PVA-Based Mixed Matrix Membranes Comprising ZSM-5 for Cations Separation
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of ZSM-5/PVA-Based MMMs
2.3. Characterization of ZSM-5/PVA-Based MMMs
2.4. Current-Voltage (I-V) Curves
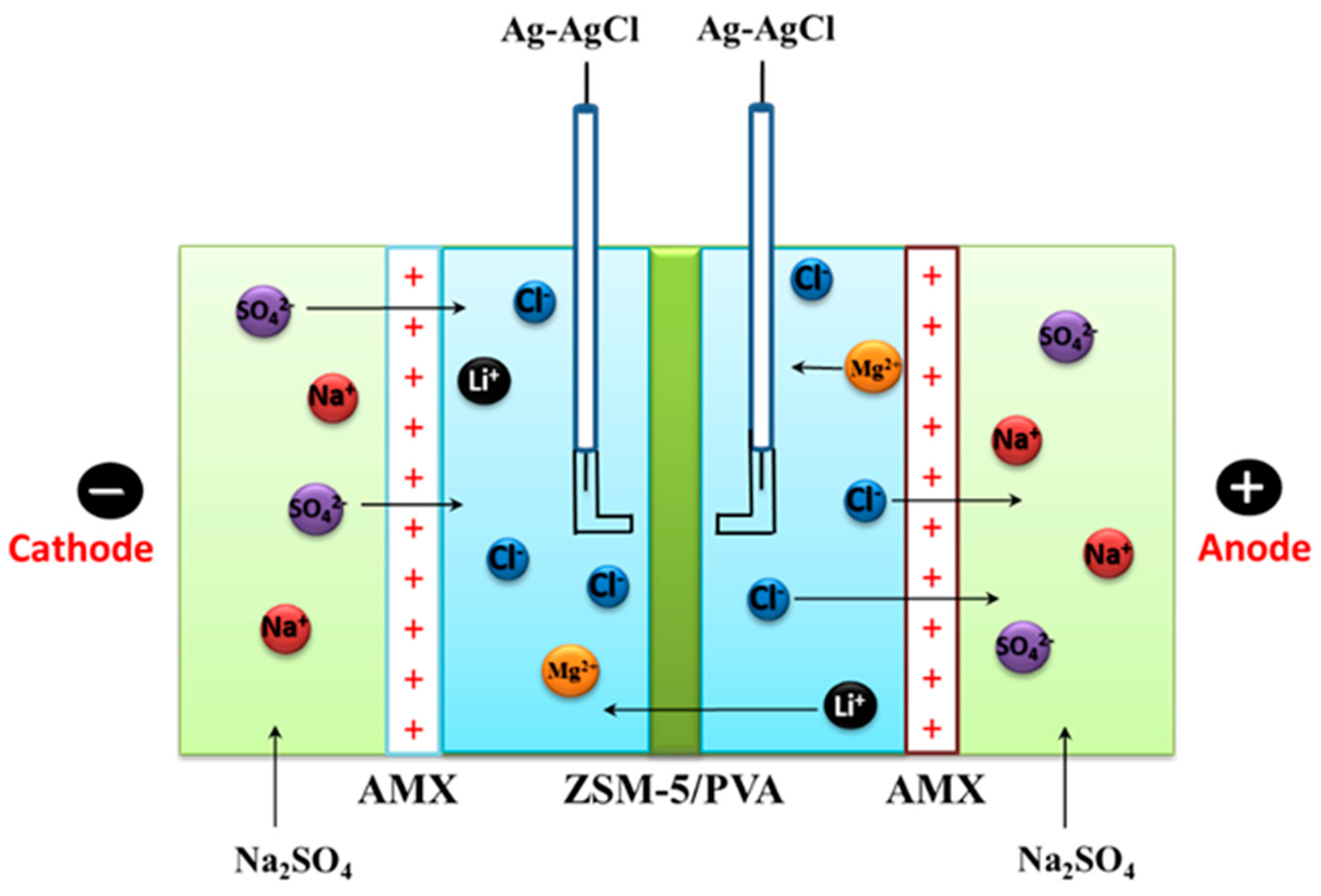
2.5. Evaluation of the Cations Permselectivity
3. Results and Discussion
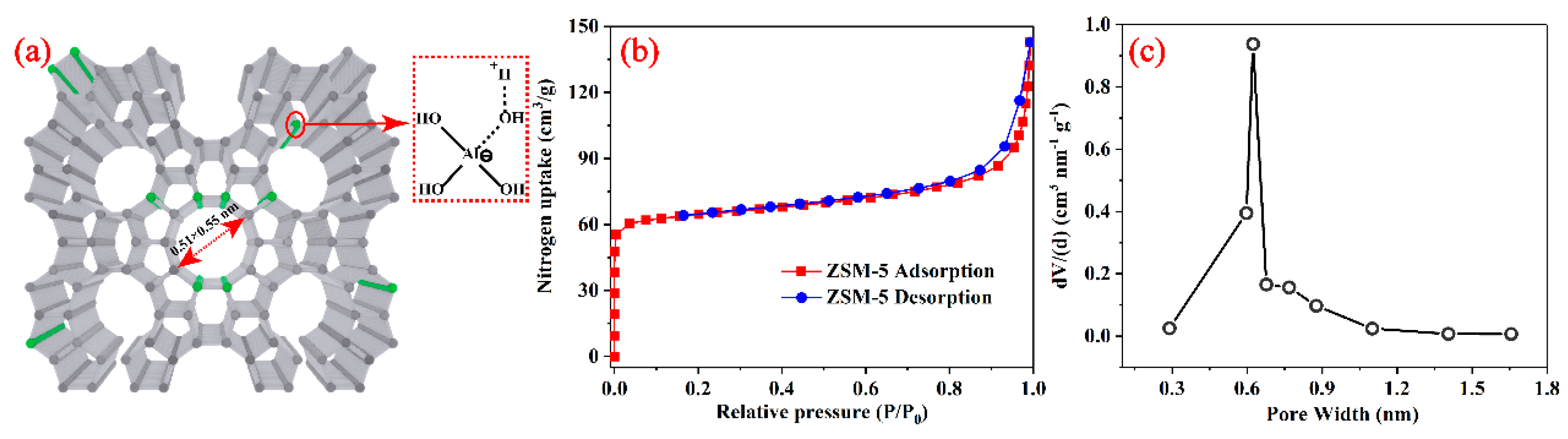
3.1. Surface Area and Pore Size Distribution Analysis of the ZSM-5
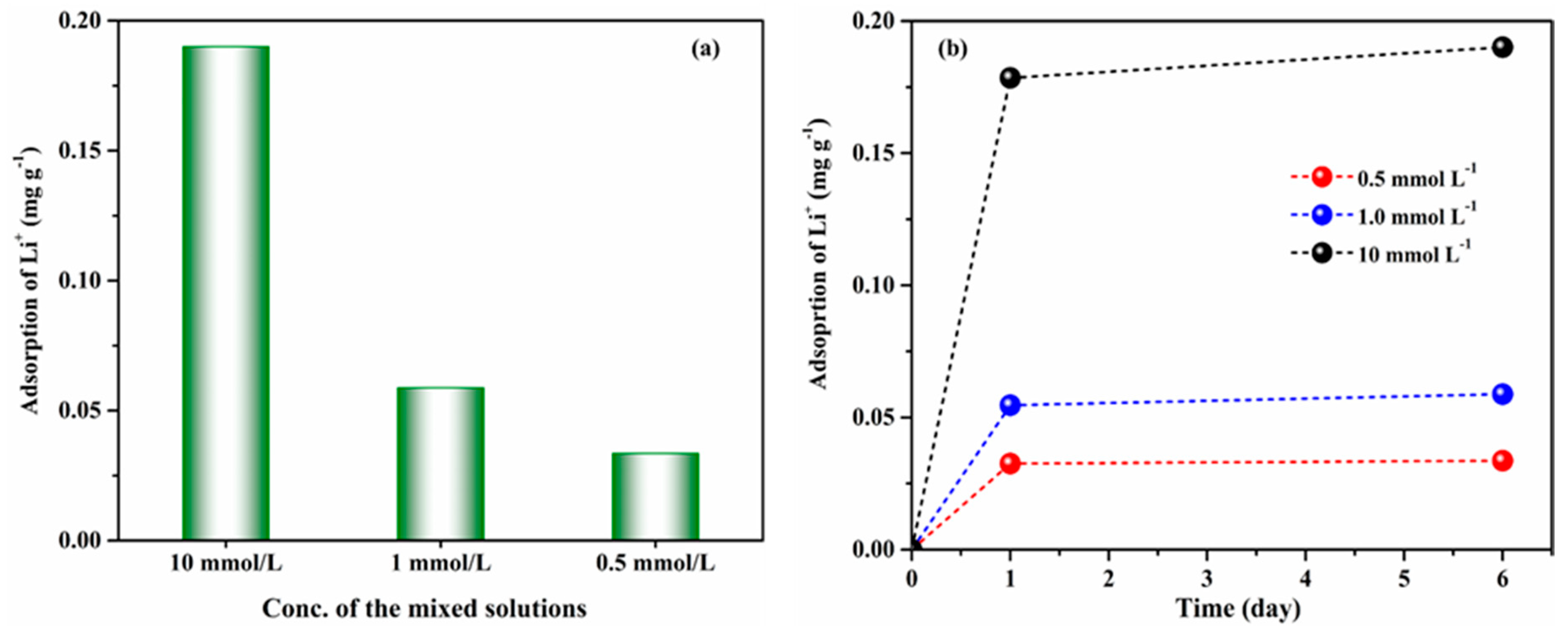
3.2. Adsorption Capacity of ZSM-5
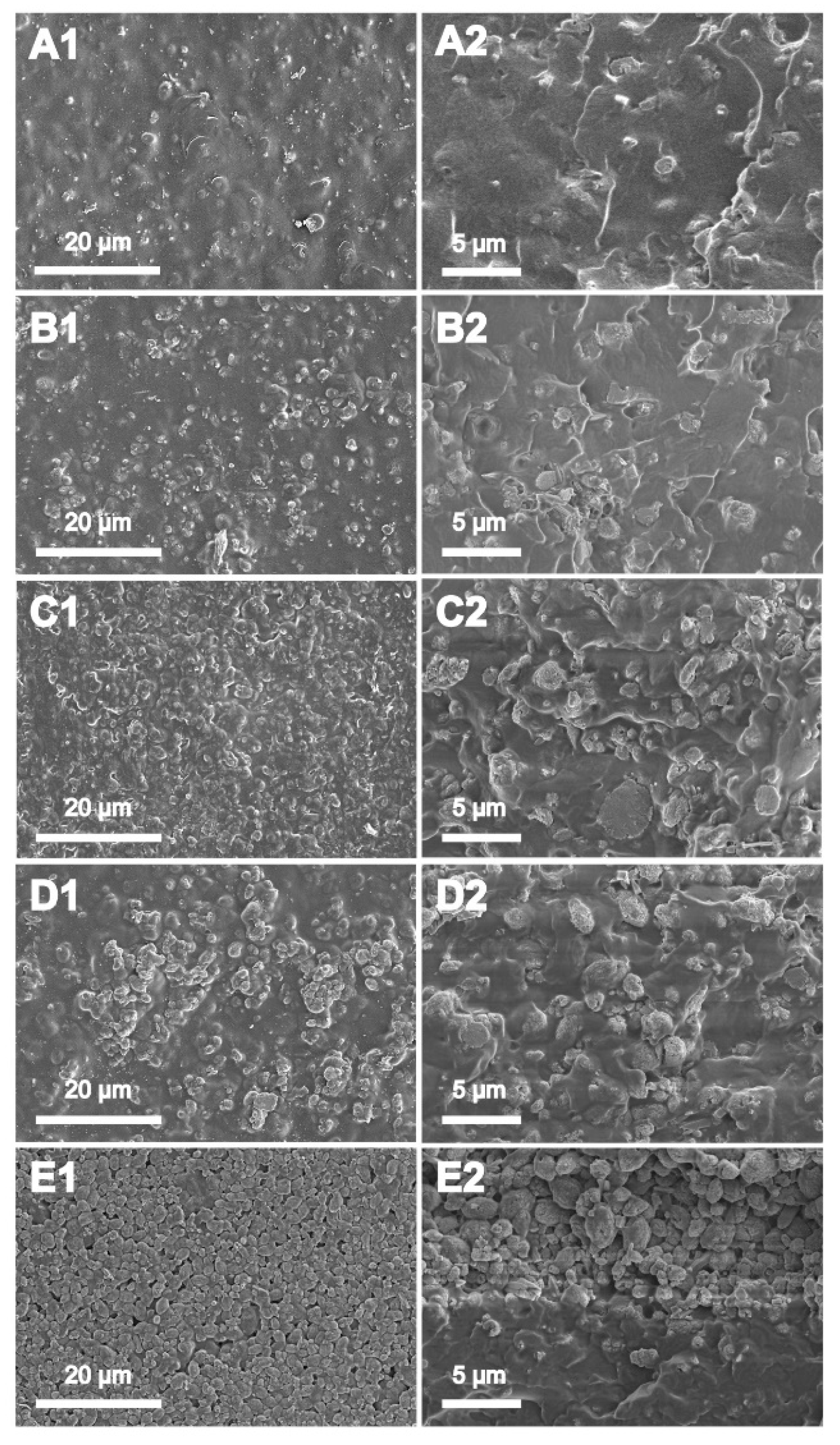
3.3. Morphology
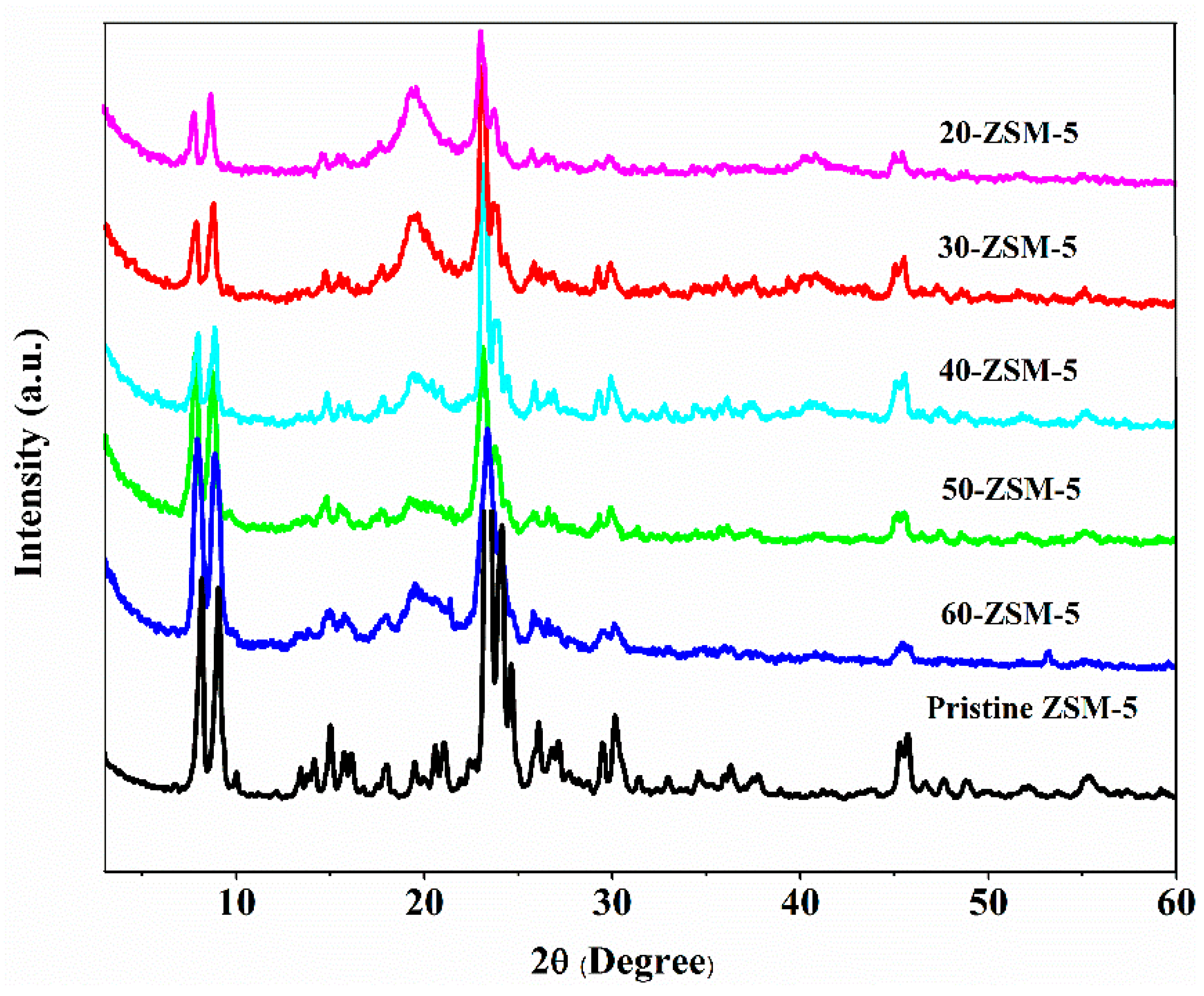
3.4. XRD Analysis
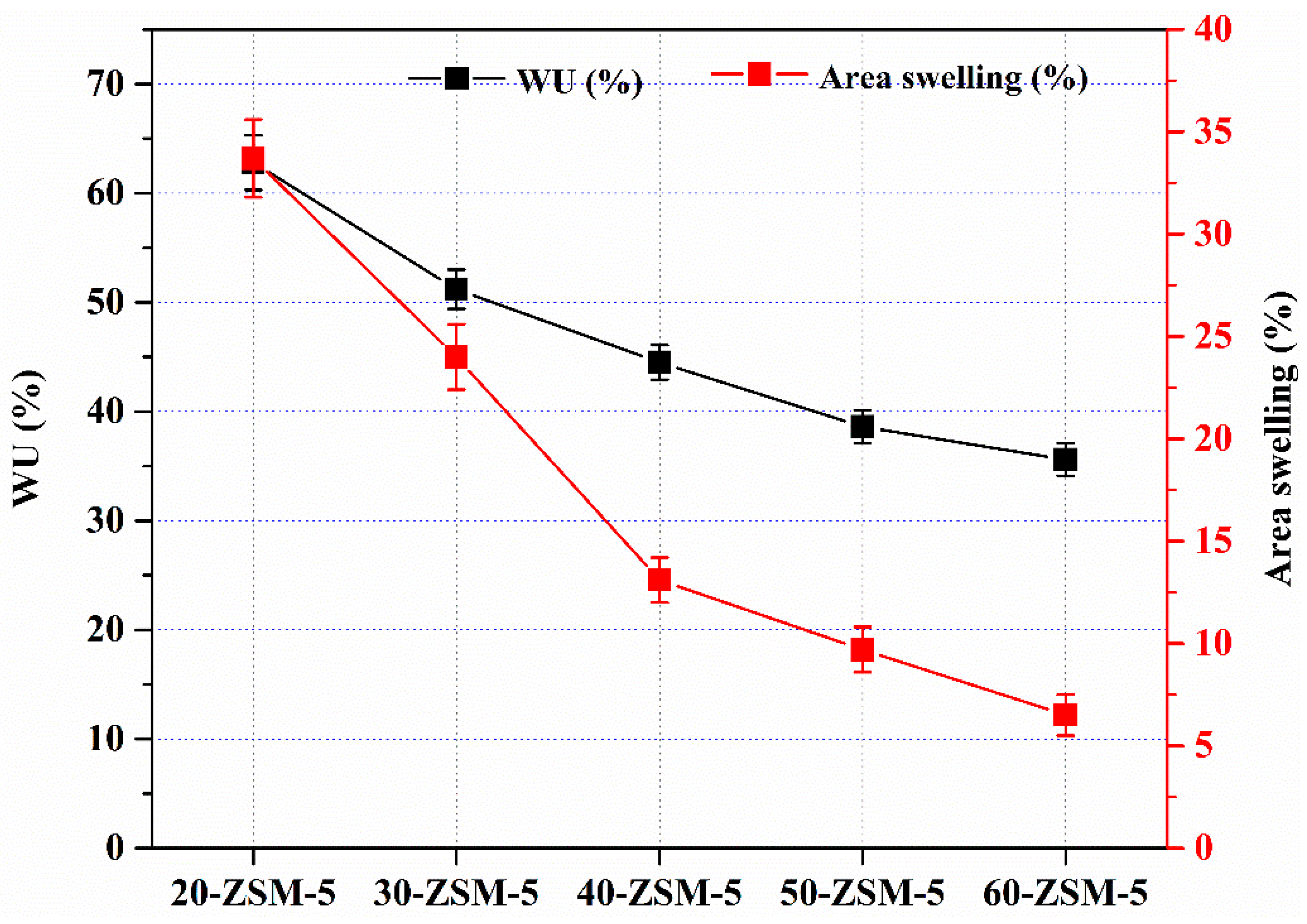
3.5. Water Uptake (WU) and Area Swelling
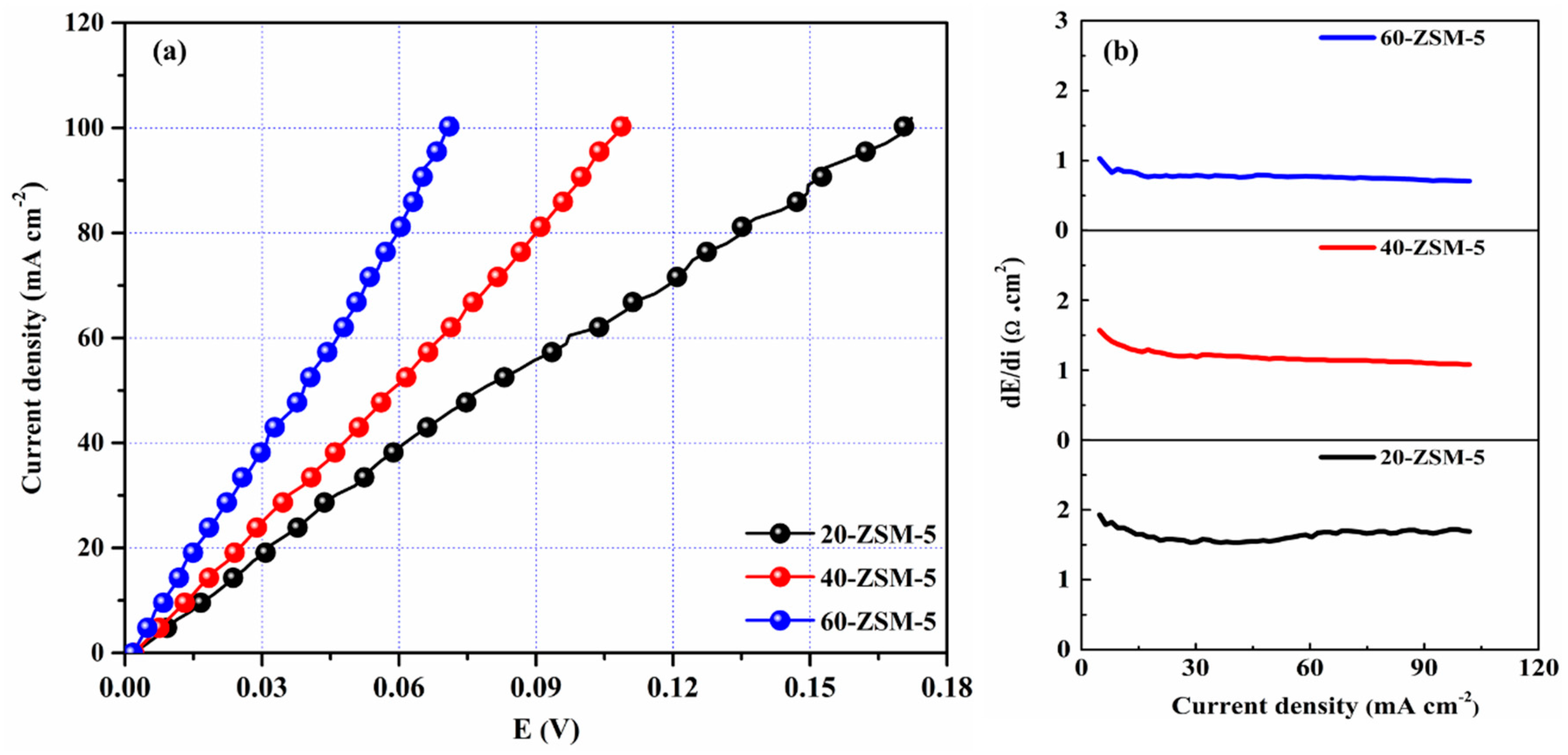
3.6. Current-Voltage (I–V) Curves
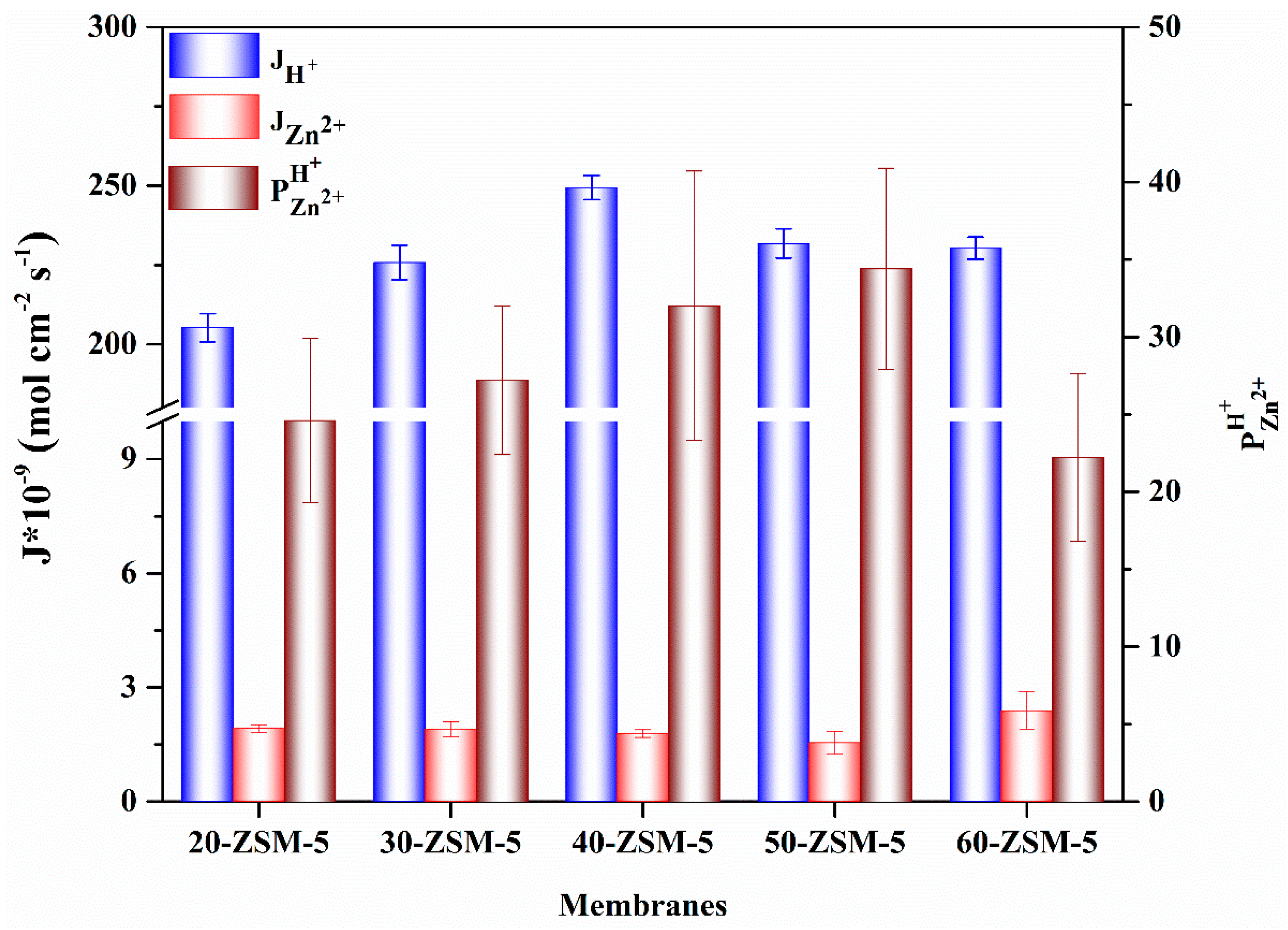
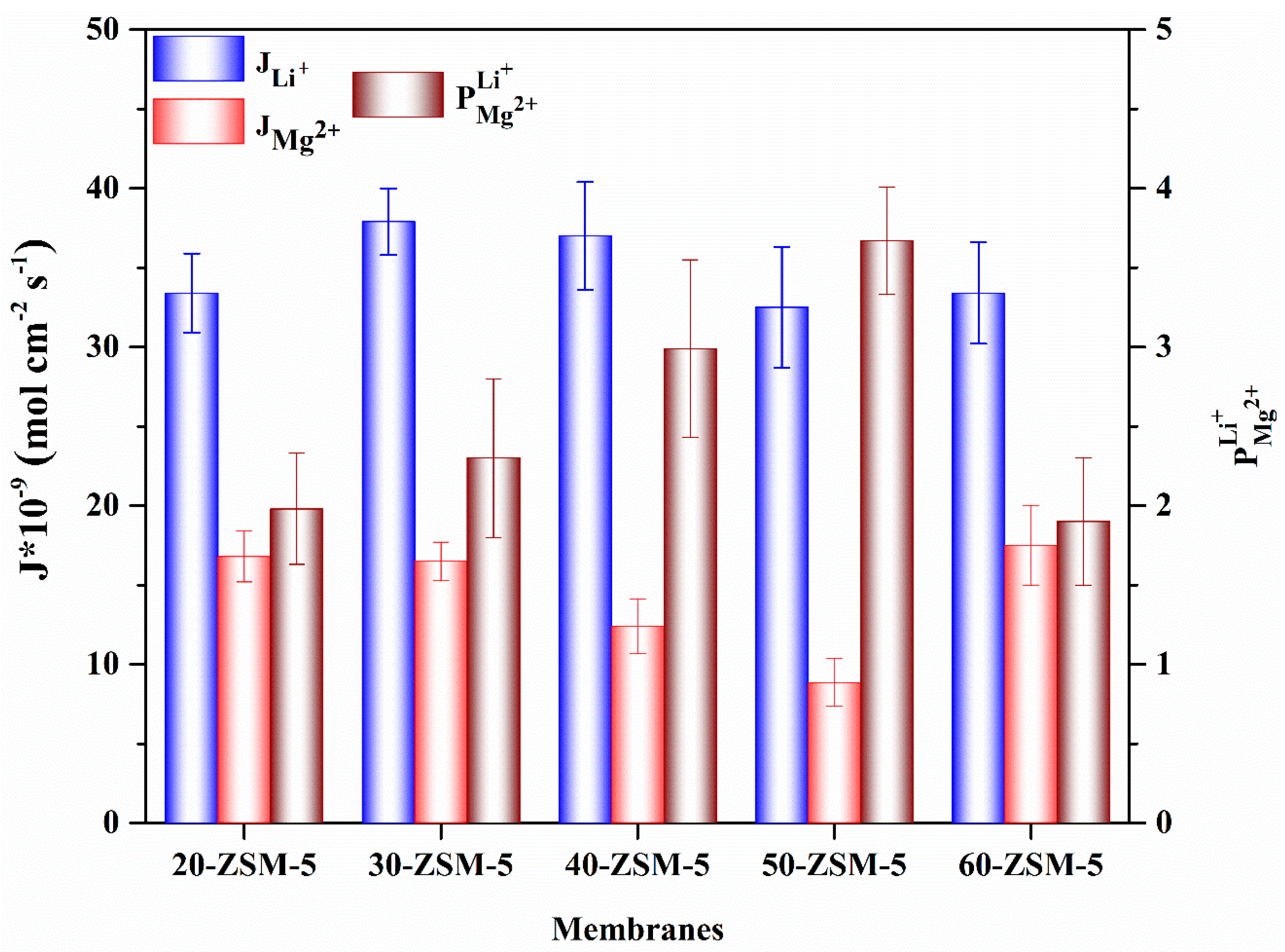
3.7. Electrodialysis (ED) Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Hou, L.; Wu, B.; Yu, D.; Wang, S.; Shehzad, M.A.; Fu, R.; Liu, Z.; Li, Q.; He, Y.; Afsar, N.U.; et al. Asymmetric porous monovalent cation perm-selective membranes with an ultrathin polyamide selective layer for cations separation. J. Membr. Sci. 2018, 557, 49–57. [Google Scholar] [CrossRef]
- Amor, Z.; Bariou, B.; Mameri, N.; Taky, M.; Nicolas, S.; Elmidaoui, A. Fluoride removal from brackish water by electrodialysis. Desalination 2001, 133, 215–223. [Google Scholar] [CrossRef]
- Lee, H.-J.; Sarfert, F.; Strathmann, H.; Moon, S.-H. Designing of an electrodialysis desalination plant. Desalination 2002, 142, 267–286. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Ahdab, Y.D.; Rehman, D. Brackish water desalination for greenhouses: Improving groundwater quality for irrigation using monovalent selective electrodialysis reversal. J. Membr. Sci. 2020, 118072. [Google Scholar] [CrossRef]
