Improved Performance of Polysulfone Ultrafiltration Membrane Using TCPP by Post-Modification Method
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
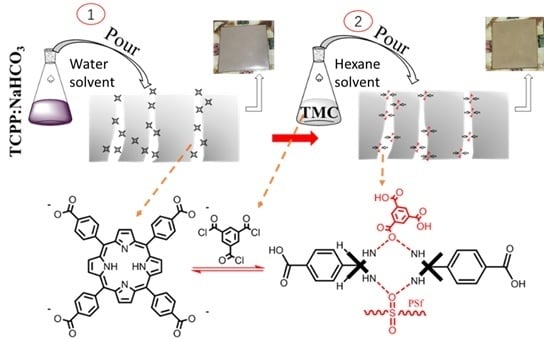
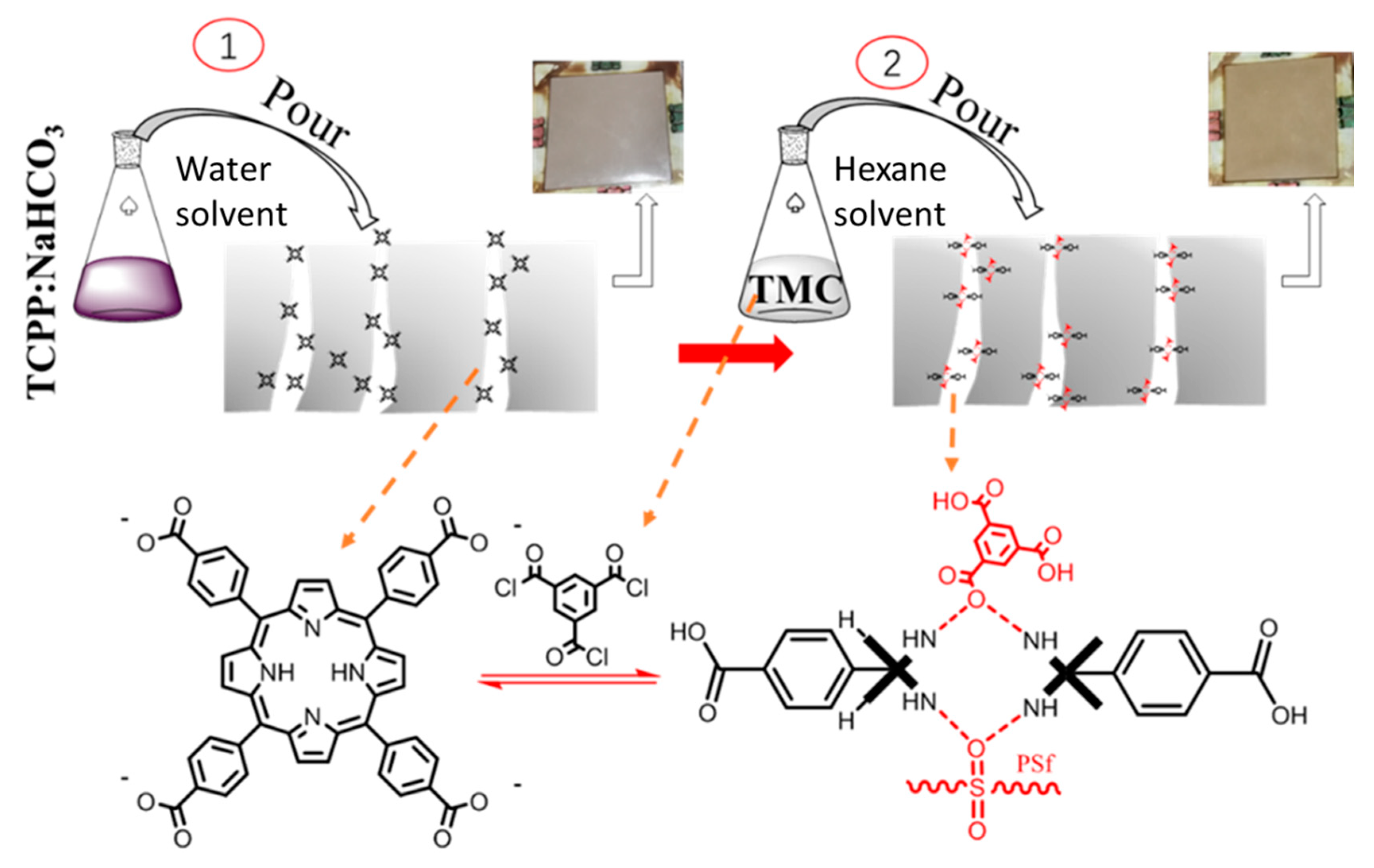
2.2. Preparation and Characterization of TCPP (tetrakis (4-carboxyphenyl) porphyrin)
2.3. Preparation of Base Membrane
2.4. Preparation of TCPP/PSf Ultrafiltration Membrane
2.5. Characterization of Base Membrane and TCPP/PSf Ultrafiltration Membranes
2.6. Test of TCPP/PSf Ultrafiltration Membranes Properties
3. Results and Discussion
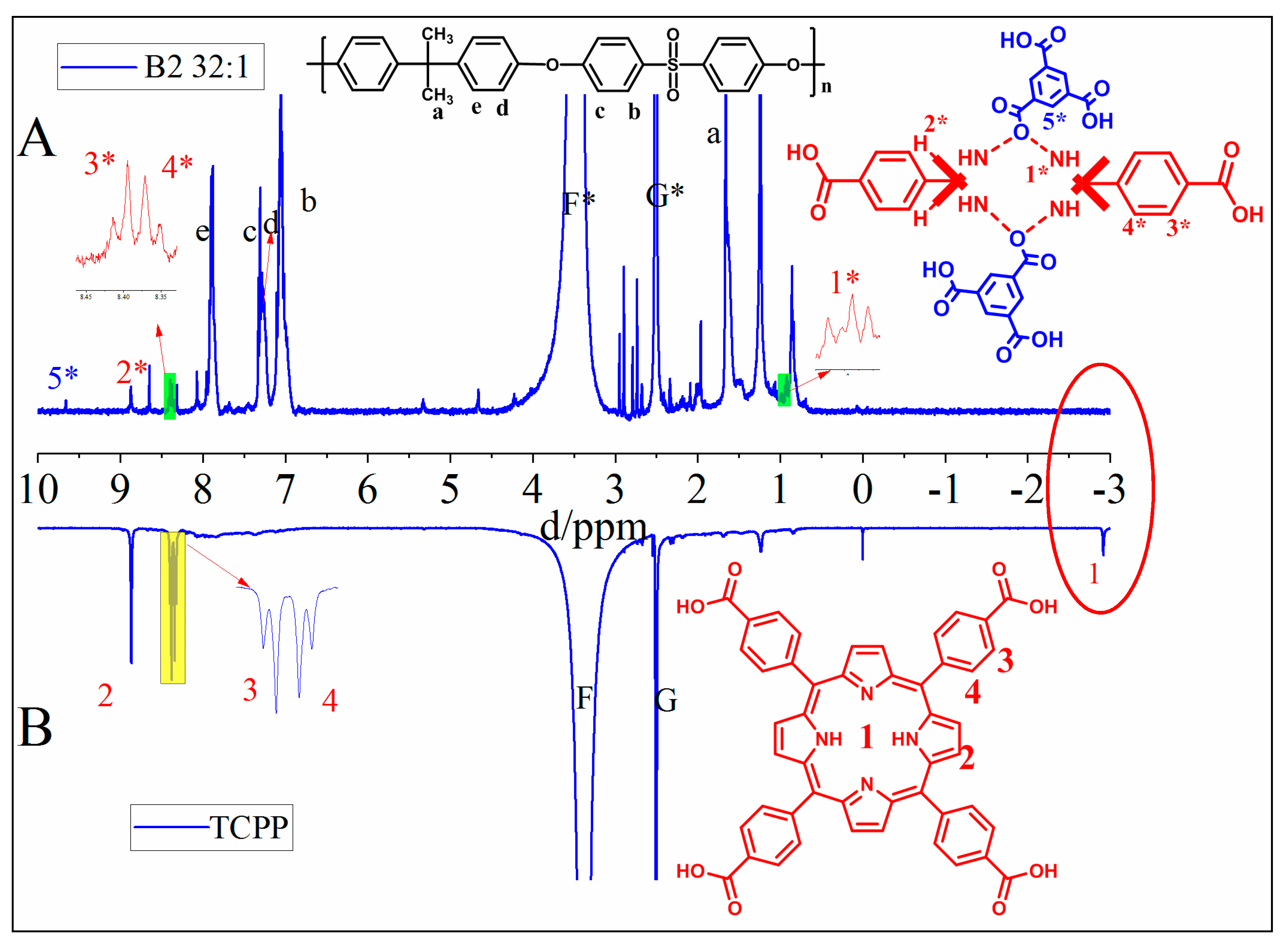
3.1. Characterization of TCPP and Its Protonation in the Membrane
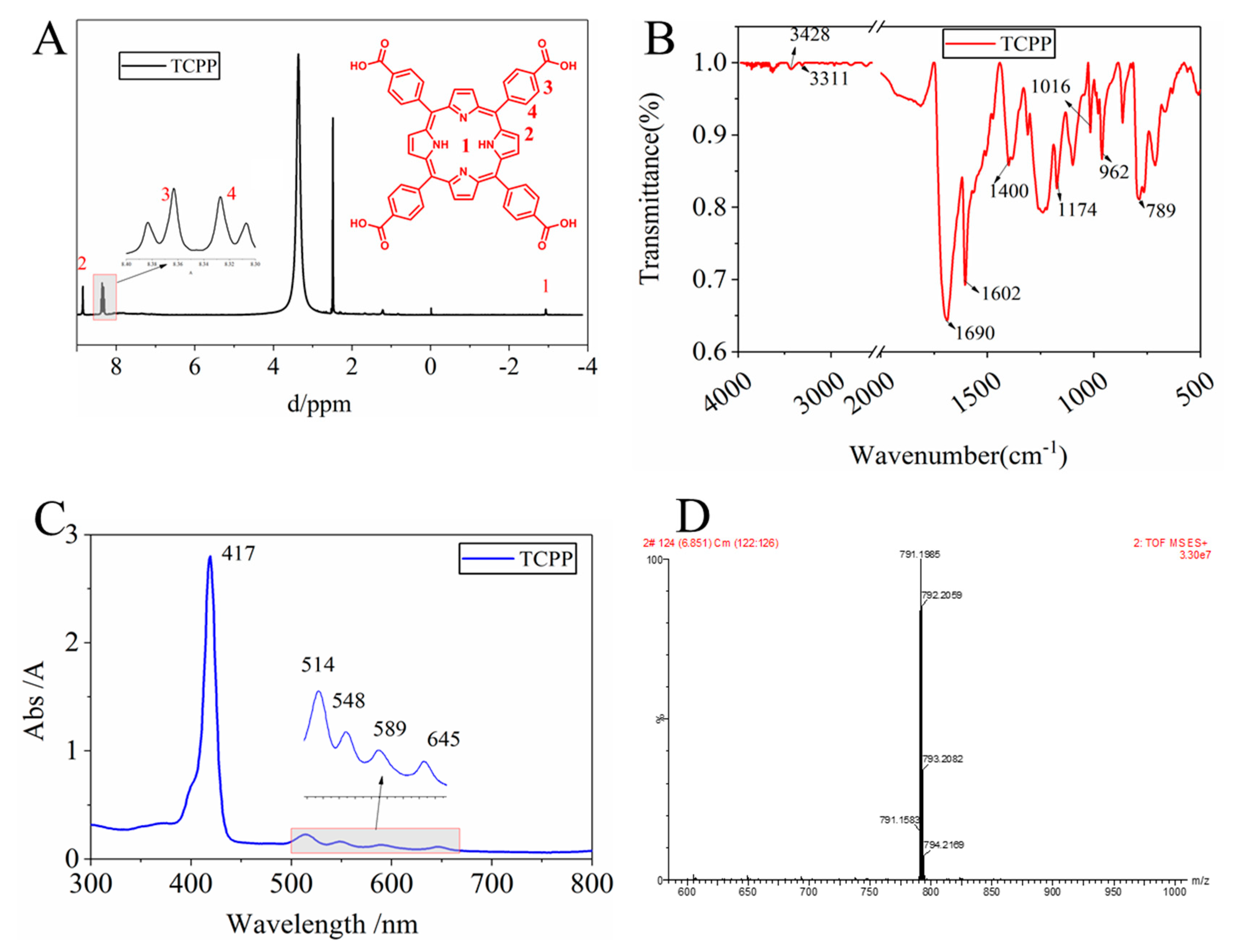
3.1.1. Characterization of as-Prepared TCPP
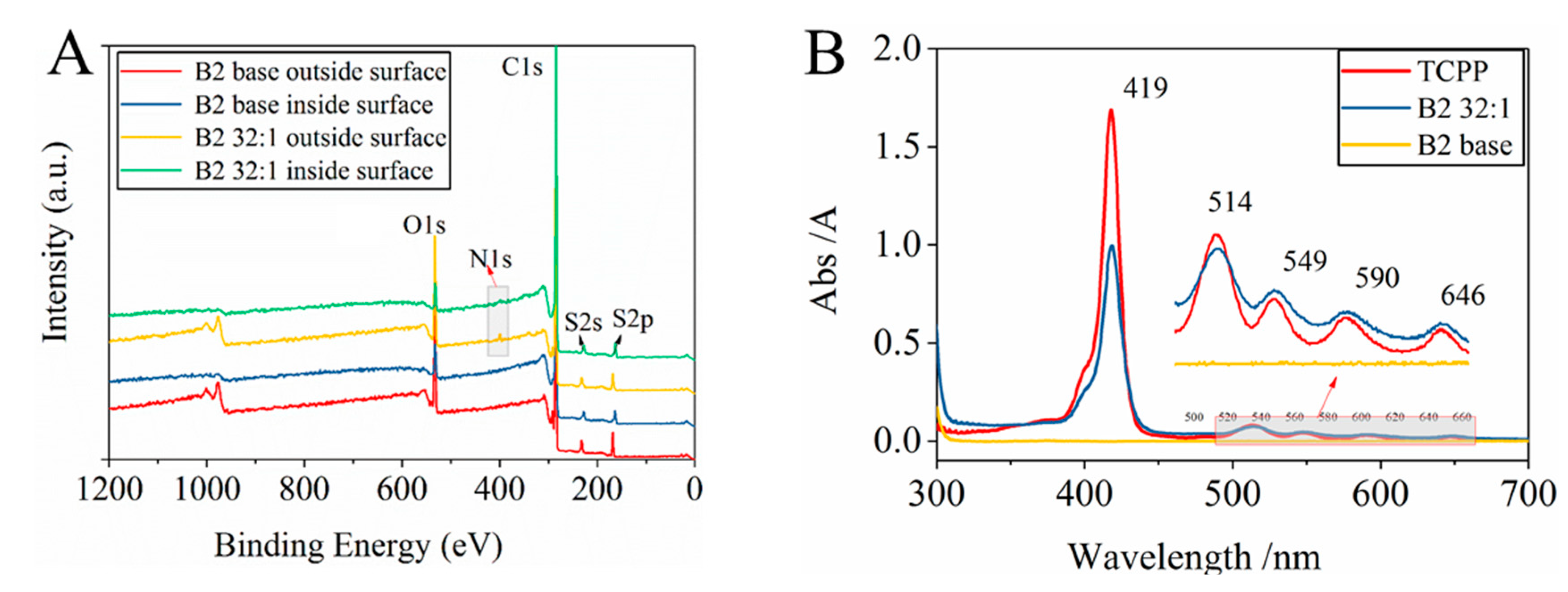
3.1.2. Determination of the Existence and Protonation of TCPP in the TCPP/PSf Membranes
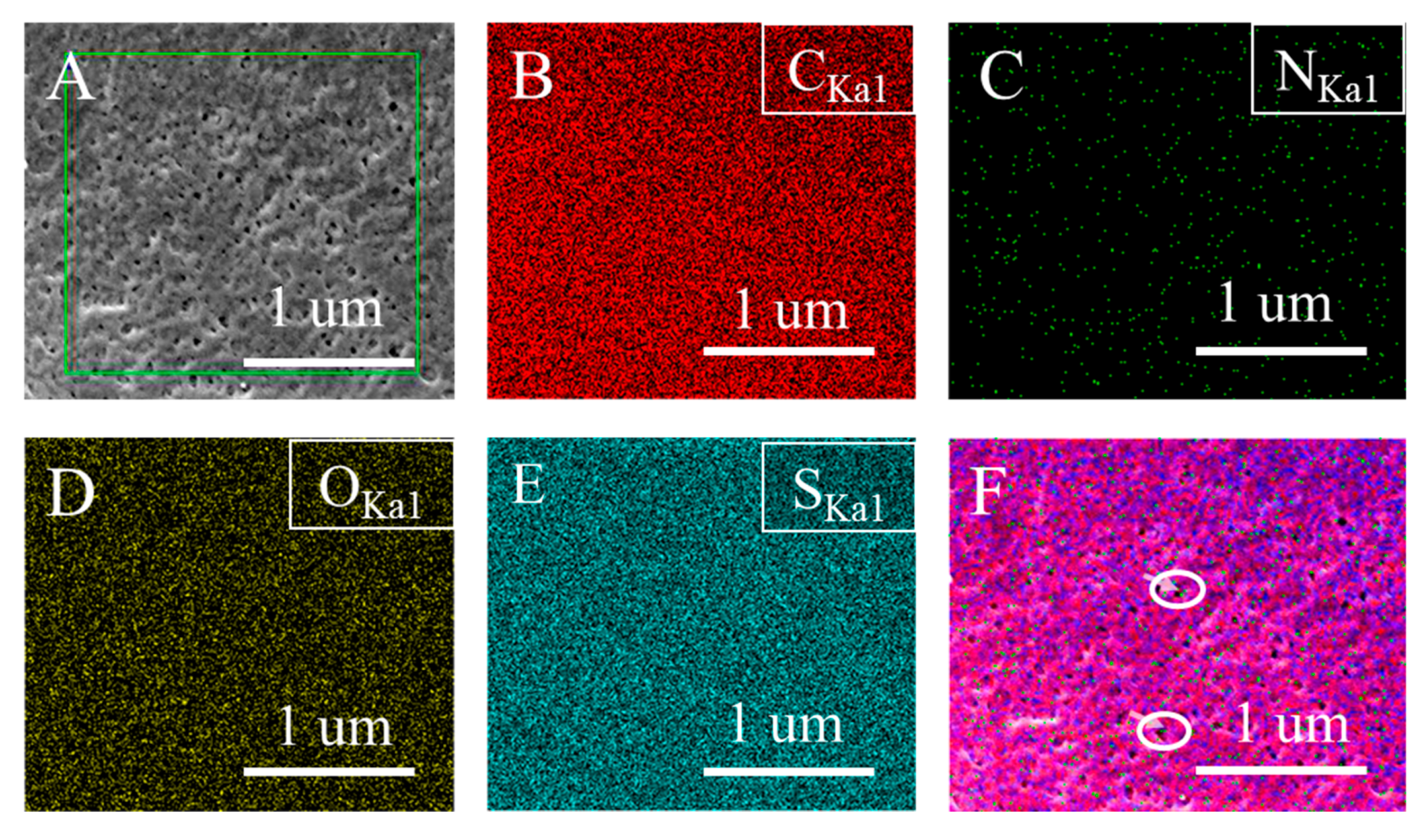
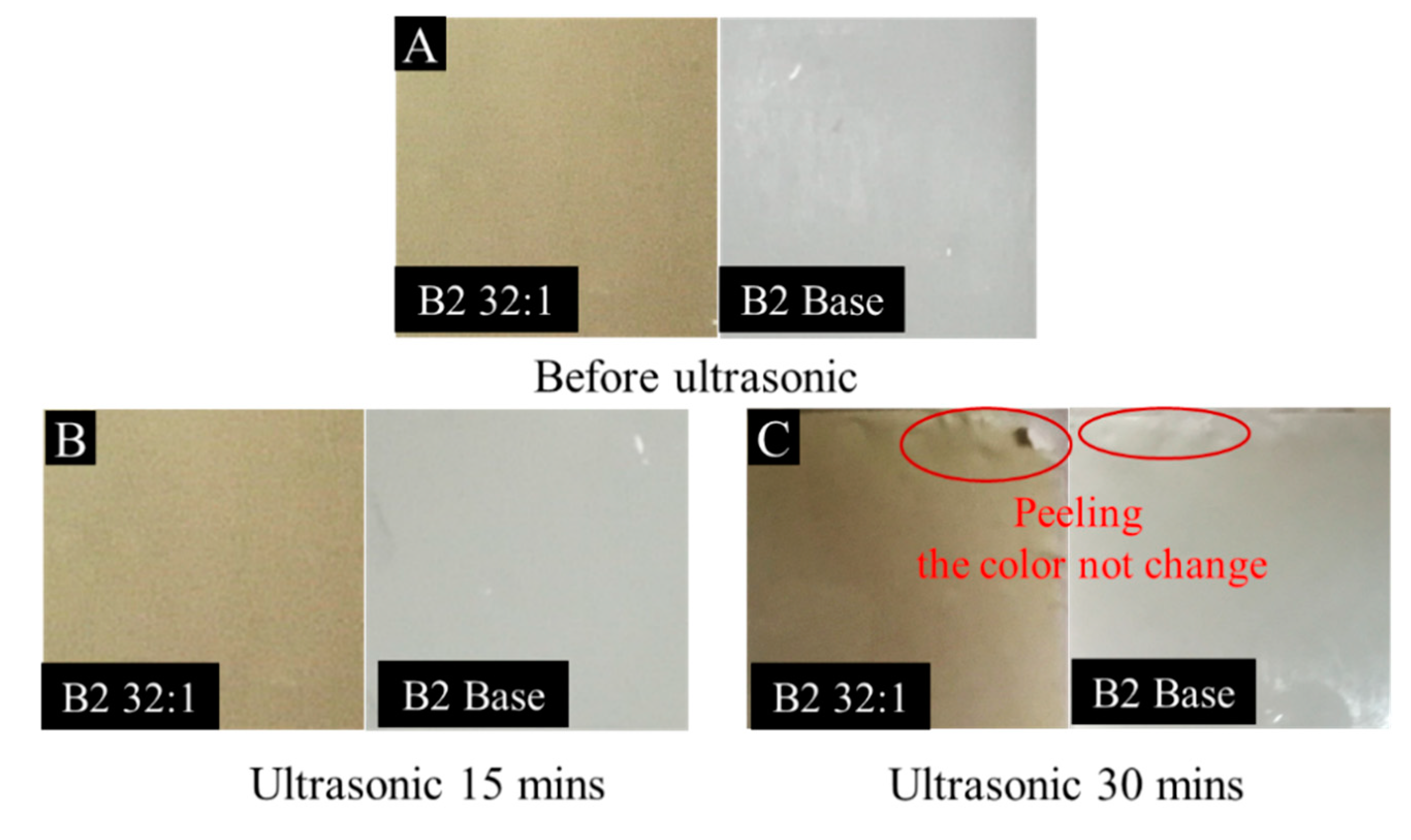
3.1.3. Distribution and Adhesion of Protonated TCPP in the Membrane
3.2. Characterization of TCPP/PSf Membranes
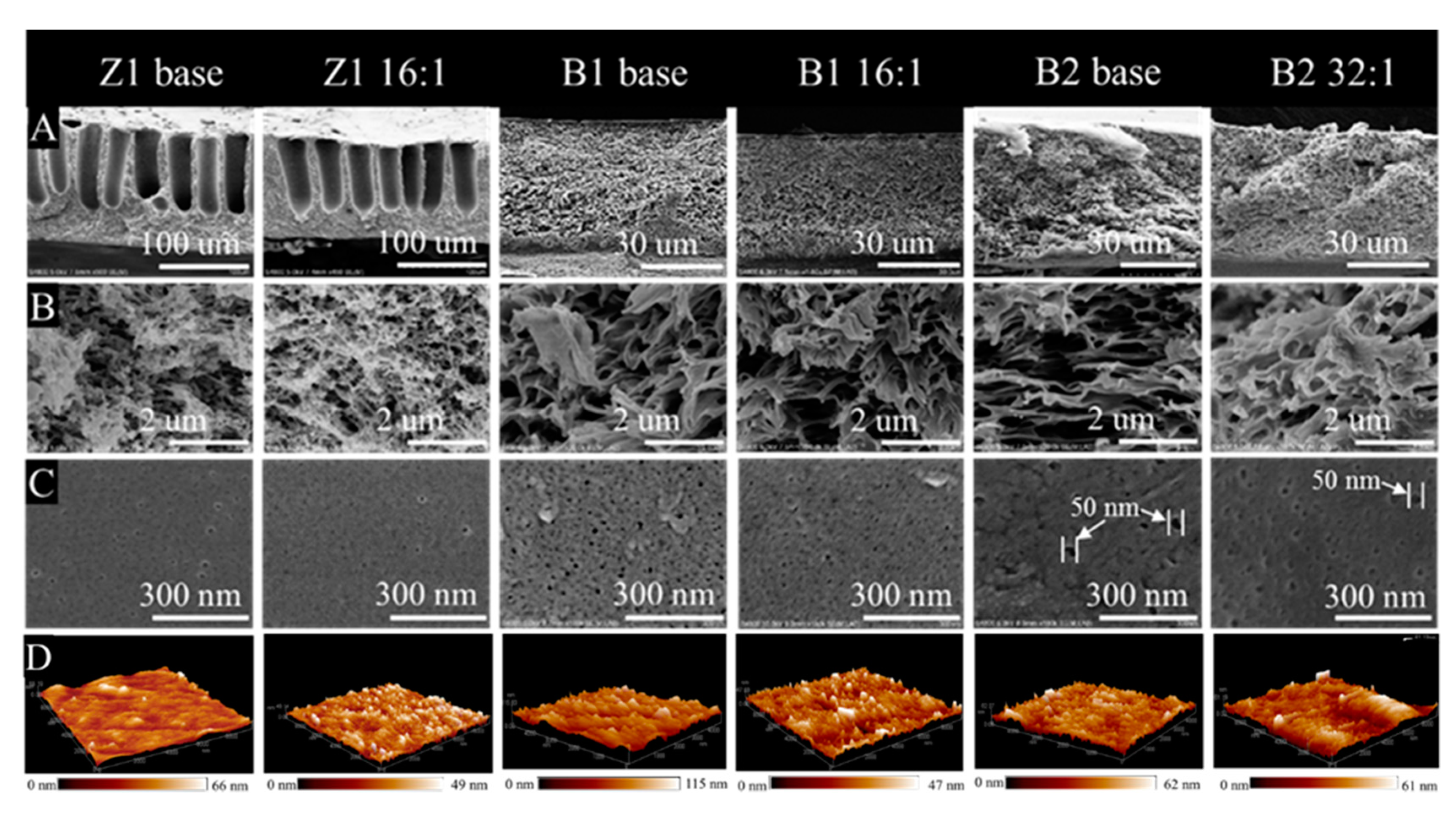
3.2.1. Morphology and of TCPP/PSf Membranes
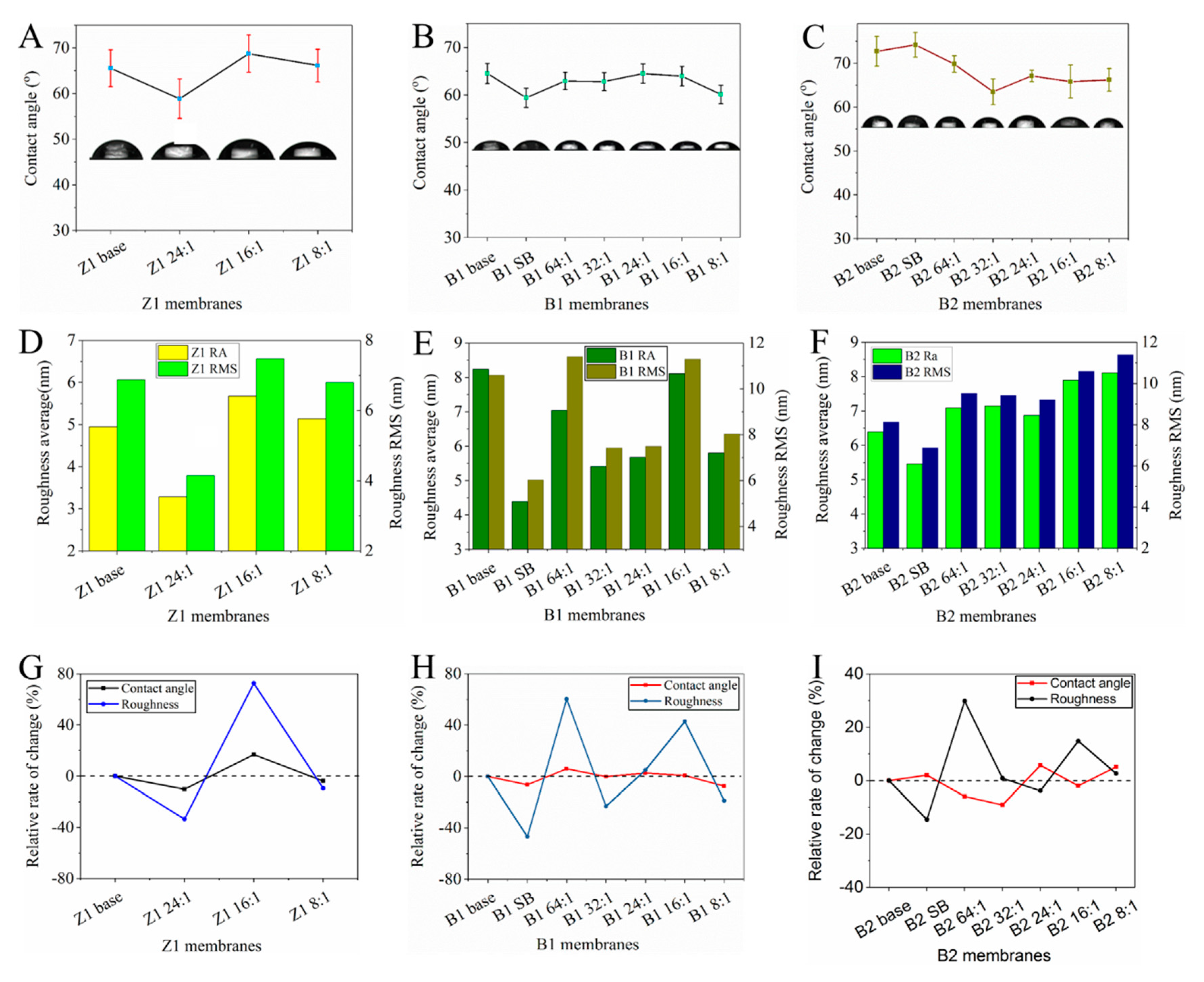
3.2.2. Hydrophilicity and Surface Roughness of TCPP/PSf Membranes
3.3. Properties of TCPP/PSf Membranes
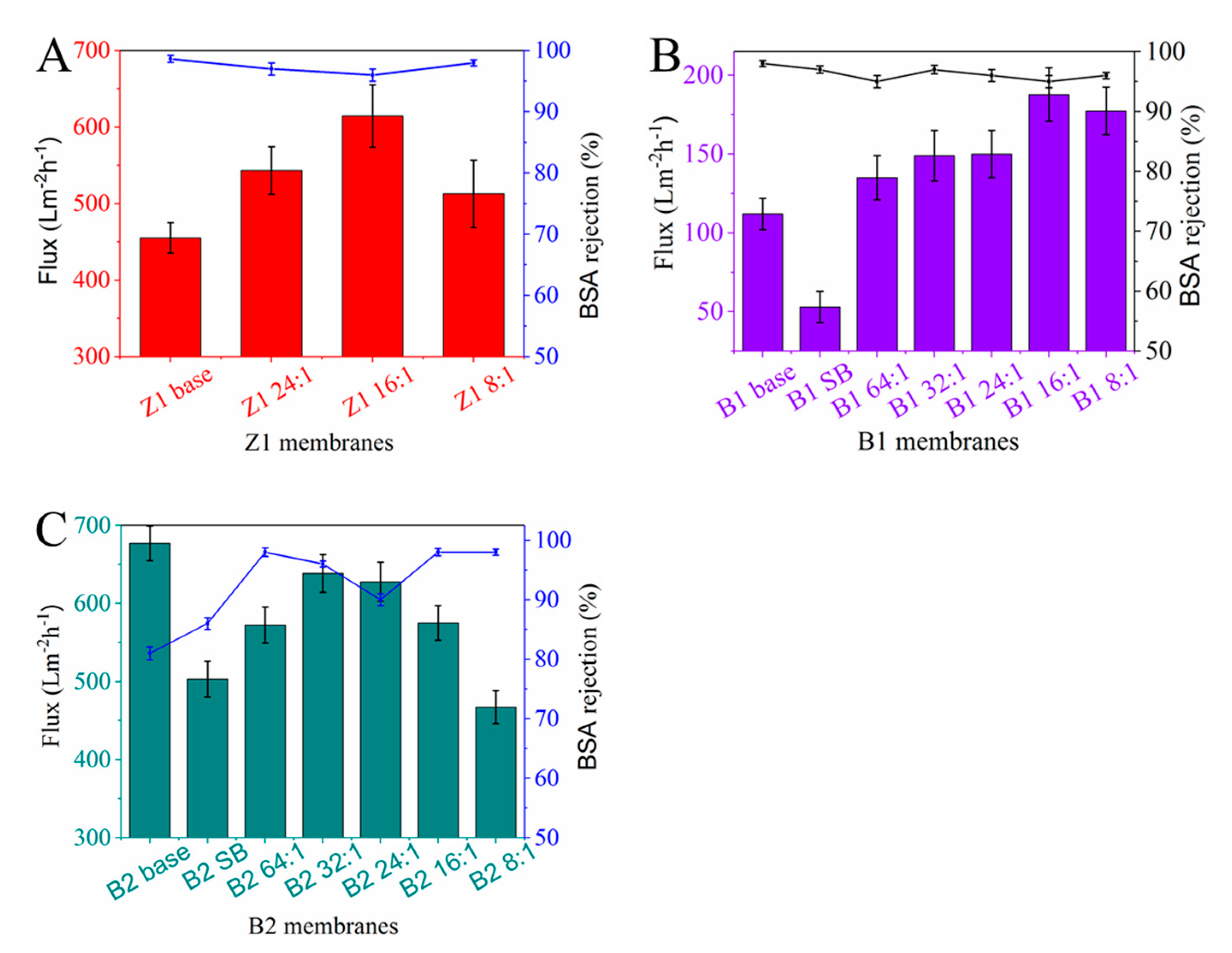
3.3.1. Water flux and BSA rejection of TCPP/PSf Membranes
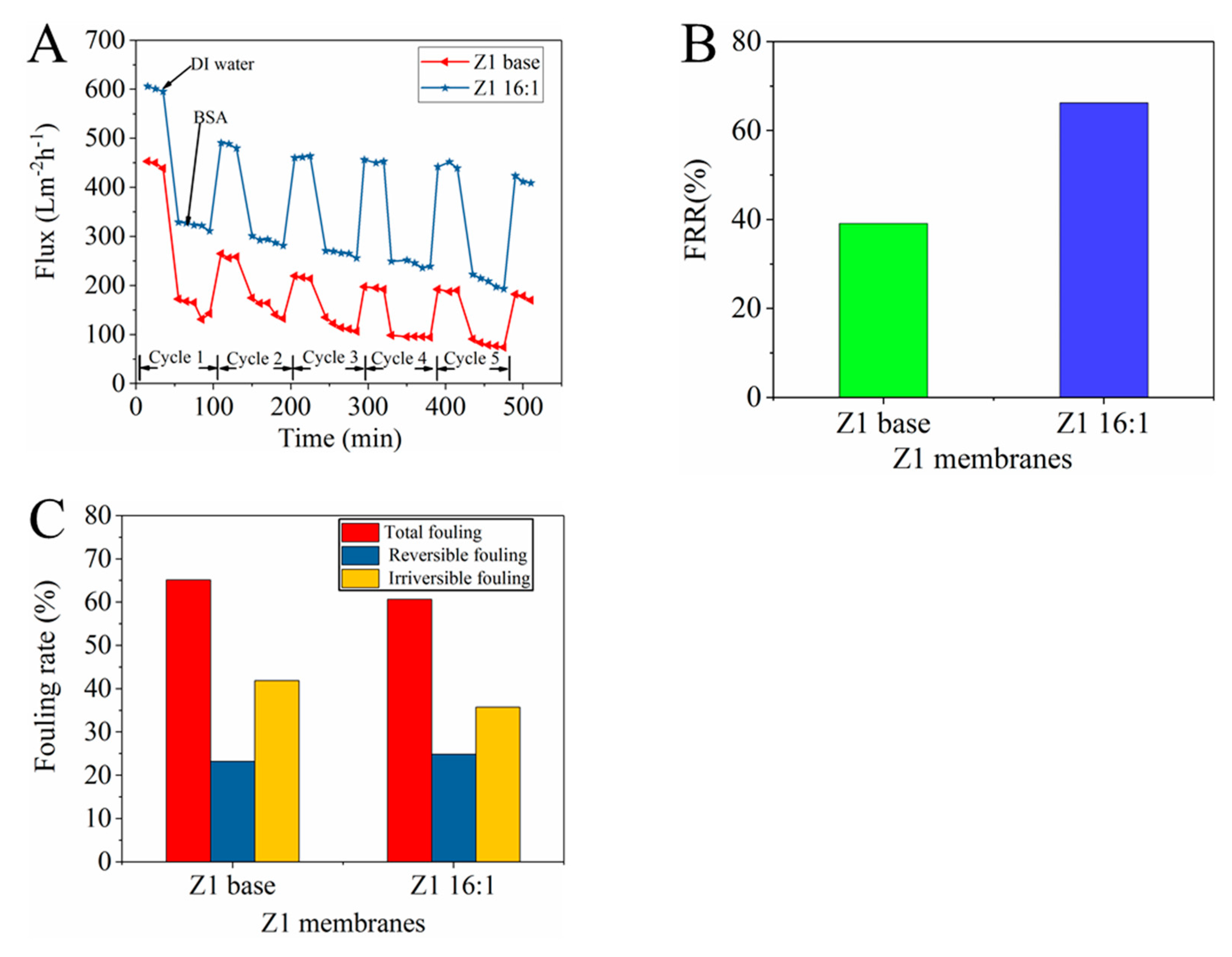
