Electrochemical Characteristics of Glycerolized PEO-Based Polymer Electrolytes
Abstract
1. Introduction
2. Experimental Methodology
2.1. Materials
2.2. Sample Preparation
2.3. Electrochemical Impedance Spectroscopy (EIS)
2.4. LSV and TNM Measurements
2.5. EDLC Fabrication
2.6. CV Measurements
3. Results and Discussion
3.1. Electrical Properties
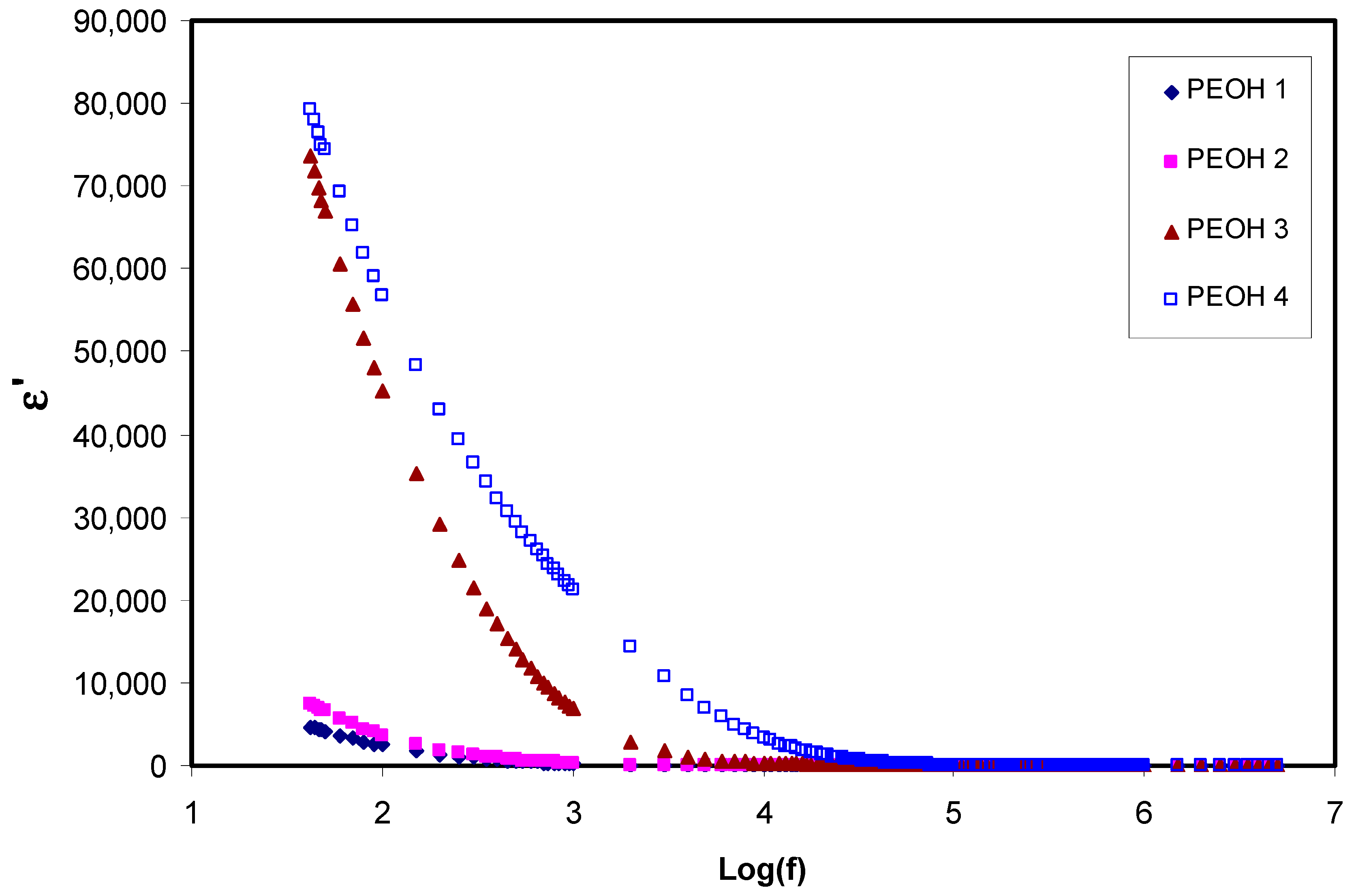
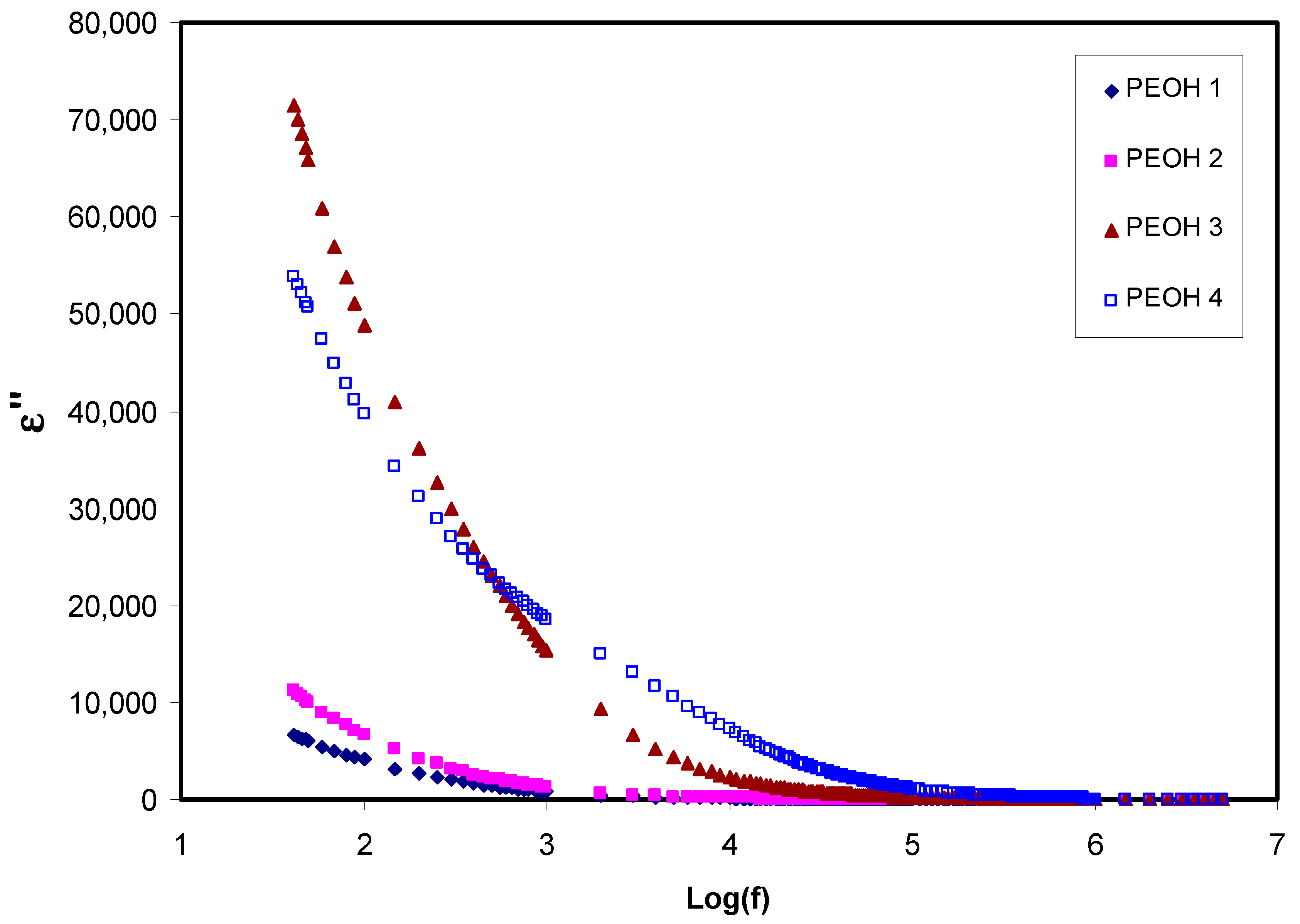
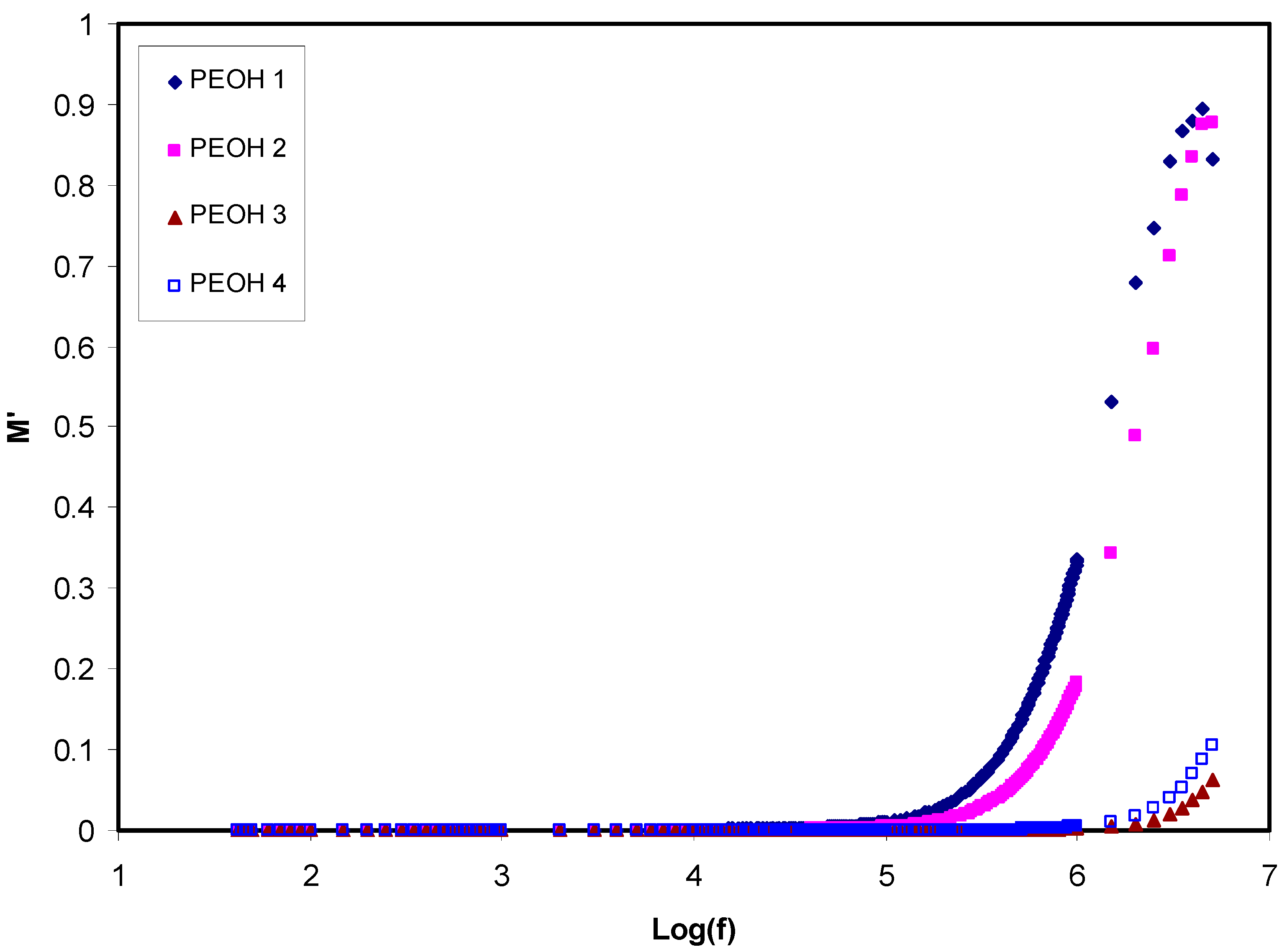
3.1.1. Dielectric and Electric Modulus Study
3.1.2. Impedance Spectroscopy Study
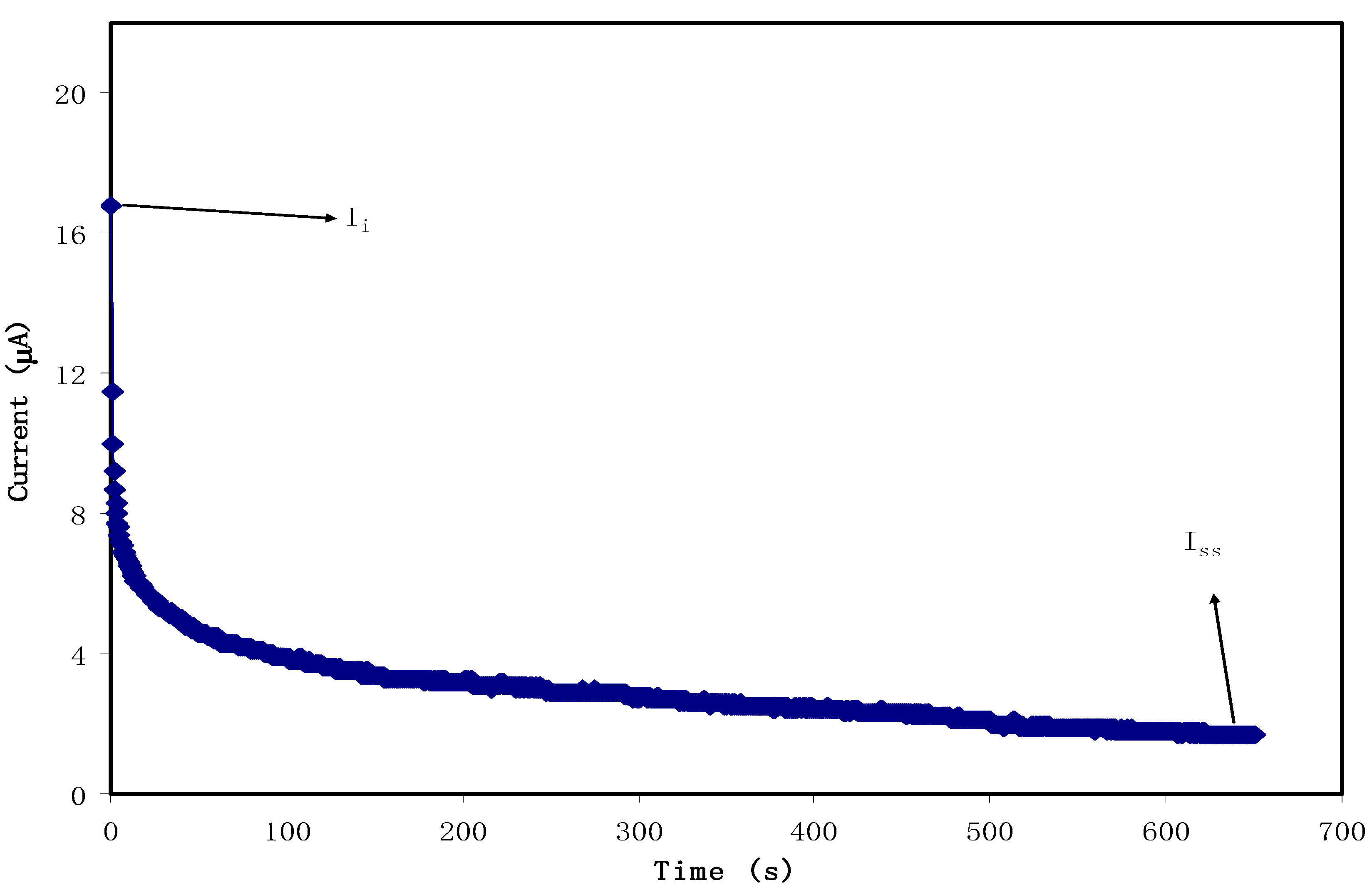
3.2. Transference Number Measurement (TNM)
3.3. Linear Sweep Voltammetry (LSV)
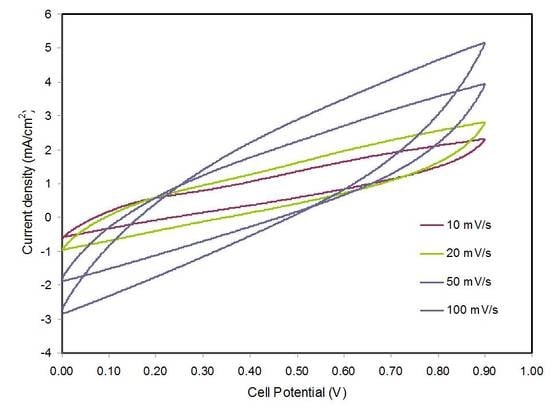
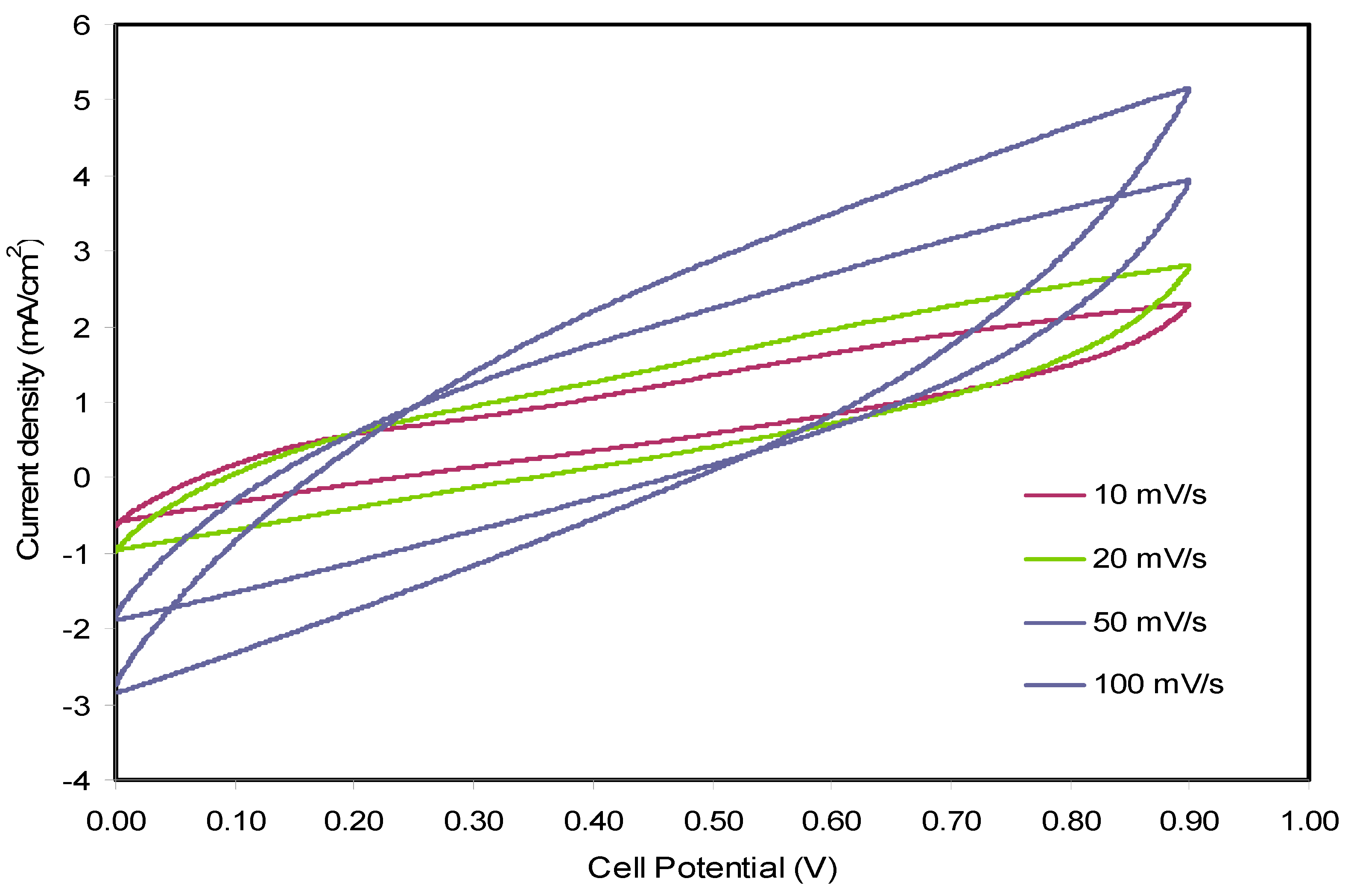
3.4. Cyclic Voltammetry (CV)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Vliet, O.; Brouwer, A.S.; Kuramochi, T.; van den Broek, M.; Faaij, A. Energy use, cost and CO2 emissions of electric cars. J. Power Sources 2011, 196, 2298–2310. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; de Angelis, F.; di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Vincent, C.A.; Scrosati, B. Modern Batteries: An Introduction to Electrochemical Power Sources; Butterworth-Heinemann: London, UK, 1997. [Google Scholar]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kalhammer, F.R. Polymer electrolytes and the electric vehicle. Solid State Ion. 2000, 135, 315–323. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Wang, X.J.; Hou, Y.Y.; Gao, X.W.; Liu, L.L.; Wu, Y.P.; Shimizu, M. A new single-ion polymer electrolyte based on polyvinyl alcohol for lithium ion batteries. Electrochimicaacta 2013, 87, 113–118. [Google Scholar] [CrossRef]
- Radha, K.P.; Selvasekarapandian, S.; Karthikeyan, S.; Hema, M.; Sanjeeviraja, C. Synthesis and impedance analysis of proton-conducting polymer electrolyte PVA: NH4F. Ionics 2013, 19, 1437–1448. [Google Scholar] [CrossRef]
- Ji, J.; Li, B.; Zhong, W.-H. Effects of a block copolymer as multifunctional fillers on ionic conductivity, mechanical properties, and dimensional stability of solid polymer electrolytes. J. Phys. Chem. B 2010, 114, 13637–13643. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Polypyrrole-derived activated carbons for high-performance electrical double-layer capacitors with ionic liquid electrolyte. Adv. Funct. Mater. 2012, 22, 827–834. [Google Scholar] [CrossRef]
