Influence of Surface Modification of MK-40 Membrane with Polyaniline on Scale Formation under Electrodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Membranes
2.2. Analysis Methods
2.2.1. Membrane Thickness
2.2.2. Membrane Electrical Conductivity
2.2.3. Contact Angle Measurements
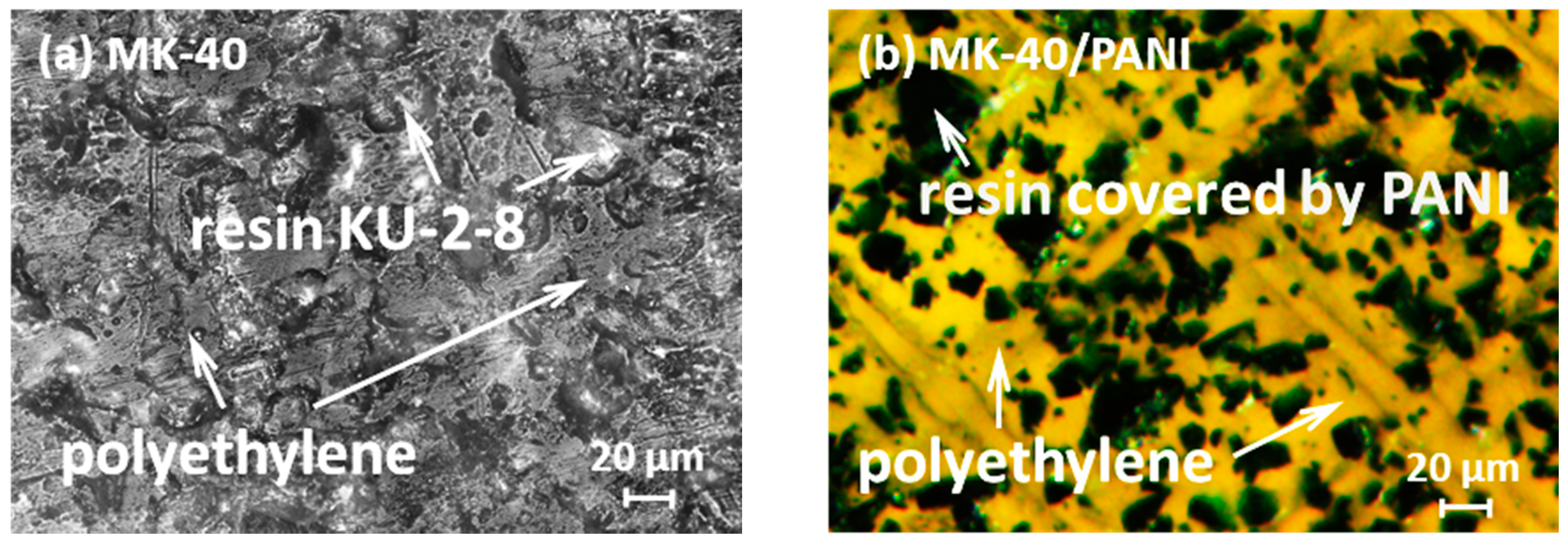
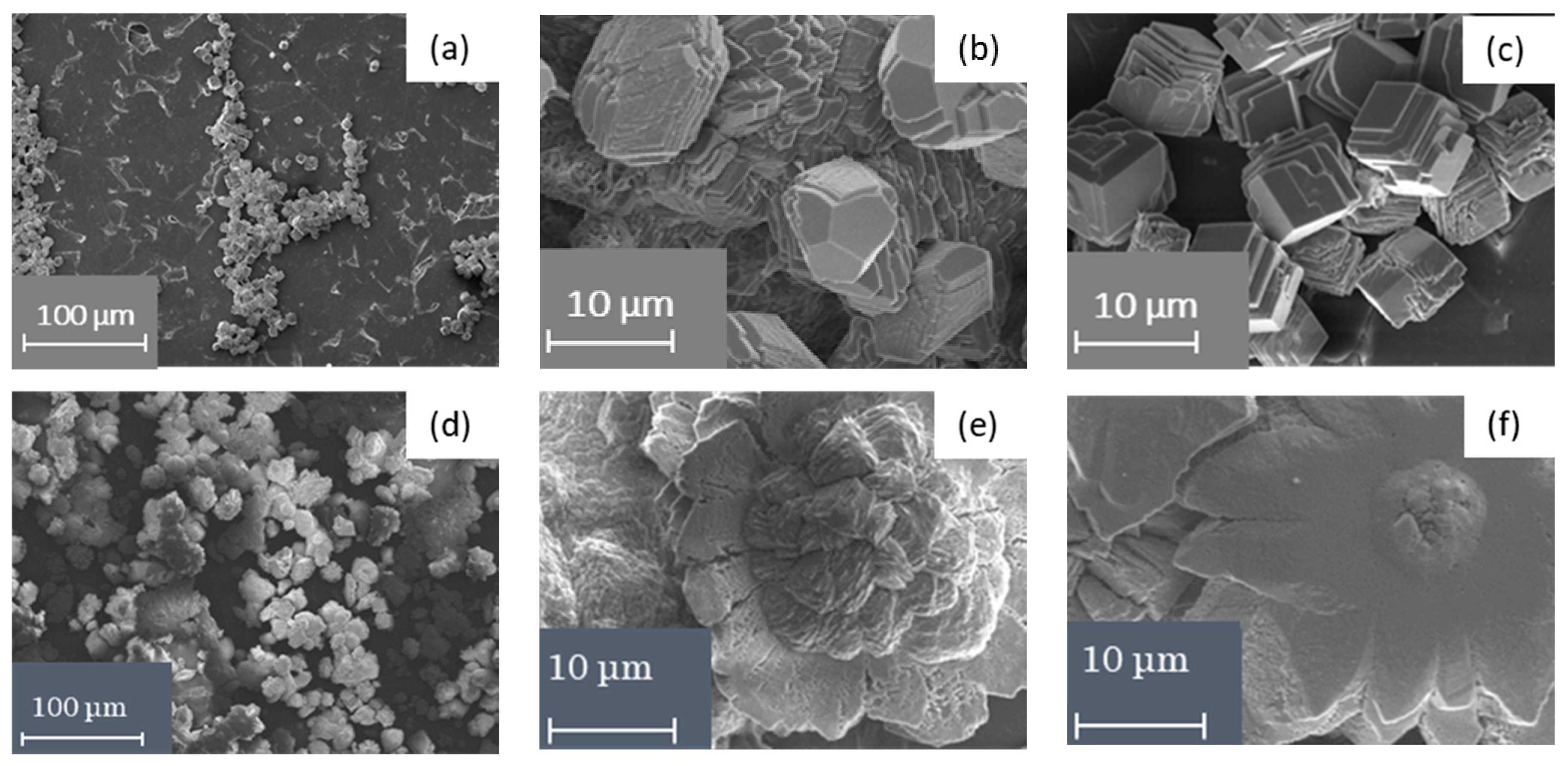
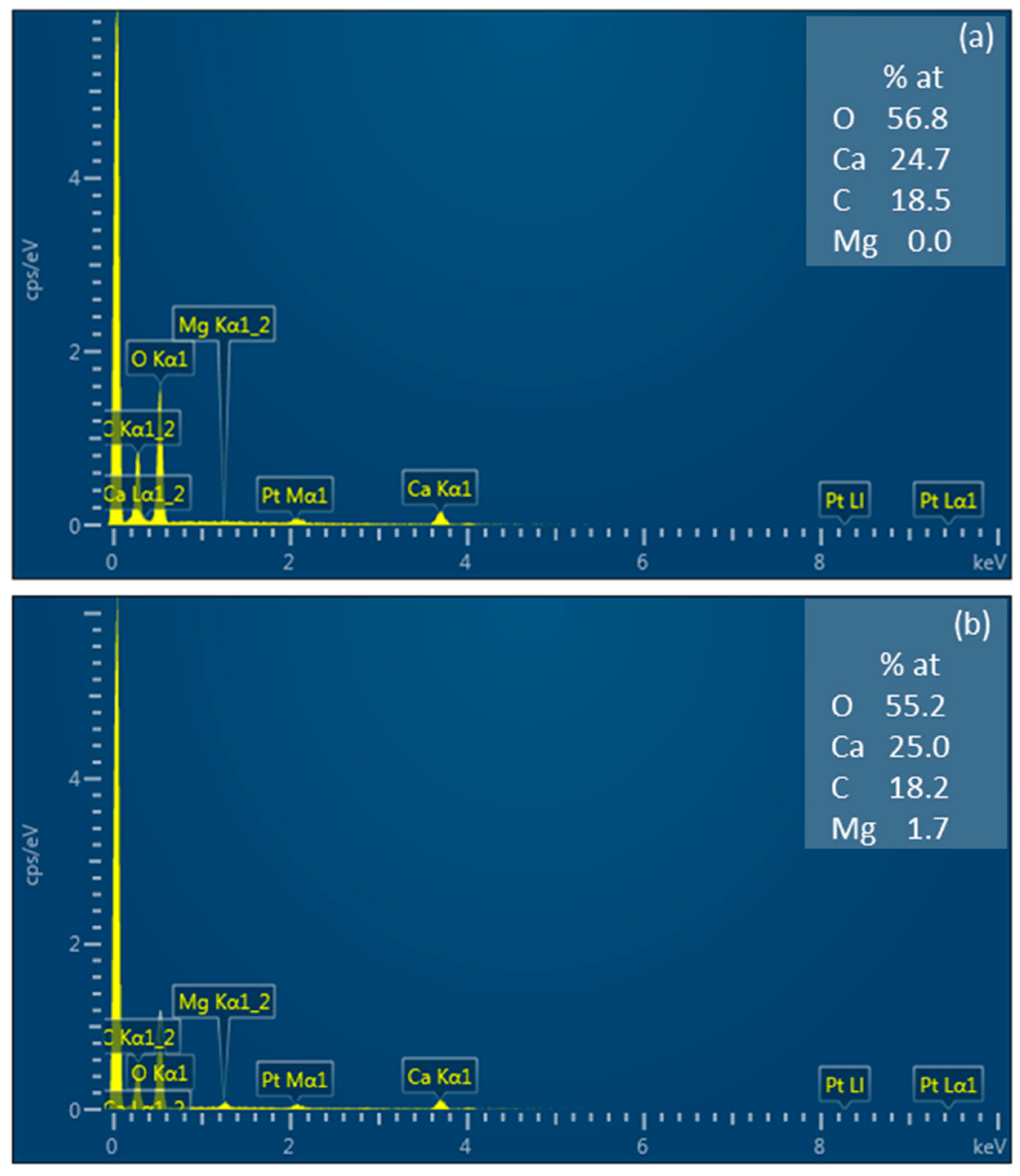
2.2.4. Scanning Electron Microscopy
2.3. Electrodialysis Cell and Experimental Setup
2.4. Protocol
3. Results and Discussion
3.1. Physico-Chemical Characteristics of Ion-Exchange Membranes
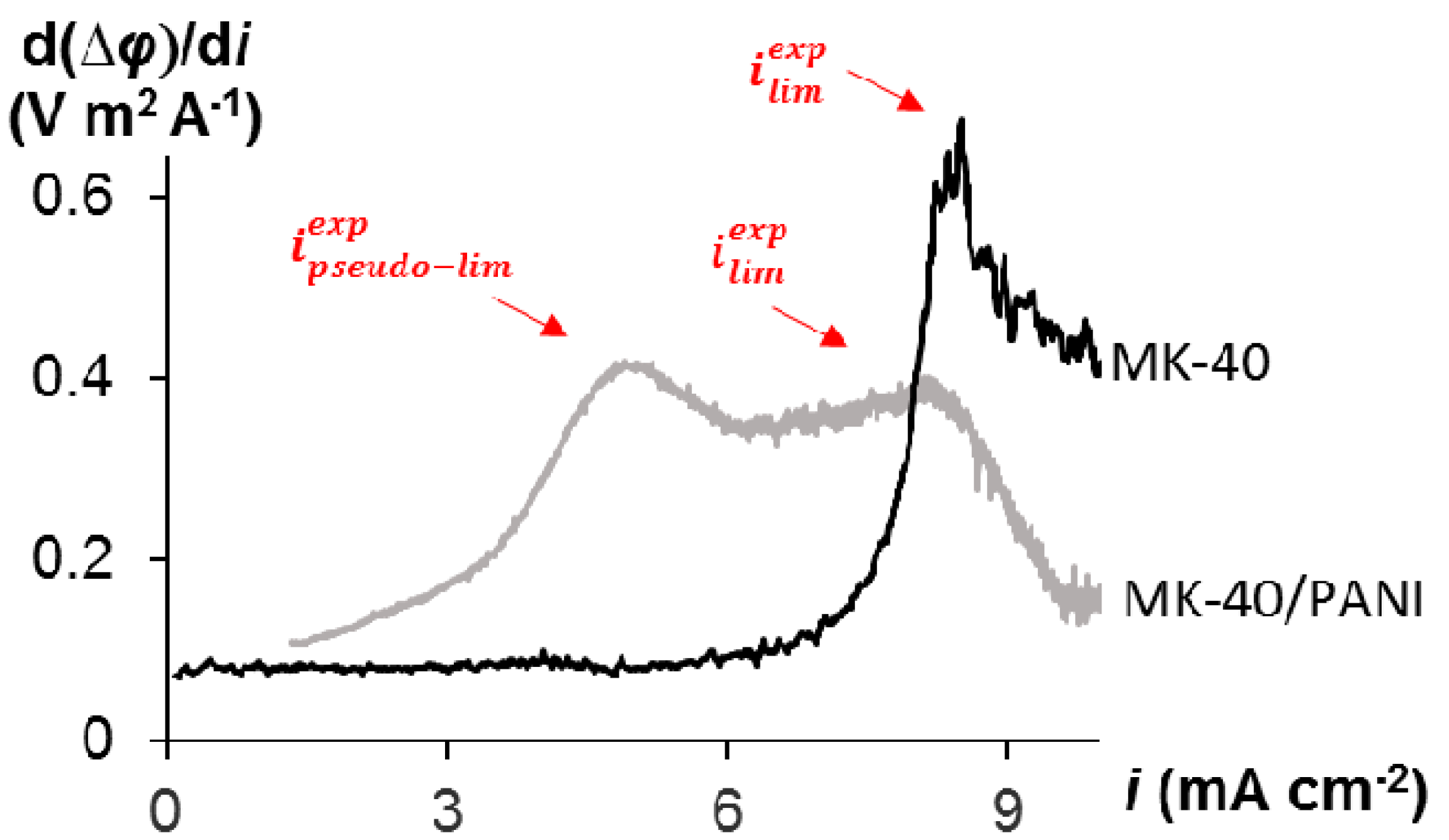
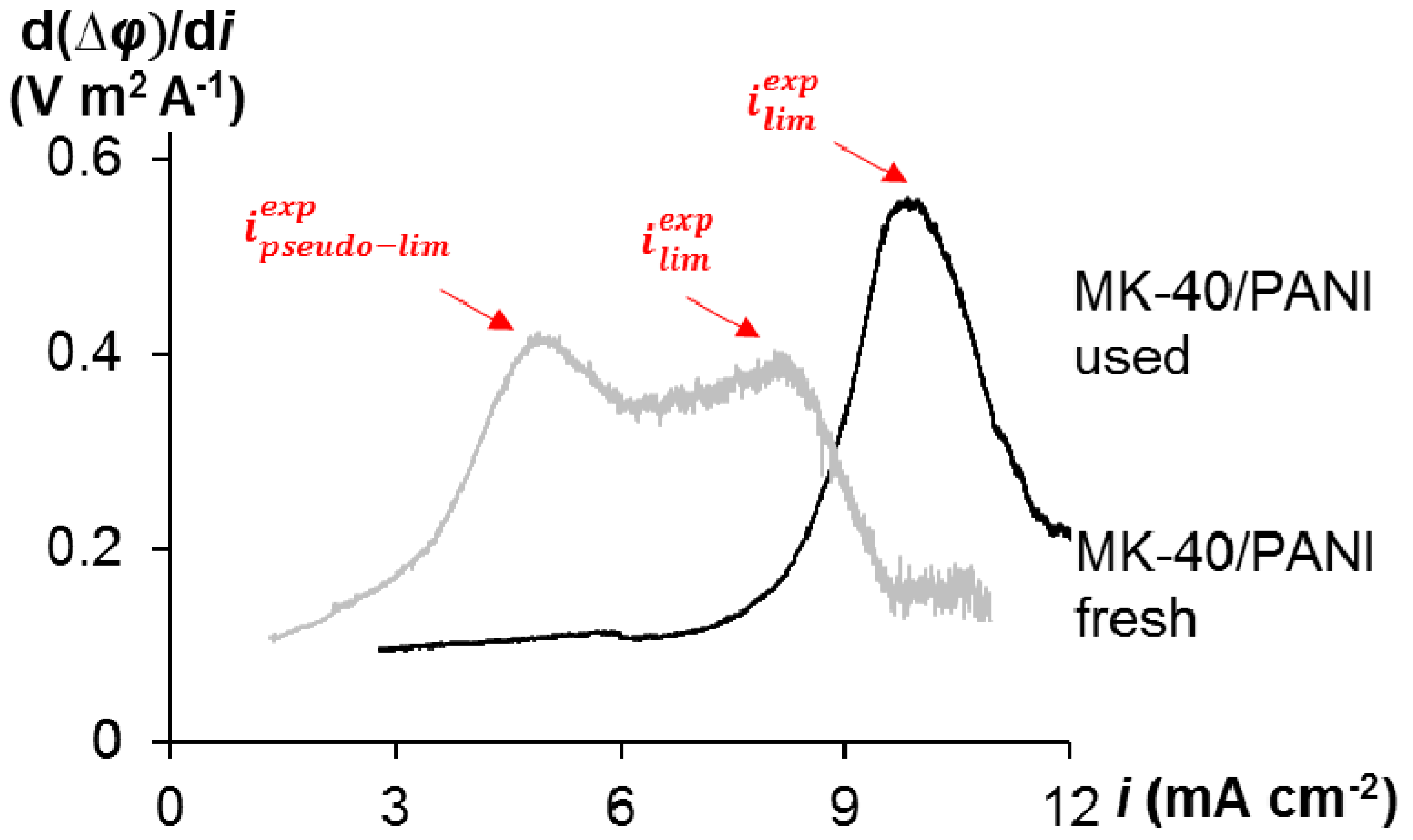
3.2. Voltammetry
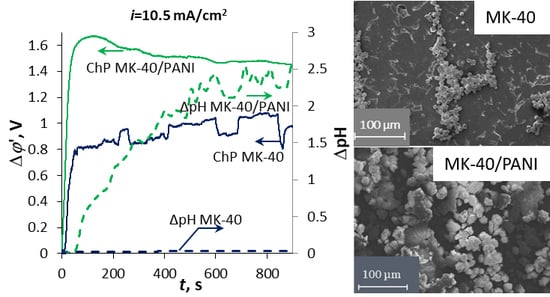
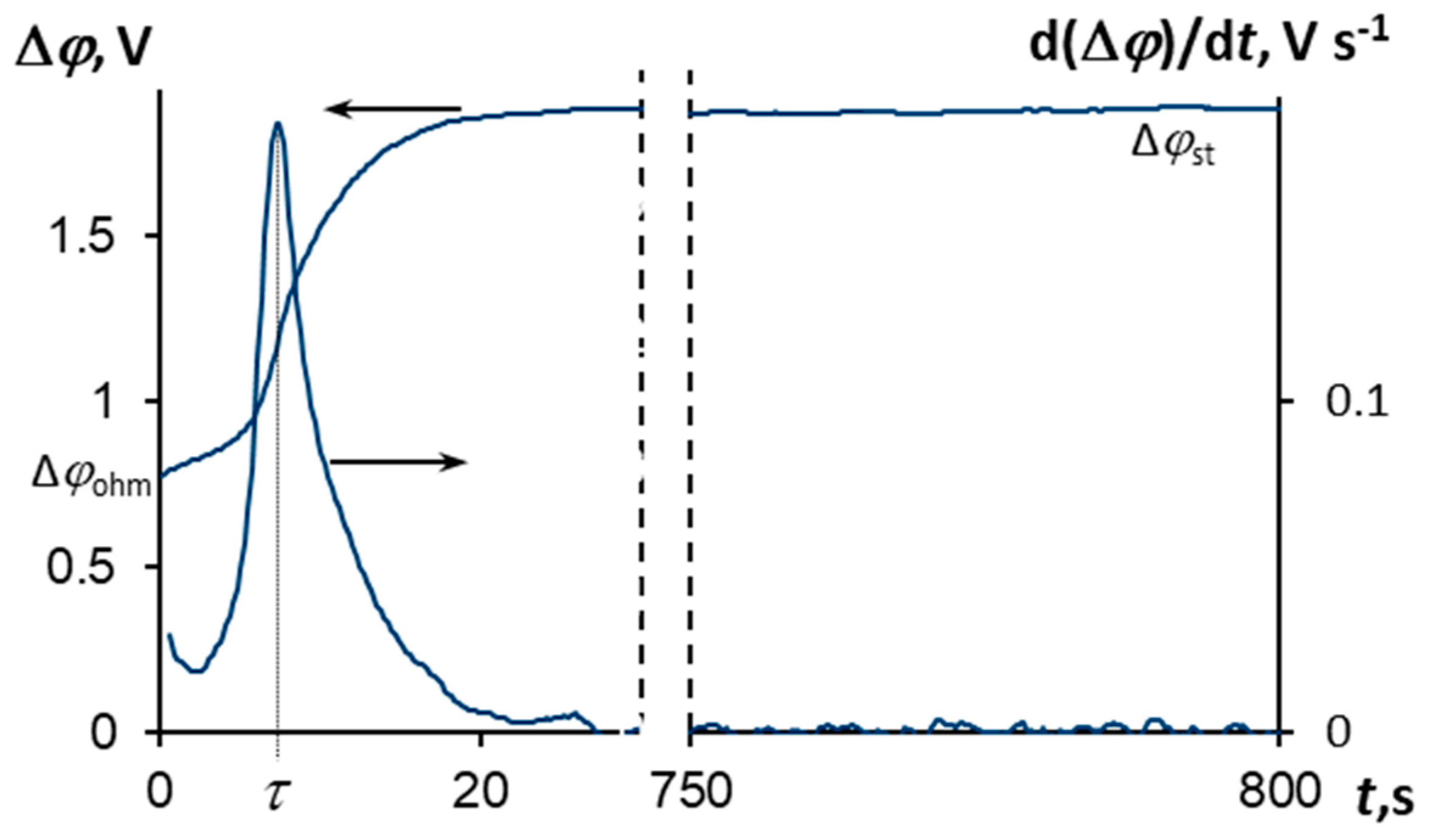
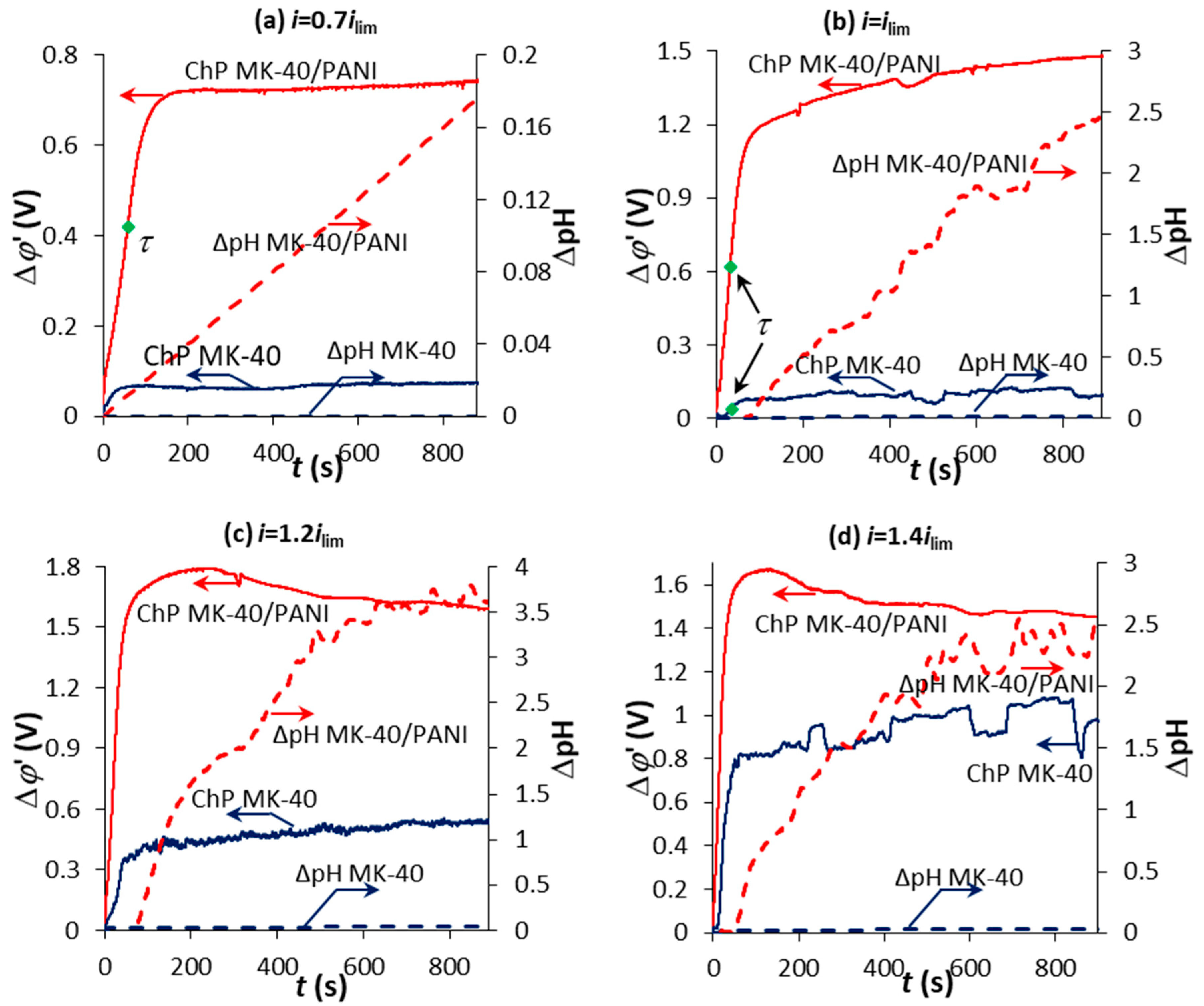
3.3. Chronopotentiometry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Kravtsov, V.; Kulikova, I.; Mikhaylin, S.; Bazinet, L. Alkalinization of acid whey by means of electrodialysis with bipolar membranes and analysis of induced membrane fouling. J. Food Eng. 2020, 277, 109891. [Google Scholar] [CrossRef]
- Merkel, A.; Ashrafi, A.M.; Ečer, J. Bipolar membrane electrodialysis assisted pH correction of milk whey. J. Memb. Sci. 2018, 555, 185–196. [Google Scholar] [CrossRef]
- Fehér, J.; Červeňanský, I.; Václavík, L.; Markoš, J. Electrodialysis applied for phenylacetic acid separation from organic impurities: Increasing the recovery. Sep. Purif. Technol. 2020. [Google Scholar] [CrossRef]