- Davis, J.R.; Chen, Y.; Baygents, J.C.; Farrell, J. Production of Acids and Bases for Ion Exchange Regeneration from Dilute Salt Solutions Using Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2015, 3, 2337–2342. [Google Scholar] [CrossRef]
- Ghalloussi, R.; Garcia-Vasquez, W.; Chaabane, L.; Dammak, L.; Larchet, C.; Deabatea, S.; Nevakshenova, E.; Nikonenko, V.; Grande, D. Ageing of ion-exchange membranes in electrodialysis: A structural and physicochemical investigation. J. Membr. Sci. 2013, 436, 68–78. [Google Scholar] [CrossRef]
- Scarazzato, T.; Panossian, Z.; Tenório, J.; Pérez-Herranz, V.; Espinosa, D. A review of cleaner production in electroplating industries using electrodialysis. J. Clean. Prod. 2017, 168, 1590–1602. [Google Scholar] [CrossRef]
- Giacalone, F.; Catrini, P.; Tamburini, A.; Cipollina, A.; Piacentino, A.; Micale, G. Exergy analysis of reverse electrodialysis. Energy Convers. Manag. 2018, 164, 588–602. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Bazinet, L.; Lamarche, F.; Ippersiel, D. Bipolar-membrane electrodialysis: Applications of electrodialysis in the food industry. Trends Food Sci. Technol. 1998, 9, 107–113. [Google Scholar] [CrossRef]
- Nemati, M.; Hosseini, S.M.; Shabanian, M. Novel electrodialysis cation exchange membrane prepared by 2-acrylamido-2-methylpropane sulfonic acid; heavy metal ions removal. J. Hazard. Mater. 2017, 337, 90–104. [Google Scholar] [CrossRef] [PubMed]