- Huo, P.; Ni, S.; Hou, P.; Xun, Z.; Liu, Y.; Gu, J. A cross linked soybean protein isolate gel polymer electrolyte based on neutral aqueous electrolyte for a high-energy-density supercapacitor. Polymers 2019, 11, 863. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Aziz, S.B.; Yusof, Y.M.; Kadir, M.F.Z. Influence of Br as an ionic source on the structural/electrical properties of dextran-based biopolymer electrolytes and EDLC application. Bull. Mater. Sci. 2020, 43, 30. [Google Scholar] [CrossRef]
- Wang, J.A.; Lu, Y.T.; Lin, S.C.; Wang, Y.S.; Ma, C.C.M.; Hu, C.C. Designing a novel polymer electrolyte for improving the electrode/electrolyte interface in flexible all-solid-state electrical double-layer capacitors. ACS Appl. Mater. Interfaces 2018, 10, 17871–17882. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Abdulwahid, R.T.; Brza, M.A.; Marif, A.S.; Kadir, M.F.Z. Protonic EDLC cell based on chitosan (CS): Methylcellulose (MC) solid polymer blend electrolytes. Ionics 2020, 26, 1829–1840. [Google Scholar] [CrossRef]
- Lee, D.-Y.; An, G.-H.; Ahn, H.-J. High-surface-area tofu based activated porous carbon for electrical double-layer capacitors. J. Ind. Eng. Chem. 2017, 52, 121–127. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F.Z.; Muzakir, S.K.; Abdulwahid, R.T. Effect of ohmic-drop on electrochemical performance of EDLC fabricated from PVA: Dextran: NH4I based polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 3734–3745. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Azha, M.A.S.; Azli, A.A.; Shukur, M.F.; Yusof, Y.M.; Muzakir, S.K.; Manan, N.S.A.; Kadir, M.F.Z. Solid-state double layer capacitors and protonic cell fabricated with dextran from Leuconostocmesenteroides based green polymer electrolyte. Mater. Chem. Phys. 2020, 241, 122290. [Google Scholar] [CrossRef]
- Staiti, P.; Minutoli, M.; Lufrano, F. All solid electric double layer capacitors based on Nafionionomer. Electrochim. Acta 2002, 47, 2795–2800. [Google Scholar] [CrossRef]
- Lim, C.-S.; Teoh, K.H.; Liew, C.-W.; Ramesh, S. Capacitive behavior studies on electrical double layer capacitor using poly (vinyl alcohol)-lithium perchlorate based polymer electrolyte incorporated with TiO2. Mater. Chem. Phys. 2014, 143, 661–667. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte selection for supercapacitive devices: A critical review. Nanoscale Adv. 2019. [Google Scholar] [CrossRef]
- Andres, B.; Dahlstrom, C.; Blomquist, N.; Norgen, M.; Olin, H. Cellulose binders for electric double-layer capacitor electrodes: The influence of cellulose quality on electrical properties. Mater. Des. 2018, 141, 342–349. [Google Scholar] [CrossRef]
