Xu, T. Preparation of Pyrrolidinium-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis. Sep. Sci. Technol. 2016, 51, 1881–1890. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, Q.; Zhao, Y.; Lu, W.; Li, X.; Zhang, H. Polypyrrole modified porous poly(ether sulfone) membranes with high performance for vanadium flow batteries. J. Mater. Chem. A 2016, 4, 12955–12962. [Google Scholar] [CrossRef]
- Zhao, Y.; Mai, Z.; Shen, P.; Ortega, E.; Shen, J.; Gao, C.; Van Der Bruggen, B. Nanofiber Based Organic Solvent Anion Exchange Membranes for Selective Separation of Monovalent anions. ACS Appl. Mater. Interfaces 2020, 12, 7539–7547. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, C.; Yao, N.; Zhang, P.; Hong, T.; Xu, C.; Cheng, J. Quaternary Ti3C2Tx enhanced ionic conduction in quaternized polysulfone membrane for alkaline anion exchange membrane fuel cells. J. Membr. Sci. 2018, 563, 882–887. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Jackson, A.C.; Savage, A.M.; Ertem, S.P.; Tsai, T.-H.; Seifert, S.; Beyer, F.L.; Liberatore, M.W.; Herring, A.M.; et al. Achieving Continuous Anion Transport Domains Using Block Copolymers Containing Phosphonium Cations. Macromolecules 2016, 49, 4714–4722. [Google Scholar] [CrossRef]
- Cho, H.; Krieg, H.M.; Kerres, J.A. Performances of Anion-Exchange Blend Membranes on Vanadium Redox Flow Batteries. Membranes 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.K.; Reddy, N.N.; Nimiwal, R.; Singh, P.S.; Adimurthy, S.; Nagarale, R. Polyaniline@porous polypropylene for efficient separation of acid by diffusion dialysis. Sep. Purif. Technol. 2020, 233, 115989. [Google Scholar] [CrossRef]
- Xu, T.; Yang, W. Sulfuric acid recovery from titanium white (pigment) waste liquor using diffusion dialysis with a new series of anion exchange membranes-static runs. J. Membr. Sci. 2001, 183, 193–200. [Google Scholar]
- Gu, F.; Dong, H.; Li, Y.; Sun, Z.; Yan, F. Base Stable Pyrrolidinium Cations for Alkaline Anion Exchange Membrane Applications. Macromolecules 2014, 47, 6740–6747. [Google Scholar] [CrossRef]
- Döbbelin, M.; Azcune, I.; Bedu, M.; De Luzuriaga, A.R.; Genua, A.; Jovanovski, V.; Cabañero, G.; Odriozola, I. Synthesis of Pyrrolidinium-Based Poly(ionic liquid) Electrolytes with Poly(ethylene glycol) Side Chains. Chem. Mater. 2012, 24, 1583–1590. [Google Scholar] [CrossRef]
- Lin, X.; Wu, L.; Liu, Y.; Ong, A.L.; Poynton, S.; Varcoe, J.R.; Xu, T. Alkali resistant and conductive guanidinium-based anion-exchange membranes for alkaline polymer electrolyte fuel cells. J. Power Sources 2012, 217, 373–380. [Google Scholar] [CrossRef]
- Khan, M.I.; Khraisheh, M.; Almomani, F. Fabrication and characterization of pyridinium functionalized anion exchange membranes for acid recovery. Sci. Total. Environ. 2019, 686, 90–96. [Google Scholar] [CrossRef]
- Ji, W.; Wu, B.; Zhu, Y.; Irfan, M.; Afsar, N.U.; Ge, L.; Xu, T. Self-organized nanostructured anion exchange membranes for acid recovery. Chem. Eng. J. 2020, 382, 122838. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Yao, L.; Wu, C.; Mao, F.; Xu, T. PVA/SiO2 anion exchange hybrid membranes from multisilicon copolymers with two types of molecular weights. J. Membr. Sci. 2012, 399, 16–27. [Google Scholar] [CrossRef]
