Treatment of Electroplating Wastewater Using NF pH-Stable Membranes: Characterization and Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Membranes
2.2. Chemicals and Solutions
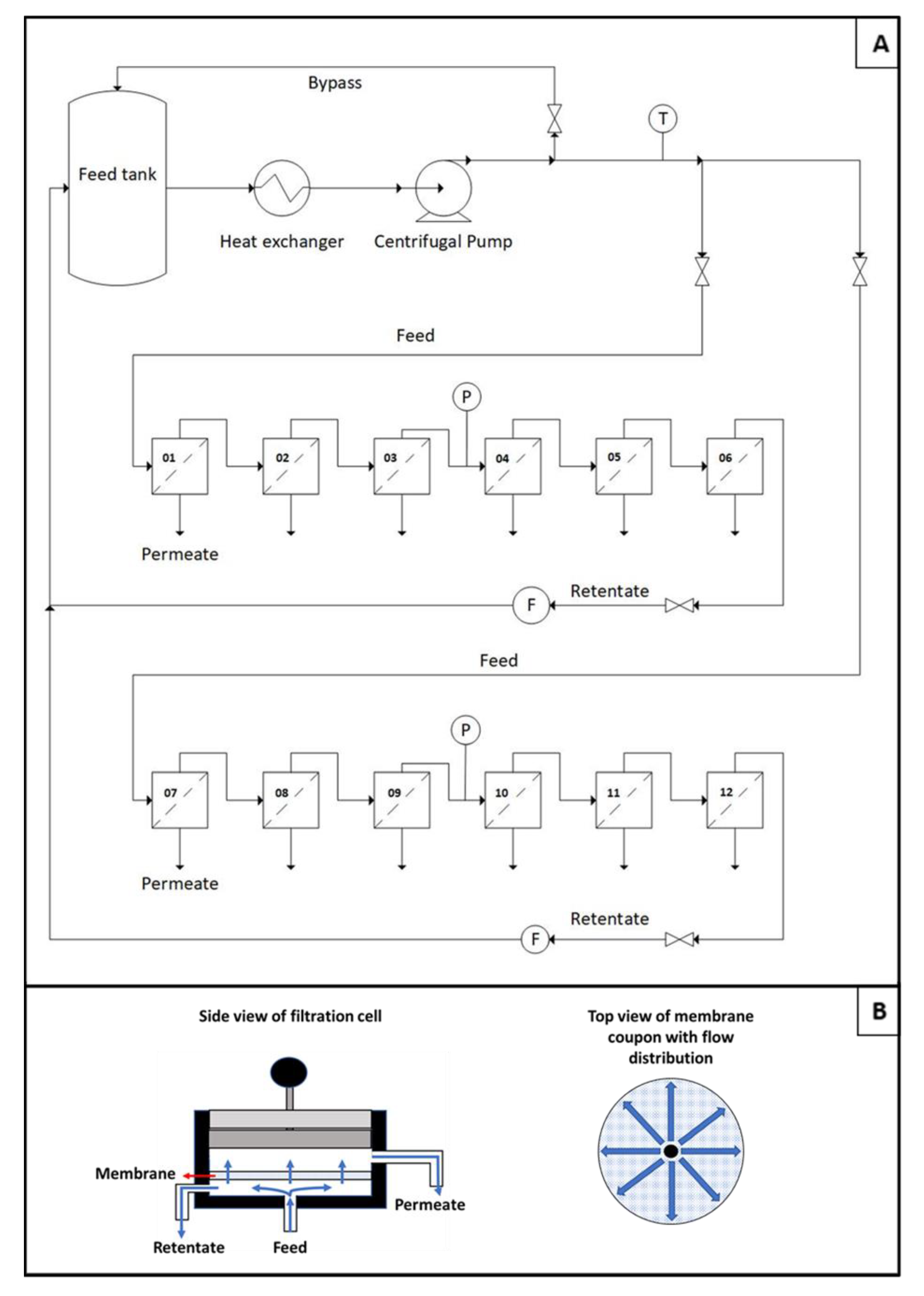
2.3. Filtration Tests
2.4. Membrane Performance Calculations
2.5. MWCO Determination
2.6. Model EPWW
2.6.1. Criteria for Defining Solution Composition
2.6.2. Single-Salt Experiments
2.6.3. Mixed-Salt Experiments
3. Analytical Methods
3.1. Membrane Characterization
3.1.1. Scanning Electron Microscopy
3.1.2. Membrane Thickness
3.1.3. Electrokinetic Characterization
3.1.4. Water Contact Angle
3.2. Concentration of Ionic Solutes
3.2.1. Conductivity and pH Measurements
3.2.2. Automated Photometric Analyzer
3.2.3. UV–Vis Spectrophotometer
3.2.4. Ion Chromatography
3.2.5. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
3.3. Determination of MWCO by Gel Permeation Chromatography (GPC)
4. Results and Discussion
4.1. Membrane Morphology
4.2. Hydrophilicity and Thickness
4.3. Surface Charge
4.4. MWCO Dependence on pH
4.5. Filtration of EPWW
4.5.1. Single Salts
4.5.2. Electroplating Mixture
5. Conclusions
- SEM images of Membrane A reveal an asymmetric polymeric structure composed of a dense few μm-thick thin top layer on its surface, turning into finger-like macrovoids in the membrane bulk. The membrane has a thickness of ca. 100 μm without considering the non-woven fabric support. The top surface shows uniformly distributed nano-pores.
