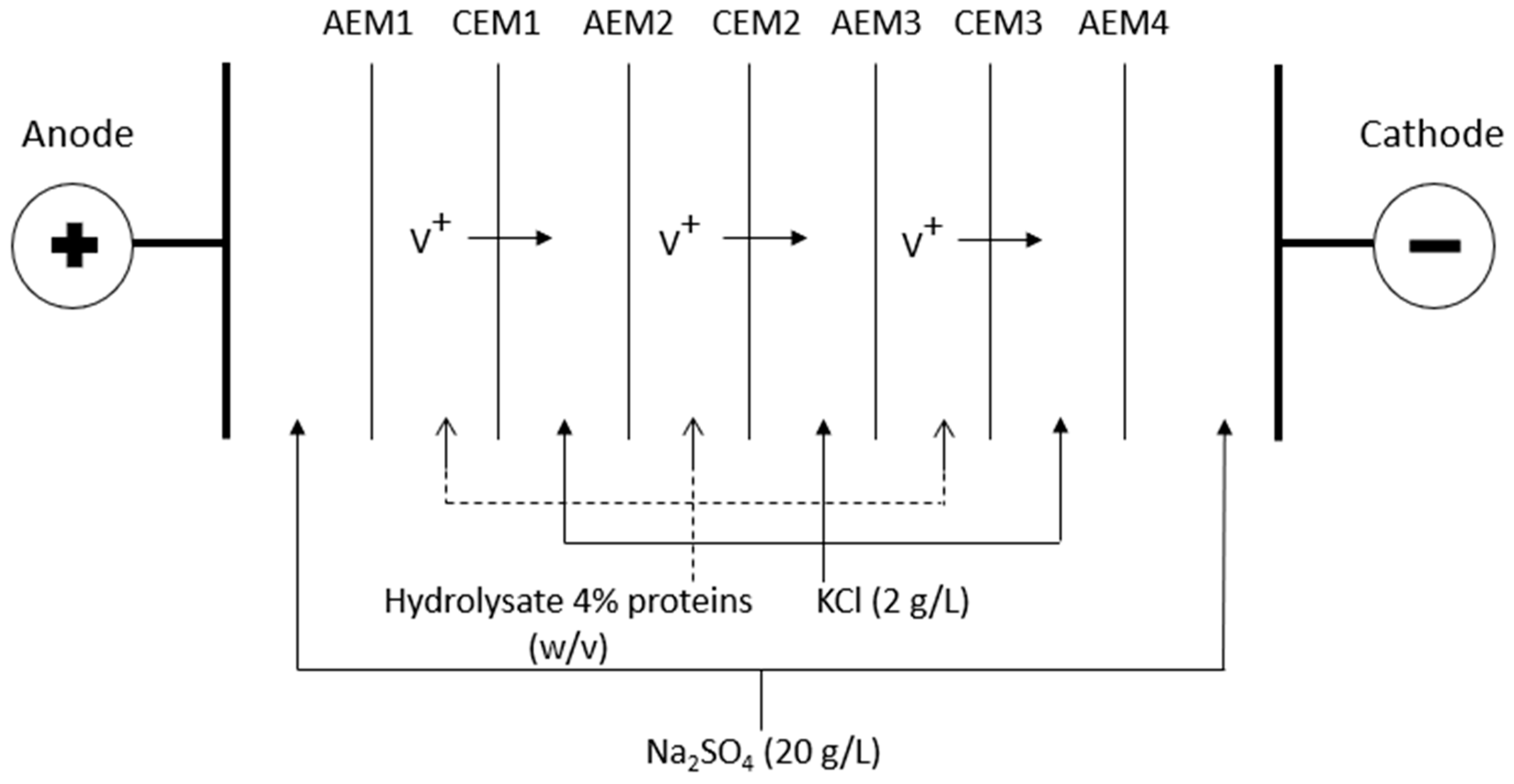
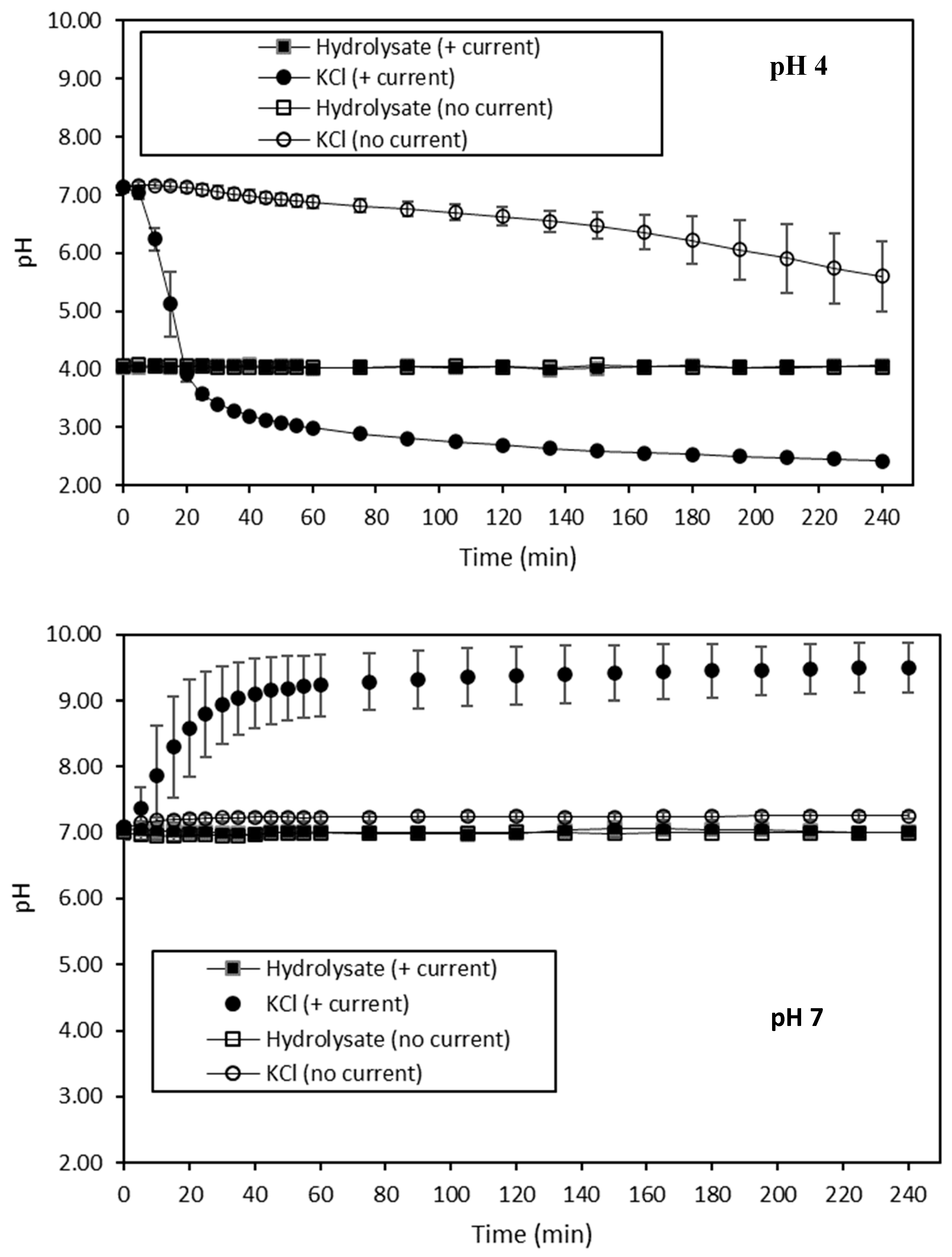
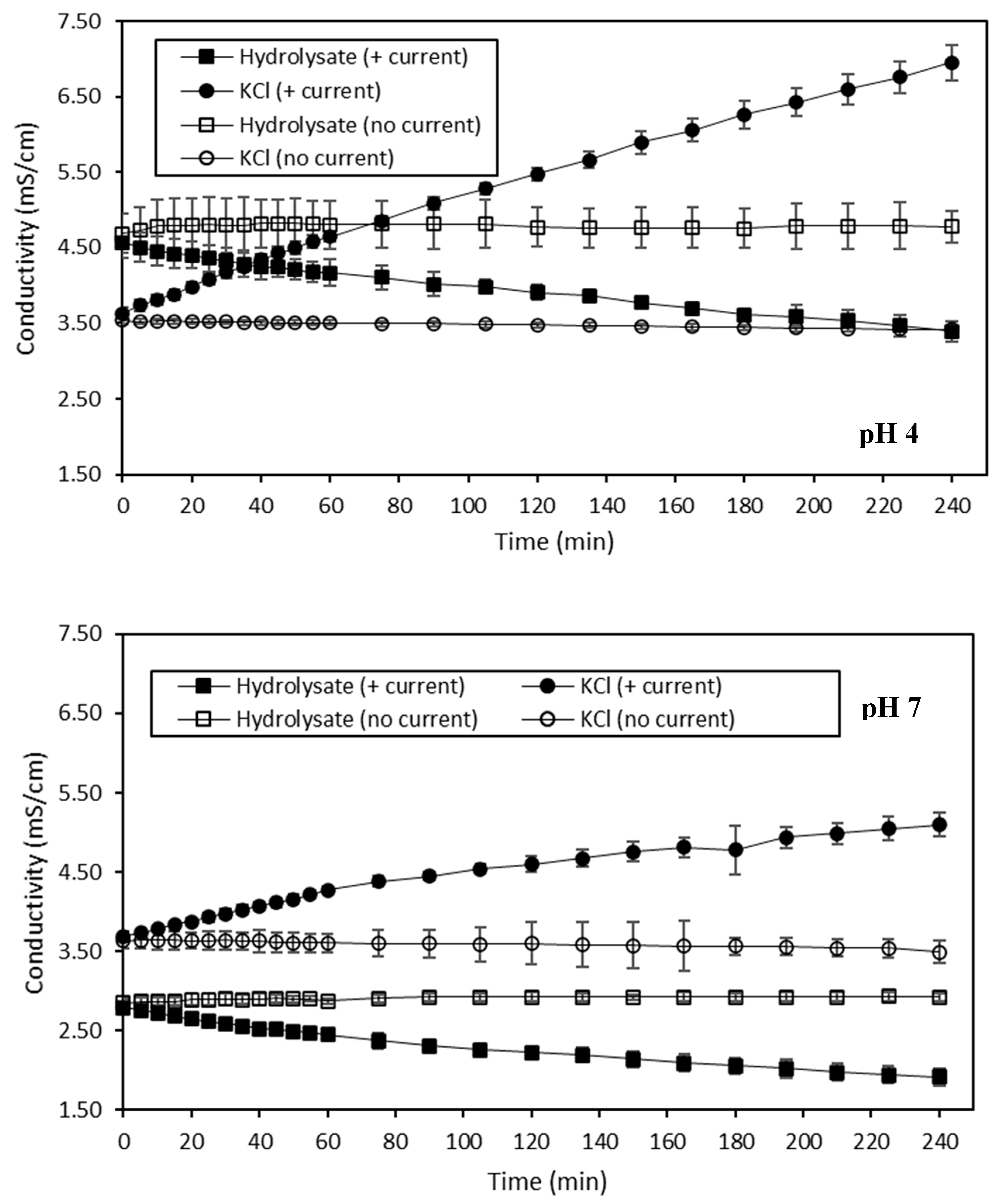
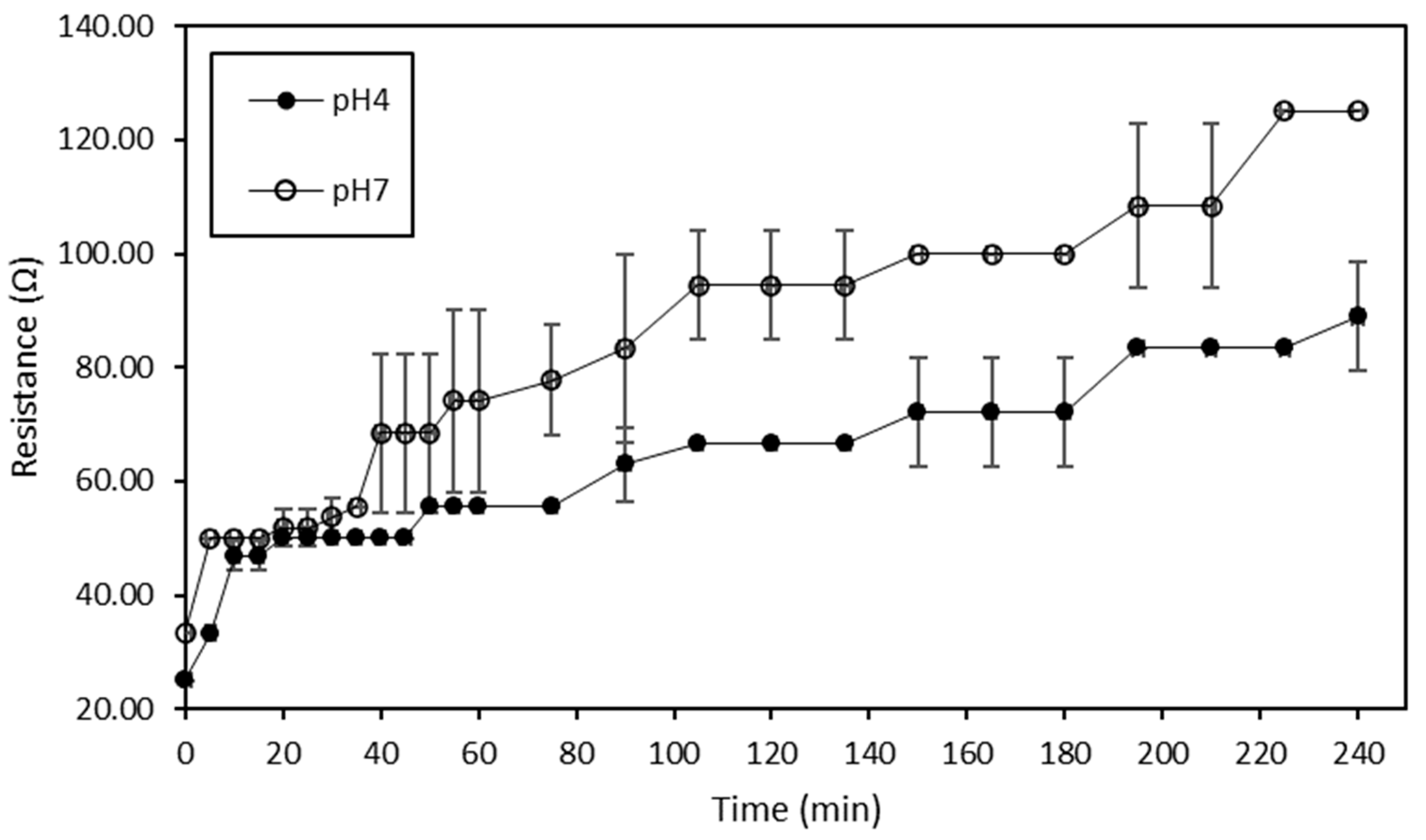
Volatile Compound Analysis
Most Potent Odor-Active Compounds
The abundance of compounds determined to be the most potent odorants of the HMH over the different ED and deaerator treatments is shown in
Table 6. Firstly, concerning the composition of the hydrolysate at the initial time, the results indicate that pH had an important impact on the volatile compounds’ abundance. It appeared that the abundance of the majority of the most potent odor-active molecules significantly decreased while pH increased from 4 to 10. For example, the abundance of 3-methylbutanal, 2,3-pentanedione, pentanal, hexanal, (Z)-4-heptenal, heptanal, methional, (Z)-6-octen-2-one, (E,E)-2,4-heptadienal, octanal, 2-nonanone and (E,Z)-2,6-nonadienal dropped by at least 50% between the hydrolysate at pH 4 and the hydrolysate at pH 10 at the initial time (
p < 0.05). 1-methyl-1H-tetrazole was the only compound that did not follow this trend. In fact, it was identified in the hydrolysate at pH 7, but it was not present in the hydrolysate at pH 4 and pH 10. In general, pH is known to be a major factor influencing the content of volatile compounds. Indeed, volatile compounds are able to interact with molecules like lipids through hydrophobic interactions, and amino acids constituents such as proteins, peptides and free amino acids through covalent irreversible bindings, in addition to hydrophobic and ionic interactions [