- Yang, I.; Kim, S.G.; Kwon, S.H.; Lee, J.H.; Kim, M.S.; Jung, J.C. Pore size-controlled carbon aerogels for EDLC electrodes in organic electrolytes. Curr. Appl. Phys. 2016, 16, 665–672. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. J. Power Sources 2013, 235, 289–296. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Wu, Y.; Cao, J.P.; Zhuang, Q.Q.; Wan, X.; He, S.; Wei, X.Y. Preparation and characterization of activated carbons from oxygen-rich lignite for electric double-layer capacitor. Int. J. Electrochem. Sci. 2018, 13, 2800–2816. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Mishra, K.; Hamsan, M.H.; Karim, W.O.; Abdullah, R.M.; Kadir, M.F.Z.; Abdulwahid, R.T. Fabrication of high performance energy storage EDLC device from proton conducting methylcellulose: Dextran polymer blend electrolytes. J. Mater. Res. Technol. 2020, 9, 1137–1150. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Kiamahalleh, M.V.; Zein, S.H.S.; Najafpour, G.; Sata, S.A.; Buniran, S. Multiwalled carbon nanotubes based nanocomposites for supercapacitors: A review of electrode materials. Nano 2012, 7, 1230002. [Google Scholar] [CrossRef]
- Shobana, V.; Parthiban, P.; Balakrishnan, K. Lithium based battery-type cathode material for hybrid supercapacitor. J. Chem. Pharm. Res. 2015, 7, 207–212. [Google Scholar]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141–16145. [Google Scholar] [CrossRef]
- Bhide, A.; Hariharan, K. A new polymer electrolyte system (PEO) n: NaPO3. J. Power Sources 2006, 159, 1450–1457. [Google Scholar] [CrossRef]
- Gray, F. Solid Polymer Electrolytes: Fundamentals and Technological Applications; VCH Publishers: New York, NY, USA, 1991. [Google Scholar]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Bonino, F.; Scrosati, B.; Selvaggi, A. The lithium-polymer electrolyte interface. I. Lithium Cyclability. Solid State Ion. 1986, 18–19, 1050–1053. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-L.; Liang, W.-J.; Chen, T.-Y. Solid polymer electrolytes V: Microstructure and ionic conductivity of epoxide crosslinked polyether networks doped with LiClO4. Polymer 2003, 44, 2957–2964. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic solid Li ion conductors: An overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Kim, Y.T.; Smotkin, E.S. The effect of plasticizers on transport and electrochemical properties of PEO-based electrolytes for lithium rechargeable batteries. Solid State Ion. 2002, 149, 29–37. [Google Scholar] [CrossRef]