- López, J.; Reig, M.; Gibert, O.; Cortina, J. Increasing sustainability on the metallurgical industry by integration of membrane nanofiltration processes: Acid recovery. Sep. Purif. Technol. 2019, 226, 267–277. [Google Scholar] [CrossRef]
- Ji, P.-Y.; Ji, Z.; Chen, Q.-B.; Liu, J.; Zhao, Y.-Y.; Wang, S.-Z.; Li, F.; Yuan, J. Effect of coexisting ions on recovering lithium from high Mg2+/Li+ ratio brines by selective-electrodialysis. Sep. Purif. Technol. 2018, 207, 1–11. [Google Scholar] [CrossRef]
- Nayar, K.G.; Fernandes, J.; McGovern, R.K.; Dominguez, K.P.; McCance, A.; Al-Anzi, B. Cost and energy requirements of hybrid RO and ED brine concentration systems for salt production. Desalination 2019, 456, 97–120. [Google Scholar] [CrossRef]
- Kim, S.; Joo, H.; Moon, T.; Kim, S.-H.; Yoon, J. Rapid and selective lithium recovery from desalination brine using an electrochemical system. Environ. Sci. Process. Impacts 2019, 21, 667–676. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; He, L.; Zhao, Z.-W. Study on extraction of lithium from salt lake brine by membrane electrolysis. Desalination 2015, 376, 35–40. [Google Scholar] [CrossRef]
- Wen, X.; Ma, P.; Zhu, C.; He, Q.; Deng, X. Preliminary study on recovering lithium chloride from lithium-containing waters by nanofiltration. Sep. Purif. Technol. 2006, 49, 230–236. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Cai, L.-J.; Nie, X.-Y.; Song, X.; Yu, J. Separation of magnesium and lithium from brine using a Desal nanofiltration membrane. J. Water Process. Eng. 2015, 7, 210–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Paepen, S.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. Selectrodialysis: Fractionation of divalent ions from monovalent ions in a novel electrodialysis stack. Sep. Purif. Technol. 2012, 88, 191–201. [Google Scholar] [CrossRef]
