Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering
Abstract
1. Introduction
2. Articular Cartilage and Clinical Strategies for Treatment
2.1. Articular Cartilage: Characteristics, Roles, Joint Diseases, and Traumatic Lesions
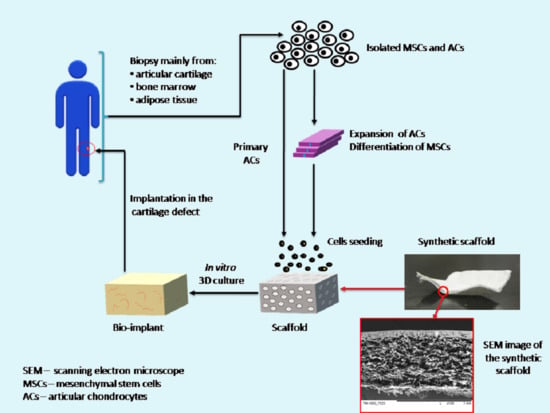
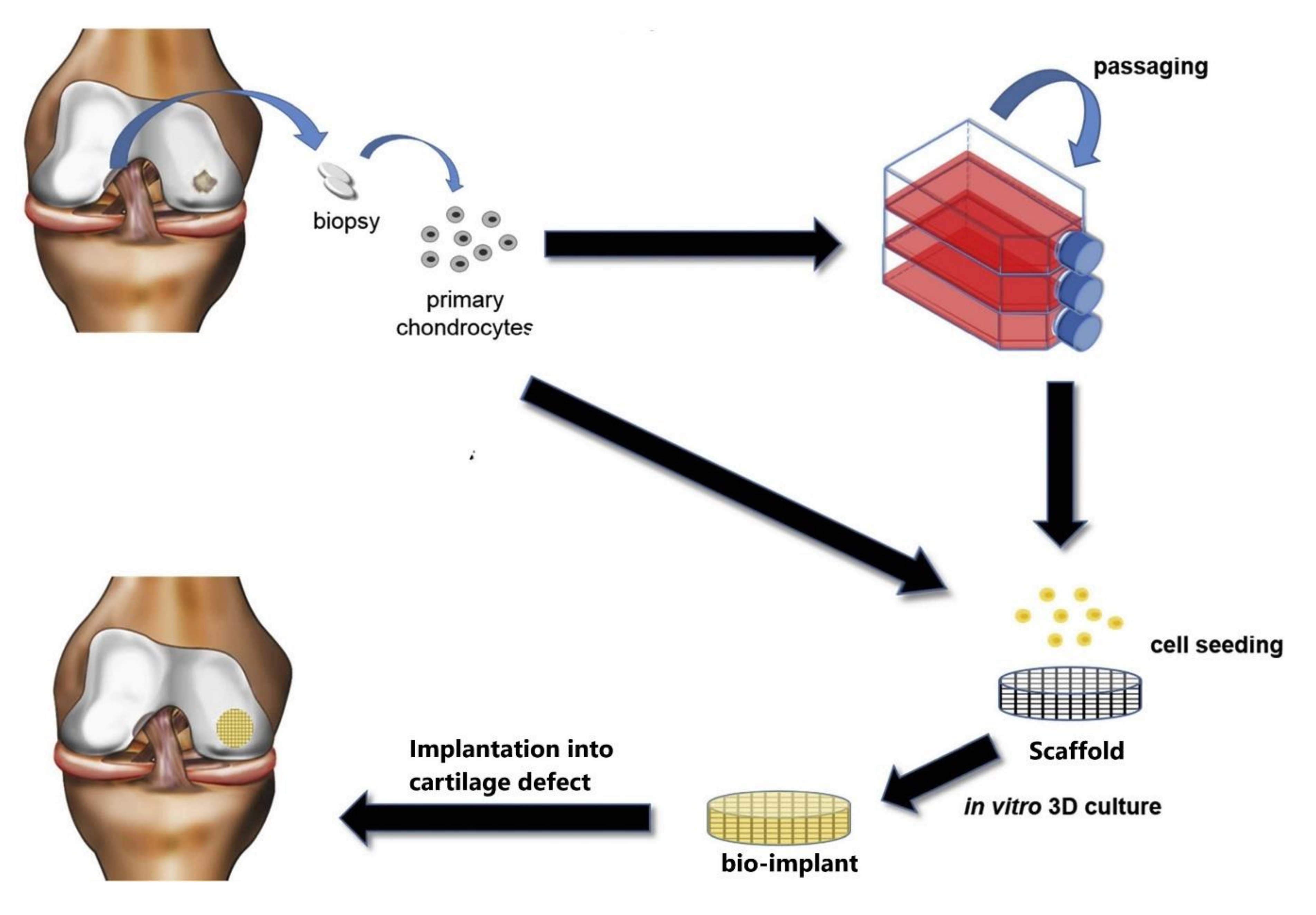
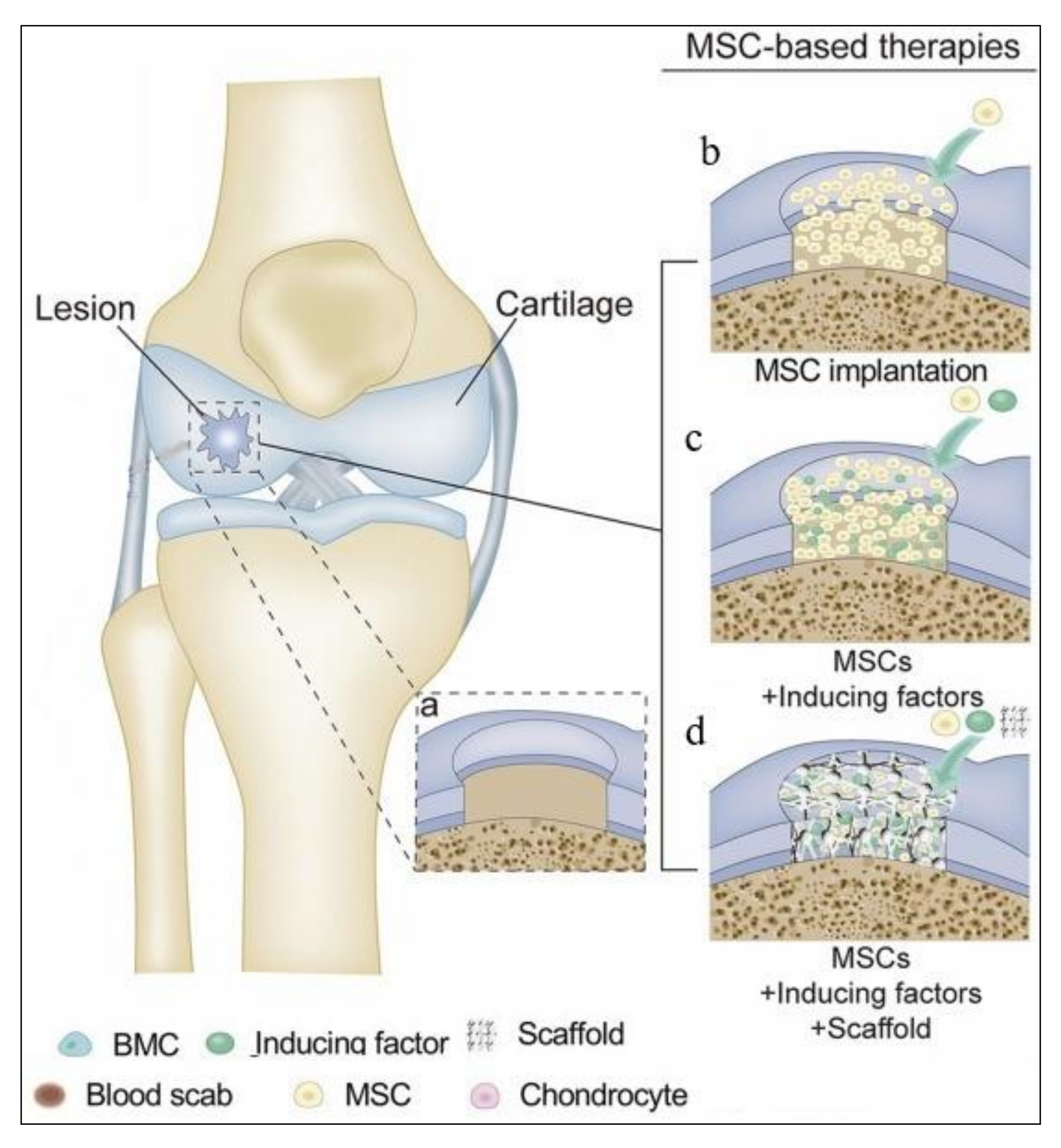
2.2. Treatment Methods for Cartilage Regeneration
3. Scaffold for Articular Cartilage Repair: Requirements, Materials, and Method for Obtaining
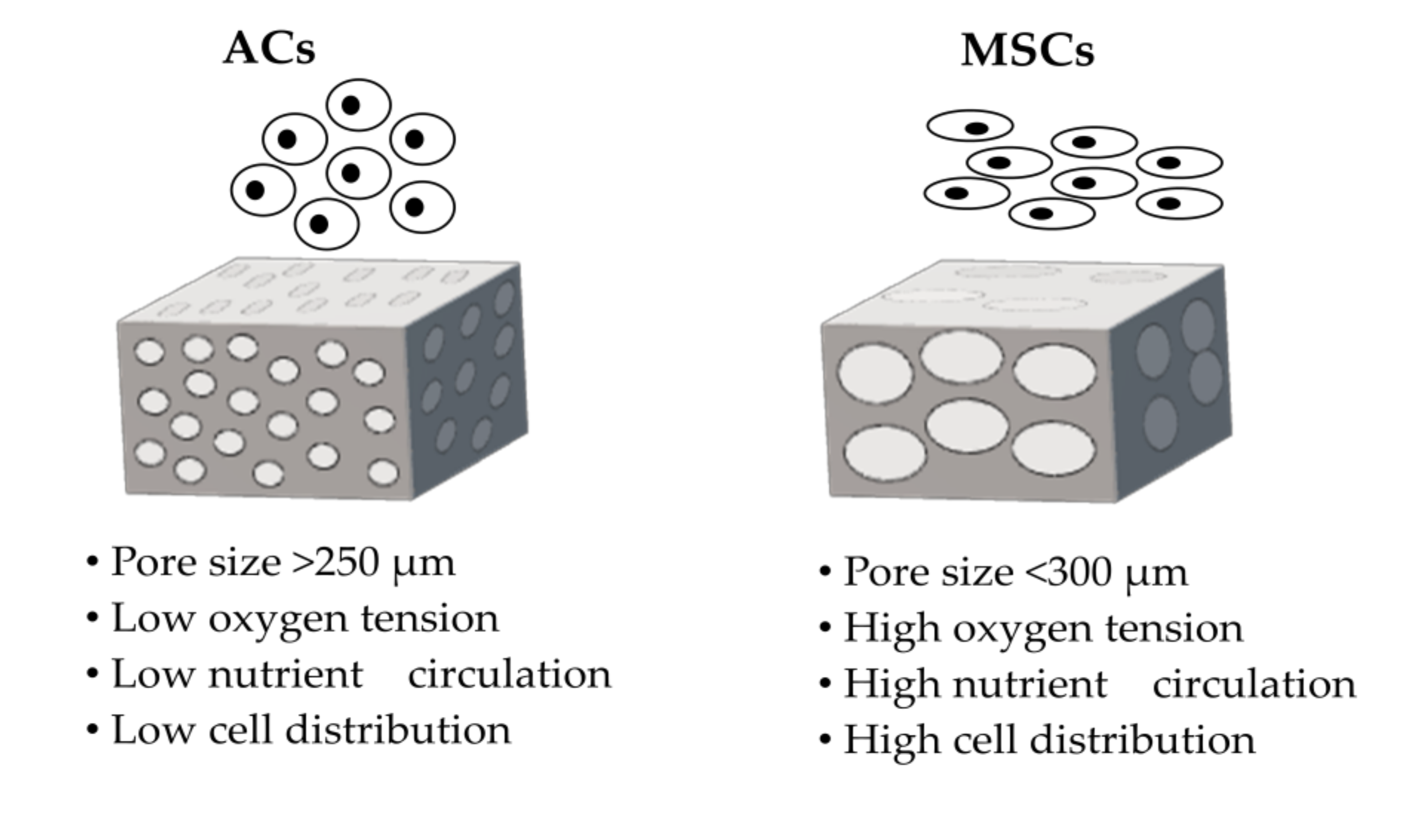
3.1. Requirements for Scaffolds
3.2. Materials Intended for Scaffolds
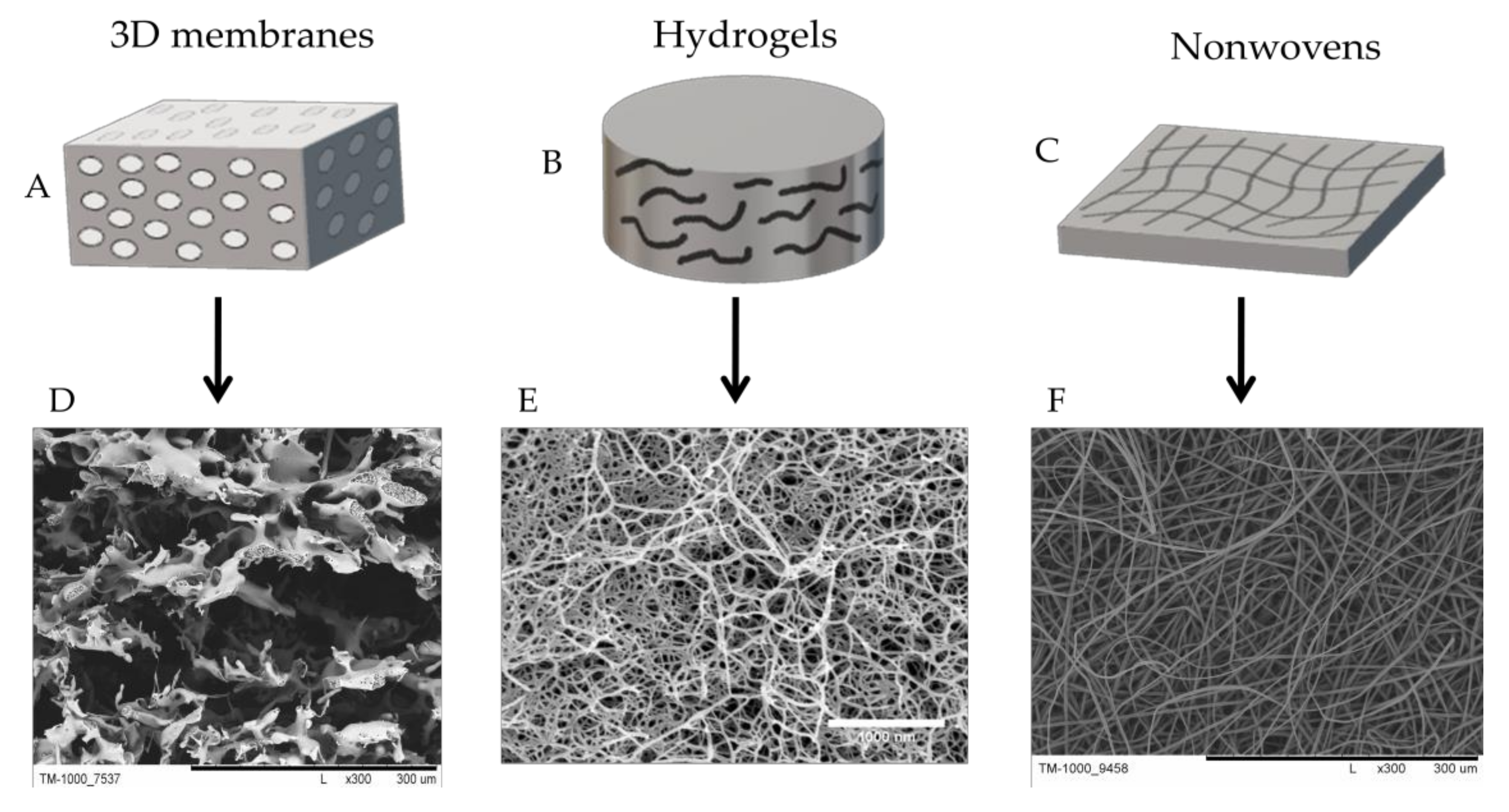
3.3. Methods for Obtaining Scaffolds
4. Scaffolds for Cartilage Treatment
4.1. Natural Scaffolds
4.2. Hydrogel Scaffolds
4.3. Synthetic Scaffolds
4.4. Hybrid Scaffolds
5. Conclusions
Funding
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Duan, B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Scarritt, M.E.; Pashos, N.C.; Bunnell, B.A. A review of cellularization strategies for tissue engineering of whole organs. Front. Bioeng. Biotechnol. 2015, 3, 43. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Giwa, S.; Lewis, J.K.; Alvarez, L.; Langer, R.; Roth, A.E.; Church, G.M.; Markmann, J.F.; Sachs, D.H.; Chandraker, A.; Wertheim, J.A.; et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 2017, 35, 530–542. [Google Scholar] [CrossRef]
- Popoola, J.; Greene, H.; Kyegombe, M.; MacPhee, I.A. Patient involvement in selection of immunosuppressive regimen following transplantation. Patient Prefer. Adherence 2014, 8, 1705–1712. [Google Scholar] [CrossRef]
- Feinberg, A.W. Engineered tissue grafts: Opportunities and challenges in regenerative medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 207–220. [Google Scholar] [CrossRef]
- Park, K.M.; Shin, Y.M.; Kim, K.; Shin, H. Tissue Engineering and Regenerative Medicine 2017: A Year in Review. Tissue Eng. Part B Rev. 2018, 24, 327–344. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. Organ engineering—Combining stem cells, biomaterials, and bioreactors to produce bioengineered organs for transplantation. BioEssays 2013, 35, 163–172. [Google Scholar] [CrossRef]
- Mazza, G.; Al-Akkad, W.; Rombouts, K.; Pinzani, M. Liver tissue engineering: From implantable tissue to whole organ engineering. Hepatol. Commun. 2018, 2, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Alexander, J.F.; Thekkedath, U.; Ferrari, M.; Grattoni, A. Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev. 2019, 139, 92–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications; Springer: Singapore, 2019; Volume 11, ISBN 0123456789. [Google Scholar]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L. Fabrication of scaffolds in tissue engineering: A review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Walter, S.G.; Ossendorff, R.; Schildberg, F.A. Articular cartilage regeneration and tissue engineering models: A systematic review. Arch. Orthop. Trauma Surg. 2019, 139, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fan, C.; Chen, F.; Sun, Y.; Xia, Y.; Ji, A.; Wang, D.A. Progress in Articular Cartilage Tissue Engineering: A Review on Therapeutic Cells and Macromolecular Scaffolds. Macromol. Biosci. 2020, 20, 1900278. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Collins, K.H.; Herzog, W.; Macdonald, G.Z.; Reimer, R.A. Obesity, Metabolic Syndrome, and Musculoskeletal Disease: Common Inflammatory Pathways Suggest a Central Role for Loss of Muscle Integrity. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Ph, D.; Vullings, J.; van de Loo, F.A.J.; Ph, D. Osteoporosis and osteoarthritis are two sides of the same coin paid for obesity. Nutrition 2020, 70, 110486. [Google Scholar] [CrossRef]
- Accadbled, F.; Vial, J.; Gauzy, J.S. De Osteochondritis dissecans of the knee. Orthop. Traumatol. Surg. Res. 2018, 104, S97–S105. [Google Scholar] [CrossRef]
- Barendregt, A.M.; Mazzoli, V.; Van Den Berg, J.M.; Kuijpers, T.W.; Maas, M. T 1 ρ-mapping for assessing knee joint cartilage in children with juvenile idiopathic arthritis—Feasibility and repeatability. Pediatric Radiol. 2020, 50, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Brody, L.T. Knee osteoarthritis: Clinical connections to articular cartilage structure and function. Phys. Ther. Sport 2015, 16, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.I.; Argyle, D.J.; Clements, D.N. In vitro models for the study of osteoarthritis. Vet. J. 2016, 209, 40–49. [Google Scholar] [CrossRef]
- Appleton, C.T. Osteoarthritis year in review 2017: Biology. Osteoarthr. Cartil. 2018, 26, 296–303. [Google Scholar] [CrossRef]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage—Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef]
- Bs, C.S.; Kweon, C.Y. Classifications in Brief: Outerbridge Classification of Chondral Lesions. Clin. Orthop. Relat. Res. 2018, 2101–2104. [Google Scholar] [CrossRef]
- Posadzy, M.; Desimpel, J.; Vanhoenacker, F. Staging of Osteochondral Lesions of the Talus: MRI and Cone Beam CT. J. Belg. Soc. Radiol. 2017. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Mirza, U.; Shubeena, S.; Shah, M.S.; Zaffer, B. Microfracture: A technique for repair of chondral defects. J. Entomol. Zool. Stud. 2018, 6, 1092–1097. [Google Scholar]
- Dunkin, B.S.; Lattermann, C. New and emerging techniques in cartilage repair: Matrix-induced autologous chondrocyte implantation. Oper. Tech. Sports Med. 2013, 21, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M. Symposium Scaffold based Autologous Chondrocyte Implantation: The Surgical Technique. Asian J. Arthrosc. 2019, 4, 23–26. [Google Scholar] [CrossRef]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.F. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am. J. Sports Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J. Orthop. Res. 2018, 36, 52–63. [Google Scholar] [CrossRef]