- Kattan Readi, O.M.; Gironès, M.; Nijmeijer, K. Separation of complex mixtures of amino acids for biorefinery applications using electrodialysis. J. Memb. Sci. 2013, 429, 338–348. [Google Scholar] [CrossRef]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Sheldeshov, N.V.; Zabolotsky, V.I.; Kovalev, N.V.; Karpenko, T.V. Electrochemical characteristics of heterogeneous bipolar membranes and electromembrane process of recovery of nitric acid and sodium hydroxide from sodium nitrate solution. Sep. Purif. Technol. 2020, 241, 116648. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Tanaka, Y. Chapter 8 Water Dissociation. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 12, pp. 139–186. ISBN 9780444519825. [Google Scholar]
- Krol, J.J.; Wessling, M.; Strathmann, H. Concentration polarization with monopolar ion exchange membranes: Current-voltage curves and water dissociation. J. Memb. Sci. 1999. [Google Scholar] [CrossRef]
- Tong, T.; Wallace, A.F.; Zhao, S.; Wang, Z. Mineral scaling in membrane desalination: Mechanisms, mitigation strategies, and feasibility of scaling-resistant membranes. J. Memb. Sci. 2019, 579, 52–69. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Vasil’eva, V.I.; Zhil’tsova, A.V.; Malykhin, M.D.; Zabolotskii, V.I.; Lebedev, K.A.; Chermit, R.K.; Sharafan, M.V. Effect of the chemical nature of the ionogenic groups of ion-exchange membranes on the size of the electroconvective instability region in high-current modes. Russ. J. Electrochem. 2014, 50, 120–128. [Google Scholar] [CrossRef]
- Balster, J.; Yildirim, M.H.; Stamatialis, D.F.; Ibanez, R.; Lammertink, R.G.H.; Jordan, V.; Wessling, M. Morphology and microtopology of cation-exchange polymers and the origin of the overlimiting current. J. Phys. Chem. B 2007, 111, 2152–2165. [Google Scholar] [CrossRef]
