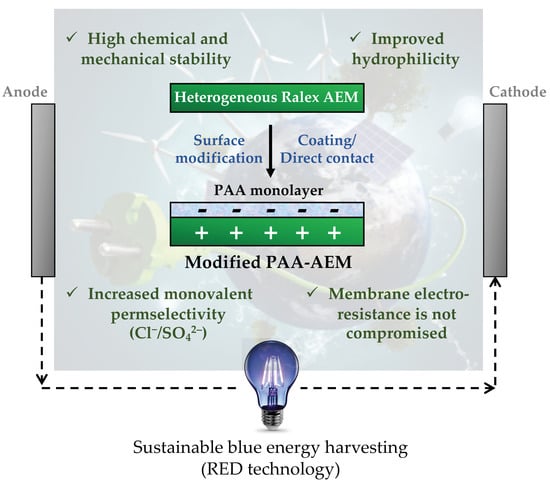
Characterization of Poly(Acrylic) Acid-Modified Heterogenous Anion Exchange Membranes with Improved Monovalent Permselectivity for RED
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane Preparation
2.2. Membrane Surface Characterization
2.3. Electrochemical Characterization
2.4. Mass Transport Experiments
3. Results and Discussion
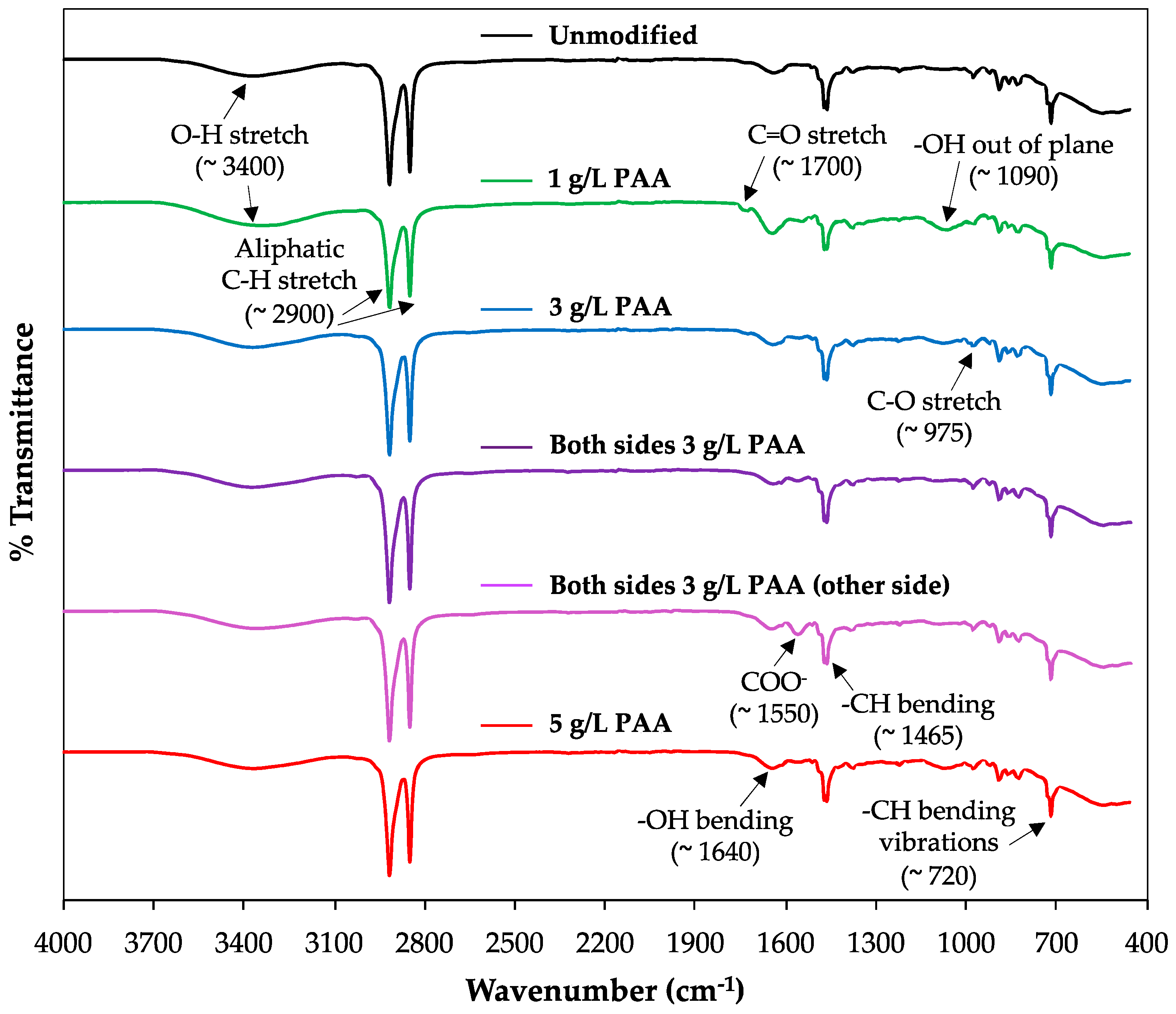
3.1. Membrane Surface Characterization: Contact Angle, Water Uptake, Ion Exchange Capacity, Fixed Charge Density, Swelling, and Fourier Attenuated Atomic Force Microscopy
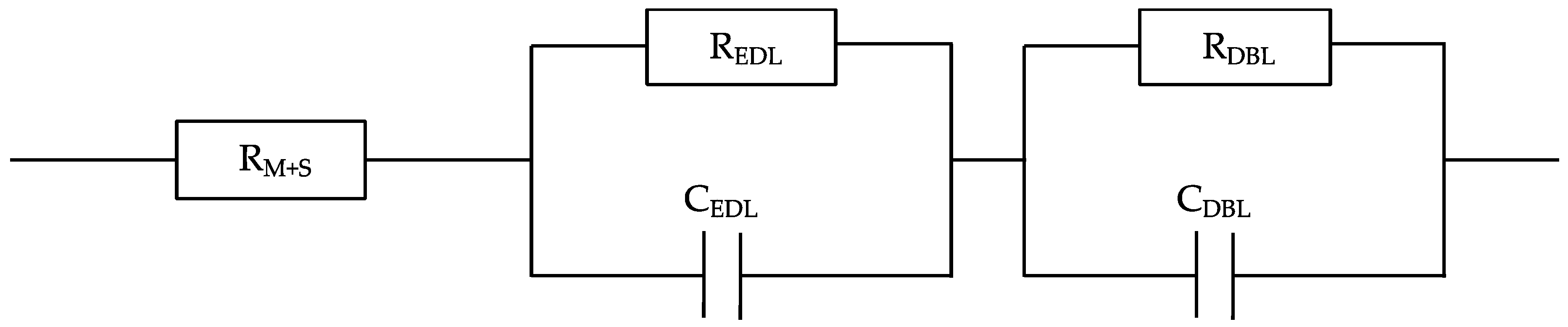
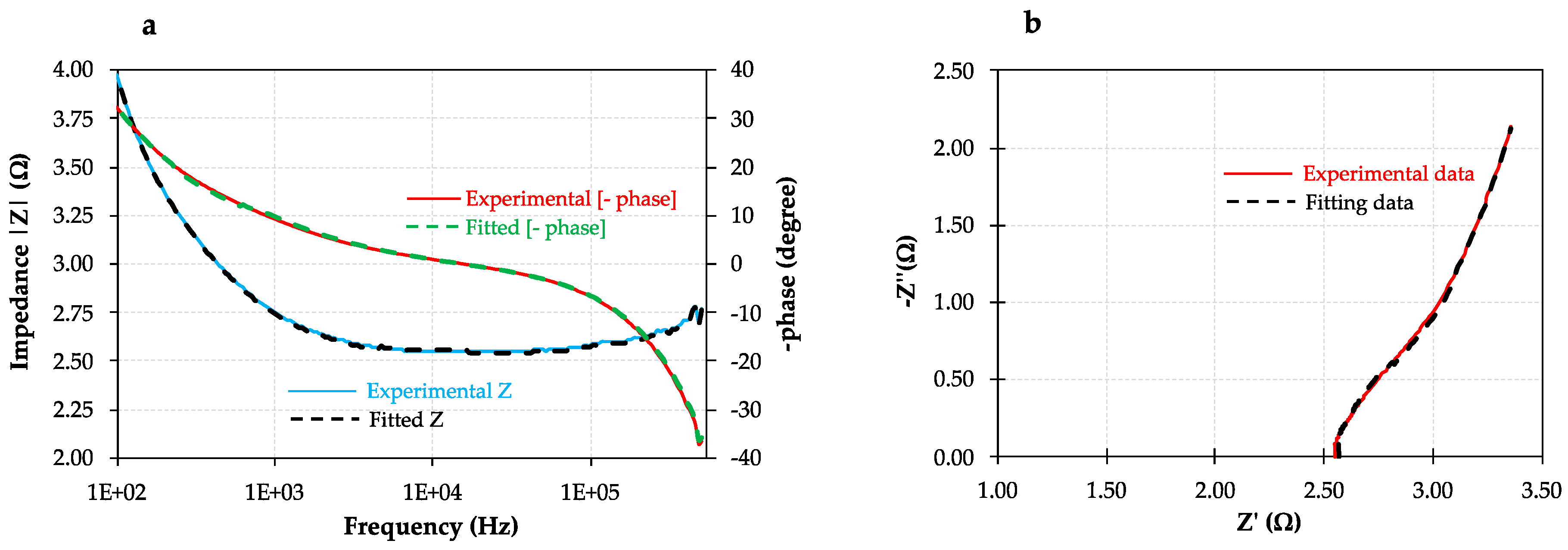
3.2. Electrochemical Characterization: Cyclic Voltammetry and Impedance Spectroscopy Measurements