- Irfan, M.; Afsar, N.U.; Ge, L.; Xu, T. Investigation of key process parameters in acid recovery for diffusion dialysis using novel (MDMH-QPPO) anion exchange membranes. J. Taiwan Inst. Chem. Eng. 2018, 93, 405–413. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, Z.; He, Y.; Mondal, A.N.; Bakangura, E.; Xu, T. Diffusion dialysis membranes with semi-interpenetrating network for acid recovery. J. Membr. Sci. 2015, 493, 645–653. [Google Scholar] [CrossRef]
- Cheng, C.; Li, P.; He, Y.; Hu, X.; Emmanuel, K. Branched Polyvinyl Alcohol Hybrid Membrane for Acid Recovery via Diffusion Dialysis. Chem. Eng. Technol. 2019, 42, 1180–1187. [Google Scholar] [CrossRef]
- Emmanuel, K.; Cheng, C.; Erigene, B.; Mondal, A.N.; Afsar, N.U.; Khan, M.I.; Hossain, M.; Jiang, C.; Ge, L.; Wu, L.; et al. Novel synthetic route to prepare doubly quaternized anion exchange membranes for diffusion dialysis application. Sep. Purif. Technol. 2017, 189, 204–212. [Google Scholar] [CrossRef]
- Yadav, S.; Soontarapa, K.; Jyothi, M.S.; Padaki, M.; Balakrishna, R.G.; Lai, J.-Y. Supplementing multi-functional groups to polysulfone membranes using Azadirachta indica leaves powder for effective and highly selective acid recovery. J. Hazard. Mater. 2019, 369, 1–8. [Google Scholar] [CrossRef]
- González, M.I.; Alvarez, S.; Riera, F.A.; Alvarez, R. Lactic acid recovery from whey ultrafiltrate fermentation broths and artificial solutions by nanofiltration. Desalination 2008, 228, 84–96. [Google Scholar] [CrossRef]
- Stachera, D.M.; Childs, R.F. Tuning the acid recovery performance of poly(4-vinylpyridine)-filled membranes by the introduction of hydrophobic groups. J. Membr. Sci. 2001, 187, 213–225. [Google Scholar] [CrossRef]
- Stachera, D.M.; Childs, R.; Mika, A.; Dickson, J.M. Acid recovery using diffusion dialysis with poly(4-vinylpyridine)-filled microporous membranes. J. Membr. Sci. 1998, 148, 119–127. [Google Scholar] [CrossRef]
- Prajapati, P.K.; Nimiwal, R.; Singh, P.S.; Nagarale, R. Polyaniline-co-epichlorohydrin nanoporous anion exchange membranes for diffusion dialysis. Polymer 2019, 170, 168–178. [Google Scholar] [CrossRef]
- Chavan, V.; Agarwal, C.; Adya, V.; Pandey, A.K. Hybrid organic-inorganic anion-exchange pore-filled membranes for the recovery of nitric acid from highly acidic aqueous waste streams. Water Res. 2018, 133, 87–98. [Google Scholar] [PubMed]
- Kim, D.-H.; Park, J.-H.; Seo, S.-J.; Park, J.-S.; Jung, S.P.; Kang, Y.S.; Choi, J.-H.; Kang, M.-S. Development of thin anion-exchange pore-filled membranes for high diffusion dialysis performance. J. Membr. Sci. 2013, 447, 80–86. [Google Scholar] [CrossRef]
- Xiarchos, I.; Doulia, D.; Gekas, V.; Trägårdh, G. Polymeric Ultrafiltration Membranes and Surfactants. Sep. Purif. Rev. 2003, 32, 215–278. [Google Scholar] [CrossRef]
- Tang, B.; Xu, T.; Gong, M.; Yang, W. A novel positively charged asymmetry membranes from poly(2,6-dimethyl-1,4-phenylene oxide) by benzyl bromination and in situ amination: Membrane preparation and characterization. J. Membr. Sci. 2005, 248, 119–125. [Google Scholar] [CrossRef]
- Lin, X.; Shamsaei, E.; Kong, B.; Liu, J.Z.; Hu, Y.; Xu, T.; Wang, H. Porous diffusion dialysis membranes for rapid acid recovery. J. Membr. Sci. 2016, 502, 76–83. [Google Scholar] [CrossRef]
- Sun, F.; Wu, C.; Wu, Y.; Xu, T. Porous BPPO-based membranes modified by multisilicon copolymer for application in diffusion dialysis. J. Membr. Sci. 2014, 450, 103–110. [Google Scholar]