- Belashova, E.; Mikhaylin, S.; Pismenskaya, N.; Nikonenko, V.; Bazinet, L. Impact of cation-exchange membrane scaling nature on the electrochemical characteristics of membrane system. Sep. Purif. Technol. 2017, 189, 441–448. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Nikonenko, V.; Pismenskaya, N.; Pourcelly, G.; Choi, S.; Kwon, H.J.; Han, J.; Bazinet, L. How physico-chemical and surface properties of cation-exchange membrane affect membrane scaling and electroconvective vortices: Influence on performance of electrodialysis with pulsed electric field. Desalination 2016, 393, 102–114. [Google Scholar] [CrossRef]
- Andreeva, M.A.; Gil, V.V.; Pismenskaya, N.D.; Nikonenko, V.V.; Dammak, L.; Larchet, C.; Grande, D.; Kononenko, N.A. Effect of homogenization and hydrophobization of a cation-exchange membrane surface on its scaling in the presence of calcium and magnesium chlorides during electrodialysis. J. Memb. Sci. 2017, 540, 183–191. [Google Scholar] [CrossRef]
- Suwal, S.; Amiot, J.; Beaulieu, L.; Bazinet, L. Effect of pulsed electric field and polarity reversal on peptide/amino acid migration, selectivity and fouling mitigation. J. Memb. Sci. 2016, 510, 405–416. [Google Scholar] [CrossRef]
- Persico, M.; Bazinet, L. Fouling prevention of peptides from a tryptic whey hydrolysate during electromembrane processes by use of monovalent ion permselective membranes. J. Memb. Sci. 2018, 549, 486–494. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Memb. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K.; Trivedi, G.S.; Rangarajan, R. Preparation and electrochemical characterization of cation- and anion-exchange/polyaniline composite membranes. J. Colloid Interface Sci. 2004. [Google Scholar] [CrossRef] [PubMed]
- Sata, T. Composite Membranes Prepared from Cation Exchange Membranes and Polyaniline and Their Transport Properties in Electrodialysis. J. Electrochem. Soc. 1999, 146, 585. [Google Scholar] [CrossRef]