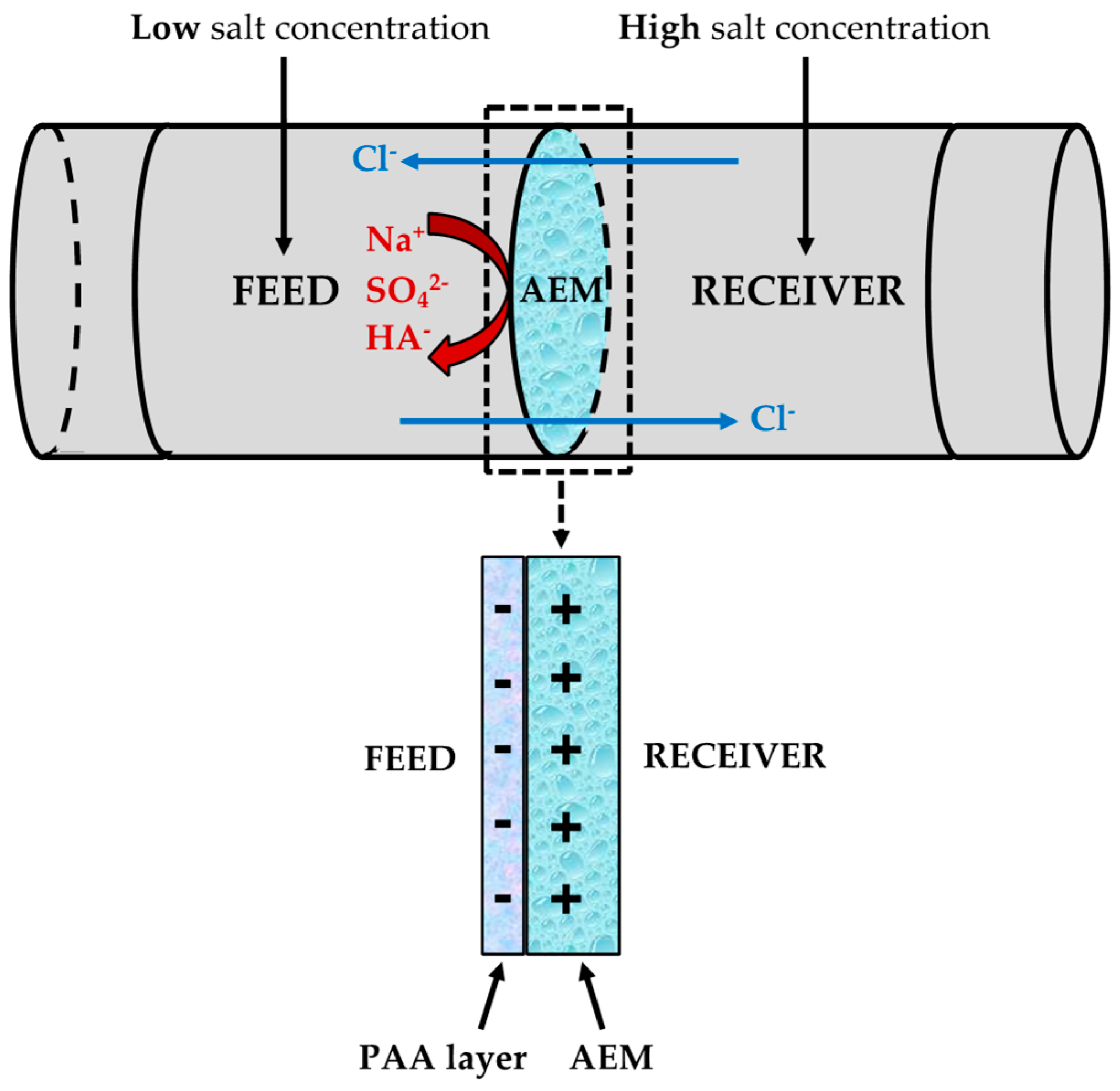
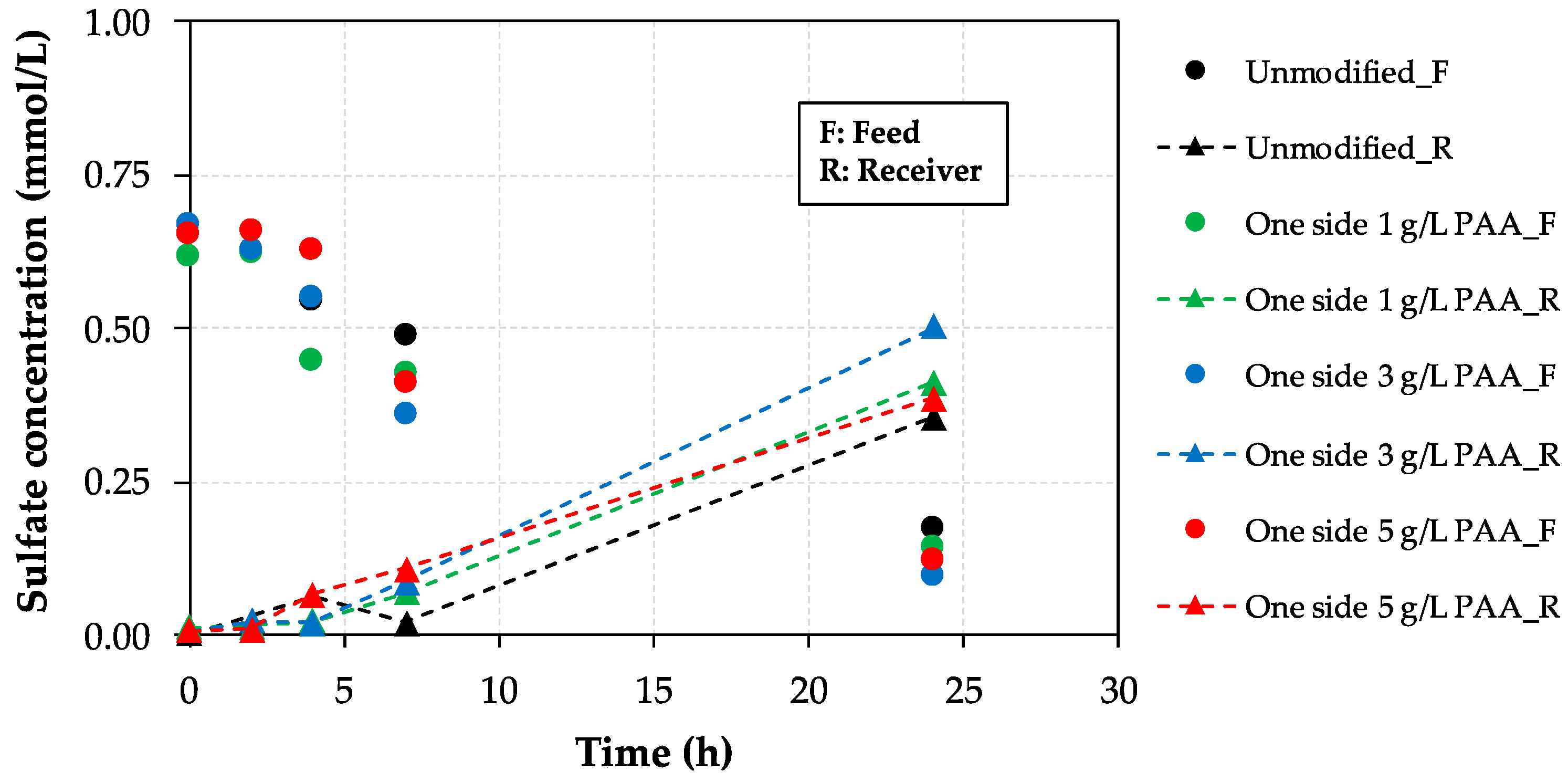
3.3. Mass Transport Experiments: Sulfate Rejection Study
4. Conclusions
- Improved membrane hydrophilicity properties are shown via contact angle analyses for the poly(acrylic) acid (optimal concentration of 3 g/L) modified membranes in comparison with the behavior of the unmodified one.