- Mastrolia, I.; Foppiani, E.M.; Murgia, A.; Candini, O.; Samarelli, A.V.; Grisendi, G.; Veronesi, E.; Horwitz, E.M.; Dominici, M. Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem Cells Transl. Med. 2019, 8, 1135–1148. [Google Scholar] [CrossRef]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Lam, A.T.L.; Reuveny, S.; Oh, S.K.W. Human mesenchymal stem cell therapy for cartilage repair: Review on isolation, expansion, and constructs. Stem Cell Res. 2020, 44, 101738. [Google Scholar] [CrossRef]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Et Biophys. Acta Gen. Subj. 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Ahmadi, F.; Giti, R.; Mohammadi-Samani, S.; Mohammadi, F. Biodegradable Scaffolds for Cartilage Tissue Engineering. Galen Med. J. 2017, 6, 70–80. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, J.; Han, J.; Zhang, W.; Ma, J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am. J. Transl. Res. 2019, 11, 6275–6289. [Google Scholar] [PubMed]
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Choi, Y.J.; Kwon, O.R. Second-Look Arthroscopic Evaluation of Cartilage Lesions after Mesenchymal Stem Cell Implantation in Osteoarthritic Knees. Am. J. Sports Med. 2014, 42, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.L.; Duchi, S.; Onofrillo, C.; Bella, C.D.; Choong, P.F.M. Adipose-Derived Mesenchymal Stem Cells in the Use of Cartilage Tissue Engineering: The Need for a Rapid Isolation Procedure. Stem Cells Int. 2018, 2018, 13–16. [Google Scholar] [CrossRef]
- Augustyniak, E.; Trzeciak, T. The role of growth factors in stem cell-directed chondrogenesis: A real hope for damaged cartilage regeneration. Int. Orthop. 2015, 39, 995–1003. [Google Scholar] [CrossRef]
- Kalkan, R.; Nwekwo, C.W.; Adali, T. The Use of Scaffolds in Cartilage Regeneration. Eukaryot. Gene Expr. 2018, 28, 343–348. [Google Scholar] [CrossRef]
- Panadero, J.A.; Lanceros-Mendez, S.; Ribelles, J.L.G. Differentiation of mesenchymal stem cells for cartilage tissue engineering: Individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016, 33, 1–12. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019. [Google Scholar] [CrossRef]
- Okubo, R.; Asawa, Y.; Watanabe, M.; Nagata, S.; Nio, M. Proliferation medium in three-dimensional culture of auricular chondrocytes promotes effective cartilage regeneration in vivo. Regen. Ther. 2019, 11, 306–315. [Google Scholar] [CrossRef]
- Takahashi, T.; Ogasawara, T.; Asawa, Y.; Mori, Y.; Uchinuma, E.; Takato, T.; Hoshi, K. Three-Dimensional Microenvironments Retain Chondrocyte Phenotypes During Proliferation Culture. Tissue Eng. 2007, 13, 1583–1592. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G.; De Souza, P.; Villegas Castrejon, H.; John, T.; Merker, H.J.; Scheid, A.; Shakibaei, M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002, 308, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Lee, J.A.; Kim, Y.S.; Lee, H.Y.; Kim, H.J.; Kang, K.T. Optimal mechanical properties of a scaffold for cartilage regeneration using finite element analysis. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Irawan, V.; Sung, T.C.; Higuchi, A.; Ikoma, T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Chen, W.C.; Huang, C.H.; Cheng, C.K.; Chan, K.K.; Chang, T.K. The effect of graft strength on knee laxity and graft in-situ forces after posterior cruciate ligament reconstruction. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Conoscenti, G.; Schneider, T.; Stoelzel, K.; Carfì Pavia, F.; Brucato, V.; Goegele, C.; La Carrubba, V.; Schulze-Tanzil, G. PLLA scaffolds produced by thermally induced phase separation (TIPS) allow human chondrocyte growth and extracellular matrix formation dependent on pore size. Mater. Sci. Eng. C 2017, 80, 449–459. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, K.; Zhou, Y.; Ye, Z.; Tan, W.S. A combinatorial variation in surface chemistry and pore size of three-dimensional porous poly(ε-caprolactone) scaffolds modulates the behaviors of mesenchymal stem cells. Mater. Sci. Eng. C 2016, 59, 193–202. [Google Scholar] [CrossRef]
- Nava, M.M.; Draghi, L.; Giordano, C.; Pietrabissa, R. The effect of scaffold pore size in cartilage tissue engineering. J. Appl. Biomater. Funct. Mater. 2016, 14, e223–e229. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 2015, 21, 486–497. [Google Scholar] [CrossRef]
- Chwojnowski, A.; Kruk, A.; Wojciechowski, C.; Łukowska, E.; Dulnik, J.; Sajkiewicz, P. The dependence of the membrane structure on the non-woven forming the macropores in the 3D scaffolds preparation. Desalin. Water Treat. 2017, 64, 324–331. [Google Scholar] [CrossRef]
- Kruk, A.; Gadomska-Gajadhur, A.; Ruśkowski, P.; Chwojnowski, A.; Dulnik, J.; Synoradzki, L. Preparation of biodegradable semi-permeable membranes as 3D scaffolds for cell cultures. Desalin. Water Treat. 2017, 64, 317–323. [Google Scholar] [CrossRef]
- Przytulska, M.; Kulikowski, J.L.; Wasyłeczko, M.; Chwojnowski, A.; Piętka, D. The evaluation of 3D morphological structure of porous membranes based on a computer-aided analysis of their 2D images. Desalin. Water Treat. 2018, 128. [Google Scholar] [CrossRef]
- Sikorska, W.; Wojciechowski, C.; Przytulska, M.; Rokicki, G.; Wasyłeczko, M.; Kulikowski, J.L.; Chwojnowski, A. Polysulfone–polyurethane (PSf-PUR) blend partly degradable hollow fiber membranes: Preparation, characterization, and computer image analysis. Desalin. Water Treat. 2018, 128. [Google Scholar] [CrossRef]
- Malik, T.; Razzaq, H.; Razzaque, S.; Nawaz, H.; Siddiqa, A.; Siddiq, M.; Qaisar, S. Design and synthesis of polymeric membranes using water-soluble pore formers: An overview. Polym. Bull. 2019, 76, 4879–4901. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Rares, H.; Benea, C.; Earar, K.; Lattanzi, W.; Quercia, V.; Berce, C.; Mohan, A. Collagen Scaffold and Lipoaspirate Fluid—Derived Stem Cells for the Treatment of Cartilage Defects in a Rabbit Model. Rev. Chim. 2015, 69, 515–520. [Google Scholar]
- Gobbi, A.; Whyte, G.P. Long-term Clinical Outcomes of One-Stage Cartilage Repair in the Knee With Hyaluronic Acid—Based Scaffold Embedded With Mesenchymal Stem Cells Sourced From Bone Marrow Aspirate Concentrate. Am. J. Sports Med. 2019, 47, 1621–1628. [Google Scholar] [CrossRef]