68,
69,
70]. Among these different interactions, those taking place between volatile compounds and amino-acid containing compounds are the most impacted by pH, as this factor modifies the conformation and charge of proteins, peptides and free amino acids, and thus the ability of binding of volatile compounds [
69]. Based on this fact, two hypotheses can be made concerning the general decrease in volatile compounds observed while pH increased. The first could be that alkaline pH might be responsible for the breaking of interactions taking place between volatile compounds and amino groups. As the targeted compounds are volatile, breaking these interactions could promote their loss. On the contrary, the second could be that the lower abundance of volatile compounds observed in the HMH at pH 10 at the initial time may be representative of a higher interaction with amino acid materials. As no study has been carried out regarding the impact of pH on the retention of volatile compounds by amino acid constituents from HMH materials so far, it was not obvious to clearly validate one hypothesis rather than the other. Nevertheless, some studies with similar purposes were already conducted on milk proteins [
71,
72], as well as on animal tissues proteins and peptides [
69,
73]. These studies showed that there was a general trend of amino acid containing molecules, such as peptides, to retain volatile compounds to a higher extent while pH increased. Several explanations are involved, depending on the proteins. For example, the milk protein β-lactoglobulin is reported to bind a larger proportion of volatile compounds at pH 9 than at pH 3. The increase in retention ability is, in this case, explained by better access to the hydrophobic amino acid residues of β-lactoglobulin due to conformation changes occurring under alkaline conditions [
70,
72]. Interestingly, leucine, a hydrophobic amino acid, is present in both β-lactoglobulin and HMH. Leucine is even present in high proportion in the latter (
Table 1). It could be suggested that leucine might participate in the retention of volatile compounds, and while pH increases, the loss of the proton H
+ on the amine group could promote this phenomenon. Independently of pH, Meynier et al. (2004) observed the unavailability of lysine and histidine of milk proteins in the presence of aldehydes, suggesting a potential interaction occurring between these amino acids and volatile compounds. It was proposed to explain this loss that the carbonyl group of aldehydes could react with the primary amine of lysine either by Michael addition or by Schiff base formation. Concerning histidine, it was suggested that aldehyde and, preferentially, alkenal could react with the imidazole ring of histidine [
74]. Since among the 15 compounds identified as being the most potent odor-active of HMH, 11 are aldehydes and one of them is an alkenal, namely (Z)-4-heptenal, and since lysine and histidine are both present in this product (