- Membrane A is thicker with an average thickness of ca. 261 μm versus ca. 229 μm of Membrane B. Membrane A is more hydrophobic (water contact angle of 64.3°) than Membrane B (water contact angle of 53.6°). These values are comparable to those reported in the literature and are consistent with the lower pure water permeability of Membrane A compared to that of Membrane B (1.26 ± 0.27 versus 4.8 ± 1.0 ).
- The IEP of Membrane A (at a pH of ca. 4.2) is higher than that of Membrane B (at a pH of ca. 3.5) and Membrane A has more positive surface charges than Membrane B in the pH range of 3 to 3.5.
- Membrane A has an improved low pH stability, showing only a slight change in its MWCO value, from ca. 510 to ca. 490 Da, compared to a significant MWCO shift for Membrane B from ca. 680 to ca. 880 Da. This means that Membrane A barely changes its rejection performance in terms of neutral molecules such as PEGs, while Membrane B becomes significantly more permeable to low-molecular weight (MW < ca. 490 Da) PEGs fractions, thus demonstrating the utmost importance of MWCO determination at the pH value of the targeted application.
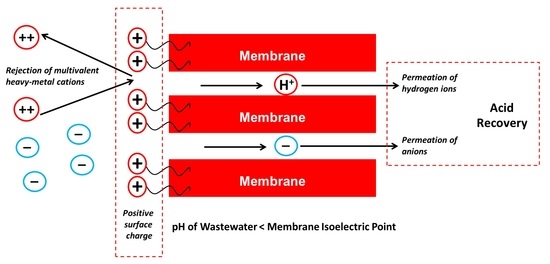
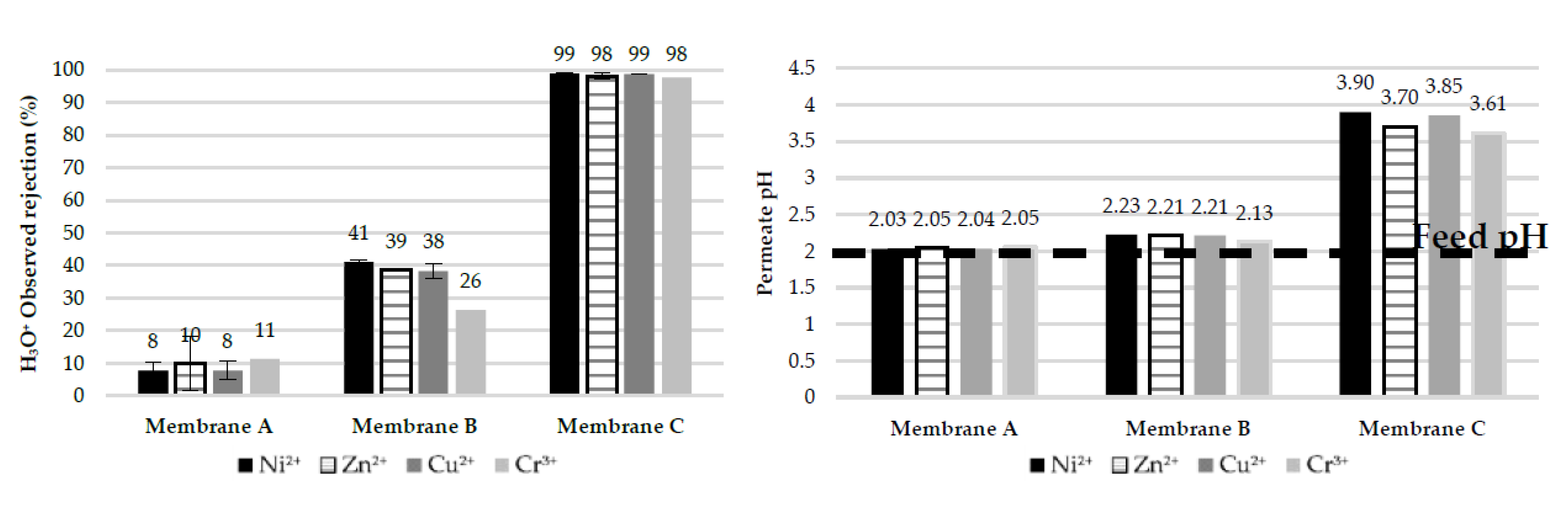
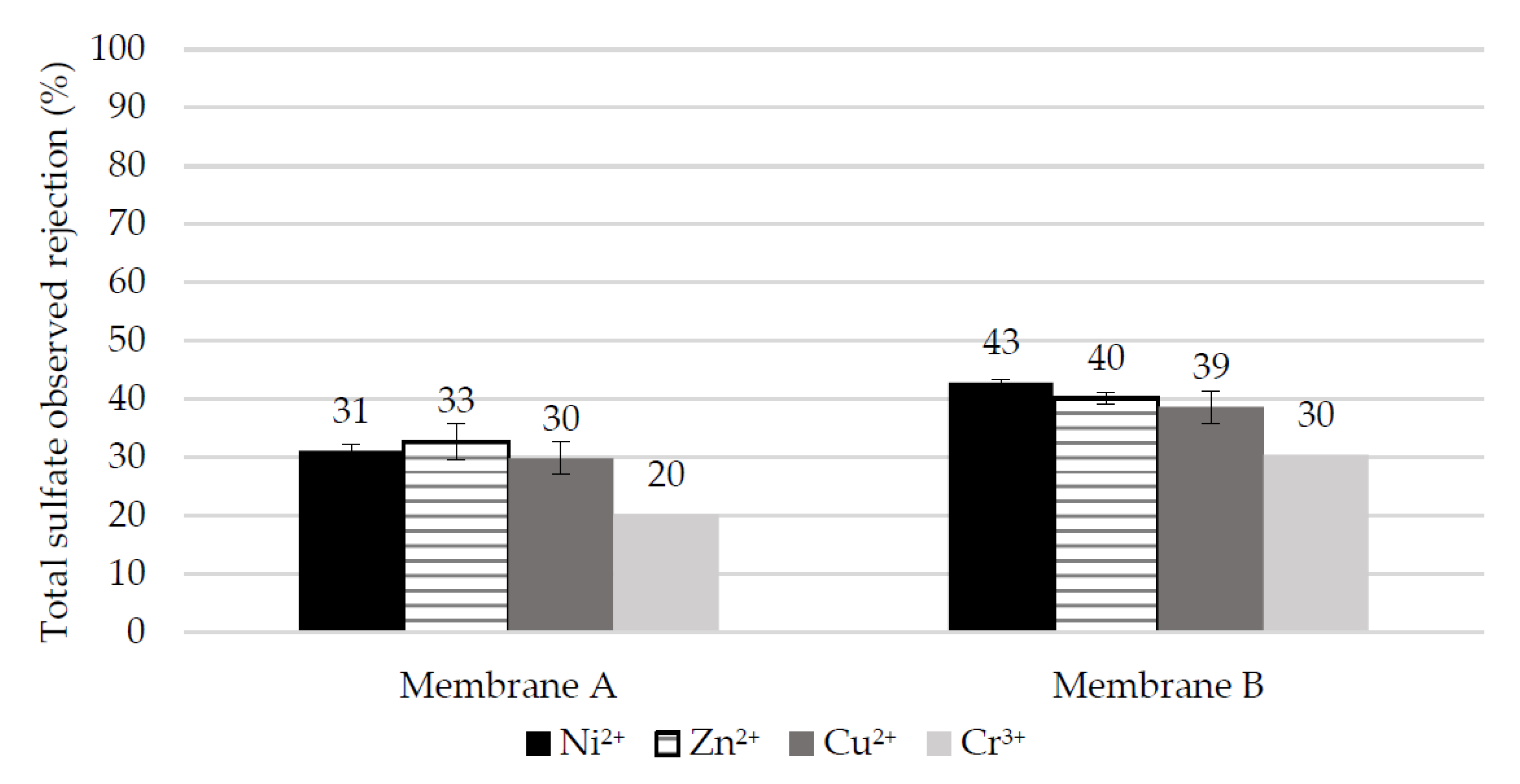
- Membrane A shows much higher (by two to three times) rejection of heavy metals compared to Membrane B at the spontaneous pH of 3.25 of the simulated EPWW mixture which becomes even higher (by > 10%) at a pH of 2, which can be attributed to the higher positive surface charge of Membrane A at low pH. Membrane A also enables a higher hydronium ions concentration in the permeate than Membrane B, which is due to the more pronounced Donnan exclusion effects for the cations at the solution pH below the membrane IEP.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CA | Cellulose acetate |
| CT | Coupon tester |
| DI | Deionized water |
| EPWW | Electroplating wastewater |
| GPC | Gel permeation chromatography |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| IEP | Isoelectric point |
| IP | Interfacial polymerization |