- Lin, H.; Beck, A.M.; Fritch, M.R.; Tuan, R.S.; Deng, Y.; Kilroy, E.J.; Tang, Y.; Alexander, P.G. Optimization of photocrosslinked gelatin/hyaluronic acid hybrid scaffold for the repair of cartilage defect. J. Tissue Eng. Regen. Med. 2019, 13, 1418–1429. [Google Scholar] [CrossRef]
- Wen, S.; Hung, K.; Hsieh, K.; Chen, C.; Tsai, C.; Hsu, S. In vitro and in vivo evaluation of chitosan—Gelatin scaffolds for cartilage tissue engineering. Mater. Sci. Eng. C 2013, 33, 2855–2863. [Google Scholar] [CrossRef]
- Mittal, H.; Sinha, S.; Singh, B.; Kaur, J.; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Chen, S.; Chen, W.; Chen, Y.; Mo, X.; Fan, C. Chondroitin sulfate modified 3D porous electrospun nano fi ber scaffolds promote cartilage regeneration. Mater. Sci. Eng. C 2020, 118, 1–12. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, X.; Cai, D.; Li, J.; Mu, Q.; Zhang, W.; Zhu, S. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Drobnic, M.; Perdisa, F.; Kon, E.; Hribernik, M.; Marcacci, M. Fibrin glue improves osteochondral scaffold fi xation: Study on the human cadaveric knee exposed to continuous passive motion. Osteoarthr. Cartil. 2014, 22, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hyung, T.; Lim, D.; Yeon, S.; Lee, Y.; Koh, Y.I. Biochemical and Biophysical Research Communications Chondrogenic differentiation of human ASCs by stiffness control in 3D fi brin hydrogel. Biochem. Biophys. Res. Commun. 2020, 522, 213–219. [Google Scholar] [CrossRef]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Fan, C.; Wang, D. A biodegradable PEG-based micro-cavitary hydrogel as scaffold for cartilage tissue engineering. Eur. Polym. J. 2015, 72, 651–660. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nourbakhsh, M.S. International Journal of Polymeric Materials and Electrospun polycaprolactone scaffolds for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2018, 1–13. [Google Scholar] [CrossRef]
- Dao, T.T.; Vu, N.B.; Pham, L.H.; Bui, T.; Le, P.T.; Van Pham, P. In Vitro Production of Cartilage Tissue from Rabbit Bone Marrow-Derived Mesenchymal Stem Cells and Polycaprolactone Scaffold. Adv. Exp. Med. Biol. 2017, 1804, 45–60. [Google Scholar] [CrossRef]
- Singhvi, M.S. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2012, 127, 1612–1626. [Google Scholar] [CrossRef]
- Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-roitman, J.; Schroeder, A. Mini Review Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Wen, Y.; Dai, N.; Hsu, S. Acta Biomaterialia Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019, 88, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.; Tseng, C.; Hsu, S. Synthesis and 3D Printing of Biodegradable Polyurethane Elastomer by a Water-Based Process for Cartilage Tissue Engineering Applications. Adv. Healthc. Mater. 2014, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Budak, K.; Sogut, O.; Sezer, U.A. A review on synthesis and biomedical applications of polyglycolic acid. J. Polym. Res. 2020, 27, 1–19. [Google Scholar] [CrossRef]
- Mahboudi, H.; Soleimani, M.; Enderami, S.E.; Kehtari, M.; Hanaee-Ahvaz, H.; Ghanbarian, H.; Bandehpour, M.; Nojehdehi, S.; Mirzaei, S.; Kazemi, B. The effect of nanofibre-based polyethersulfone (PES) scaffold on the chondrogenesis of human induced pluripotent stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1948–1956. [Google Scholar] [CrossRef]
- Dudziński, K.; Chwojnowski, A.; Gutowska, M.; Płończak, M.; Czubak, J.; Łukowska, E.; Wojciechowski, C. Three dimensional polyethersulphone scaffold for chondrocytes cultivation—The future supportive material for articular cartilage regeneration. Biocybern. Biomed. Eng. 2010, 30, 65–76. [Google Scholar]
- Plończak, M.; Czubak, J.; Hoser, G.; Chwojnowskl, A.; Kawiak, J.; Dudzińskp, K.; Czumińska, K. Repair of articular cartilage full thickness defects with cultured chondrocytes placed on polysulphonic membrane—Experimental studies in rabbits. Biocybern. Biomed. Eng. 2008, 28, 87–93. [Google Scholar]
- Irfan, M.; Idris, A. Overview of PES biocompatible/hemodialysis membranes: PES-blood interactions and modification techniques. Mater. Sci. Eng. C 2015, 56, 574–592. [Google Scholar] [CrossRef]
- Filimon, A.; Olaru, N.; Doroftei, F.; Logigan, C.; Dunca, S. Design of Biologically Active Surfaces Based on Functionalized Polysulfones by Electrospinning. Proceedings 2019, 41, 35. [Google Scholar] [CrossRef]
- Filimon, A.; Avram, E.; Dunca, S. Surface and Interface Properties of Functionalized Polysulfones: Cell-Material Interaction and Antimicrobial Activity. Polym. Eng. Sci. 2015, 55, 2184–2894. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester elastomers for soft tissue engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Progress in Polymer Science Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Janoušková, O. Synthetic Polymer Scaffolds for Soft Tissue Engineering. Physiol. Res. 2018, 67, S335–S348. [Google Scholar] [CrossRef]
- He, Y.; Wang, W.R.; Ding, J.D. Effects of L-lactic acid and D,L-lactic acid on viability and osteogenic differentiation of mesenchymal stem cells. Chin. Sci. Bull. 2013, 58, 2404–2411. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug—Polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1692–1716. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, L.; Ma, B.; Ding, H.; Tang, C. Effect of component and surface structure on poly (L-lactide-co-ε-caprolactone) (PLCA)-based composite membrane. Compos. Part B 2019, 174, 107031. [Google Scholar] [CrossRef]
- Prasanna, S.; Narayan, B.; Rastogi, A.; Srivastava, P. Design and evaluation of chitosan/poly (L-lactide)/pectin based composite scaffolds for cartilage tissue regeneration. Int. J. Biol. Macromol. 2018, 112, 909–920. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Jeuken, R.M.; Roth, A.K.; Peters, R.J.R.W.; van Donkelaar, C.C.; Thies, J.C.; van Rhijn, L.W.; Emans, P.J. Polymers in cartilage defect repair of the knee: Current status and future prospects. Polymers 2016, 8, 219. [Google Scholar] [CrossRef]