- The importance of the method used to evaluate the ion exchange capacity of anion exchange membranes is demonstrated, depending on the nature of the replacing anions.
- The swelling effect was investigated in terms of dimension changes (i.e., thickness and diameter). The thickness of swelled membranes is increased by 27%, whereas the diameter is widened by 5% in most of the cases at the same conditions. This analysis highlights the essential role of both thickness (higher thicknesses might result in increased membrane electro-resistance) and diameter (modifications in membrane area may affect process efficiency) to optimize membrane design for RED applications.
- The analysis of functional groups present on membrane surfaces demonstrates the high chemical stability of the polyester-based anion exchange membranes after adding a negative poly(acrylic) acid monolayer onto their surfaces, suggesting the absence of chemical reactions between the modifying agent and the membrane, thus demonstrating that the attachment is electrostatic.
- The use of silver electrodes in cyclic voltammetry measurements allowed to reach ideal resistor behavior. The modified membranes present higher current-voltage responses due to improved hydrophilic properties, which involves a higher overall transport of anions through the corresponding modified membrane, even though the presence of humic acid as model foulant involved a certain decrease in this overall transport owing to its attachment onto the membrane surface.
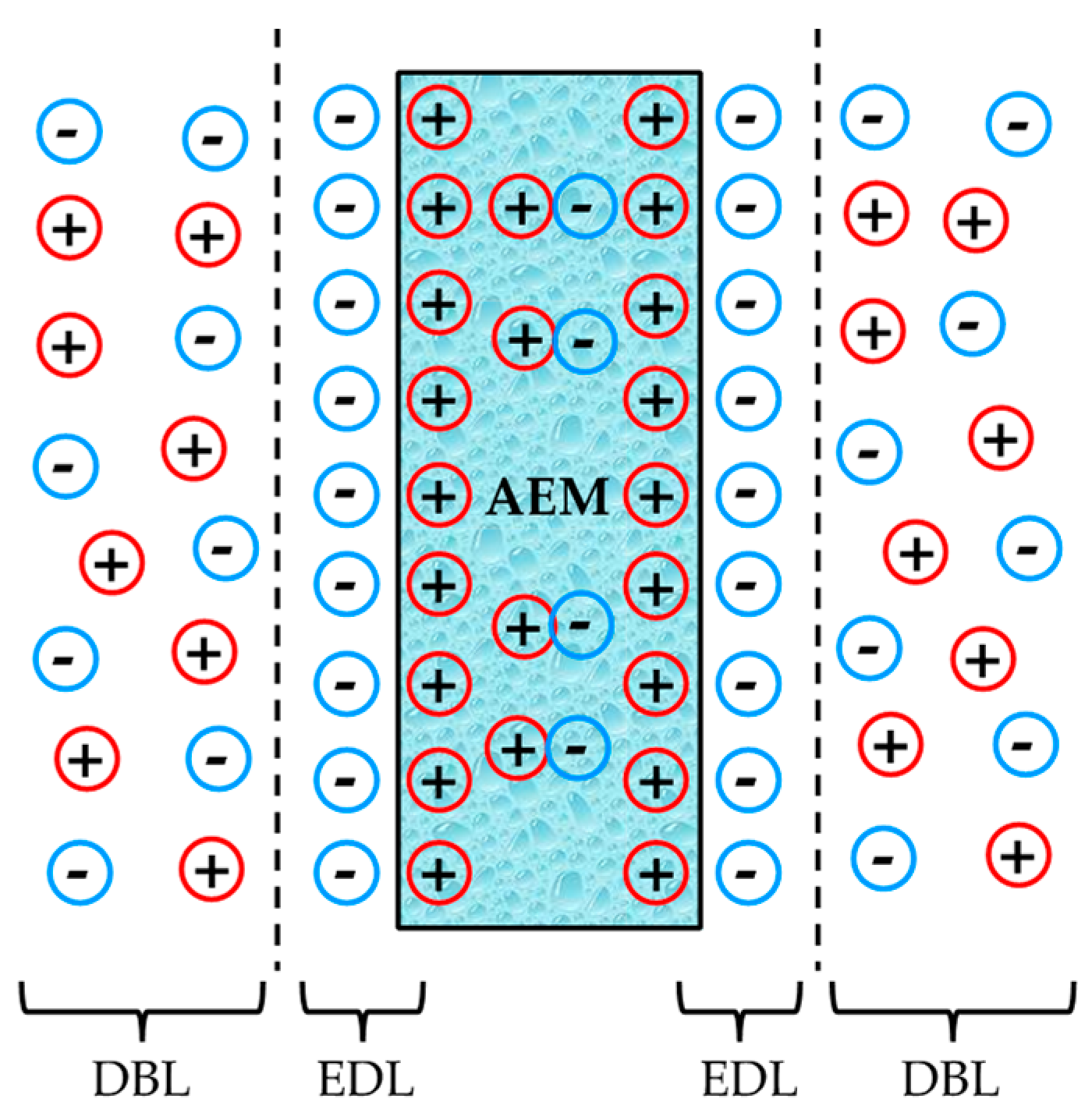
- The membrane electro-resistances, double-layer resistances, and diffusion boundary layer resistances of the different modified membranes were in the same order of magnitude compared to the unmodified anion exchange membrane (i.e., 5.0–5.4 Ω·cm2, 1.6–2.0 Ω·cm2, and 0.5–0.9 Ω·cm2, respectively) in 0.5 M NaCl aqueous solutions. The small difference observed in the modified ones might be associated with their higher membrane thicknesses. Therefore, the electrical conductivity of the different prepared modified membranes is not compromised by the addition of a negative monolayer onto their surfaces with uniform characteristics, which might involve a reduction of both surface heterogeneity and disorderliness. The membrane electro-resistances decrease in external electrolytes following the order LiCl > NaCl > KCl, owing to the increasing hydrated radii (decreased ionic mobility) in the order K+ < Na+ < Li+. The membrane electro-resistance results obtained in the present study after membrane modification are comparable to those reported in literature for both modified heterogeneous and homogeneous anion exchange membranes.