- Nie, X.-Y.; Sun, S.-Y.; Sun, Z.; Song, X.; Yu, J. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 2017, 403, 128–135. [Google Scholar] [CrossRef]
- Afsar, N.U.; Ji, W.; Wu, B.; Shehzad, M.A.; Ge, L.; Xu, T. SPPO-based cation exchange membranes with a positively charged layer for cation fractionation. Desalination 2019, 472, 114145. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Golubenko, D.; Shevlyakova, N.; D’Yakova, M.; Tverskoi, V.; Dammak, L.; Grande, D.; Yaroslavtsev, A. New cation-exchange membranes based on cross-linked sulfonated polystyrene and polyethylene for power generation systems. J. Membr. Sci. 2016, 515, 196–203. [Google Scholar] [CrossRef]
- Roghmans, F.; Evdochenko, E.; Martí-Calatayud, M.; Garthe, M.; Tiwari, R.; Walther, A.; Wessling, M. On the permselectivity of cation-exchange membranes bearing an ion selective coating. J. Membr. Sci. 2020, 600, 117854. [Google Scholar] [CrossRef]
- Sulaiman, K.O.; Sajid, M.; Alhooshani, K. Application of porous membrane bag enclosed alkaline treated Y-Zeolite for removal of heavy metal ions from water. Microchem. J. 2020, 152, 104289. [Google Scholar] [CrossRef]
- Ji, W.; Afsar, N.U.; Wu, B.; Sheng, F.; Shehzad, M.A.; Ge, L.; Xu, T. In-situ crosslinked SPPO/PVA composite membranes for alkali recovery via diffusion dialysis. J. Membr. Sci. 2019, 590, 117267. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Xu, R. Needs and trends in rational synthesis of zeolitic materials. Chem. Soc. Rev. 2012, 41, 1729–1741. [Google Scholar] [CrossRef]