- Daranarong, D.; Techaikool, P.; Intatue, W.; Daengngern, R.; Thomson, K.A.; Molloy, R.; Kungwan, N.; Foster, L.J.R.; Boonyawan, D.; Punyodom, W. Effect of surface modification of poly(L-lactide-co-ε-caprolactone) membranes by low-pressure plasma on support cell biocompatibility. Surf. Coat. Technol. 2016, 306, 328–335. [Google Scholar] [CrossRef]
- Guo, C.; Cai, N.; Dong, Y. Duplex surface modification of porous poly (lactic acid) scaffold. Mater. Lett. 2013, 94, 11–14. [Google Scholar] [CrossRef]
- Tsurumi, T.; Fuse, M. Enhancement of apatite precipitation on an alkaline hydrolyzed poly (lactic acid-ε-Caprolactone) film in simulated body fluid. J. Hard Tissue Biol. 2014, 23, 15–19. [Google Scholar] [CrossRef][Green Version]
- Setayeshmehr, M.; Esfandiari, E.; Rafieinia, M.; Hashemibeni, B.; Taheri-kafrani, A.; Samadikuchaksaraei, A. Hybrid and Composite Scaffolds Based on Extracellular. Tissue Eng. Part B Rev. 2019, 25, 202–224. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Pan, Z.; Sun, H.; Wang, J.; Yu, D.; Zhu, S.; Dai, J.; Chen, Y.; Tian, N.; et al. The effects of lactate and acid on articular chondrocytes function: Implications for polymeric cartilage scaffold design. Acta Biomater. 2016, 42, 329–340. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques of fabrication and construction three-dimensional scaffold. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef]
- Mannella, G.A.; Conoscenti, G.; Pavia, F.C.; Carrubba, V.L.; Brucato, V. Preparation of polymeric foams with a pore size gradient via Thermally Induced Phase Separation (TIPS). Mater. Lett. 2015, 160, 31–33. [Google Scholar] [CrossRef]
- Buzarovska, A.; Gualandi, C.; Parrilli, A.; Scandola, M. Effect of TiO2 nanoparticle loading on Poly(L-lactic acid) porous scaffolds fabricated by TIPS. Compos. Part B 2015. [Google Scholar] [CrossRef]
- Sultana, N.; Hassana, M.I.; Ridzuana, N.; Ibrahima, Z.; Soonc, C.F. Fabrication of Gelatin Scaffolds using Thermally Induced Phase Separation Technique. Int. J. Eng. 2018, 31, 1302–1307. [Google Scholar] [CrossRef]
- Structure, M.M.; Mechanical, H.; Kim, J.; Shin, K.; Koh, Y.; Hah, M.J.; Moon, J.; Kim, H. Production of Poly(ε-Caprolactone)/Hydroxyapatite Composite Scaffolds with a Tailored Macro/Micro-Porous Structure, High Mechanical Properties, and Excellent Bioactivity. Materials 2017, 10, 1123. [Google Scholar] [CrossRef]
- Georgiadou, S.; Katsogiannis, K.A.G.; Vladisavljevic, G.T. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Huang, C.; Thomas, N.L. Fabricating Porous Poly(lactic acid) Fibres via Electrospinning. Eur. Polym. J. 2017. [Google Scholar] [CrossRef]
- Prasad, A.; Sankar, M.R.; Katiyar, V. ScienceDirect State of Art on Solvent Casting Particulate Leaching Method for Orthopedic Scaffolds Fabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Plisko, T.V.; Penkova, A.V.; Burts, K.S.; Bildyukevich, A.V.; Dmitrenko, M.E.; Melnikova, G.B.; Atta, R.R.; Mazur, A.S.; Zolotarev, A.A.; Missyul, A.B. Effect of Pluronic F127 on porous and dense membrane structure formation via non-solvent induced and evaporation induced phase separation. J. Membr. Sci. 2019, 580, 336–349. [Google Scholar] [CrossRef]
- Gadomska-Gajadhur, A.; Kruk, A.; Ruśkowski, P.; Sajkiewicz, P.; Dulnik, J.; Chwojnowski, A. Original method of imprinting pores in scaffolds for tissue engineering. Polym. Adv. Technol. 2020, 1–13. [Google Scholar] [CrossRef]
- Taylor, P.; Yang, Q.; Chen, L.; Shen, X.; Tan, Z. Preparation of Polycaprolactone Tissue Engineering Scaffolds by Improved Solvent Casting/Particulate Leaching Method Preparation of Polycaprolactone Tissue Engineering Scaffolds by Improved Solvent Casting/Particulate Leachin. J. Macromol. Sci. Part B Phys. 2006, 45, 1171–1181. [Google Scholar] [CrossRef]
- Sharifi, F.; Irani, S.; Azadegan, G.; Pezeshki-Modaress, M. Bioactive Carbohydrates and Dietary Fibre Co-electrospun gelatin-chondroitin sulfate/polycaprolactone nanofibrous scaffolds for cartilage tissue engineering. Bioact. Carbohydr. Diet. Fibre 2020, 22, 100215. [Google Scholar] [CrossRef]
- Zhou, Y.; Chyu, J.; Zumwalt, M. Recent Progress of Fabrication of Cell Scaffold by Electrospinning Technique for Articular Cartilage Tissue Engineering. Int. J. Biomater. 2018, 2018. [Google Scholar] [CrossRef]
- Girão, A.F.; Semitela, Â.; Ramalho, G.; Completo, A.; Marques, P.A.A.P. Mimicking nature- Fabrication of 3D anisotropic electrospun polycaprolactone scaffolds for cartilage tissue engineering applications. Compos. Part B 2018. [Google Scholar] [CrossRef]
- Bdikin, I.; Marques, P.A.A.P. Electrospinning of bioactive polycaprolactone-gelatin nanofibres with increased pore size for cartilage tissue engineering applications. J. Biomater. Appl. 2020, 35, 459–470. [Google Scholar] [CrossRef]
- Wang, Z.; Wan, L.; Liu, Z.; Huang, X.; Xu, Z. Enzymatic Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B 2009, 56, 189–195. [Google Scholar] [CrossRef]
- Yang, Z.; Si, J.; Cui, Z.; Ye, J.; Wang, X.; Wang, Q.; Peng, K.; Chen, W.; Chen, S. Biomimetic composite scaffolds based on surface modification of polydopamine on electrospun poly (lactic acid)/cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Canton, T.T.; Kunert, L.R.; Suellen, B.; Ana, I.; Serafini, P. Nonwoven membranes for tissue engineering: An overview of cartilage, Nonwoven membranes for tissue engineering: An overview of cartilage, epithelium, and bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1026–1049. [Google Scholar] [CrossRef]
- Abdelaal, O.A.M.; Darwish, S.M.H. Review of Rapid Prototyping Techniques for Tissue Engineering Scaffolds Fabrication. In Characterization and Development of Biosystems and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2013; Volume 29, pp. 33–54. [Google Scholar] [CrossRef]