- Jyothi, M.; Yadav, S.; Balakrishna, G. Effective recovery of acids from egg waste incorporated PSf membranes: A step towards sustainable development. J. Membr. Sci. 2018, 549, 227–235. [Google Scholar] [CrossRef]
- Pan, J.; He, Y.; Wu, L.; Jiang, C.; Wu, B.; Mondal, A.N.; Cheng, C.; Xu, T. Anion exchange membranes from hot-pressed electrospun QPPO–SiO2 hybrid nanofibers for acid recovery. J. Membr. Sci. 2015, 480, 115–121. [Google Scholar] [CrossRef]
- Dakashev, A.D.; Stancheva, K.A. Quantitative chemical analysis of electrolytes in aqueous solutions exploiting the Donnan dialysis process. J. Anal. Chem. 2008, 63, 69–74. [Google Scholar] [CrossRef]
- Emmanuel, K.; Erigene, B.; Cheng, C.; Mondal, A.N.; Hossain, M.; Khan, M.I.; Afsar, N.U.; Ge, L.; Wu, L.; Xu, T. Facile synthesis of pyridinium functionalized anion exchange membranes for diffusion dialysis application. Sep. Purif. Technol. 2016, 167, 108–116. [Google Scholar] [CrossRef]
- Emmanuel, K.; Cheng, C.; Ge, L.; Mondal, A.N.; Hossain, M.; Khan, M.I.; Afsar, N.U.; Liang, G.; Wu, L.; Xu, T. Imidazolium functionalized anion exchange membrane blended with PVA for acid recovery via diffusion dialysis process. J. Membr. Sci. 2016, 497, 209–215. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, Z.; Pan, J.; Tong, B.; Xu, T. Facile and cost effective PVA based hybrid membrane fabrication for acid recovery. Sep. Purif. Technol. 2014, 136, 250–257. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, H.-S.; Seo, S.-J.; Park, J.-S.; Moon, S.-H.; Choi, Y.-W.; Jiong, Y.S.; Kim, N.H.; Kang, M.-S. Facile surface modification of anion-exchange membranes for improvement of diffusion dialysis performance. J. Colloid Interface Sci. 2014, 416, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Mondal, A.N.; Liu, X.; Wu, B.; Yu, D.; Li, Q.; Miao, J.; Ge, Q.; Xu, T. Advanced charged porous membranes with ultrahigh selectivity and permeability for acid recovery. J. Membr. Sci. 2017, 536, 11–18. [Google Scholar] [CrossRef]
- Ge, Q.; Ning, Y.; Wu, L.; Ge, L.; Liu, X.; Yang, Z.; Xu, T. Enhancing acid recovery efficiency by implementing oligomer ionic bridge in the membrane matrix. J. Membr. Sci. 2016, 518, 263–272. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Sun, P.; Wang, K.; Wei, J.; Zhong, M.; Wu, D.; Zhu, H. Effective recovery of acids from iron-based electrolytes using graphene oxide membrane filters. J. Mater. Chem. A 2014, 2, 7734–7737. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, J.; Hu, Y.; Wang, P.; Ou, R.; Jiang, L.; Liu, J.Z.; Freeman, B.; Hill, A.J.; Wang, H. Ultrafast selective transport of alkali metal ions in metal organic frameworks with subnanometer pores. Sci. Adv. 2018, 4, 66. [Google Scholar] [CrossRef]
- Huang, H.-H.; Joshi, R.; De Silva, K.K.H.; Badam, R.; Yoshimura, M.; De Silva, K. Fabrication of reduced graphene oxide membranes for water desalination. J. Membr. Sci. 2019, 572, 12–19. [Google Scholar] [CrossRef]
- Liu, D.; Zhong, C. Understanding gas separation in metal–organic frameworks using computer modeling. J. Mater. Chem. 2010, 20, 10308. [Google Scholar] [CrossRef]
- Peng, F.; Lu, L.; Sun, H.; Wang, Y.; Liu, J.; Jiang, Z. Hybrid Organic−Inorganic Membrane: Solving the Tradeoff between Permeability and Selectivity. Chem. Mater. 2005, 17, 6790–6796. [Google Scholar] [CrossRef]