Table 1), it could be possible that these interactions occur between these volatile compounds and these amino acids. Histidine is also a constituent of carnosine, a dipeptide found in animal tissues, and whose ability to retain volatile compounds is also known to increase while pH increases [
69]. In that case, as pH affects the retention of volatile compounds, it could be proposed, similarly to leucine, that the loss of the proton H
+ on the imidazole ring occurring under alkaline conditions could promote the interaction between histidine and aldehydes. Interestingly, HMH contains a high amount of arginine (
Table 1), which is an amino acid with an amine-containing side-chain similar to lysine and histidine. Based on this, it should be proposed that arginine was also involved in the interactions, explaining partially the decrease in volatile compound abundance observed. Therefore, the hypothesis that seemed to be the most plausible regarding the decrease in the abundance of volatile compounds at pH 10 compared to pH 4 and pH 7 would be those implying a higher degree of interaction occurring between volatile compounds and amino acid containing compounds, such as peptides present in the hydrolysate. In that case, the fact that 1-methyl-1H-tetrazole was not detected in the hydrolysate at pH 4 and 10 might suggest that this compound could have more interactions at these pH values than at pH 7, allowing its detection at this pH value only. Also, it is of interest to mention that, while volatile compounds are bound to other components, both their release and perception are hindered [
69]. This means that HMH should be globally less odorous at pH 10 than at pH 4 and pH 7.