- Berezina, N.P.; Shkirskaya, S.A.; Kolechko, M.V.; Popova, O.V.; Senchikhin, I.N.; Roldugin, V.I. Barrier effects of polyaniline layer in surface modified MF-4SK/Polyaniline membranes. Russ. J. Electrochem. 2011, 47, 995–1005. [Google Scholar] [CrossRef]
- Demina, O.A.; Shkirskaya, S.A.; Kononenko, N.A.; Nazyrova, E.V. Assessing the selectivity of composite ion-exchange membranes within the framework of the extended three-wire model of conduction. Russ. J. Electrochem. 2016. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Dolgopolov, S.V.; Loza, N.V.; Shel’deshov, N.V. Effects of ph variation in solutions under the polarization conditions of the MF-4SK membrane with surface modified by polyaniline. Russ. J. Electrochem. 2015, 51, 19–24. [Google Scholar] [CrossRef]
- Shestakov, K.V.; Lazarev, S.I.; Polyanskiy, K.K. Study of kinetic and structural characteristics of membranes in purification process of copper-containing solutions by electrodialysis. Izv. Vyss. Uchebnykh Zaved. Seriya Khimiya i Khimicheskaya Tekhnologiya 2019. [Google Scholar] [CrossRef]
- Asraf-Snir, M.; Gilron, J.; Oren, Y. Scaling of cation exchange membranes by gypsum during Donnan exchange and electrodialysis. J. Memb. Sci. 2018, 567, 28–38. [Google Scholar] [CrossRef]
- Melnikov, S.; Sheldeshov, N.; Zabolotsky, V.; Loza, S.; Achoh, A. Pilot scale complex electrodialysis technology for processing a solution of lithium chloride containing organic solvents. Sep. Purif. Technol. 2017, 189, 74–81. [Google Scholar] [CrossRef]
- Melnikov, S.; Loza, S.; Sharafan, M.; Zabolotskiy, V. Electrodialysis treatment of secondary steam condensate obtained during production of ammonium nitrate. Technical and economic analysis. Sep. Purif. Technol. 2016. [Google Scholar] [CrossRef]