- Sun, X.G.; Liu, G.; Xie, J.B.; Han, Y.B.; Kerr, J.B. New gel polyelectrolytes for rechargeable lithium batteries. Solid State Ion. 2004, 175, 713–716. [Google Scholar] [CrossRef][Green Version]
- Pawlicka, A.; Danczuk, M.; Wieczorek, W.; Zygadło-Monikowska, E. Influence of plasticizer type on the properties of polymer electrolytes based on Chitosan. J. Phys. Chem. A 2008, 112, 8888–8895. [Google Scholar] [CrossRef]
- Aziz, S.B.; Karim, O.W.; Ghareeb, H.O. The deficiency of chitosan: AgNO3 polymer electrolyte incorporated with titanium dioxide filler for device fabrication and membrane separation technology. J. Mater. Res. Technol. 2020, 3, 4692–4705. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Mustafa, M.S.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F.Z.; Ghareeb, H.O.; Hussein, S.A. Electrochemical impedance study of proton conducting polymer electrolytes based on PVC doped with Thiocyanate and plasticized with glycerol. Int. J. Electrochem. Sci. 2020, 15, 4671–4683. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z. Ion-transport study in nanocomposite solid polymer electrolytes based on chitosan: Electrical and dielectric analysis. J. Appl. Polym. Sci. 2015, 132, 41774. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M.; Rasheed, M.A.; Ahmed, H.M. Role of ion dissociation on DC conductivity and silver nanoparticle formation in PVA: AgNt based polymer electrolytes: Deep insights to ion transport mechanism. Polymers 2017, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Abdullah, R.M. Crystalline and amorphous phase identification from the tanδ relaxation peaks and impedance plots in polymer blend electrolytes based on [CS:AgNt]x:PEO(x − 1) (10 ≤ x ≤ 50). Electrochim. Acta 2018, 285, 30–46. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Aziz, S.B.; Kadir, M.F.Z. Dextran from Leuconostoc mesenteroides-doped ammonium salt-based green polymer electrolyte. Bull. Mater. Sci. 2019, 42, 57. [Google Scholar] [CrossRef]
- Baskaran, R.; Selvasekarapandian, S.; Kuwata, N.; Kawamura, J.; Hattori, T. ac impedance, DSC and FT-IR investigations on (x)PVAc–(1 − x)PVdF blends with LiClO4. Mater. Chem. Phys. 2006, 98, 55–61. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Teo, L.P.; Majid, S.R.; Arof, A.K. Ionic conductivity by correlated barrier hopping in NH4I doped chitosan solid electrolyte. Phys. B Condens. Matter 2009, 404, 1373–1379. [Google Scholar] [CrossRef]