3.3.2. Anti-Fouling Performance of TCPP/PSf membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nanosci. Technol. Collect. Rev. Nat. J. 2010, 337–346. [Google Scholar]
- Santos, M.J.; Teixeira, J.A.; Rodrigues, L.R. Fractionation of the major whey proteins and isolation of β-Lactoglobulin variants by anion exchange chromatography. Sep. Purif. Technol. 2012, 90, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic membranes for water purification: Status and future. Angew. Chem. Int. Edit. 2015, 54, 3368–3386. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, H.W.; Nam, S.Y.; Park, H.B. Fouling-tolerant polysulfone–poly (ethylene oxide) random copolymer ultrafiltration membranes. J. Membr. Sci. 2011, 379, 296–306. [Google Scholar] [CrossRef]
- Sun, M.; Su, Y.; Mu, C.; Jiang, Z. Improved antifouling property of PES ultrafiltration membranes using additive of silica PVP nanocomposite. Ind. Eng. Chem. Res. 2009, 49, 790–796. [Google Scholar] [CrossRef]
- Kumar, M.; Ulbricht, M. Low fouling negatively charged hybrid ultrafiltration membranes for protein separation from sulfonated poly (arylene ether sulfone) block copolymer and functionalized multiwalled carbon nanotubes. Sep. Purif. Technol. 2014, 127, 181–191. [Google Scholar] [CrossRef]