| MWCO | Molecular weight cut-off |
| NF | Nanofiltration |
| PEG | Polyethylene glycol |
| PES | Polyethersulfone |
| PS | Polysulfone |
| RO | Reverse osmosis |
| SEM | Scan electron microscopy |
| SPEEK | Sulfonated poly(ether ether ketone) |
| TFC | Thin film composite |
| UF | Ultrafiltration |
Symbols and Nomenclature
| Symbols | Parameter | SI Units |
| P | Pressure | |
| ΔP | Pressure gradient | Pa |
| Observed rejection of component i | ||
| Molar concentration of component i in permeate | ||
| Molar concentration of component i in feed | ||
| Volumetric flux | ||
| Volume | ||
| Area of membrane filtration | ||
| Time of permeate collection | ||
| Π | Osmotic pressure | |
| Molar concentration of component i | ||
| R | Ideal gas constant | |
| T | Temperature | |
| K | Conductivity |
References
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Fane, A.G.; Waite, T.D. Nanofiltration: Principles and Applications; Elsevier Advanced Technology: Oxford, UK, 2005; ISBN 9781856174053. [Google Scholar]
- Lee, K.P.; Zheng, J.; Bargeman, G.; Kemperman, A.J.B.; Benes, N.E. pH stable thin film composite polyamine nanofiltration membranes by interfacial polymerisation. J. Membr. Sci. 2015, 478, 75–84. [Google Scholar] [CrossRef]
- Zhu, Y.; Dou, P.; He, H.; Lan, H.; Xu, S.; Zhang, Y.; He, T.; Niu, J. Improvement of permeability and rejection of an acid resistant polysulfonamide thin-film composite nanofiltration membrane by a sulfonated poly(ether ether ketone) interlayer. Sep. Purif. Technol. 2020, 239, 116528. [Google Scholar] [CrossRef]
- Linder, C.; Kedem, O. History of nanofiltration membranes: 1960 to 1990. In Nanofiltration: Principles and Applications; Elsevier Advanced Technology: Oxford, UK, 2005; ISBN 9781856174053. [Google Scholar]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters—A review. Sep. Purif. Technol. 2018, 198–220. [Google Scholar] [CrossRef]
- Product Specification—Nadir® NP010 P Flat Sheet Nanofiltration Membrane. Available online: https://www.microdyn-nadir.com/wp-content/uploads/NP010-P-Flat-Sheet-Membrane.pdf (accessed on 16 October 2020).