- Deraman, S.K.; Mohamed, N.S.; Subban, R.H.Y. Conductivity and dielectric properties of proton conducting poly (Vinyl) Chloride (PVC) based gel polymer electrolytes. Sains Malays. 2013, 42, 475–479. [Google Scholar]
- Abdullah, O.G.H.; Aziz, S.B.; Rasheed, M.A. Incorporation of NH4NO3 into MC-PVA blend-based polymer to prepare proton-conducting polymer electrolyte films. Ionics 2018, 24, 777–785. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, R.M.; Kadir, M.F.Z.; Ahmed, H.M. Non suitability of silver ion conducting polymer electrolytes based on chitosan mediated by barium titanate (BaTiO3) for electrochemical device applications. Electrochim. Acta 2019, 296, 494–507. [Google Scholar] [CrossRef]
- Aziz, S.B. The mixed contribution of ionic and electronic carriers to conductivity in chitosan based solid electrolytes mediated by CuNt salt. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1942–1952. [Google Scholar] [CrossRef]
- Aziz, S.B.; Karim, W.O.; Brza, M.A.; Abdulwahid, R.T.; Saeed, S.R.; Al-Zangana, S.; Kadir, M.F.Z. Ion transport study in CS: POZ based polymer membrane electrolytes using Trukhan model. Int. J. Mol. Sci. 2019, 20, 5265. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abidin, Z.H.Z.; Arof, A.K. Influence of silver ion reduction on electrical modulus parameters of solid polymer electrolyte based on chitosan-silver triflate electrolyte membrane. Express Polym. Lett. 2010, 4, 300–310. [Google Scholar] [CrossRef]
- Aziz, S.B.; Marif, R.B.; Brza, M.A.; Hamsan, M.H.; Kadir, M.F.Z. Employing of trukhan model to estimate ion transport parameters in PVA based solid polymer electrolyte. Polymers 2019, 11, 1694. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Karim, W.O.; Qadir, K.W.; Zafar, Q. Proton ion conducting solid polymer electrolytes based on chitosan incorporated with various amounts of barium titanate (BaTiO3). Int. J. Electrochem. Sci. 2018, 13, 6112–6125. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Kadir, M.F.Z. NH4NO3 as charge carrier contributor in glycerolized potato starch-methyl cellulose blend-based polymer electrolyte and the application in electrochemical double-layer capacitor. Ionics 2017, 23, 3429–3453. [Google Scholar] [CrossRef]
- Siti, K.D.; Mohamed, N.S.; Subban, R.H.Y. Ionic liquid incorporated PVC based polymer electrolytes: Electrical and dielectric properties. Sains Malays. 2014, 43, 877–883. [Google Scholar]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Kadir, M.F.Z. A promising polymer blend electrolytes based on chitosan: Methyl cellulose for EDLC application with high specific capacitance and energy density. Molecules 2019, 24, 2503. [Google Scholar] [CrossRef]