- Sarapulova, V.; Shkorkina, I.; Mareev, S.; Pismenskaya, N.; Kononenko, N.; Larchet, C.; Dammak, L.; Nikonenko, V. Transport characteristics of fujifilm ion-exchange membranes as compared to homogeneous membranes АМХ and СМХ and to heterogeneous membranes MK-40 and MA-41. Membranes (Basel) 2019, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Akberova, E.M.; Vasil’eva, V.I.; Zabolotsky, V.I.; Novak, L. Effect of the sulfocation-exchanger dispersity on the surface morphology, microrelief of heterogeneous membranes and development of electroconvection in intense current modes. J. Memb. Sci. 2018, 566, 317–328. [Google Scholar] [CrossRef]
- Falina, I.V.; Demina, O.A.; Zabolotskii, V.I. Verification of a capillary model for the electroosmotic transport of a free solvent in ion-exchange membranes of different natures. Colloid J. 2017. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs structure. Adv. Colloid Interface Sci. 2008. [Google Scholar] [CrossRef]
- Gnusin, N.P.; Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A. Transport structural parameters to characterize ion exchange membranes. J. Memb. Sci. 2004. [Google Scholar] [CrossRef]
- Loza, N.V.; Dolgopolov, S.V.; Kononenko, N.A.; Andreeva, M.A.; Korshikova, Y.S. Effect of surface modification of perfluorinated membranes with polyaniline on their polarization behavior. Russ. J. Electrochem. 2015, 51, 538–545. [Google Scholar] [CrossRef]
- Lteif, R.; Dammak, L.; Larchet, C.; Auclair, B. Conductivitéélectrique membranaire: Étude de l’effet de la concentration, de la nature de l’électrolyte et de la structure membranaire. Eur. Polym. J. 1999, 35, 1187–1195. [Google Scholar] [CrossRef]