- Mondal, A.N.; Cheng, C.; Khan, M.I.; Hossain, M.; Emmanuel, K.; Ge, L.; Wu, B.; He, Y.; Ran, J.; Ge, X.; et al. Improved acid recovery performance by novel Poly(DMAEM-co-γ-MPS) anion exchange membrane via diffusion dialysis. J. Membr. Sci. 2017, 525, 163–174. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Li, Z.; Liao, J.; Huang, Y.; Lei, Y.; Li, N. Mixed-charge poly(2,6-dimethyl-phenylene oxide)anion exchange membrane for diffusion dialysis in acid recovery. J. Membr. Sci. 2018, 549, 543–549. [Google Scholar] [CrossRef]
- Ran, J.; Hu, M.; Yu, D.; He, Y.; Shehzad, M.A.; Wu, L.; Xu, T. Graphene oxide embedded “three-phase” membrane to beat “trade-off” in acid recovery. J. Membr. Sci. 2016, 520, 630–638. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Mao, F.; Zhang, G.; Tong, J.; Xu, T.; Wu, Y. Anion exchange membranes used in diffusion dialysis for acid recovery from erosive and organic solutions. Sep. Purif. Technol. 2014, 122, 376–383. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Luo, J.; Xu, T.; Fu, Y. Anion exchange hybrid membranes from PVA and multi-alkoxy silicon copolymer tailored for diffusion dialysis process. J. Membr. Sci. 2010, 356, 96–104. [Google Scholar] [CrossRef]
- Irfan, M.; Bakangura, E.; Afsar, N.U.; Xu, T.; Ran, J. Augmenting acid recovery from different systems by novel Q-DAN anion exchange membranes via diffusion dialysis. Sep. Purif. Technol. 2018, 201, 336–345. [Google Scholar] [CrossRef]
- Khan, M.I.; Luque, R.; Prinsen, P.; Rehman, A.U.; Anjum, S.; Nawaz, M.; Shaheen, A.; Zafar, S.; Mustaqeem, M. BPPO-Based Anion Exchange Membranes for Acid Recovery via Diffusion Dialysis. Materials 2017, 10, 266. [Google Scholar] [CrossRef]
- Irfan, M.; Afsar, N.U.; Bakangura, E.; Mondal, A.N.; Khan, M.I.; Emmanuel, K.; Yang, Z.; Wu, L.; Xu, T. Development of novel PVA-QUDAP based anion exchange membranes for diffusion dialysis and theoretical analysis therein. Sep. Purif. Technol. 2017, 178, 269–278. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Preparation of anion exchange membrane by efficient functionalization of polysulfone for electrodialysis. J. Membr. Sci. 2020, 596, 117591. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Wang, J. Crosslinked anion exchange membrane with improved membrane stability and conductivity for alkaline fuel cells. J. Appl. Polym. Sci. 2019, 136, 48169. [Google Scholar] [CrossRef]
- Tuan, C.M.; Tinh, V.D.C.; Kim, D. Anion Exchange Membranes Prepared from Quaternized Polyepichlorohydrin Cross-Linked with 1-(3-aminopropyl)imidazole Grafted Poly(arylene ether ketone) for Enhancement of Toughness and Conductivity. Membranes 2020, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, C.; Li, Y.; Xu, T.; Fu, Y. PVA–silica anion-exchange hybrid membranes prepared through a copolymer crosslinking agent. J. Membr. Sci. 2010, 350, 322–332. [Google Scholar] [CrossRef]
- Lin, X.; Shamsaei, E.; Kong, B.; Wang, H.; Liu, J.Z.; Xu, T. Fabrication of asymmetrical diffusion dialysis membranes for rapid acid recovery with high purity. J. Mater. Chem. A 2015, 3, 24000–24007. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhao, L.; Zhang, G.; Wu, C.; Xu, T. QPPO/PVA anion exchange hybrid membranes from double crosslinking agents for acid recovery. J. Membr. Sci. 2013, 428, 95–103. [Google Scholar] [CrossRef]