- Muchakayala, R.; Song, S.; Gao, S.; Wang, X.; Fan, Y. Structure and ion transport in an ethylene carbonate-modified biodegradable gel polymer electrolyte. Polym. Test. 2017, 58, 116–125. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Rasheed, M.A.; Ahmed, H.M. Effect of high salt concentration (HSC) on structural, morphological and electrical characteristics of chitosan based solid polymer electrolytes. Polymers 2017, 9, 187. [Google Scholar] [CrossRef]
- Ahad, N.; Saion, E.; Gharibshahi, E. Structural, thermal, and electrical properties of PVA-Sodium salicylate solid composite polymer electrolyte. J. Nanomater. 2012. [Google Scholar] [CrossRef]
- Sampathkumar, L.; Selvin, P.C.; Selvasekarapandian, S.; Perumal, P.; Chitra, R.; Muthukrishnan, M. Synthesis and characterization of biopolymer electrolyte based on tamarind seed polysaccharide, lithium perchlorate and ethylene carbonate for electrochemical applications. Ionics 2019, 25, 1067–1082. [Google Scholar] [CrossRef]
- Monisha, S.; Mathavan, T.; Selvasekarapandian, S.; Benial, A.M.; Latha, M.P. Preparation and characterization of cellulose acetate and lithium nitrate for advanced electrochemical devices. Ionics 2016, 23, 2697–2706. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Song, S.; Ma, Q.; Liu, R. High performance poly (vinyl alcohol)-based li-ion conducting gel polymer electrolyte films for electric double-layer capacitors. Polymers 2018, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sekhon, S.S. Role of plasticizer’s dielectric constant on conductivity modification of PEO-NH4F polymer electrolytes. Eur. Polym. J. 2002, 38, 1297–1304. [Google Scholar] [CrossRef]
- Pandey, G.P.; Kumar, Y.; Hashmi, S.A. Ionic liquid incorporated PEO based polymer electrolyte for electrical double layer capacitors: A comparative study with lithium and magnesium systems. Solid State Ion. 2011, 190, 93–98. [Google Scholar] [CrossRef]
- Bandara, L.R.A.K.; Dissanayake, M.A.K.L.; Mellander, B. Ionic conductivity of plasticized (PEO)-LiCF3SO3 electrolytes. Electrochim. Acta 1998, 43, 10–14. [Google Scholar] [CrossRef]