- Belashova, E.D.; Melnik, N.A.; Pismenskaya, N.D.; Shevtsova, K.A.; Nebavsky, A.V.; Lebedev, K.A.; Nikonenko, V.V. Overlimiting mass transfer through cation-exchange membranes modified by Nafion film and carbon nanotubes. Electrochim. Acta 2012, 59, 412–423. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Nikonenko, V.; Pourcelly, G.; Bazinet, L. Intensification of demineralization process and decrease in scaling by application of pulsed electric field with short pulse/pause conditions. J. Memb. Sci. 2014, 468, 389–399. [Google Scholar] [CrossRef]
- Shkirskaya, S.A.; Senchikhin, I.N.; Kononenko, N.A.; Roldugin, V.I. Effect of polyaniline on the stability of electrotransport characteristics and thermochemical properties of sulfocationite membranes with different polymer matrices. Russ. J. Electrochem. 2017, 53, 78–85. [Google Scholar] [CrossRef]
- Barragán, V.M.; Ruíz-Bauzá, C. Current–Voltage Curves for Ion-Exchange Membranes: A Method for Determining the Limiting Current Density. J. Colloid Interface Sci. 1998, 205, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, N.; Berezina, N.; Loza, N. Interaction of surfactants with ion-exchange membranes. Colloids Surfaces A Physicochem. Eng. Asp. 2004, 239, 59–64. [Google Scholar] [CrossRef]
- Zerdoumi, R.; Oulmi, K.; Benslimane, S. Electrochemical characterization of the CMX cation exchange membrane in buffered solutions: Effect on concentration polarization and counterions transport properties. Desalination 2014, 340, 42–48. [Google Scholar] [CrossRef]
- Strathmann, H. Preparation and Characterization of Ion-Exchange Membranes. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9, pp. 89–146. [Google Scholar]
- Melnikov, S.S.; Sheldeshov, N.V.; Zabolotskii, V.I. Theoretical and experimental study of current-voltage characteristics of asymmetric bipolar membranes. Desalin. Water Treat. 2018, 123, 1–13. [Google Scholar] [CrossRef]