- Sharma, P.P.; Yadav, V.; Rajput, A.; Kulshrestha, V. Poly (triethoxyvinylsilane-co-quaternaryvinylbenzylchloride)/fGNR based anion exchange membrane and its application towards salt and acid recovery. J. Membr. Sci. 2018, 556, 303–311. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Gibert, O.; Cortina, J.-L. Increasing sustainability on the metallurgical industry by integration of membrane nanofiltration processes: Acid recovery. Sep. Purif. Technol. 2019, 226, 267–277. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Ge, L.; Luo, J.; Xu, T. Recovery of acetic acid from simulated acetaldehyde wastewaters: Bipolar membrane electrodialysis processes and membrane selection. J. Membr. Sci. 2011, 379, 184–190. [Google Scholar] [CrossRef]
- Yun, T.; Chung, J.W.; Kwak, S.-Y. Recovery of sulfuric acid aqueous solution from copper-refining sulfuric acid wastewater using nanofiltration membrane process. J. Environ. Manag. 2018, 223, 652–657. [Google Scholar] [CrossRef]
- Yun, T.; Kwak, S.-Y. Recovery of hydrochloric acid using positively-charged nanofiltration membrane with selective acid permeability and acid resistance. J. Environ. Manag. 2020, 260, 110001. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, M.; Li, W.; Wu, C.; Han, X.; Zhong, S.; Chen, Y. Application and modeling of pressure-concentration double-driven diffusion dialysis. J. Membr. Sci. 2020, 595, 117478. [Google Scholar] [CrossRef]
- Ipekçi, D.; Kabay, N.; Bunani, S.; Altıok, E.; Arda, M.; Yoshizuka, K.; Nishihama, S. Application of heterogeneous ion exchange membranes for simultaneous separation and recovery of lithium and boron from aqueous solution with bipolar membrane electrodialysis (EDBM). Desalination 2020, 479, 114313. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Q.; Li, Y.; Ge, L.; Xu, T. Water electro-transport with hydrated cations in electrodialysis. Desalination 2015, 365, 204–212. [Google Scholar] [CrossRef]
- Duan, X.; Wang, C.; Wang, T.; Xie, X.; Zhou, X.; Ye, Y. A polysulfone-based anion exchange membrane for phosphoric acid concentration and purification by electro-electrodialysis. J. Membr. Sci. 2018, 552, 86–94. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wang, X.; Ge, L.; Xu, T. Recovery of hydrochloric acid from simulated chemosynthesis aluminum foils wastewater: An integration of diffusion dialysis and conventional electrodialysis. J. Membr. Sci. 2012, 409, 257–263. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Li, C.; Feng, H.; Li, Q.; Ge, L.; Wang, G.; Xu, T. A preliminary study on electrically assisted diffusion dialysis. Sep. Purif. Technol. 2014, 122, 331–340. [Google Scholar] [CrossRef]
- Palatý, Z.; Bendová, H. Transport of nitric acid through anion-exchange membrane in the presence of sodium nitrate. J. Membr. Sci. 2011, 372, 277–284. [Google Scholar] [CrossRef]
- Carstensen, F.; Klement, T.; Büchs, J.; Melin, T.; Wessling, M. Continuous production and recovery of itaconic acid in a membrane bioreactor. Bioresour. Technol. 2013, 137, 179–187. [Google Scholar] [CrossRef]
- Gueccia, R.; Aguirre, A.R.; Randazzo, S.; Cipollina, A.; Micale, G. Diffusion Dialysis for Separation of Hydrochloric Acid, Iron and Zinc Ions from Highly Concentrated Pickling Solutions. Membranes 2020, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Shin, C.-H.; Choi, H.; Bae, W. Recovery of phosphoric acid from mixed waste acids of semiconductor industry by diffusion dialysis and vacuum distillation. Sep. Purif. Technol. 2012, 90, 64–68. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Huang, J.; Zhu, X.; Wang, Y. Separation and recovery of sulfuric acid from acidic vanadium leaching solution by diffusion dialysis. Sep. Purif. Technol. 2012, 96, 44–49. [Google Scholar] [CrossRef]