- Mass transport tests finally prove that the rejection of sulfate (monovalent permselectivity) is improved in the absence of humic acid as the concentration of poly(acrylic) acid increases up to 3 g/L. In this respect, when both sides of the membrane are modified (3 g/L), sulfate rejection is enhanced by 54% compared to the performance of the unmodified membrane, thus suggesting an improved reverse electrodialysis process performance. Nevertheless, the behavior of the modified samples is clearly negatively affected by the presence of organic foulants such as humic acid. The sulfate flux results show that the optimal modifying agent concentration is equal to 3 g/L of poly(acrylic) acid.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pawlowski, S.; Huertas, R.M.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. On operation of reverse electrodialysis (RED) and membrane capacitive deionisation (MCDI) with natural saline streams: A critical review. Desalination 2020, 476, 114183. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, B.; Tong, X.; Chen, Y. Monovalent-anion selective and antifouling polyelectrolytes multilayer anion exchange membrane for reverse electrodialysis. J. Membr. Sci. 2018, 567, 68–75. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Kunteng, D.; Veerman, J.; Saakes, M.; Nijmeijer, K. Periodic feedwater reversal and air sparging as antifouling strategies in reverse electrodialysis. Environ. Sci. Technol. 2014, 48, 3065–3073. [Google Scholar] [CrossRef]
- Gómez-coma, L.; Ortiz-martínez, V.M.; Carmona, J.; Palacio, L.; Prádanos, P.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Modeling the influence of divalent ions on membrane resistance and electric power in reverse electrodialysis. J. Membr. Sci. 2019, 592, 117385. [Google Scholar] [CrossRef]
- Veerman, J.; Vermaas, D.A. Reverse electrodialysis: Fundamentals. In Sustainable Energy from Salinity Gradients; Elsevier Ltd: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Pintossi, D.; Saakes, M.; Borneman, Z.; Nijmeijer, K. Electrochemical impedance spectroscopy of a reverse electrodialysis stack: A new approach to monitoring fouling and cleaning. J. Power Source 2019, 444, 227302. [Google Scholar] [CrossRef]
- Güler, E.; van Baak, W.; Saakes, M.; Nijmeijer, K. Monovalent-ion-selective membranes for reverse electrodialysis. J. Membr. Sci. 2014, 455, 254–270. [Google Scholar] [CrossRef]
- Pintossi, D.; Chen, C.; Saakes, M.; Nijmeijer, K.; Borneman, Z. Influence of sulfate on anion exchange membranes in reverse electrodialysis. NPJ Clean Water 2020, 3, 29. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.M.; Gómez-Coma, L.; Tristán, C.; Pérez, G.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. A comprehensive study on the effects of operation variables on reverse electrodialysis performance. Desalination 2020, 482, 114389. [Google Scholar] [CrossRef]
- Ortiz-Imedio, R.; Gomez-Coma, L.; Fallanza, M.; Ortiz, A.; Ibañez, R.; Ortiz, I. Comparative performance of Salinity Gradient Power-Reverse Electrodialysis under different operating conditions. Desalination 2019, 457, 8–21. [Google Scholar] [CrossRef]
- Luque Di Salvo, J.; Cosenza, A.; Tamburini, A.; Micale, G.; Cipollina, A. Long-run operation of a reverse electrodialysis system fed with wastewaters. J. Environ. Manag. 2018, 217, 871–887. [Google Scholar] [CrossRef]
- Pawlowski, S.; Crespo, J.G.; Velizarov, S. Profiled ion exchange membranes: A comprehensible review. Int. J. Mol. Sci. 2019, 20, 165. [Google Scholar] [CrossRef] [PubMed]
- Rijnaarts, T.; Moreno, J.; Saakes, M.; de Vos, W.M.; Nijmeijer, K. Role of anion exchange membrane fouling in reverse electrodialysis using natural feed waters. Colloids Surf. A Physicochem. Eng. Asp. 2019, 560, 198–204. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloids Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; de Hart, N.; Saakes, M.; Nijmeijer, K. CO2 saturated water as two-phase flow for fouling control in reverse electrodialysis. Water Res. 2017, 125, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, H.J.; Choi, S.J.; Geckeler, K.E.; Cho, J.; Moon, S.H. Fouling mitigation of anion exchange membrane by zeta potential control. J. Colloids Interface Sci. 2003, 259, 293–300. [Google Scholar] [CrossRef]
- Hao, L.; Liao, J.; Jiang, Y.; Zhu, J.; Li, J.; Zhao, Y.; Van der Bruggen, B.; Sotto, A.; Shen, J. “Sandwich”-like structure modified anion exchange membrane with enhanced monovalent selectivity and fouling resistant. J. Membr. Sci. 2018, 556, 98–106. [Google Scholar] [CrossRef]