- Product Specification—Nadir® NP030 P Flat Sheet Nanofiltration Membrane. Available online: https://www.microdyn-nadir.com/wp-content/uploads/NP030-P-Flat-Sheet-Membrane.pdf (accessed on 16 October 2020).
- Boussu, K.; Van der Bruggen, B.; Volodin, A.; Van Haesendonck, C.; Delcour, J.A.; Van der Meeren, P.; Vandecasteele, C. Characterization of commercial nanofiltration membranes and comparison with self-made polyethersulfone membranes. Desalination 2006, 191, 245–253. [Google Scholar] [CrossRef]
- Daems, N.; Milis, S.; Verbeke, R.; Szymczyk, A.; Pescarmona, P.P.; Vankelecom, I.F.J. High-performance membranes with full pH-stability. RSC Adv. 2018, 8, 8813–8827. [Google Scholar] [CrossRef]
- HYDRACoRe70pHT Series—Product Data Sheet. Available online: http://membranes.com/wp-content/uploads/2017/02/HYDRACoRe70pHT-Series.pdf. (accessed on 16 October 2020).
- Zhang, Y.; Guo, M.; Yan, H.; Pan, G.; Xu, J.; Shi, Y.; Liu, Y. Novel organic–inorganic hybrid composite membranes for nanofiltration of acid and alkaline media. RSC Adv. 2014, 4, 57522–57528. [Google Scholar] [CrossRef]
- Duracid Series—SUEZ—Water Technology & Solutions. Available online: https://pdfslide.net/documents/duracid-series-suez-water-technologies-solutions-mar-16-water-technologies-solutions.html (accessed on 16 October 2020).
- Schlesinger, R.; Götzinger, G.; Sixta, H.; Friedl, A.; Harasek, M. Evaluation of alkali resistant nanofiltration membranes for the separation of hemicellulose from concentrated alkaline process liquors. Desalination 2006, 192, 303–314. [Google Scholar] [CrossRef]
- Data Sheet—SelRO® MPS-34—pH Stable Membrane. Available online: https://www.kochseparation.com/wp-content/uploads/2020/10/Selro-NF-MPS-34-2-5-and-4-inch-elements.pdf (accessed on 16 October 2020).
- Data Sheet—SelRO® MPS-36—pH Stable Membrane. Available online: https://www.kochseparation.com/wp-content/uploads/2020/10/Selro-NF-MPS-36-2-5-and-4-inch-elements.pdf (accessed on 16 October 2020).
- Product Data Sheet—FILMTECTM NF270-400/34i Element. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/45-D01699-en.pdf (accessed on 16 October 2020).