- Krol, J. Chronopotentiometry and overlimiting ion transport through monopolar ion exchange membranes. J. Memb. Sci. 1999, 162, 155–164. [Google Scholar] [CrossRef]
- Pismenskaia, N.; Sistat, P.; Huguet, P.; Nikonenko, V.; Pourcelly, G. Chronopotentiometry applied to the study of ion transfer through anion exchange membranes. J. Memb. Sci. 2004, 228, 65–76. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; García-Gabaldón, M.; Pérez-Herranz, V. Effect of the equilibria of multivalent metal sulfates on the transport through cation-exchange membranes at different current regimes. J. Memb. Sci. 2013, 443, 181–192. [Google Scholar] [CrossRef]
- Paul, D.; Halder, S.; Das, G. Whey protein directed in vitro vaterite biomineralization: Influence of external parameters on phase transformation. Colloid Interface Sci. Commun. 2020, 36, 100255. [Google Scholar] [CrossRef]
- Huang, F.; Liang, Y.; He, Y. On the Pickering emulsions stabilized by calcium carbonate particles with various morphologies. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 580, 123722. [Google Scholar] [CrossRef]
- Casademont, C.; Pourcelly, G.; Bazinet, L. Bilayered self-oriented membrane fouling and impact of magnesium on CaCO3 formation during consecutive electrodialysis treatments. Langmuir 2010. [Google Scholar] [CrossRef] [PubMed]
- Casademont, C.; Sistat, P.; Ruiz, B.; Pourcelly, G.; Bazinet, L. Electrodialysis of model salt solution containing whey proteins: Enhancement by pulsed electric field and modified cell configuration. J. Memb. Sci. 2009, 328, 238–245. [Google Scholar] [CrossRef]
- Casademont, C.; Farias, M.; Pourcelly, G.; Bazinet, L. Impact of electrodialytic parameters on cation migration kinetics and fouling nature of ion-exchange membranes during treatment of solutions with different magnesium/calcium ratios. J. Memb. Sci. 2008, 325, 570–579. [Google Scholar] [CrossRef]