- Qu, F.; Liang, H.; Zhou, J.; Nan, J.; Shao, S.; Zhang, J.; Li, G. Ultrafiltration membrane fouling caused by extracellular organic matter (EOM) from Microcystis aeruginosa: Effects of membrane pore size and surface hydrophobicity. J. Membr. Sci. 2014, 449, 58–66. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Development of novel SiO2–GO nanohybrid/polysulfone membrane with enhanced performance. J. Membr. Sci. 2014, 451, 94–102. [Google Scholar] [CrossRef]
- Loh, C.H.; Wang, R.; Shi, L.; Fane, A.G. Fabrication of high performance polyethersulfone UF hollow fiber membranes using amphiphilic Pluronic block copolymers as pore-forming additives. J. Membr. Sci. 2011, 380, 114–123. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.; Aziz, M. Polyethersulfone (PES) ultrafiltration (UF) membranes loaded with silver nitrate for bacteria removal. Membr. Water Treat. 2011, 2, 25–37. [Google Scholar] [CrossRef]
- Liang, H.Q.; Wu, Q.Y.; Wan, L.S.; Huang, X.J.; Xu, Z.K. Thermally induced phase separation followed by in situ sol–gel process: A novel method for PVDF/SiO2 hybrid membranes. J. Membr. Sci. 2014, 465, 56–67. [Google Scholar] [CrossRef]
- Tang, Y.P.; Cai, T.; Loh, D.; O’Brien, G.S.; Chung, T.S. Construction of antifouling lumen surface on a poly (vinylidene fluoride) hollow fiber membrane via a zwitterionic graft copolymerization strategy. Sep. Purif. Technol. 2017, 176, 294–305. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Wang, J.; Wang, S. The effect of pH of coagulation bath on tailoring the morphology and separation performance of polysulfone/polyaniline ultrafiltration membrane. J. Membr. Sci. 2014, 469, 316–325. [Google Scholar] [CrossRef]