- Li, K.; Wang, D.; Zhao, K.; Song, K.; Liang, J. Electrohydrodynamic jet 3D printing of PCL/PVP composite scaffold for cell culture. Talanta 2020, 120750. [Google Scholar] [CrossRef]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700298. [Google Scholar] [CrossRef]
- Seung, J.; Sang, H.; Jung, H.; Lee, H.; Hong, H.; Jin, Y.; Ji, Y.; Joo, O.; Hee, S.; Hum, C. 3D-printable photocurable bioink for cartilage regeneration of tonsil-derived mesenchymal stem cells. Addit. Manuf. 2020, 33, 101136. [Google Scholar] [CrossRef]
- Marycz, K.; Smieszek, A.; Targonska, S.; Walsh, A.; Szustakiewicz, K.; Wiglusz, R.J. Three dimensional (3D) printed PLA with nano-hydroxyapatite doped with europium(III) ions (nHAp-PLLA@Eu3+) composite for osteochondral defect regeneration and theranostics. Mater. Sci. Eng. C 2020, 110634. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Zheng, J.; Pan, L.; Liu, X. Fabrication and improvement of PCL/alginate/PAAm scaffold via selective laser sintering for tissue engineering. Micro Nano Lett. 2019, 14, 852–855. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Tomaschke, A.; Kleinjan, E.; Muralidharan, A.; Pascual-Garrido, C.; Mcleod, R.R.; Ferguson, V.L.; Bryant, S.J. A Stereolithography-Based 3D Printed Hybrid Scaffold for In Situ Cartilage Defect Repair. Macromol. Biosci. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.; Woo, J.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Biomaterials Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef] [PubMed]
- Chartrain, N.A.; Williams, C.B.; Whittington, A.R. A review on fabricating tissue scaffolds using vat photopolymerization. Acta Biomater. 2018, 74, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.; Ferreira, A.M.; Gentile, P. Recent Approaches to the Manufacturing of Biomimetic Multi-Phasic Scaffolds for Osteochondral Regeneration. Int. J. Mol. Sci. 2018, 19, 1755. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K. Improving the Impact Strength and Heat Resistance of 3D Printed Models: Structure, Property, and Processing Correlationships during Fused Deposition Modeling (FDM) of Poly(Lactic Acid). ACS Omega 2018. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H.; Antonio, S.; et al. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2018, 25, 14–29. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.; Liu, Y.; Wang, Z.; Li, Y.; Jiang, G.; Mo, X.; Zhou, G. Three-dimensional printed electrospun fi ber-based scaffold for cartilage regeneration. Mater. Des. 2019, 179, 107886. [Google Scholar] [CrossRef]
- Garrigues, N.W.; Little, D.; Sanchez-Adams, J.; Ruch, D.S.; Guilak, F. Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2014, 59784, 28–30. [Google Scholar] [CrossRef]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013, 5, 1–11. [Google Scholar] [CrossRef]
- Nielsen, L.E. Polymer Reviews Cross-Linking—Effect on Physical Properties of Polymers. J. Macromol. Sci. Part C 2008, 3, 69–103. [Google Scholar] [CrossRef]
- Rofiqoh, N.; Putri, E.; Wang, X.; Chen, Y.; Li, X.; Kawazoe, N.; Chen, G. Preparation of PLGA-collagen hybrid scaffolds with controlled pore structures for cartilage tissue engineering. Prog. Nat. Sci. Mater. Int. 2020. [Google Scholar] [CrossRef]
- Laurent, P. Suitability of a PLCL fibrous scaffold for soft tissue engineering applications: A combined biological and mechanical characterisation. J. Biomater. Appl. 2018, 32, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Bistolfi, A.; Ferracini, R.; Galletta, C.; Tosto, F.; Sgarminato, V.; Digo, E.; Vernè, E.; Massè, A. Regeneration of articular cartilage: Scaffold used in orthopedic surgery. A short handbook of available products for regenerative joints surgery. Clin. Sci. Res. Rep. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Müller, S. Repair of Focal Cartilage Defects With Scaffold-Assisted Autologous Chondrocyte Grafts. Am. J. Sports Med. 2011, 39, 1697–1705. [Google Scholar] [CrossRef]
- Tsai, M.; Hung, K.; Hung, S.; Hsu, S. Evaluation of biodegradable elastic scaffolds made of anionic polyurethane for cartilage tissue engineering. Colloids Surf. B Biointerfaces 2015, 125, 34–44. [Google Scholar] [CrossRef]
- Borsøe, B.; Casper, C.; Foldager, B.; Møller, O.; Lind, M. A novel nano-structured porous polycaprolactone scaffold improves hyaline cartilage repair in a rabbit model compared to a collagen type I/III scaffold: In vitro and in vivo studies. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1192–1204. [Google Scholar] [CrossRef]
- Theodoridis, K.; Aggelidou, E.; Vavilis, T.; Manthou, M.E.; Tsimponis, A.; Demiri, E.C.; Boukla, A.; Salpistis, C. Hyaline cartilage next generation implants from adipose—Tissue—Derived mesenchymal stem cells: Comparative study on 3D—Printed polycaprolactone scaffold patterns. J. Tissue Eng. Regen. Med. 2019, 342–355. [Google Scholar] [CrossRef]
- Płończak, M.; Czubak, J. Culture of Human Autologous Chondrocytes on Polysulphonic Membrane—Preliminary Studies. Biocybern. Biomed. Eng. 2012, 32, 63–67. [Google Scholar] [CrossRef]
- Płończak, M. The Value of Autogenous Cartilage Cell Transplants in the Experimental Treatment of Articular Cartilage Defects in Rabbits. Ph.D. Thesis, Medical Centre of Postgraduate Education in Warsaw, Warsaw, Poland, 28 May 2008. [Google Scholar]
- Taylor, P.; Jung, Y.; Kim, S.H.; You, H.J. Application of an elastic biodegradable poly (L-lactide-co-ε-caprolactone) scaffold for cartilage tissue regeneration. J. Biomater. Sci. Polym. Ed. 2012, 1073–1085. [Google Scholar] [CrossRef]