- Conidi, C.; Cassano, A.; Drioli, E. A membrane-based study for the recovery of polyphenols from bergamot juice. J. Membr. Sci. 2011, 375, 182–190. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Zhang, L.; Yu, J.Q. An acid resistant nanofiltration membrane prepared from a precursor of poly(s-triazine-amine) by interfacial polymerization. J. Membr. Sci. 2018, 546, 225–233. [Google Scholar] [CrossRef]
- Dalwani, M.; Bargeman, G.; Hosseiny, S.S.; Boerrigter, M.; Wessling, M.; Benes, N.E. Sulfonated poly(ether ether ketone) based composite membranes for nanofiltration of acidic and alkaline media. J. Membr. Sci. 2011, 381, 81–89. [Google Scholar] [CrossRef]
- Lopez, J.; Reig, M.; Gibert, O.; Valderrama, C.; Cortina, J.L. Evaluation of NF membranes as treatment technology of acid mine drainage: Metals and sulfate removal. Desalination 2018, 440, 122–134. [Google Scholar] [CrossRef]
- Al-Zoubi, H.; Rieger, A.; Steinberger, P.; Pelz, W.; Haseneder, R.; Härtel, G. Optimization Study for Treatment of Acid Mine Drainage Using Membrane Technology. Sep. Sci. Technol. 2010, 45, 2004–2016. [Google Scholar] [CrossRef]
- Aguiar, A.O.; Andrade, L.H.; Ricci, B.C.; Pires, W.L.; Miranda, G.A.; Amaral, M.C.S. Gold acid mine drainage treatment by membrane separation processes: An evaluation of the main operational conditions. Sep. Purif. Technol. 2016, 170, 360–369. [Google Scholar] [CrossRef]
- Chesters, S.; Morton, P.; Fazel, M. Membranes and minewater—Waste or revenue stream. In Proceedings of the IMWA, Freiberg, Germany, 11–15 July 2016; Carsten, D., Michael, P., Eds.; pp. 1310–1322. Available online: https://www.imwa.info/docs/imwa_2016/IMWA2016_Chesters_93.pdf (accessed on 19 November 2020).
- Meschke, K.; Daus, B.; Haseneder, R.; Repke, J.-U. Strategic elements from leaching solutions by nanofiltration—Influence of pH on separation performance. Sep. Purif. Technol. 2017, 184, 264–274. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Fan, Z.; Yang, X.; Wang, J.; Wang, S. Experimental study on treatment of electroplating wastewater by nanofiltration. J. Membr. Sci. 2007, 305, 185–195. [Google Scholar] [CrossRef]
- Wei, X.; Kong, X.; Wang, S.; Xiang, H.; Wang, J.; Chen, J. Removal of Heavy Metals from Electroplating Wastewater by Thin-Film Composite Nanofiltration Hollow-Fiber Membranes. Ind. Eng. Chem. Res. 2013, 52, 17583–17590. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Nazif, A.; Alaei Shahmirzadi, M.A.; Ortiz, I. Fabrication, tuning and optimization of poly (acrilonitryle) nanofiltration membranes for effective nickel and chromium removal from electroplating wastewater. Sep. Purif. Technol. 2017, 187, 46–59. [Google Scholar] [CrossRef]
- Siew, Y.W.; Zedda, K.L.; Velizarov, S. Nanofiltration of Simulated Acid Mine Drainage: Effect of pH and Membrane Charge. Appl. Sci. 2020, 10, 400. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Yaroshchuk, A.; Licon, E.; Gibert, O.; Cortina, J.L. Experimental and theoretical study of nanofiltration of weak electrolytes: SO42–/HSO4–/H+ system. J. Membr. Sci. 2018, 550, 389–398. [Google Scholar] [CrossRef]
- Rinsing. Available online: http://www.plating.com/platingtechnical/rinsing.html (accessed on 19 November 2020).
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metals Toxicity and the Environment. EXS 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Dalwani, M.; Benes, N.E.; Bargeman, G.; Stamatialis, D.; Wessling, M. A method for characterizing membranes during nanofiltration at extreme pH. J. Membr. Sci. 2010, 363, 188–194. [Google Scholar] [CrossRef]
- Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics; European Commission: Sevilla, Spain, 2006; Available online: https://eippcb.jrc.ec.europa.eu/sites/default/files/2019-11/stm_bref_0806.pdf (accessed on 19 November 2020).
- Schöngen, G. Chromium (III)—A Genuine Alternative to Chromium (VI)? Available online: https://www.kiesow.co.uk/fileadmin/redakteure/dokumente/1_Englische_Dokumente/News/SAPHIR_2000_JOT_issue_November_2014.pdf (accessed on 19 November 2020).
- List of Substances Included in Annex XIV of REACH. Available online: https://echa.europa.eu/authorisation-list (accessed on 19 November 2020).