- Yang, L.; Tang, B.; Wu, P. UF membrane with highly improved flux by hydrophilic network between graphene oxide and brominated poly (2, 6-dimethyl-1, 4-phenylene oxide). J. Mater. Chem. A 2014, 2, 18562–18573. [Google Scholar] [CrossRef]
- Lee, S.J.; Dilaver, M.; Park, P.K.; Kim, J.H. Comparative analysis of fouling characteristics of ceramic and polymeric microfiltration membranes using filtration models. J. Membr. Sci. 2013, 432, 97–105. [Google Scholar] [CrossRef]
- Zhou, R.; Ren, P.F.; Yang, H.C.; Xu, Z.K. Fabrication of antifouling membrane surface by poly (sulfobetaine methacrylate)/polydopamine co-deposition. J. Membr. Sci. 2014, 466, 18–25. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.J.; Paul, D.R.; Freeman, B.D. An improved method for surface modification of porous water purification membranes. Polymer 2014, 55, 1375–1383. [Google Scholar] [CrossRef]
- Miller, D.J.; Kasemset, S.; Wang, L.; Paul, D.R.; Freeman, B.D. Constant flux crossflow filtration evaluation of surface-modified fouling-resistant membranes. J. Membr. Sci. 2014, 452, 171–183. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface modification of water purification membranes. Angew. Chem. Int. Edit. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [Green Version]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Bryjak, M.; Gancarz, I.; Poźniak, G.; Tylus, W. Modification of polysulfone membranes 4. Ammonia plasma treatment. Eur. Polym. J. 2002, 38, 717–726. [Google Scholar] [CrossRef]