- Casademont, C.; Pourcelly, G.; Bazinet, L. Effect of magnesium/calcium ratio in solutions subjected to electrodialysis: Characterization of cation-exchange membrane fouling. J. Colloid Interface Sci. 2007, 315, 544–554. [Google Scholar] [CrossRef] [PubMed]

| Membrane | L, mm | κ, S m−1 | θ, ° |
|---|---|---|---|
| MK-40 | 0.470 ± 0.005 | 0.36 ± 0.03 | 55 ± 3 |
| MK-40/PANI | 0.490 ± 0.005 | 0.30 ± 0.02 | 54 ± 3 |
| Membrane | ipseudo-lim, mA cm−2 | ilim, mA cm−2 |
|---|---|---|
| MK-40 | - | 7.7 |
| MK-40/PANI | 3.2 | 7.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, M.A.; Loza, N.V.; Pis’menskaya, N.D.; Dammak, L.; Larchet, C. Influence of Surface Modification of MK-40 Membrane with Polyaniline on Scale Formation under Electrodialysis. Membranes 2020, 10, 145. https://doi.org/10.3390/membranes10070145
Andreeva MA, Loza NV, Pis’menskaya ND, Dammak L, Larchet C. Influence of Surface Modification of MK-40 Membrane with Polyaniline on Scale Formation under Electrodialysis. Membranes. 2020; 10(7):145. https://doi.org/10.3390/membranes10070145
Chicago/Turabian StyleAndreeva, Marina A., Natalia V. Loza, Natalia D. Pis’menskaya, Lasaad Dammak, and Christian Larchet. 2020. "Influence of Surface Modification of MK-40 Membrane with Polyaniline on Scale Formation under Electrodialysis" Membranes 10, no. 7: 145. https://doi.org/10.3390/membranes10070145
APA StyleAndreeva, M. A., Loza, N. V., Pis’menskaya, N. D., Dammak, L., & Larchet, C. (2020). Influence of Surface Modification of MK-40 Membrane with Polyaniline on Scale Formation under Electrodialysis. Membranes, 10(7), 145. https://doi.org/10.3390/membranes10070145