- Xu, J.; Fu, D.; Lu, S. The recovery of sulphuric acid from the waste anodic aluminum oxidation solution by diffusion dialysis. Sep. Purif. Technol. 2009, 69, 168–173. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wang, H.; Xu, T. Recovery of hydrochloric acid from simulated chemosynthesis aluminum foil wastewater by spiral wound diffusion dialysis (SWDD) membrane module. J. Membr. Sci. 2011, 384, 219–225. [Google Scholar] [CrossRef]
- Palatý, Z.; Bendová, H. Continuous dialysis of sulphuric acid and sodium sulphate mixture. J. Membr. Sci. 2016, 497, 36–46. [Google Scholar] [CrossRef]
- Luo, F.; Zhang, X.; Pan, J.; Mondal, A.N.; Feng, H.; Xu, T. Diffusion dialysis of sulfuric acid in spiral wound membrane modules: Effect of module number and connection mode. Sep. Purif. Technol. 2015, 148, 25–31. [Google Scholar] [CrossRef]
- Ye, H.; Zou, L.; Wu, C.; Wu, Y. Tubular membrane used in continuous and semi-continuous diffusion dialysis. Sep. Purif. Technol. 2020, 235, 116147. [Google Scholar] [CrossRef]
- Xu, T.; Liu, Z.; Huang, C.; Wu, Y.; Wu, L.; Yang, W. Preparation of a Novel Hollow-Fiber Anion-Exchange Membrane and Its Preliminary Performance in Diffusion Dialysis. Ind. Eng. Chem. Res. 2008, 47, 6204–6210. [Google Scholar] [CrossRef]


| Methods | Description | Advantages | Disadvantages |
|---|---|---|---|
| Crystallization [3,4] | The solubility of saline, such as FeCl2 or AlCl3, in the waste solutions reduces at low temperature, resulting in crystallization and separation. | Less investment in equipment simple to install | Long production period Low processing capacity High energy cost |
| Solvent extraction [5,6,7] | Extraction agents are used to extract acid or metal ions selectively from waste solutions. Then, the acid or metal ions could be collected via back-extraction. | High yield and selectivity Pure product | Complicated operation Bad for environmental owing to the extraction agents. |
| Ion exchange resin [8,9,10] | Ion exchange materials are used to absorb acids or metal ions in the waste solution, and then the acids or metal ions could be desorbed from the solid phases. | High selectivity Simple to operation | High costs Low adsorption capacities |
| Membrane technology [13,18,19] | Membrane technologies contain the reverse osmosis process, electrodialysis and diffusion dialysis which correspond to pressure, an electric field or activity as driving forces, respectively. Acids are transported through membranes from feed side to the receiving side under the driving forces. | High efficiency Reliable Simple to install and scale up. | Limited processing capacities |
| Material | Price ($) |
|---|---|
| Diffusion dialysis unit | 170,000–1,350,000 |
| Membranes replacement | 15,000–300,000 |
| Auxiliary (pump, circuit, valve, and tank) | 150,000 |
| Power, labor, and others | 3000–5000 |
| Total | 338,000–1,805,000 |
| Write-off (investment-recovery period): 4.8–26.4 months | |
| Membrane | Structure | UH (10−3 m/h) | S | Simulated Solution System |
|---|---|---|---|---|