- Siclari, A.; Mascaro, G.; Kaps, C.; Boux, E. A 5-Year Follow-Up After Cartilage Repair in the Knee Using a Platelet—Rich Plasma-Immersed Polymer-Based Implant. Open Orthop. J. 2014, 8, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Alizadeh, E.; Rahmani, A.; Bakhshayesh, D.; Mostafavi, E.; Akbarzadeh, A.; Davaran, S. Fabrication and in Vitro Evaluation of Nanocomposite Hydrogel Sca ff olds Based on Gelatin/PCL—PEG—PCL for Cartilage Tissue Engineering. ACS Omega 2019, 4, 449–457. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.; Guan, L.; Chen, J.; Duan, L.; Jia, Z.; Huang, J.; Li, W.; Liu, J.; Xiong, J.; et al. A 3D-Printed PLCL Scaffold Coated with Collagen Type I and Its Biocompatibility. Biomed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haaparanta, A.; Ja, E.; Fatih, I.; Ville, C.; Kiviranta, I.; Kelloma, M. Preparation and characterization of collagen/PLA, chitosan/PLA, and collagen/chitosan/PLA hybrid scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Nogami, M.; Kimura, T.; Seki, S.; Matsui, Y.; Yoshida, T.; Koike-Soko, C.; Okabe, M.; Motomura, H.; Gejo, R.; Nikaido, T. A human amnion derived extracellular matrix coated cell free scaffold for cartilage repair: In vitro and in vivo studies. Tissue Eng. Part A 2016, 22, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Yamamoto, K.; Sakai, Y.; Suda, Y.; Shigemitsu, Y. Seeding of mesenchymal stem cells into inner part of interconnected porous biodegradable scaffold by a new method with a filter paper. Dent. Mater. J. 2015, 34, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shyu, V.B.; Chen, J.; Lee, M. Selective laser sintered poly-ε-caprolactone scaffold hybridized with collagen hydrogel for cartilage tissue engineering. Biofabrication 2014, 015004. [Google Scholar] [CrossRef]
- Taylor, P.; Li, C.; Wang, L.; Yang, Z.; Kim, G. A Viscoelastic Chitosan-Modified Three-Dimensional Porous Poly (L-Scaffold for Cartilage Tissue Engineering. Biomater. Sci. 2012, 405–424. [Google Scholar] [CrossRef]
- Liao, J.; Qu, Y.; Chu, B.; Zhang, X.; Qian, Z. Biodegradable CSMA/PECA/Graphene Porous Hybrid Scaffold for Cartilage Tissue Engineering. Sci. Rep. 2015, 5, 9879. [Google Scholar] [CrossRef]
- Urbanek, O.; Kołbuk, D.; Wróbel, M. International Journal of Polymeric Materials and Articular cartilage: New directions and barriers of scaffolds development—Review. Int. J. Polym. Mater. Polym. Biomater. 2018, 1–15. [Google Scholar] [CrossRef]
- Tan, S.I.; Jun, S.; Tho, W.; Tho, K.S. Biological resurfacing of grade IV articular cartilage ulcers in knee joint with Hyalofast. J. Orthop. Surg. 2020, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sofu, H.; Camurcu, Y.; Ucpunar, H.; Ozcan, S.; Yurten, H.; Sahin, V. Clinical and radiographic outcomes of chitosan-glycerol phosphate / blood implant are similar with hyaluronic acid-based cell-free scaffold in the treatment of focal osteochondral lesions of the knee joint. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from Trial for Safety and Results From a a Clinical Clinical Trial for Safety and Concept. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar]
- Negoro, T.; Takagaki, Y.; Okura, H.; Matsuyama, A. Trends in clinical trials for articular cartilage repair by cell therapy. NPJ Regen. Med. 2018. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Ren, K.; He, C.; Xiao, C.; Li, G.; Chen, X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds for cartilage tissue engineering. Biomaterials 2015, 51, 238–249. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, J.; Koshut, W.J.; Watt, J.; Riboh, J.C.; Gall, K.; Wiley, B.J. A Synthetic Hydrogel Composite with the Mechanical Behavior and Durability of Cartilage. Adv. Funct. Mater. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Rosenzweig, D.H.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-printed ABS and PLA scaffolds for cartilage and nucleus pulposustissue regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef]
- Cristian, M.; Conde, M.; Demarco, F.F.; Alcazar, J.C.; Nör, J.E.; Beatriz, S. Influence of Poly-L-Lactic Acid Scaffold ’ s Pore Size on the Proliferation and Differentiation of Dental Pulp Stem Cells. Braz. Dent. J. 2015, 26, 93–98. [Google Scholar]
- Oh, S.H.; Kim, T.H.; Im, G.I.; Lee, J.H. Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using a pore size gradient scaffold. Biomacromolecules 2010, 11, 1948–1955. [Google Scholar] [CrossRef]
- Moura, C.S.; Silva, J.C.; Fernandes, P.R.; Lobato, C.; Manuel, J.; Cabral, S.; Linhardt, R.; Bártolo, P.J.; Ferreira, F.C. Chondrogenic differentiation of mesenchymal stem/stromal cells on 3D porous poly (ε-caprolactone) scaffolds: Effects of material alkaline treatment and chondroitin sulfate supplementation. J. Biosci. Bioeng. 2020, 129, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Sonomoto, K.; Yamaoka, K.; Kaneko, H.; Yamagata, K. Spontaneous Differentiation of Human Mesenchymal Stem Cells on Poly-Lactic-Co-Glycolic Acid Nano-Fiber Scaffold. PLoS ONE 2016, 11, e0153231. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Liu, L.; Luo, X.; Liu, Y.; Liu, Y.; Liu, F.; Wang, X. Repair of osteochondral defects with in vitro engineered cartilage based on autologous bone marrow stromal cells in a swine model. Sci. Rep. 2017, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Liu, K.; Shen, H.; Zhu, Y.; Zhang, W.; Liu, W.; Wang, S.; Cao, Y.; Zhou, G. The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials 2012, 33, 2926–2935. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Pan, Z.; Cao, L.; He, Y.; Wang, H.; Qu, Z.; Dong, J. The effects of pore size in bilayered poly (lactide- co -glycolide) scaffolds on restoring osteochondral defects in rabbits. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2013, 102, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Jonnalagadda, J.B.; Rivero, I.V.; Dertien, J.S. In vitro chondrocyte behavior on porous biodegradable poly (e-caprolactone)/polyglycolic acid scaffolds for articular chondrocyte adhesion and proliferation. J. Biomater. Sci. 2015, 37–41. [Google Scholar] [CrossRef]
- Safinsha, S.; Ali, M.M. Composite scaffolds in tissue engineering. Mater. Today Proc. 2020, 24, 2318–2329. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Guo, J.; Zhang, H.; Zhang, X.; Yin, C. 3D Molecularly Functionalized Cell-Free Biomimetic Scaffolds for Osteochondral Regeneration. Adv. Funct. Mater. 2019, 29, 1807356. [Google Scholar] [CrossRef]