- Michael, M.S.; Jacob, M.M.E.; Prabaharan, S.R.S.; Radhakrishna, S. Enhanced lithium ion transport in PEO-based solid polymer electrolytes employing a novel class of plasticizers. Solid State Ion. 1997, 98, 167–174. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Karim, W.O.; Kadir, M.F.Z.; Brza, M.A.; Abdullah, O.G. High proton conducting polymer blend electrolytes based on chitosan: Dextran with constant specific capacitance and energy density. Biomolecular 2019, 9, 267. [Google Scholar] [CrossRef]
- Mishra, K.; Hashmi, S.A.; Rai, D.K. Investigations on poly (ethylene oxide) + NH4PF6 solid polymer electrolyte system. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 663–670. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Kadir, M.F.Z.; Karim, W.O.; Abdullah, R.M. Development of polymer blend electrolyte membranes based on chitosan: Dextran with high ion transport properties for EDLC application. Int. J. Mol. Sci. 2019, 20, 3369. [Google Scholar] [CrossRef]
- Marf, A.S.; Abdullah, R.M.; Aziz, S.B. Structural, morphological, electrical and electrochemical properties of PVA: CS-based proton-conducting polymer blend electrolytes. Membranes 2020, 10, 71. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Shukur, M.F.; Kadir, M.F.Z. Protonic cell performance employing electrolytes based on plasticized methylcellulose-potato starch-NH4NO3. Ionics 2019, 25, 559–572. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Arof, A.K. Application of PVA-chitosan blend polymer electrolyte membrane in electrical double layer capacitor. Mater. Res. Innov. 2011, 15, 217–220. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Illias, H.A.; Kadir, M.F.Z. Proton conducting polymer electrolyte based on plasticized chitosan-PEO blend and application in electrochemical devices. Opt. Mater. 2013, 35, 1834–1841. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Teo, L.P.; Woo, H.J.; Majid, S.R.; Arof, A.K. Electrical double-layer capacitors with plasticized polymer electrolyte based on methyl cellulose. Polym. Bull. 2012, 69, 807–826. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Brza, M.A.; Kadir, M.F.Z.; Abdulwahid, R.T.; Ghareeb, H.O.; Woo, H.J. Fabrication of energy storage EDLC device based on CS: PEO polymer blend electrolytes with high Li+ ion transference number. Results Phys. 2019, 15, 102584. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdulwahid, R.T.; Hamsan, M.H.; Brza, M.A.; Abdullah, R.M.; Kadir, M.F.Z.; Muzakir, S.K. Structural, impedance, and EDLC characteristics of proton conducting chitosan-based polymer blend electrolytes with high electrochemical stability. Molecules 2019, 24, 3508. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Z.; Yun, G.; Shi, K.; Lv, X.; Yang, B. High-performance solid-state supercapacitors based on graphene-ZnO hybrid nanocomposites. Nanoscale Res. Lett. 2013, 8, 473–482. [Google Scholar] [CrossRef]