- Yamagishi, H.; Crivello, J.V.; Belfort, G. Development of a novel photochemical technique for modifying poly (arylsulfone) ultrafiltration membranes. J. Membr. Sci. 1995, 105, 237–247. [Google Scholar] [CrossRef]
- Han, M.J.; Nam, S.T. Thermodynamic and rheological variation in polysulfone solution by PVP and its effect in the preparation of phase inversion membrane. J. Membr. Sci. 2002, 202, 55–61. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.; Purkait, M. Preparation, characterization and performance studies of polysulfone membranes using PVP as an additive. J. Membr. Sci. 2008, 315, 36–47. [Google Scholar] [CrossRef]
- Zheng, Q.Z.; Wang, P.; Yang, Y.N. Rheological and thermodynamic variation in polysulfone solution by PEG introduction and its effect on kinetics of membrane formation via phase-inversion process. J. Membr. Sci. 2006, 279, 230–237. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, F.; Ma, J.; Wu, M.; Zhang, J.; Gao, C. Effect of PEG additive on the morphology and performance of polysulfone ultrafiltration membranes. Desalination 2011, 272, 51–58. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Membr. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Ahmad, A.; Majid, M.; Ooi, B. Functionalized PSf/SiO2 nanocomposite membrane for oil-in-water emulsion separation. Desalination 2011, 268, 266–269. [Google Scholar] [CrossRef]