- Schneider, A.C.; Pasel, C.; Luckas, M.; Schmidt, K.G.; Herbell, J.-D. Determination of Hydrogen Single Ion Activity Coefficients in Aqueous HCl Solutions at 25 °C. J. Solut. Chem. 2004, 33, 257–273. [Google Scholar] [CrossRef]
- Maghsoudy-Louyeh, S.; Ju, H.S.; Tittmann, B.R. Surface roughness study in relation with hydrophilicity/hydrophobicity of materials using atomic force microscopy. AIP Conf. Proc. 2010, 1211, 1487–1492. [Google Scholar] [CrossRef]
- Mänttäri, M.; Pihlajamäki, A.; Nyström, M. Effect of pH on hydrophilicity and charge and their effect on the filtration efficiency of NF membranes at different pH. J. Membr. Sci. 2006, 280, 311–320. [Google Scholar] [CrossRef]
- Baek, Y.; Kang, J.; Theato, P.; Yoon, J. Measuring hydrophilicity of RO membranes by contact angles via sessile drop and captive bubble method: A comparative study. Desalination 2012, 303, 23–28. [Google Scholar] [CrossRef]
- Xu, F.; Wei, M.; Zhang, X.; Song, Y.; Zhou, W.; Wang, Y. How Pore Hydrophilicity Influences Water Permeability? Research 2019, 2019, 2581241. [Google Scholar] [CrossRef]
- Nicolini, J.V.; Borges, C.P.; Ferraz, H.C. Selective rejection of ions and correlation with surface properties of nanofiltration membranes. Sep. Purif. Technol. 2016, 171, 238–247. [Google Scholar] [CrossRef]
- Coday, B.D.; Luxbacher, T.; Childress, A.E.; Almaraz, N.; Xu, P.; Cath, T.Y. Indirect determination of zeta potential at high ionic strength: Specific application to semipermeable polymeric membranes. J. Membr. Sci. 2015, 478, 58–64. [Google Scholar] [CrossRef]
- Ernst, M.; Bismarck, A.; Springer, J.; Jekel, M. Zeta-potential and rejection rates of a polyethersulfone nanofiltration membrane in single salt solutions. J. Membr. Sci. 2000, 165, 251–259. [Google Scholar] [CrossRef]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Coronell, O.; González, M.I.; Mariñas, B.J.; Cahill, D.G. Ionization Behavior, Stoichiometry of Association, and Accessibility of Functional Groups in the Active Layers of Reverse Osmosis and Nanofiltration Membranes. Environ. Sci. Technol. 2010, 44, 6808–6814. [Google Scholar] [CrossRef] [PubMed]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Abri, M.; Al-Hinai, H.; Abu-Arabi, M. Characterization and retention of NF membranes using PEG, HS and polyelectrolytes. Desalination 2008, 221, 284–293. [Google Scholar] [CrossRef]
- Luo, J.; Wan, Y. Effects of pH and salt on nanofiltration—A critical review. J. Membr. Sci. 2013, 438, 18–28. [Google Scholar] [CrossRef]
- Vezzani, D.; Bandini, S. Donnan equilibrium and dielectric exclusion for characterization of nanofiltration membranes. Desalination 2002, 149, 477–483. [Google Scholar] [CrossRef]
- Visser, T.J.K.; Modise, S.J.; Krieg, H.M.; Keizer, K. The removal of acid sulphate pollution by nanofiltration. Desalination 2001, 140, 79–86. [Google Scholar] [CrossRef]
- Verbych, S.; Hilal, N.; Sorokin, G.; Leaper, M. Ion Exchange Extraction of Heavy Metal Ions from Wastewater. Sep. Sci. Technol. 2005, 39, 2031–2040. [Google Scholar] [CrossRef]






| Model | Material | Configuration | pH Range | Permeance () | Rejection (%) | Max T (°C) | Drawback | References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MWCO | NaCl | MgSO4 | Na2SO4 | ||||||||
| Microdyn-Nadir NP010 | Sulfonated PES | Asymmetric | 0–14 * | >5 * | 1000–1200 * | - | - | 35–75 * | - | High MWCO | [7] |
| Microdyn-Nadir NP030 | Sulfonated PES | Asymmetric | 0–14 | 1.7 | 520 | 30 | - | 80–95* | 95 | Low flux | [3,8,9,10] |