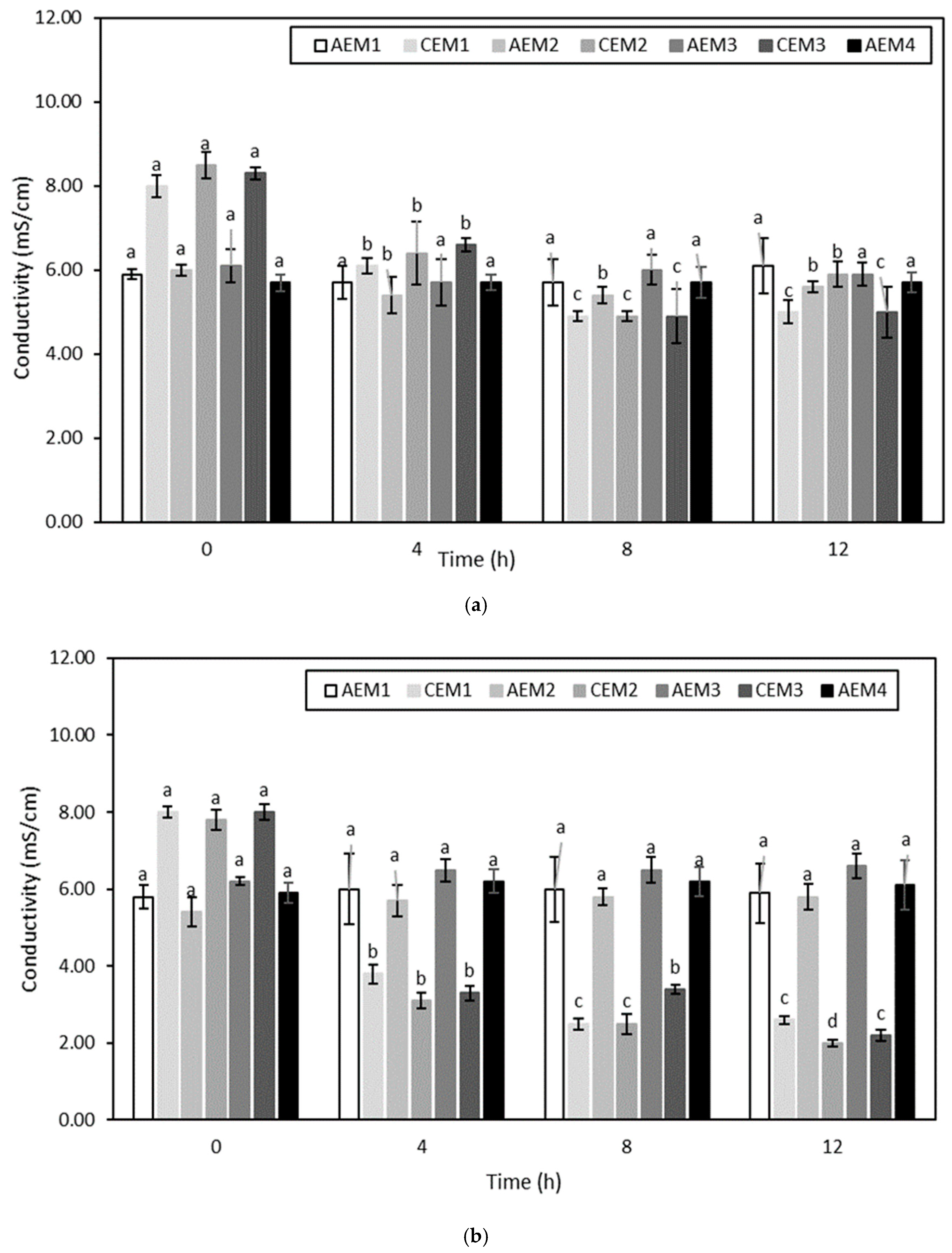
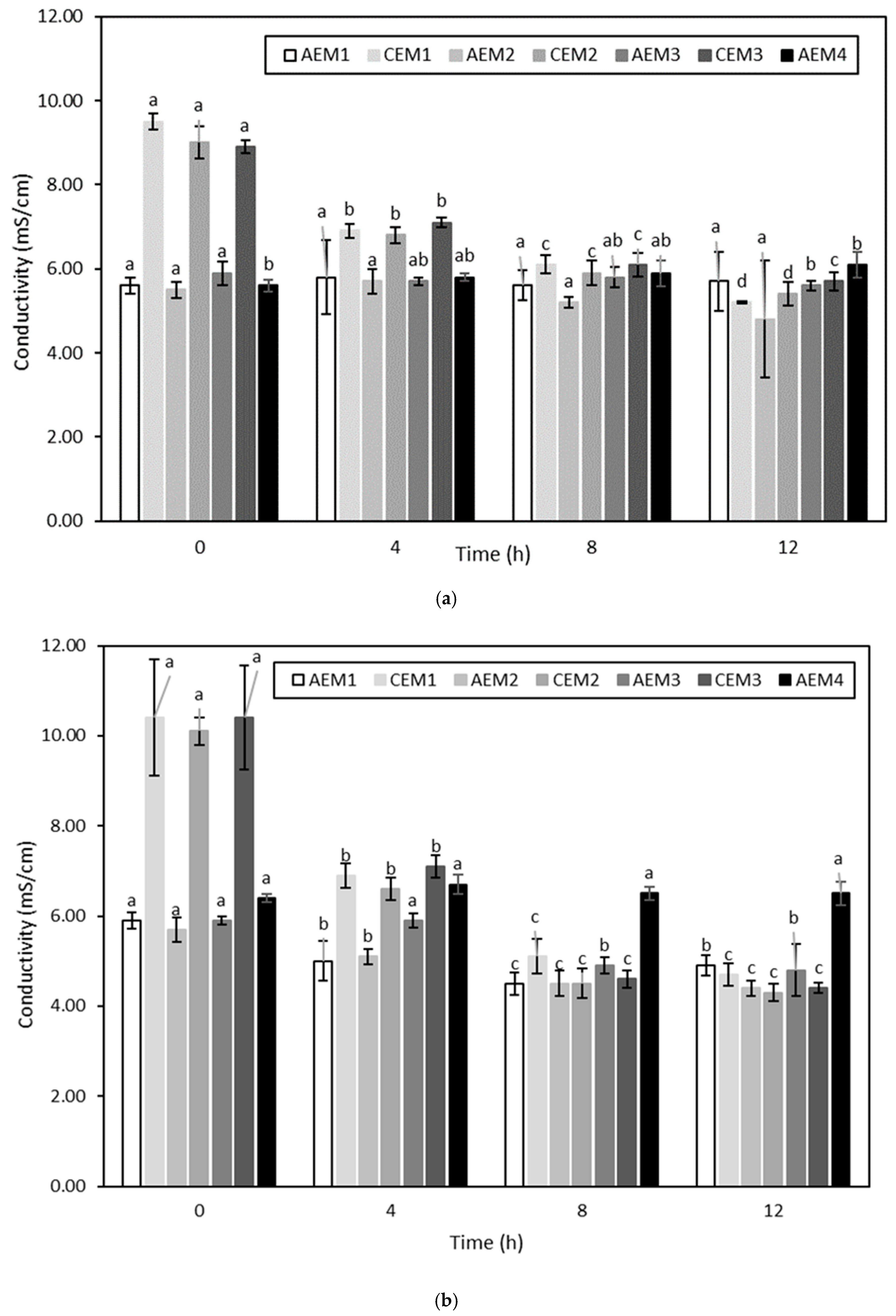
Then, regarding the ED treatments, no significant difference was globally observed between the hydrolysate at a given pH at the initial time and the hydrolysate treated with or without current at the final time. More precisely, if attention is paid to the ED treatments conducted without current, the fact that no decrease in the content of the targeted volatile compounds was observed would suggest that a simple circulation of the hydrolysate solution for 240 min, independently of its pH, was not sufficient enough to allow a loss of these molecules due to their volatile state. Concerning ED conducted with current, no change in the content of volatile compounds occurred except for (Z)-6-octen-2-one, (E,E)-2,4-heptadienal and (E,Z)-2,6-nonadienal whose abundance was inferior at final time for ED on the hydrolysate at pH 4 (
p < 0.05). These results could be representative of the non-migration of volatile compounds. Since ED is a process based on the migration of charged compounds, and since the targeted compounds were not supposed to be charged under the conditions tested, it was not surprising, at the first glance, to obtain such results. However, in their studies, Cros et al. (2005) and Chindapan et al. (2011) observed that an ED treatment could lead to a drop of volatile compounds even if they are not charged [
25,
26]. Several points could explain the discrepancy between these two studies and the present one. The first could be that the compounds whose abundance dropped during ED treatments in the studies of Cros et al. (2005) and Chindapan et al. (2011) were not the same compounds targeted in the present study. Indeed, Cros et al. (2005) observed a significant decrease in the non ionizable (Z)-4-heptenal, 2,3-butanedione, 3-octen-2-one and limonene compounds. The only compound that this study and the present one had in common was (Z)-4-heptenal. Nevertheless, it is noteworthy to mention that Cros et al. (2005) noticed the important decrease of the compounds listed before only while the LCD was reached. Different hypotheses were formulated to explain such a decrease under this specific condition in this study. The first was that the formation of protons H
+ and hydroxyls OH
- resulting from water dissociation under this critical condition could have altered volatile compounds, explaining their decrease. Another was that the LCD could have also brought about a local membrane heating, potentially leading to a thermal degradation of volatile compounds. Or, simply, the volatile compounds could have been adsorbed on the membranes through hydrophobic and ionic interactions [