- Chen, Z.; Holmberg, B.; Li, W.; Wang, X.; Deng, W.; Munoz, R.; Yan, Y. Nafion/Zeolite Nanocomposite Membrane by in Situ Crystallization for a Direct Methanol Fuel Cell. Chem. Mater. 2006, 18, 5669–5675. [Google Scholar] [CrossRef]
- Csicsery, S.M. Shape-selective catalysis in zeolites. Zeolites 1984, 4, 202–213. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhu, X.; Li, M.; Lu, W.; Li, X.; Zhang, H. A Highly Ion-Selective Zeolite Flake Layer on Porous Membranes for Flow Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 3058–3062. [Google Scholar] [CrossRef] [PubMed]
- Moliner, M.; Martínez, C.; Corma, A. Multipore Zeolites: Synthesis and Catalytic Applications. Angew. Chem. Int. Ed. 2015, 54, 3560–3579. [Google Scholar] [CrossRef] [PubMed]
- Nur, H.; Kee, G.L.; Hamdan, H.; Mahlia, T.M.I.; Efendi, J.; Metselaar, H.S.C. Organosulfonic acid functionalized zeolite ZSM-5 as temperature tolerant proton conducting material. Int. J. Hydrog. Energy 2012, 37, 12513–12521. [Google Scholar] [CrossRef]
- Qiao, X.; Chung, T.-S.; Rajagopalan, R.; Chung, T.-S. Zeolite filled P84 co-polyimide membranes for dehydration of isopropanol through pervaporation process. Chem. Eng. Sci. 2006, 61, 6816–6825. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, L.; Zhang, L.; Chen, H.; Gao, C.; Ho, W.W. High-flux reverse osmosis membranes incorporated with NaY zeolite nanoparticles for brackish water desalination. J. Membr. Sci. 2015, 476, 373–383. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A. Effect of added NaX nano-zeolite into polyamide as a top thin layer of membrane on water flux and salt rejection in a reverse osmosis process. J. Membr. Sci. 2011, 375, 88–95. [Google Scholar] [CrossRef]