| Commercial Neosepta-AFX [85] | Dense | 4 | 25 | 0.05 M FeCl3–2 M H2SO4 |
| Commercial DF-120 [44] | Dense | 4 | 19 | 0.25 M FeCl2–1.0 M HCl |
| Neosepta-AFX modified with 5 vol.% pyrrole [85] | Dense | 4 | 48 | 0.05 M FeCl3–2 M H2SO4 |
| BPPO-based AEM crosslinked with a multi-amine oligomer [87] | Dense | 9 | 2074 | 0.59 M FeSO4–1.03 M H2SO4 |
| The pore-filled AEM with PE and polypyrrole [74] | Porous | 10–11 | 36–54 | 0.05 M FeCl3–2 M H2SO4 |
| The quaternized BPPO AEM [57] | Dense | 3–13 | H/Fe: 40 | 0.15 M TiO2–0.17 M FeSO4–0.25 M H2SO4 |
| H/Ti: 70 | ||||
| The PPO-based AEM with quaternized nitrogen and –COOH groups [64] | Dense | 5–19 | 73–390 | 0.25 M FeCl2–1.0 M HCl |
| The PVA-based AEM modified by pyridinium [82] | Dense | 17–25 | 31–58 | 0.25 M FeCl2–1.0 M HCl |
| The BPPO-based AEM with sponge-like pores [86] | porous | 15–20 | 81–665 | 0.21 M FeCl2–1 M HCl |
| 22–28 | 100–2033 | 0.46 M AlCl3–2.12 M HCl | ||
| The PECs/PVA AEM [44] | Dense | 3–23 | 40–90 | 0.25 M FeCl2–1.0 M HCl |
| The PPO-based ultrafiltration AEM containing –COOH groups and quaternary ammonium [78] | Porous | 20–25 | 28–46 | 0.25 M FeCl2–1.0 M HCl |
| The PVC-based AEM immobilized by DMAM and DVB [65] | Dense | 12–40 | 36–61 | 0.18 M FeCl2–0.81 M HCl |
| The nanofiber AEM [80] | Porous | 41 | 50 | 0.225 M FeCl2–1 M HCl |
| PANI-based AEM [56] | Porous | 32 | 20 | 5% FeCl3–3.5 M HCl - |
| A-PANI-based AEM [56] | Porous | 42 | 17 | 5% FeCl3–3.5 M HCl - |
| The PVA-based AEM modified by multisilicon copolymers [63] | Dense | 10–43 | 22–39 | 0.12 M FeCl2–1 M HCl |
| The double quaternization PVA-based membrane [67] | Dense | 30–45 | 21–32 | 0.25 M FeCl2–1.0 M HCl |
| ESM/PSF membrane [79] | Porous | 10–46 | 33–93 | 0.125 M FeCl2–0.5 M HCl |
| NP/PSF membrane [68] | Porous | 47 | 154 | 0.125 M FeCl2–0.5 M HCl |
| Imidazolium functionalized PVA-based AEM [83]. | Dense | 19–48 | 13–53 | 0.25 M FeCl2–1.0 M HCl |
| The BPPO-based AEM modified pyrrolidinium [50] | Dense | 18–49 | 36–66 | 0.18 M FeCl2–0.81 M HCl |
| PVA-based AEMs by grafting different contents of allyltrimethylammonium chloride [66]. | Dense | 17–60 | 8–26 | 0.18 M FeCl2–0.81 M HCl |
| The PSF-based AEM [47] | Porous | 65 | 34 | 0.2 M FeCl2–1 M HCl |
| The BPPO-based AEM modified MP [61] | Dense | 11–66 | 25–78 | 0.25 M FeCl2–1 M HCl |
| The PPO-based ultrafiltration AME modified by PEI and TMA [77] | Porous | 56–70 | 11–21 | 0.2 M FeCl2–1 M HCl |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, W.; Wang, Y. Diffusion Dialysis for Acid Recovery from Acidic Waste Solutions: Anion Exchange Membranes and Technology Integration. Membranes 2020, 10, 169. https://doi.org/10.3390/membranes10080169
Zhang C, Zhang W, Wang Y. Diffusion Dialysis for Acid Recovery from Acidic Waste Solutions: Anion Exchange Membranes and Technology Integration. Membranes. 2020; 10(8):169. https://doi.org/10.3390/membranes10080169
Chicago/Turabian StyleZhang, Chengyi, Wen Zhang, and Yuxin Wang. 2020. "Diffusion Dialysis for Acid Recovery from Acidic Waste Solutions: Anion Exchange Membranes and Technology Integration" Membranes 10, no. 8: 169. https://doi.org/10.3390/membranes10080169
APA StyleZhang, C., Zhang, W., & Wang, Y. (2020). Diffusion Dialysis for Acid Recovery from Acidic Waste Solutions: Anion Exchange Membranes and Technology Integration. Membranes, 10(8), 169. https://doi.org/10.3390/membranes10080169