26]. The migration of these molecules was not considered to be a potential explanation, as none of them were found in the recovery solution. Regarding (Z)-4-heptenal only, Cros et al. (2005) hypothesized its hydrogenation in heptanal as a possible explanation for its decrease [
26]. Since not all the ED treatments conducted in the present study seemed to have evidenced reaching LCD, it was not possible to totally verify all the hypotheses formulated by Cros et al. (2005). However, as suggested by the analyses of the parameters of the ED treatment conducted on the hydrolysate at pH 7, it seems that this condition experienced the reaching of LCD. As no change regarding the volatile compound content was observed in that case, it may indicate that the LCD was not a sufficient condition to lead to a decrease in the abundance of these compounds. In addition, the fact that both ED treatments conducted with current presented water dissociation in the present study could show that the generation of H
+ and OH
- species could not be effectively responsible for the alteration of the volatile compounds. On the contrary to Cros et al. (2005), Chindapan et al. (2011) did not reach the LCD condition in their study. Despite this fact, they observed a significant decrease in 2,6-dimethylpyrazine, phenol and carboxylic acids (acetic acid, butanoic acid, 2-methylbutanoic acid, pentanoic acid, 4-methylpentanoic acid) while the ED treatment was performed, to reach a salt concentration of 2% in the treated fish sauce. Chindapan et al. (2011) gave two main reasons for the loss of these compounds: either their adsorption on the membranes or their transport through the membranes occurring at the same time as electroosmosis. Nevertheless, as no mention concerning the composition of the recovery solution was made, it was not possible to know if the latter reason was plausible in that case [
25]. Concerning the decreased in (Z)-6-octen-2-one, (E,E)-2,4-heptadienal and (E,Z)-2,6-nonadienal observed in the hydrolysate at pH 4 treated with current at final time, three hypotheses could be made, based on those previously mentioned. The first one would be that a slight loss of these molecules due to their volatile state happened during the ED process. However, as no decrease of these compounds was observed for other conditions, this hypothesis does not seem highly plausible. The second hypothesis would be that preferential interactions occurred at pH 4 between these three volatile compounds and other constituents of the HMH. Finally, the last hypothesis would be that these compounds preferentially adsorb on the membranes due to hydrophobic interactions. This last hypothesis appears to be the most probable, based on the membrane conductivity evolution discussed previously.