- Leo, C.; Lee, W.C.; Ahmad, A.; Mohammad, A.W. Polysulfone membranes blended with ZnO nanoparticles for reducing fouling by oleic acid. Sep. Purif. Technol. 2012, 89, 51–56. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Webb, M.J.; Bampos, N. Noncovalent interactions in acid–porphyrin complexes. Chem. Sci. 2012, 3, 2351. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J-and H-aggregates of porphyrin—Surfactant complexes: Time-resolved fluorescence and other spectroscopic studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Zhang, L.; Meng, Q.; Shen, C.; Zhang, J. Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g-HEMA and its antifouling characteristics. J. Membr. Sci. 2013, 436, 163–173. [Google Scholar] [CrossRef]
- Wang, M.; Yan, F.; Zhao, L.; Zhang, Y.; Sorci, M. Preparation and characterization of a pH-responsive membrane carrier for meso-tetraphenylsulfonato porphyrin. RSC Adv. 2017, 7, 1687–1696. [Google Scholar] [CrossRef] [Green Version]
- Eskelsen, J.R.; Wang, Y.; Qui, Y.; Ray, M.; Handlin, M.; Hipps, K.W.; Mazur, U. Protonation state of core nitrogens in the meso-tetra(4-carboxyphenyl)porphyrin impacts the chemical and physical properties of nanostructures formed in acid solutions. J. Porphyr. Phthalocyanines 2012, 16, 1233–1243. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Akbari, A.; Mehrnia, M.R. Preparation of polysulfone nanofiltration membranes by UV-assisted grafting polymerization for water softening. Desalination 2010, 263, 217–225. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, D.; Wang, L.; Guo, L.; Chen, W.; Yu, F.; Wang, Y.; Yang, Y. Two-dimensional superstructures filled into polysulfone membranes for highly improved ultrafiltration: The case of cuprous iodide nanosheets. J. Membr. Sci. 2019, 576, 142–149. [Google Scholar] [CrossRef]
- Duong, P.H.H.; Kuehl, V.A.; Mastorovich, B.; Hoberg, J.O.; Parkinson, B.A.; Oakey, K.D. Carboxyl-functionalized covalent organic framework as a two-dimensional nanofiller for mixed-matrix ultrafiltration membranes. J. Membr. Sci. 2019, 574, 338–348. [Google Scholar] [CrossRef]
| Type | Pure Water Flux (Lm−2h−1) | BSA Rejection Rate (%) | Total Thickness (μm) | Porosity (%) |
|---|---|---|---|---|
| B1 | 112.36 (low) | 98.00 (high) | 43.80 | 68.16 ± 3.3 |
| Z1 | 455.30 (medium) | 98.67 (high) | 137.60 | 72.13 ± 4.8 |
| B2 | 677.52 (high) | 81.00 (low) | 34.73 | 77.28 ± 5 |
| Labels of TCPP/PSf Membranes | Aqueous Solution Concentration (wt.%) | ||
|---|---|---|---|
| Water | NaHCO3 | TCPP | |
| Z1 24:1 | 95.84 | 4.00 | 0.17 |
| Z1 16:1 | 95.75 | 4.00 | 0.25 |
| Z1 8:1 | 95.50 | 4.00 | 0.50 |
| B1 SB | 96.00 | 4.00 | 0.00 |
| B1 64:1 | 95.94 | 4.00 | 0.06 |
| B1 32:1 | 95.88 | 4.00 | 0.13 |
| B1 24:1 | 95.84 | 4.00 | 0.17 |
| B1 16:1 | 95.75 | 4.00 | 0.25 |
| B1 8:1 | 95.50 | 4.00 | 0.50 |
| B2 SB | 96.00 | 4.00 | 0.00 |
| B2 64:1 | 95.94 | 4.00 | 0.06 |
| B2 32:1 | 95.88 | 4.00 | 0.13 |