- Setayeshmehr, M.; Esfandiari, E.; Hashemibeni, B.; Hossein, A. Chondrogenesis of human adipose-derived mesenchymal stromal cells on the [devitalized costal cartilage matrix/poly (vinyl alcohol)/fi brin] hybrid scaffolds. Eur. Polym. J. 2019, 118, 528–541. [Google Scholar] [CrossRef]
- Tavakoli, E.; Mehdikhani-Nahrkhalaji, M.; Hashemi-Beni, B.; Zargar-Kharazi, A. Preparation, Characterization and Mechanical Assessment of Poly (Lactide-Co-Glycolide)/Hyaluronic Acid/Fibrin/Bioactive Glass Nano-Composite Scaffolds for Cartilage Tissue Engineering Applications. Procedia Mater. Sci. 2015, 11, 124–130. [Google Scholar] [CrossRef]
- Mintz, B.R.; Cooper, A.C., Jr. Hybrid hyaluronic acid hydrogel/poly (e-caprolactone) scaffold provides mechanically favorable platform for cartilage tissue engineering studies. J. Biomed. Mater. Res. Part A 2013, 102, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fu, W.; Feng, B.; Wang, H.; Liu, Z.; Yin, M.; Wang, W. Electrospun collagen—poly (l-lactic acid-co-e-caprolactone) membranes for cartilage tissue engineering. Regen. Med. 2013, 8, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Paatela, T.; Meller, A.; Muhonen, V.; Salonius, E.; Haaparanta, A.; Elina, J. Articular Cartilage Repair With Recombinant Human Type II Collagen/Polylactide Scaffold in a Preliminary Porcine Study. J. Orthop. Res. 2016, 34, 745–753. [Google Scholar] [CrossRef]
- Levorson, E.J.; Hu, O.; Mountziaris, P.M.; Kasper, F.K.; Mikos, A.G. Cell Derived Polymer / Extracellular Matrix Composite Scaffolds for Cartilage Regeneration, Part 2: Construct Devitalization and Determination of Chondroinductive Capacity. Tissue Eng. Part C Methods 2014, 20, 358–372. [Google Scholar] [CrossRef] [PubMed]
| Grade of Damage | Description |
|---|---|
| Grade 0 | Normal AC with a smooth surface |
| Grade I | Soft and swollen cartilage with a reduced amount of proteoglycans and increased water content. |
| Grade II | The surface is cracked up to half the thickness of the cartilage, a so-called “Blemish” of cartilage. Swelling or fraying is visible via Magnetic Resonance Imaging (MRI) imaging. The area of the damage does not exceed 1.25 cm2 (less than 50%) of the surface. This corresponds to damage of an intermediate thickness. |
| Grade III | The damage exceeds half the thickness of the cartilage and may reveal the subchondral bone; the surface of the damage exceeds 1.25 cm2. The deep defect comprises more than 50%. |
| Grade IV | Full thickness defect(s). Destruction with complete exposure of the subchondral bone. |
| Technique | Advantages | Disadvantages |
|---|---|---|
| 3D printing (3DP) |
|
|
| Selective laser sintering (SLS) |
|
|
| Stereolithography (SLA) |
|
|
| Fused deposition modeling (FDM) |
|
|
| Bioprinting |
|
|
| Electrospinning |
|
|
| Freeze-drying |
|
|
| Thermal-induced phase separation (TIPS) |
|
|
| Solvent-casting particulate leaching (SCPL) |
|
|
| Scaffold Name [Ref.] | Component | Method | Properties (Porosity (%), Pore Size (µm), Mechanical Properties) | Cell Source/Animal Model | Results |
|---|---|---|---|---|---|
| Synthetic scaffolds | |||||
| BioSeed®-C (Biotissue) [156,157] | PGA/PLA, PDS | Thermoplastic process | Good mechanical properties and adequate structure for cells | Human articular chondrocytes | Assessed in clinical trials. In the results, the scaffolds featured significantly improved final postoperative values. This highlights their effectiveness in cartilage regeneration. |
| Spongy PU scaffold [158] | PU | Freeze-drying | 96.9% 126–186 µm Storage modulus: ~60.36 kPa | Chondrocytes, human MSCs | Biodegradable PU scaffold had better outcomes than PLA 3D membranes during culturing. |
| NSP-PCL scaffold [159] | PCL | Freeze-drying | The porosity of the scaffold was designed to promote cartilage ingrowth | Rabbit articular chondrocytes | The NSP-PCL scaffold indicated better results during in vitro and in vivo studies compared to the Chondro-Gide® scaffold. |
| RO45 3DHC [160] | PCL | 3D printing | RO45: 84.6% 135–285 µm Compressive modulus: 25.6 MPa 3DHC 83.8% 150–700 µm Compressive modulus: 3 MPa | Human adipose-derived MSCs | The RO45 scaffold was preferable for chondrogenic differentiation compared to 3DHC, which indicated better cell proliferation, scaffold penetration, and more favorable mechanical properties in the final construct. |
| Polysulphonic scaffold [97,98,161,162] | PES | Non-solvent induced phase separation and porogen- leaching | 98.5% 60–300 µm | Rabbit model and human articular chondrocytes | A study with a rabbit model suggested that the scaffold is effective in repairing articular cartilage defects. In vitro study with human cells gave promise results. |
| PLLA-100 scaffolds [66] | PLLA | Thermally induced phase separation | 93% 100 ± 20 μm | Human articular chondrocytes | The scaffold promoted the secretion of chondrogenic genes. It was better than the PLLA scaffold with larger pores (~200 μm). |
| PLCL-2 scaffold [163] | PLCL | Gel-pressing | 80% 300–500 µm Young’s modulus: ~0.7 MPa | Rabbit articular chondrocytes and mice model | The adequate structure of the scaffold showed that chondrocytes did not change their phenotypes during the in vitro study. The in vivo study indicated that the scaffold would maintain mechanical integrity and guide cartilaginous tissue formation. |
| Hybrid scaffold | |||||
| Chondrotissue® (Biotissue) [156,164] | PGA, HA | Freeze-drying | Platelet-rich plasma and bone marrow concentrate | The one-step cartilage repair method is available for clinical use. Treatment results follow up to 5 years of good outcomes with the potential for future benefits. | |
| IC scaffold [153] | PLGA, COL | Freeze-drying and cross-linking | 99.1% 50–400 µm Young’s modulus: ~9 kPa | Bovine articular chondrocytes (BACs) and mice model | IC scaffold promoted cartilaginous gene expression, chondrocyte proliferation, and the regeneration of cartilage tissue with high mechanical properties. It seems to be promising for cartilage tissue applications. |