- Kingsbury, R.S.; Liu, F.; Zhu, S.; Boggs, C.; Armstrong, M.D.; Call, D.F.; Coronell, O. Impact of natural organic matter and inorganic solutes on energy recovery from five real salinity gradients using reverse electrodialysis. J. Membr. Sci. 2017, 541, 621–632. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, C.; Kavanagh, J.; Dominguez-Ramos, A.; Ibañez, R.; Irabien, A.; Chen, Y.; Coster, H. Electrochemical impedance spectroscopy of enhanced layered nanocomposite ion exchange membranes. J. Membr. Sci. 2017, 541, 611–620. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, J.; Ding, J.; Van der Brugge, B.; Shen, J.; Gao, C. Electric-pulse layer-by-layer assembled of anion exchange membrane with enhanced monovalent selectivity. J. Membr. Sci. 2018, 548, 81–90. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Matsuyama, H. Simultaneous improvement of the monovalent anion selectivity and antifouling properties of an anion exchange membrane in an electrodialysis process, using polyelectrolyte multilayer deposition. J. Membr. Sci. 2013, 431, 113–120. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Liu, L.; Yang, W. Developments and new applications of UV-induced surface graft polymerizations. Prog. Polym. Sci. 2009, 34, 156–193. [Google Scholar] [CrossRef]
- Saracco, G.; Zanetti, M.C. Ion Transport Through Monovalent-Anion-Permselective Membranes. Ind. Eng. Chem. Res. 1994, 33, 96–101. [Google Scholar] [CrossRef]
- Nebavskaya, X.; Sarapulova, V.; Butylskii, D.; Larchet, C.; Pismenskaya, N. Electrochemical properties of homogeneous and heterogeneous anion exchange membranes coated with cation exchange polyelectrolyte. Membranes 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ding, R.; Zhang, Y.; Shi, B.; Wang, J.; Liu, J. Stably coating loose and electronegative thin layer on anion exchange membrane for efficient and selective monovalent anion transfer. Desalination 2017, 410, 55–65. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Cao, H.; Zhao, Z.; Su, C.; Wen, H. Improvement of the antifouling performance and stability of an anion exchange membrane by surface modification with graphene oxide (GO) and polydopamine (PDA). J. Membr. Sci. 2018, 566, 44–53. [Google Scholar] [CrossRef]
- An, Y.; Jiang, G.; Ren, Y.; Zhang, L.; Qi, Y.; Ge, Q. An environmental friendly and biodegradable shale inhibitor based on chitosan quaternary ammonium salt. J. Pet. Sci. Eng. 2015, 135, 253–260. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Liu, H.; Van der Bruggen, B.; Sotto Díaz, A.; Shen, J.; Gao, C. An anion exchange membrane modified by alternate electro-deposition layers with enhanced monovalent selectivity. J. Membr. Sci. 2016, 520, 262–271. [Google Scholar] [CrossRef]
- Besha, A.T.; Tsehaye, M.T.; Aili, D.; Zhang, W.; Tufa, R.A. Design of monovalent ion selective membranes for reducing the impacts of multivalent ions in reverse electrodialysis. Membranes 2020, 10, 7. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Takagi, R.; Matsuyama, H. Surface modification of an anion exchange membrane to improve the selectivity for monovalent anions in electrodialysis - experimental verification of theoretical predictions. J. Membr. Sci. 2015, 490, 301–310. [Google Scholar] [CrossRef]
- Kedem, O.; Schechtmann, L.; Mirsky, Y.; Saveliev, G.; Daltrophe, N. Low-polarisation electrodialysis membranes. Desalination 1998, 118, 305–314. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Ahmadi Monfared, H.; Khadivi, M.A. Fabrication of PES nanofiltration membrane by simultaneous use of multi-walled carbon nanotube and surface graft polymerization method: Comparison of MWCNT and PAA modified MWCNT. Sep. Purif. Technol. 2013, 104, 32–44. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Membr. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Sarapulova, V.; Shkorkina, I.; Mareev, S.; Pismenskaya, N.; Kononenko, N.; Larchet, C.; Dammak, L.; Nikonenko, V. Transport characteristics of fujifilm ion-exchange membranes as compared to homogeneous membranes AMX and CMX and to heterogeneous membranes MK-40 and MA-41. Membranes 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, S.; Shkirskaya, S. Transport properties of bilayer and multilayer surface-modified ion-exchange membranes. J. Membr. Sci. 2019, 590, 117272. [Google Scholar] [CrossRef]
- Guler, E.; Zhang, Y.; Saakes, M.; Nijmeijer, K. Tailor-made anion-exchange membranes for salinity gradient power generation using reverse electrodialysis. ChemSusChem. 2012, 5, 2262–2270. [Google Scholar] [CrossRef]
- Karas, F.; Hnát, J.; Paidar, M.; Schauer, J.; Bouzek, K. Determination of the ion-exchange capacity of anion-selective membranes. Int. J. Hydrogen Energy 2014, 39, 5054–5062. [Google Scholar] [CrossRef]
- Østedgaard-Munck, D.N.; Catalano, J.; Birch Kristensen, M.; Bentien, A. Data on flow cell optimization for membrane-based electrokinetic energy conversion. Data BR 2017, 15, 1–11. [Google Scholar] [CrossRef]