| B2 24:1 | 95.84 | 4.00 | 0.17 |
| B2 16:1 | 95.75 | 4.00 | 0.25 |
| B2 8:1 | 95.50 | 4.00 | 0.50 |
| Membrane | Etching Depth (nm) | Atomic Percent (atom %) | |||
|---|---|---|---|---|---|
| C | O | N | S | ||
| B2 base | 0 | 81.3 | 14.31 | 0.96 | 3.27 |
| B2 base | 10 | 91.5 | 4.94 | 0.89 | 2.46 |
| B2 32:1 | 0 | 80.9 | 11.5 | 1.71 | 2.72 |
| B2 32:1 | 10 | 92.26 | 3.65 | 1.48 | 2.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Sun, S.; Li, S.; Wang, Z.; Wen, F.; Li, C.; Matsuyama, H.; Hu, S. Improved Performance of Polysulfone Ultrafiltration Membrane Using TCPP by Post-Modification Method. Membranes 2020, 10, 66. https://doi.org/10.3390/membranes10040066
Jia Y, Sun S, Li S, Wang Z, Wen F, Li C, Matsuyama H, Hu S. Improved Performance of Polysulfone Ultrafiltration Membrane Using TCPP by Post-Modification Method. Membranes. 2020; 10(4):66. https://doi.org/10.3390/membranes10040066
Chicago/Turabian StyleJia, Yuandong, Shuangqing Sun, Shunshun Li, Zhikun Wang, Fushan Wen, Chunling Li, Hideto Matsuyama, and Songqing Hu. 2020. "Improved Performance of Polysulfone Ultrafiltration Membrane Using TCPP by Post-Modification Method" Membranes 10, no. 4: 66. https://doi.org/10.3390/membranes10040066
APA StyleJia, Y., Sun, S., Li, S., Wang, Z., Wen, F., Li, C., Matsuyama, H., & Hu, S. (2020). Improved Performance of Polysulfone Ultrafiltration Membrane Using TCPP by Post-Modification Method. Membranes, 10(4), 66. https://doi.org/10.3390/membranes10040066