| Hydranautics/Nitto Denko HYDRA-CoRe 70pHT | Sulfonated PES | TFC | 1–13.5 * | 5.8 | 720 * | 70 | - | 97.6 | 70 * | High NaCl rejection | [11,12] |
| Osmonics/GE/Suez Duracid | Proprietary | TFC | 0–10 | 7 *−8 | 400 | - | 98 * | - | 70 * | Only for acid | [10,13] |
| Koch SELRO MPS−34 | Proprietary | Composite | 0–14 | 1.75 | 200 *−300 | 35 | - | - | 50–70 * | Low flux | [10,14,15] |
| Koch SELRO MPS−36 | Proprietary | Composite | 0–14 | 8 * | 1000 | 10 * | - | - | High MWCO | [3,16] | |
| Dupont Filmtec NF−270 | Polyamide | TFC | 3–10 * | 10.6 | 200–400 | - | >97 * | - | 45 * | Only mild pH | [3,17] |
| Inopor Nano | TiO2 (α-Al2O3 support) | Composite | 0–14 | 8.6 | 750 | - | - | - | 350 | Expensive and low packing density | [18] |
| 0.77 | 450 | - | - | - | 350 | [3,18] | |||||
| Material | pH Range | Permeance () | MWCO (Da) | Rejection (%) | IEP (pH) | Year | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | MgSO4 | Na2SO4 | MgCl2 | |||||||
| TCF with PS as support, SPEEK interlayer and polysulfonamide active layer | 0–7(Alkaline stability not reported) | 1.74 | 800 | 89 | 97 | 99.7 | 79 | 4.1 | 2020 | [4] |
| Polyvinylidene fluoride grafted with polystyrene sulfonic acid | 0–14 | 2.4 | <500 | 60 | 80 | - | - | <3 | 2018 | [10] |
| IP of 1,3,5-(tris-piperazine)-triazine and trimesoyl chloride on top of PS UF support | >1 | 9 | - | 40 | 97 | 98.6 | - | 3.5 | 2018 | [19] |
| Poly(vinyl alcohol)-aminopropyl triethoxysilane | 0–14 | 0.7 | - | 50–55 | - | 98.5 | - | - | 2014 | [12] |
| Polyamine on porous PES support | 1–13 | 2.5 | 500 | 65 | 40 | - | 90 | 7.5 | 2014 | [3] |
| SPEEK on PES UF support | 1–13 | 4.5 | 500 | 60 | - | 90 | - | <2 | 2011 | [20] |
| Chemicals | Manufacturer | Purity |
|---|---|---|
| MgSO4∙7H2O | Fluka Honeywell | ≥98% |
| CaSO4∙2H2O | Sigma Aldrich | ≥99% |
| NaCl | VWR | 100% |
| Na2SO4 | Sigma Aldrich | ≥99% |
| HCl | Merck | 37% |
| NaOH | Merck | 49–51% |
| H2SO4 | Merck | 95–97% |
| NiSO4∙6H2O | Alfa Aesar | >98% |
| Cr2(SO4)3∙H2O | Sigma Aldrich | For synthesis |
| ZnSO4∙7H2O | Alfa Aesar | >98% |
| CuSO4∙5H2O | Merck | For analysis |
| PEG 200/300/400/600/1000/2000/3000 MW | Merck | For synthesis |
| Ion | Wang 2007 [26] | Wei 2013 [27] | Target of This Work | |
|---|---|---|---|---|
| Cations (ppm) | Na+ | 13.8 | 653.8 | 657 |
| K+ | 1.9 | 105.7 | --- | |
| NH4+ | 34.0 | --- | --- | |
| Ca2+ | 16.0 | 76.9 | 77 | |
| Mg2+ | 0.2 | 19.9 | 20 | |
| Zn2+ | 0.6 | 14.8 | 15 | |
| Mn2+ | 0.1 | --- | --- | |
| Ni2+ | 0.8 | 146.7 | 147 | |
| Cu2+ | 11.8 | 57.8 | 58 | |
| Total chromium | 17.1 | 123.5 | 124 | |
| Anions (ppm) | F− | 232.5 | --- | --- |
| Cl− | 27.5 | 943.8 | 1013 | |
| NO3− | 100.8 | 64.8 | --- | |
| NO2− | --- | 38.5 | --- | |
| HCO3− | --- | --- | --- | |
| SO42− | 415.8 | 971.0 | 957 | |
| pH | 2.32 | 2.20 | 2.00 | |
| Heavy Metal | Salt | pH | Feed Concentration at Spontaneous pH | Feed Ionic Strength (mmol/L) | ||
|---|---|---|---|---|---|---|
| Metal (ppm) | Metal (mmol/L) | Sulfate (mmol/L) | ||||
| Cu2+ | CuSO4 | 5.05 | 145 | 2.3 | 2.3 | 9.2 |
| Zn2+ | ZnSO4 | 5.60 | 153 | 2.4 | 2.4 | 9.6 |
| Ni2+ | NiSO4 | 5.70 | 135 | 2.3 | 2.3 | 9.2 |
| Cr3+ | Cr2(SO4)3 | 3.23 | 50 | 1.0 | 1.5 | 7.5 |
| Ion | Feed pH 3.25 | Feed pH 2 | ||
|---|---|---|---|---|
| Theoretical Amount (ppm) | Result of Analysis (ppm) | Theoretical Amount (ppm) | Result of Analysis (ppm) | |
| Na+ | 657 | 690 | 657 | 680 |
| Ca2+ | 77 | 78 | 77 | 80 |
| Mg2+ | 20 | 21 | 20 | 22 |
| Zn2+ | 15 | 16 | 15 | 16 |
| Ni2+ | 147 | 150 | 147 | 160 |