- Hamid, S.A.; Azha, S.F.; Sellaoui, L.; Bonilla-Petriciolet, A. Suzylawati Adsorption of copper (II) cation on polysulfone/zeolite blend sheet membrane: Synthesis, characterization, experiments and adsorption modelling. Colloids Surfaces A: Physicochem. Eng. Asp. 2020, 124980. [Google Scholar] [CrossRef]
- Raso, R.; Tovar, M.; Lasobras, J.; Herguido, J.; Kumakiri, I.; Araki, S.; Menéndez, M. Zeolite membranes: Comparison in the separation of H2O/H2/CO2 mixtures and test of a reactor for CO2 hydrogenation to methanol. Catal. Today 2020. [Google Scholar] [CrossRef]
- Liu, B.; Kita, H.; Yogo, K. Preparation of Si-rich LTA zeolite membrane using organic template-free solution for methanol dehydration. Sep. Purif. Technol. 2020, 239, 116533. [Google Scholar] [CrossRef]
- Sheng, F.; Hou, L.; Wang, X.; Irfan, M.; Shehzad, M.A.; Wu, B.; Ren, X.; Ge, L.; Xu, T. Electro-nanofiltration membranes with positively charged polyamide layer for cations separation. J. Membr. Sci. 2020, 594, 117453. [Google Scholar] [CrossRef]
- Abdu, S.; Martí-Calatayud, M.-C.; Wong, J.E.; García-Gabaldón, M.; Wessling, M. Layer-by-Layer Modification of Cation Exchange Membranes Controls Ion Selectivity and Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, H.; Schott, J.A.; Li, B.; Zheng, Y.-P.; Mahurin, S.M.; Jiang, D.-E.; Cui, G.; Hu, X.; Wang, Y.; et al. Porous liquid zeolites: Hydrogen bonding-stabilized H-ZSM-5 in branched ionic liquids. Nanoscale 2019, 11, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Jambulingam, R.; Jambulingam, R. Waste crab shell derived CaO impregnated Na-ZSM-5 as a solid base catalyst for the transesterification of neem oil into biodiesel. Sustain. Environ. Res. 2017, 27, 273–278. [Google Scholar] [CrossRef]