- Østedgaard-Munck, D.N.; Catalano, J.; Kristensen, M.B.; Bentien, A. Membrane-based electrokinetic energy conversion. Mater. Today Energy 2017, 5, 118–125. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, J.; Wang, P.; Wang, Z.; Shi, F.; Liu, H. Investigations on the interfacial capacitance and the diffusion boundary layer thickness of ion exchange membrane using electrochemical impedance spectroscopy. J. Membr. Sci. 2016, 502, 37–47. [Google Scholar] [CrossRef]
- Villafaña-lópez, L.; Reyes-valadez, D.M.; González-vargas, O.A. Custom-Made Ion Exchange Membranes at Laboratory Scale for Reverse Electrodialysis. Membranes 2019, 9, 145. [Google Scholar] [CrossRef]
- Manatunga, D.C.; De Silva, R.M.; De Silva, K.M.N. Double layer approach to create durable superhydrophobicity on cotton fabric using nano silica and auxiliary non fluorinated materials. Appl. Surf. Sci. 2016, 360, 777–788. [Google Scholar] [CrossRef]
- Ahmadi, E.; Zarghami, N.; Jafarabadi, M.A.; Alizadeh, L.; Khojastehfard, M.; Yamchi, M.R.; Salehi, R. Enhanced anticancer potency by combination chemotherapy of HT-29 cells with biodegradable, pH-sensitive nanoparticles for co-delivery of hydroxytyrosol and doxorubicin. J. Drug Deliv. Sci. Technol. 2019, 51, 721–735. [Google Scholar] [CrossRef]
- Memon, A.A.; Arbab, A.A.; Sahito, I.A.; Sun, K.C.; Mengal, N.; Jeong, S.H. Synthesis of highly photo-catalytic and electro-catalytic active textile structured carbon electrode and its application in DSSCs. Sol. Energy 2017, 150, 521–531. [Google Scholar] [CrossRef]
- Parvinzadeh, M.; Ebrahimi, I. Influence of atmospheric-air plasma on the coating of a nonionic lubricating agent on polyester fiber. Radiat. Eff. Defects Solids 2011, 166, 408–416. [Google Scholar] [CrossRef]
- Alvarez-Gayosso, C.; Canseco, M.A.; Estrada, R.; Palacios-Alquisira, J.; Hinojosa, J.; Castano, V. Preparation and microstructure of cobalt(III) poly (acrylate) hybrid materials. Int. J. Basic Appl. Sci. 2015, 4, 255–263. [Google Scholar] [CrossRef]
- Długołecki, P.; Ogonowski, P.; Metz, S.J.; Saakes, M.; Nijmeijer, K.; Wessling, M. On the resistances of membrane, diffusion boundary layer and double layer in ion exchange membrane transport. J. Membr. Sci. 2010, 349, 369–379. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Liang, J.; Xiang, Y.; Liu, X. An attempt for improving electrodialytic transport properties of a heterogeneous anion exchange membrane. Desalination 2014, 351, 163–170. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Takagi, R.; Matsuyama, H. Biofouling phenomena on anion exchange membranes under the reverse electrodialysis process. J. Membr. Sci. 2017, 530, 232–239. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Mulyati, S.; Takagi, R.; Matsuyama, H. Improved antifouling of anion-exchange membrane by polydopamine coating in electrodialysis process. Desalination 2014, 332, 126–133. [Google Scholar] [CrossRef]
- Cao, R.; Shi, S.; Li, Y.; Xu, B.; Zhao, Z.; Duan, F.; Cao, H.; Wang, Y. The properties and antifouling performance of anion exchange membranes modified by polydopamine and poly (sodium 4-styrenesulfonate). Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124429. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Ruan, H.; Xue, L.; Van der Bruggen, B.; Gao, C.; Shen, J. Sulfonated reduced graphene oxide modification layers to improve monovalent anions selectivity and controllable resistance of anion exchange membrane. J. Membr. Sci. 2017, 536, 167–175. [Google Scholar] [CrossRef]
- Ruan, H.; Zheng, Z.; Pan, J.; Gao, C.; Van der Bruggen, B.; Shen, J. Mussel-inspired sulfonated polydopamine coating on anion exchange membrane for improving permselectivity and anti-fouling property. J. Membr. Sci. 2018, 550, 427–435. [Google Scholar] [CrossRef]
- Grebenyuk, V.D.; Chebotareva, R.D.; Peters, S.; Linkov, V. Surface modification of anion-exchange electrodialysis membranes to enhance anti-fouling characteristics. Desalination 1998, 115, 313–329. [Google Scholar] [CrossRef]



| List of Membranes | Modification Type/Modified Sides | PAA Concentration (g/L) | Nomenclature |
|---|---|---|---|
| (1) | Unmodified | - | Unmodified |
| (2) | Monolayer/one | 1 | One side 1 g/L PAA |
| (3) | Monolayer/one | 3 | One side 3 g/L PAA |
| (4) | Monolayer/both | 3 | Both sides 3 g/L PAA |
| (5) | Monolayer/one | 5 | One side 5 g/L PAA |
| Membrane Type | WU (%) | IEC1 (mmol/g) | IEC2 (mmol/g) | CDfix1 (mmol/g) | CDfix2 (mmol/g) |
|---|---|---|---|---|---|
| (1) Unmodified | 59.0 ± 1.8 | 0.949 ± 0.18 | 1.369 ± 0.05 | 1.605 ± 0.25 | 2.321 ± 0.08 |
| (2) One side 1 g/L PAA | 67.4 ± 6.0 | 0.681 ± 0.03 | 1.625 ± 0.05 | 1.017 ± 0.14 | 2.413 ± 0.08 |
| (3) One side 3 g/L PAA | 62.7 ± 3.8 | 0.433 ± 0.01 | 1.751 ± 0.05 | 0.692 ± 0.05 | 2.793 ± 0.08 |
| (4) Both sides 3 g/L PAA | 61.5 ± 0.9 | 0.378 ± 0.01 | 1.751 ± 0.05 | 0.614 ± 0.02 | 2.847 ± 0.08 |
| (5) One side 5 g/L PAA | 63.3 ± 5.0 | 0.352 ± 0.02 | 1.760 ± 0.05 | 0.560 ± 0.08 | 2.779 ± 0.08 |