| Gel/PCEC-TGFβ1 hydrogel scaffold [165] | Gelatin, PCEC, TGFβ1 | Cross-linking, freeze-drying | ~150 μm Young’s modulus: ~0.65 MPa | Human adipose tissue (AD)-MSCs | The study showed the potential for the growth and differentiation of h-AD-MSCs and could be a promising scaffold for cartilage tissue engineering. |
| PLCL-COLI [166] | PLCL, COL | 3D printing | ~85% ~10 μm; ~450 μm Young’s modulus: ~0.21 MPa | Rabbit articular chondrocytes | Scaffold with a controlled structure, good biocompatibility, elasticity, and mechanical properties, as well as potential in cartilage regeneration. |
| C2C1H scaffold [167] | PLA, COL, CH | Freeze-drying and melt-spun | >85% Young’s modulus: 52.3 kPa | Bovine articular cartilage chondrocytes | A hybrid scaffold with high porosity, good mechanical strength, and interconnected pore network. It has potential as a scaffold for cartilage tissue engineering. |
| ECM-PLGA scaffold [168,169] | PLGA, ECM | SCPL | 90% | Rat mesenchymal stem cells (MSCs) and rat model | The in vitro study showed good properties of attachment, proliferation, and differentiation of the MSCs. Involved the implantation of a cell with MSCs and type II collagen mRNA expression. The in vivo study indicated the regeneration of tissue to hyaline cartilage. The scaffold could be promising for cartilage regeneration therapy. |
| PCL/COL1 [170] | PCL, COL | Selective laser sintering | 82.98% Young’s modulus: 3.75 MPa | Pig articular chondrocytes and nude mice model | Scaffold with high porosity and repetitive pore structure. In vitro and in vivo study showed good outcomes compared to the PCL membrane. The addition of collagen ensured the proper development of chondrocytes. |
| CH/PLLA/PC scaffold [110] | PLLA, CH, PC | Freeze-drying and cross-linking | 79–84% 49–170 μm | Rabbit articular chondrocytes | Outcomes from the in vivo study showed the suitability of the scaffold for cartilage tissue regeneration. |
| Chitosan-modified PLCL scaffold [171] | PLCL, CH | Porogen-leaching, lyophilization, and cross-linking | ~85% 200–500 µm Young’s modulus: 0.04 MPa | Pig articular chondrocytes | Biodegradable scaffolds with high porosity, good mechanical strength, and interconnected pore structure. Supplied a good environment for chondrocyte adhesion, proliferation, differentiation, and ECM secretion. The results were good but still require further research. |
| CSMA/PECA/GO (S2) scaffold [172] | CSMA, MPEG-PCL-AC (PECA), GO | ~70% Mean 175.2 μm Compressive modulus: 0.48 MPa | Rabbit articular chondrocytes | Scaffold with an appropriate structure with biological components; provided an adequate environment for cells. The in vivo results were promising with great potential for the future. | |
| Product (Company) | Materials | Characteristic |
|---|---|---|
| Hyalofast®(Anika) [110,154,174,175] | Benzyl ester of hyaluronic acid | A bioresorbable3D scaffold used through a one-step procedure aftera microfracture. It can be used even for deep cartilage lesions. The scaffold’s non-woven structure allows it to be cut and adaptively matched into uneven lesions. |
| NeoCart®(Histogenics) [44,110,154] | Bovine type I collagen | Bioresorbableelectrospun scaffold used in MACI, a two-step procedure. The patient’s chondrocytes are expanded into scaffolds. Then, they are incubated in the Tissue Engineering Processor (TEP), which simulates the variation of mechanical forces and reduces oxygen pressure, allowing the maintenance of the chondrocyte phenotype forming the appropriate proteins of the ECM. |
| ChondroGide(Geistlich) [110,154] | Type I/III collagen | The first described matrix for the ACI method. It is used in a one-step procedure. ChondroGide’s role is to support and promote the chondrogenic differentiation of MSCs released after the microfracture method. |
| ACI-MaixTM (MACI) [44,45] | Type I/III collagen | The procedure is a two-step process. Expanded autologous chondrocytes (2 or 3 passage) are cultured into the scaffold for 3 or 4 days before implantation into the patient. |
| Cartipatch®(Xizia Biotech) [44,156,173] | Agarose and alginate | The cylindrical scaffold of a single layer of hydrogel with expanded cartilage cells. The clinical procedure is the same as that for the two-step method. The alginate polymer provides elasticity to the matrix, which facilitates handling during the surgical procedure. |
| NOVOCART® 3D—AesculapOrthopaedics (BBraun) [44,64,111,156] | Type I collagen, chondroitin sulfate | A sponge scaffold with a bilayer structure and interconnected pores, used in a two-step procedure. This scaffold is desirable in young patients,<16 years old, to avoid eventual secondary injuries, such as early osteoarthritis. |
| CaReS®(Arthrokinetics) [44,64,111,156] | Type I collagen gel | The scaffold is used in a two-step clinical procedure. Isolated autologous chondrocytes are mixed with a fluid matrix. Then, after 14 days, it is set in the lesion using fibrin glue. The height, thickness, and size of the hydrogel can be easily adjusted to the lesion. |
| CARTISTEM® (Medipost) [49,176,177] | Hyaluronic acid | Allogeneic human umbilical cord blood (hUCB)-derived MSCs and HA hydrogel products for cartilage regeneration for repeated traumas or degenerative osteoarthritis. A 7-year follow-up study of 104 patients showed promising efficacy in terms of durable cartilage regeneration with no significant adverse effects. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes 2020, 10, 348. https://doi.org/10.3390/membranes10110348
Wasyłeczko M, Sikorska W, Chwojnowski A. Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes. 2020; 10(11):348. https://doi.org/10.3390/membranes10110348
Chicago/Turabian StyleWasyłeczko, Monika, Wioleta Sikorska, and Andrzej Chwojnowski. 2020. "Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering" Membranes 10, no. 11: 348. https://doi.org/10.3390/membranes10110348
APA StyleWasyłeczko, M., Sikorska, W., & Chwojnowski, A. (2020). Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering. Membranes, 10(11), 348. https://doi.org/10.3390/membranes10110348