The content in volatile compounds of the KCl recovery solutions was analyzed for each condition, and is listed in
Table 7. The results show the unchanged presence of 3-methylbutanal, hexanal, heptanal, benzaldehyde, octanal and 2-nonanone in KCl solution at final time independently of the ED treatment. It is worthwhile to mention that none of these compounds were detected in the KCl solution at the initial time. Except for benzaldehyde and hexanal, in all cases, the presence of volatile compounds in the recovery solution can be considered to be trace. This should probably be due to a punctual contamination of these compounds due to their volatility from the hydrolysate to the KCl solution. The fact that this phenomenon could be considered to be punctual was accredited by the generally high values of standard deviations proportionally to those of means, and even sometimes the higher values of standard deviations compared to the corresponding means. However, another explanation could be involved for hexanal and benzaldehyde. Regarding hexanal, its presence in the KCl solution could be due to its diffusion or migration. However, based on the membrane conductivity analysis discussed previously, it seemed that some interactions with membrane components also occurred during the different ED treatments. Therefore, another explanation could be that hexanal may have interacted with the sulfonic groups present in the CEMs, resulting in its release into the KCl compartment thereafter. Interestingly, the same trend was not found for compounds similar to hexanal, such as pentanal and heptanal. In that case, the differences observed should probably be due to the presence of hexanal in higher quantity in HMH, compared to pentanal and heptanal. Regarding benzaldehyde, the same explanations as those mentioned for hexanal could be involved. Nevertheless, on the contrary to hexanal, the fact that benzaldehyde was found in higher abundance only in KCl solution of the hydrolysate at pH 4 treated with current may indicate that a special mechanism was involved in that case. Initially, as benzaldehyde is not charged, it was not supposed to migrate. However, its recovery in the KCl solution might suggest that benzaldehyde could have either established interactions with another positively charged constituent that migrated into the CEMs, or that benzaldehyde established an interaction with the sulfonic groups of the CEMs, resulting in its release into the KCl compartment thereafter. Nonetheless, assuming that an interaction with another constituent could explain the presence of benzaldehyde in that case, this interaction could have been broken once this compound was finally in the KCl solution, as its detection was still allowed. Indeed, as mentioned previously in this study, while volatile compounds interact with other constituents, it hinders their detection [
69]. Moreover, the results could show that this potential interaction occurred only at pH 4, as a similar trend was not found at pH 7. The charged compounds in that case could be histidine, present mainly in its free form in the HMH [
1] as, at pH 4, its side-chain was totally protonated (pKa ~6.0), allowing its migration to the cathode through CEMs, while at pH 7 this latter was in its non-charged form. Interestingly, the presence of (Z)-6-octen-2-one, (E,E)-2,4-heptadienal and (E,Z)-2,6-nonadienal, whose abundance was lower in the hydrolysate at pH 4 treated with current at the final time, was not found in the corresponding KCl solution. Therefore, supposing that the hypothesis formulated before aiming that these compounds could have established interactions with membranes, this could indicate that none of these three compounds were released into the KCl compartment thereafter.
Finally, the performance of ED to decrease the abundance of the most potent odor-active compounds of the HMH was compared to that of a deaerator (
Table 4). In this case, in addition to pH 4 and 7, the treatment was also conducted on the hydrolysate at pH 10. Compared to pH 4-hydrolysate at the initial time, the deaerator allowed the decrease in seven compounds (
p < 0.05), namely 3-methylbutanal, 2-methylbutanal, 2,3-pentanedione, pentanal, hexanal, 2-nonanone and (E,Z)-2,6-nonadienal. A similar trend was observed for pH 7-hydrolysate, for which the deaerator allowed a drop in the abundance of the seven following compounds (
p < 0.05): 3-methylbutanal, 2-methylbutanal, 1-methyl-1H-tetrazole, pentanal, hexanal, (Z)-6-octen-2-one and 2-nonanone. It is of interest to note that, in this case, the deaerator conducted the total loss of 1-methyl-1H-tetrazole, pentanal and (Z)-6-octen-2-one. That could suggest that the ability of such device to remove volatile compounds was better at pH 7 than at pH 4. As it was mentioned previously, the hypothesis that looked more plausible to explain the difference in volatile compound content between the initial hydrolysate at different pH values was the following: while pH increased, higher interactions between the volatiles and other constituents occurred. This could be in line with the results of the deaerator for the hydrolysate at pH 4 and pH 7. Indeed, it seemed that this device could break weak interactions occurring between volatile compounds and other compounds present in the hydrolysate, resulting in a better decrease rate at pH 7 than at pH 4. Nevertheless, the deaerator did not lead to a decrease in the volatile content while the hydrolysate was treated at pH 10. This could indicate that, at pH 10, the chosen hypothesis was not enough to totally explain the mechanisms involved. It could be supposed that, at pH 10, a certain proportion of volatile compounds could take part in strong interactions, such as covalent bonds, but at the same time, some of them could have been lost due to their promoted passage in the headspace of the hydrolysate solution as well, or simply altered, hindering their detection.