| Membrane Type | Thickness Wet (μm) | Thickness Dry (μm) | Diameter Wet (mm) | Diameter Dry (mm) |
|---|---|---|---|---|
| (1) Unmodified | 643.3 ± 5.8 | 472.7 ± 2.3 | 45.7 ± 0.6 | 43.3 ± 1.2 |
| (2) One side 1 g/L PAA | 664.0 ± 0.0 | 487.7 ± 4.0 | 45.0 ± 1.7 | 44.8 ± 2.0 |
| (3) One side 3 g/L PAA | 654.7 ± 4.6 | 473.3 ± 2.3 | 47.3 ± 1.2 | 44.8 ± 0.8 |
| (4) Both sides 3 g/L PAA | 650.0 ± 20.4 | 473.7 ± 6.0 | 47.7 ± 1.5 | 45.5 ± 0.5 |
| (5) One side 5 g/L PAA | 654.8 ± 21.3 | 476.0 ± 5.3 | 47.3 ± 0.6 | 45.3 ± 0.6 |
| Membrane Type | Feed Solution | Current (mA) at 0.6 V |
|---|---|---|
| (1) Unmodified | KCl + K2SO4 | 3.5 |
| KCl + Na2SO4 | 3.2 | |
| NaCl + Na2SO4 | 2.7 | |
| LiCl + Na2SO4 | 2.6 | |
| (3) One side 3 g/L PAA | KCl + K2SO4 | 3.4 |
| KCl + Na2SO4 | 3.3 | |
| NaCl + Na2SO4 | 3.2 | |
| LiCl + Na2SO4 | 2.8 | |
| (3) One side 5 g/L PAA | KCl + K2SO4 | 3.8 |
| KCl + Na2SO4 | 3.4 | |
| NaCl + Na2SO4 | 3.2 | |
| LiCl + Na2SO4 | 3.0 |
| Membrane Type | RM (Ω·cm2) | REDL (Ω·cm2) | RDBL (Ω·cm2) | CEDL (µF) | CDBL (µF) | X2 Error Function |
|---|---|---|---|---|---|---|
| (1) Unmodified | 5.01 ± 0.52 | 1.83 ± 0.23 | 0.74 ± 0.15 | 115 ± 16 | 46 ± 10 | 0.0023 |
| (2) One side 1 g/L PAA | 5.14 ± 0.50 | 1.98 ± 0.08 | 0.89 ± 0.14 | 200 ± 19 | 93 ± 16 | 0.0028 |
| (3) One side 3 g/L PAA | 5.21 ± 0.04 | 1.56 ± 0.18 | 0.67 ± 0.08 | 188 ± 12 | 67 ± 8 | 0.0023 |
| (4) Both sides 3 g/L PAA | 5.33 ± 0.16 | 1.92 ± 0.17 | 0.53 ± 0.01 | 72 ± 12 | 19 ± 4 | 0.0020 |
| (5) One side 5 g/L PAA | 5.36 ± 0.18 | 1.58 ± 0.02 | 0.68 ± 0.02 | 140 ± 15 | 42 ± 5 | 0.0023 |
| Membrane Type | RM (Ω·cm2) | REDL (Ω·cm2) | RDBL (Ω·cm2) | CEDL (µF) | CDBL (µF) | X2 Error Function | |
|---|---|---|---|---|---|---|---|
| (1) Unmodified | LiCl | 5.35 ± 0.03 | 2.32 ± 0.44 | 0.35 ± 0.00 | 147 ± 4 | 59 ± 3 | 0.0013 |
| NaCl | 5.01 ± 0.52 | 1.83 ± 0.23 | 0.74 ± 0.15 | 115 ± 16 | 46 ± 10 | 0.0023 | |
| KCl | 4.88 ± 0.02 | 2.17 ± 0.57 | 0.30 ± 0.00 | 166 ± 6 | 66 ± 5 | 0.0011 | |
| (3) One side 3 g/L PAA | LiCl | 5.49 ± 0.02 | 1.79 ± 0.30 | 0.49 ± 0.00 | 145 ± 3 | 55 ± 2 | 0.0013 |
| NaCl | 5.21 ± 0.04 | 1.56 ± 0.18 | 0.67 ± 0.08 | 188 ± 12 | 67 ± 8 | 0.0023 | |
| KCl | 4.87 ± 0.01 | 1.27 ± 0.11 | 0.39 ± 0.00 | 162 ± 2 | 60 ± 1 | 0.0011 | |
| AEM Type | Modification Approach | Modifying Agent | RM Before Modification (Ω·cm2) | RM After Modification (Ω·cm2) | Reference |
|---|---|---|---|---|---|
| Heterogeneous Ralex AM-PES (Mega a.s.) | Direct contact/immersion | Poly(acrylic) acid | 5.01 | 5.1–5.4 | This study |
| Heterogeneous AEM (Zhe-jiang Qianqiu Environmental Protection & Water Treatment Co. Ltd.) | LbL deposition | Glutaraldehyde and poly(ethyleneimine) | 4.5 | 4.8 | [48] |
| Neosepta AMX (Astom Corp.) | Dip coating | Polydopamine (PDA) | 1.2 | 2.9 | [30] |
| Neosepta AMX (Astom Corp.) | Immersion | PDA | 2.5 | 5.0 | [49,50] |
| Homogeneous Neosepta ASE (Astom Corp.) | Immersion (co-deposition) | PDA and poly (sodium 4-styrene sulfonate) | 3.6 | 4.5 | [51] |
| Homogeneous JAM-II-07 (Yanrun) | Coating by deposition | Sulfonated reduced graphene oxide nanosheets | 3.1 | 3.7 | [52] |
| Homogeneous Type I (Fujifilm) | Self-adhesion deposition | Sulfonated polydopamine | 1.0 | 6.8 | [53] |
| AEM * (Ionics) | Coating by adsorption | Olygourethane surfactants and disodium salt α, ω-oligooxipropylene-bis(o-urethane-2.4, 2.6 tolueneurylbenzene sulphonic acid) | 2.5 | 5.7 | [54] |
| Membrane Type | Sulfate Flux (mmol/(m2·h)) |
|---|---|
| (1) Unmodified | 3.6 |
| (2) One side 1 g/L PAA | 2.2 |
| (3) One side 3 g/L PAA | 1.9 ± 0.1 |
| (5) One side 5 g/L PAA | 1.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino-Garcia, I.; Kotoka, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Characterization of Poly(Acrylic) Acid-Modified Heterogenous Anion Exchange Membranes with Improved Monovalent Permselectivity for RED. Membranes 2020, 10, 134. https://doi.org/10.3390/membranes10060134
Merino-Garcia I, Kotoka F, Portugal CAM, Crespo JG, Velizarov S. Characterization of Poly(Acrylic) Acid-Modified Heterogenous Anion Exchange Membranes with Improved Monovalent Permselectivity for RED. Membranes. 2020; 10(6):134. https://doi.org/10.3390/membranes10060134
Chicago/Turabian StyleMerino-Garcia, Ivan, Francis Kotoka, Carla A.M. Portugal, João G. Crespo, and Svetlozar Velizarov. 2020. "Characterization of Poly(Acrylic) Acid-Modified Heterogenous Anion Exchange Membranes with Improved Monovalent Permselectivity for RED" Membranes 10, no. 6: 134. https://doi.org/10.3390/membranes10060134
APA StyleMerino-Garcia, I., Kotoka, F., Portugal, C. A. M., Crespo, J. G., & Velizarov, S. (2020). Characterization of Poly(Acrylic) Acid-Modified Heterogenous Anion Exchange Membranes with Improved Monovalent Permselectivity for RED. Membranes, 10(6), 134. https://doi.org/10.3390/membranes10060134