| Cu2+ | 58 | 56 | 58 | 59 |
| Cr3+ | 124 | 93 | 124 | 95 |
| Cl− | 1013 | 900 | 1013 | 910 |
| SO42− total | 957 | 820 | 1801 | 1650 |
| Single-Salt | Model EPWW | |||
|---|---|---|---|---|
| Spontaneous pH | pH 2 | Both pH Conditions | ||
| Cations | Na+ | Conductivity | Charge balance | ICP-OES |
| Ca2+ | Conductivity | UV–Vis (LCK-327) | ||
| Mg2+ | Conductivity | UV–Vis (LCK-326) | ||
| Zn2+ | Conductivity | UV–Vis (LCK-360) | ||
| Ni2+ | Conductivity | UV–Vis (LCK-337) | ||
| Cu2+ | Conductivity | UV–Vis (LCK-329) | ||
| Cr3+ | ICP-OES | ICP-OES | ||
| Anions | Cl− | Conductivity | Automated photometric analyzer | Ion chromatography |
| SO42− | Conductivity | UV–Vis (LCK−153) | ||
| Membrane | Contact Angle (°) | Thickness (μm) | ||
|---|---|---|---|---|
| Mean Value | Standard Deviation | Mean Value * | Standard Deviation | |
| A | 64.3 | 3.9 | 261 | 5 |
| B | 53.6 | 5.8 | 229 | 6 |
| C | 36.4 | 4.0 | 144 | 3 |
| Membrane | Parameter | Initial Standard pH 7 | PEG pH 5.35 | PEG pH 2 | Final Standard pH 7 |
|---|---|---|---|---|---|
| A | MgSO4 Rejection (%) | 93.2 ± 0.6 | --- | --- | 89.0 ± 0.4 |
| MWCO (Da) | --- | ca. 510 | ca. 490 | --- | |
| A () | 1.07 ± 0.04 | 1.25 ± 0.01 | 1.40 ± 0.05 | 1.57 ± 0.05 | |
| B | MgSO4 Rejection (%) | 66.3 ± 0.8 | --- | --- | 61.6 ± 0.6 |
| MWCO (Da) | --- | ca. 680 | ca. 880 | --- | |
| A () | 4.04 ± 0.14 | 3.64 ± 0.10 | 3.56 ± 0.09 | 3.75 ± 0.09 |
| Ions | Electroplating Mixture | |||||
|---|---|---|---|---|---|---|
| Spontaneous pH | pH 2 (+H2SO4) | |||||
| C Feed | Membrane Rejection (%) | C Feed | Membrane Rejection (%) | |||
| mmol/L | A | B | mmol/L | A | B | |
| Na+ | 30 | 29 | 20.3 | 29.6 | 27.9 | 14.7 |
| Ca2+ | 1.9 | 66.7 | 32.1 | 2 | 77.5 | 28.8 |
| Mg2+ | 0.9 | 66.2 | 28.6 | 0.9 | 79.5 | 27.3 |
| Zn2+ | 0.2 | 65 | 31.3 | 0.2 | 76.3 | 25 |
| Ni2+ | 2.6 | 68 | 26.7 | 2.7 | 80 | 31.3 |
| Cu2+ | 0.9 | 60.7 | 32.1 | 0.9 | 72.9 | 30.5 |
| Cr3+ | 1.8 | 81.7 | 32.3 | 1.8 | 87.4 | 43.2 |
| Cl− | 25.4 | 14.4 | 0 | 25.7 | 14.3 | 2.2 |
| SO42− total | 8.5 | 90.5 | 59.8 | 17.2 | 58.8 | 39.4 |
| Feed | Perm A | Perm B | Feed | Perm A | Perm B | |
| pH | 3.3 | 3.5 | 3.4 | 2 | 2 | 2.08 |
| K (µS/cm) | 4500 | 3000 | 3700 | 8500 | 7100 | 7200 |
| Permeance () | --- | 0.81 ± 0.19 | 5.24 ± 0.4 | --- | 0.84 ± 0.19 | 4.73 ± 0.37 |
| Heavy Metal | Concentration (ppm) | |||
|---|---|---|---|---|
| Discharge Limit [33] | Model EPWW | Permeate A | Permeate B | |
| Chromium | 0.05 | 95 | 12 | 54 |
| Copper | 0.25 | 59 | 16 | 41 |
| Nickel | 0.2 | 160 | 32 | 110 |
| Zinc | 0.8 | 16 | 4 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegoburu, I.; Zedda, K.L.; Velizarov, S. Treatment of Electroplating Wastewater Using NF pH-Stable Membranes: Characterization and Application. Membranes 2020, 10, 399. https://doi.org/10.3390/membranes10120399
Hegoburu I, Zedda KL, Velizarov S. Treatment of Electroplating Wastewater Using NF pH-Stable Membranes: Characterization and Application. Membranes. 2020; 10(12):399. https://doi.org/10.3390/membranes10120399
Chicago/Turabian StyleHegoburu, Ignacio, Karina Listiarini Zedda, and Svetlozar Velizarov. 2020. "Treatment of Electroplating Wastewater Using NF pH-Stable Membranes: Characterization and Application" Membranes 10, no. 12: 399. https://doi.org/10.3390/membranes10120399
APA StyleHegoburu, I., Zedda, K. L., & Velizarov, S. (2020). Treatment of Electroplating Wastewater Using NF pH-Stable Membranes: Characterization and Application. Membranes, 10(12), 399. https://doi.org/10.3390/membranes10120399