TMAO, TMA and DMA
The TMAO, TMA and DMA contents of HMH are shown in
Table 8. Firstly, concerning the hydrolysate at the initial time for the three tested pH values, their concentration in TMAO, TMA and DMA was similar (
p > 0.05). The only difference observed was related to the content of TMAO of the hydrolysate at pH 10, which was 20 times lower (
p < 0.05) than those of the hydrolysate at pH 4 and 7 at the initial time. In this context, it is worth to mention that the procedures used for the analysis of TMAO, TMA and DMA recommend to alkalize samples of interest, to allow a better detection of these molecules based on their higher release into the sample headspace [
41]. Therefore, the huge decrease in TMAO content observed in the hydrolysate at pH 10 could be related to a loss following their release into the headspace of the hydrolysate solution due to its high volatility.
Then, concerning the content in TMAO, TMA and DMA after the four ED treatments, no difference was observed between the hydrolysate at initial and final times. ED treatments were especially designed to assess whether TMA and DMA, two positively charged compounds at pH 4 and 7, were able to migrate. However, the results indicated that no migration happened while experiments were conducted with current. As suggested by the ED parameter analyses, some water dissociation took place during the treatments conducted at pH 4 and 7 with current. Therefore, it could be hypothesized that TMA and DMA had been in competition with the generated H
+ to migrate into the CEMs, and that H
+ could have prevailed over TMA and DMA. Another explanation could be that fouling occurring on CEMs, as suggested by the membrane conductivity analysis, hindering the migration of TMA and DMA, thus explaining such results. Chindapan et al. (2011) experienced, in their study, a decrease in TMA, and explained this result by its loss occurring during ED due to its high volatility [
25]. However, the results obtained in the present study may indicate that TMA could not be lost as easily, since ED treatments carried out without current did not evidence any change in the content of this compound. Moreover, the fact that no change in the concentration of TMAO was observed between the hydrolysate at the initial and final times treated with current was more expected. Indeed, this molecule is a zwitterion, and the absence of global charge makes it less likely to migrate during an ED process.
The contents in TMAO, TMA and DMA of KCl recovery solution were analyzed (
Table 9). The results show that the initial solution was free of these compounds, while the KCl solution at the final time of all the tested conditions only evidenced the presence of TMAO. The presence of TMAO in the recovery solution of treatments conducted with current was not expected, as the global charge of this compound was neutral. However, the fact that TMAO was present in the recovery solution of treatments carried out without current as well could suggest that another mechanism than electromigration could be involved. In addition, the concentration of TMAO in the different KCl recovery solutions was surprisingly as important as those of the corresponding hydrolysate at the initial time, and since the concentration of this compound in the hydrolysate did not evolve during the different ED treatments, this could suggest that new TMAO was generated over the time. The most logical explanation at the first glance could have been that some TMA evidenced oxidation, resulting in the formation of much more TMAO. However, this was not possible in the case of this study, as the initial hydrolysate, independently of its pH, had too low a content of TMA. This means that more complex mechanisms occurred. TMAO is traditionally produced from nitrogenous compounds, such as choline, betaine or carnitine, through metabolism pathways involving enzymes and gut microbiota [
75]. Interestingly, HMH contains phospholipids whose choline can be a constituent and carnitine as well (
Table 1). Even if metabolism pathways could not be involved in that case, it could be supposed that some TMAO was generated from the choline of phospholipids and carnitine through other reactions, such as oxidation. Nevertheless, this could only explain the occurrence of much more TMAO compared to the initial time, and not its recovery in the different KCl solutions. Another hypothesis could be that reactions between constituents of HMH, such as the choline of phospholipids or carnitine, as mentioned before, and those of CEMs could have taken place. This latter hypothesis seems to be even more plausible, as the analysis of ED parameters, and more specifically those regarding membrane conductivity, revealed that some interactions happened between hydrolysate constituents and membranes. However, at this stage, it is not possible to effectively favor one hypothesis rather than another one. A last point that is worth mentioning is that the absence of TMA and DMA in the KCl recovery solutions of treatments conducted with current was effectively representative of their non-migration.
Finally, the comparison of the performance of ED with those of a deaerator was assessed. The results are presented in
Table 8. They indicate that the deaerator was only effective in decreasing the concentration of TMAO (
p < 0.05) of the hydrolysate at pH 4 and 7. Regarding the hydrolysate at pH 10, this device had no effect on its composition. These results were consistent with those obtained for the most potent odor-active compounds, as discussed before. However, in that case, the fact that no impact regarding the TMAO content of the hydrolysate was observed gave credit to its loss following its release into the headspace of the hydrolysate sample, promoted by alkaline conditions and occurring before the deaerator treatment, as mentioned previously.