- Ali, F.; Maiz, F. Structural, optical and AFM characterization of PVA:La3+ polymer films. Phys. B Condens. Matter 2018, 530, 19–23. [Google Scholar] [CrossRef]
- Puguan, J.M.C.; Kim, H.-S.; Lee, K.-J.; Kim, H. Low internal concentration polarization in forward osmosis membranes with hydrophilic crosslinked PVA nanofibers as porous support layer. Desalination 2014, 336, 24–31. [Google Scholar] [CrossRef]
- Park, M.J.; Gonzales, R.R.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Hydrophilic polyvinyl alcohol coating on hydrophobic electrospun nanofiber membrane for high performance thin film composite forward osmosis membrane. Desalination 2018, 426, 50–59. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, B.; Sun, D.; Yang, P. Preparation and characterization of PVA membrane modified by water-soluble hyperbranched polyester (WHBP) for the dehydration of n-butanol. J. Appl. Polym. Sci. 2016, 133, 133. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Zhao, L.; Zhang, G.; Wu, C.; Xu, T. QPPO/PVA anion exchange hybrid membranes from double crosslinking agents for acid recovery. J. Membr. Sci. 2013, 428, 95–103. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Wang, J.; Zhu, J.; Shen, J.; Van Der Bruggen, B. Mussel-inspired modification of ion exchange membrane for monovalent separation. J. Membr. Sci. 2018, 553, 139–150. [Google Scholar] [CrossRef]
- Liu, H.; Ruan, H.; Zhao, Y.; Pan, J.; Sotto, A.; Gao, C.; Van Der Bruggen, B.; Shen, J. A facile avenue to modify polyelectrolyte multilayers on anion exchange membranes to enhance monovalent selectivity and durability simultaneously. J. Membr. Sci. 2017, 543, 310–318. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvation of ions. Part 5.—Gibbs free energy of hydration at 298.15 K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- He, Y.; Ge, L.; Ge, Z.; Zhao, Z.; Sheng, F.; Liu, X.; Ge, X.; Yang, Z.; Fu, R.; Liu, Z.; et al. Monovalent cations permselective membranes with zwitterionic side chains. J. Membr. Sci. 2018, 563, 320–325. [Google Scholar] [CrossRef]
| Membranes | Morphology | Systems | Perm-Selectivity | [Ref.] |
|---|---|---|---|---|
| CSO | dense | H+/Zn2+, Li+/Mg2+ | 3.5, 1.6 | [40,52] |
| SBQAPPO | dense | H+/Zn2+, Na+/Mg2+ | 23.5, 7.4 | [52] |
| Neosepta CMX | dense | Na+/Mg2+ | 1.6 | [41] |
| Asymmetric porous | porous | Na+/Mg2+ | 3.3 | [2] |
| DL-2540 NF | porous | Li+/Mg2+ | 3.3 | [20] |
| ZSM-5/PVA-based MMMs | porous | H+/Zn2+, Li+/Mg2+ | 34.4, 3.7 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, F.; Afsar, N.U.; Zhu, Y.; Ge, L.; Xu, T. PVA-Based Mixed Matrix Membranes Comprising ZSM-5 for Cations Separation. Membranes 2020, 10, 114. https://doi.org/10.3390/membranes10060114
Sheng F, Afsar NU, Zhu Y, Ge L, Xu T. PVA-Based Mixed Matrix Membranes Comprising ZSM-5 for Cations Separation. Membranes. 2020; 10(6):114. https://doi.org/10.3390/membranes10060114
Chicago/Turabian StyleSheng, Fangmeng, Noor Ul Afsar, Yanran Zhu, Liang Ge, and Tongwen Xu. 2020. "PVA-Based Mixed Matrix Membranes Comprising ZSM-5 for Cations Separation" Membranes 10, no. 6: 114. https://doi.org/10.3390/membranes10060114
APA StyleSheng, F., Afsar, N. U., Zhu, Y., Ge, L., & Xu, T. (2020). PVA-Based Mixed Matrix Membranes Comprising ZSM-5 for Cations Separation. Membranes, 10(6), 114. https://doi.org/10.3390/membranes10060114