- Bandaranayake, C.M.; Weerasinghe, W.A.D.S.S.; Vidanapathirana, K.P.; Perera, K.S. A Cyclic Voltammetry study of a gel polymer electrolyte based redox-capacitor. Sri Lankan J. Phys. 2015, 16, 19–27. [Google Scholar] [CrossRef]
- Singh, A.; Chandra, A. Graphite oxide/polypyrrole composite electrodes for achieving high energy density supercapacitors. J. Appl. Electrochem. 2013, 43, 773–782. [Google Scholar] [CrossRef]



| Sample Code | Wt.(g) PEO | Wt. % NH4l | Molality (mol/kg) | Wt. % Glycerol |
|---|---|---|---|---|
| PEOH1 | 1 | 10 | 0.7666 | 0 |
| PEOH2 | 1 | 20 | 1.7248 | 0 |
| PEOH3 | 1 | 30 | 2.9568 | 0 |
| PEOH4 | 1 | 30 | 2.9568 | 10 |
| Sample Code | |
|---|---|
| PEOH1 | |
| PEOH2 | |
| PEOH3 | |
| PEOH4 |
| Electrolyte Composition | Polymer/Salt Ratio | Plasticizer | T (°C) | σDC (S cm−1) | Ref. |
|---|---|---|---|---|---|
| PEO-(NH4F)-DMA | F/O = 0.12 | DMA | 30 | [65] | |
| PEO-[Mg(Cf3SO3)2-EMITF | EO/Mg = ~25 | EMITF | ~25 | [66] | |
| PEO-LiCf3SO3-EC | - | EC | 23 | [67] | |
| (PEO)8 LiClO4:DBP (99.5:0.5) | (PEO)8 LiClO4 = 99.5 | DBP | 29 | [68] | |
| PEO:NH4I:glycerol | I/O = 2.957 | glycerol | 29 | This work |
| Scan Rate (mV s−1) | Specific Capacitance (F g−1) |
|---|---|
| 10 | 40.46 |
| 20 | 31.43 |
| 50 | 21.03 |
| 100 | 13.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, M.S.; Ghareeb, H.O.; Aziz, S.B.; Brza, M.A.; Al-Zangana, S.; Hadi, J.M.; Kadir, M.F.Z. Electrochemical Characteristics of Glycerolized PEO-Based Polymer Electrolytes. Membranes 2020, 10, 116. https://doi.org/10.3390/membranes10060116
Mustafa MS, Ghareeb HO, Aziz SB, Brza MA, Al-Zangana S, Hadi JM, Kadir MFZ. Electrochemical Characteristics of Glycerolized PEO-Based Polymer Electrolytes. Membranes. 2020; 10(6):116. https://doi.org/10.3390/membranes10060116
Chicago/Turabian StyleMustafa, Muhammad S., Hewa O. Ghareeb, Shujahadeen B. Aziz, M. A. Brza, Shakhawan Al-Zangana, Jihad M. Hadi, and M. F. Z. Kadir. 2020. "Electrochemical Characteristics of Glycerolized PEO-Based Polymer Electrolytes" Membranes 10, no. 6: 116. https://doi.org/10.3390/membranes10060116
APA StyleMustafa, M. S., Ghareeb, H. O., Aziz, S. B., Brza, M. A., Al-Zangana, S., Hadi, J. M., & Kadir, M. F. Z. (2020). Electrochemical Characteristics of Glycerolized PEO-Based Polymer Electrolytes. Membranes, 10(6), 116. https://doi.org/10.3390/membranes10060116