Cu(I/II) Metal–Organic Frameworks Incorporated Nanofiltration Membranes for Organic Solvent Separation
Abstract
1. Introduction
2. Experimental
2.1. Reagents
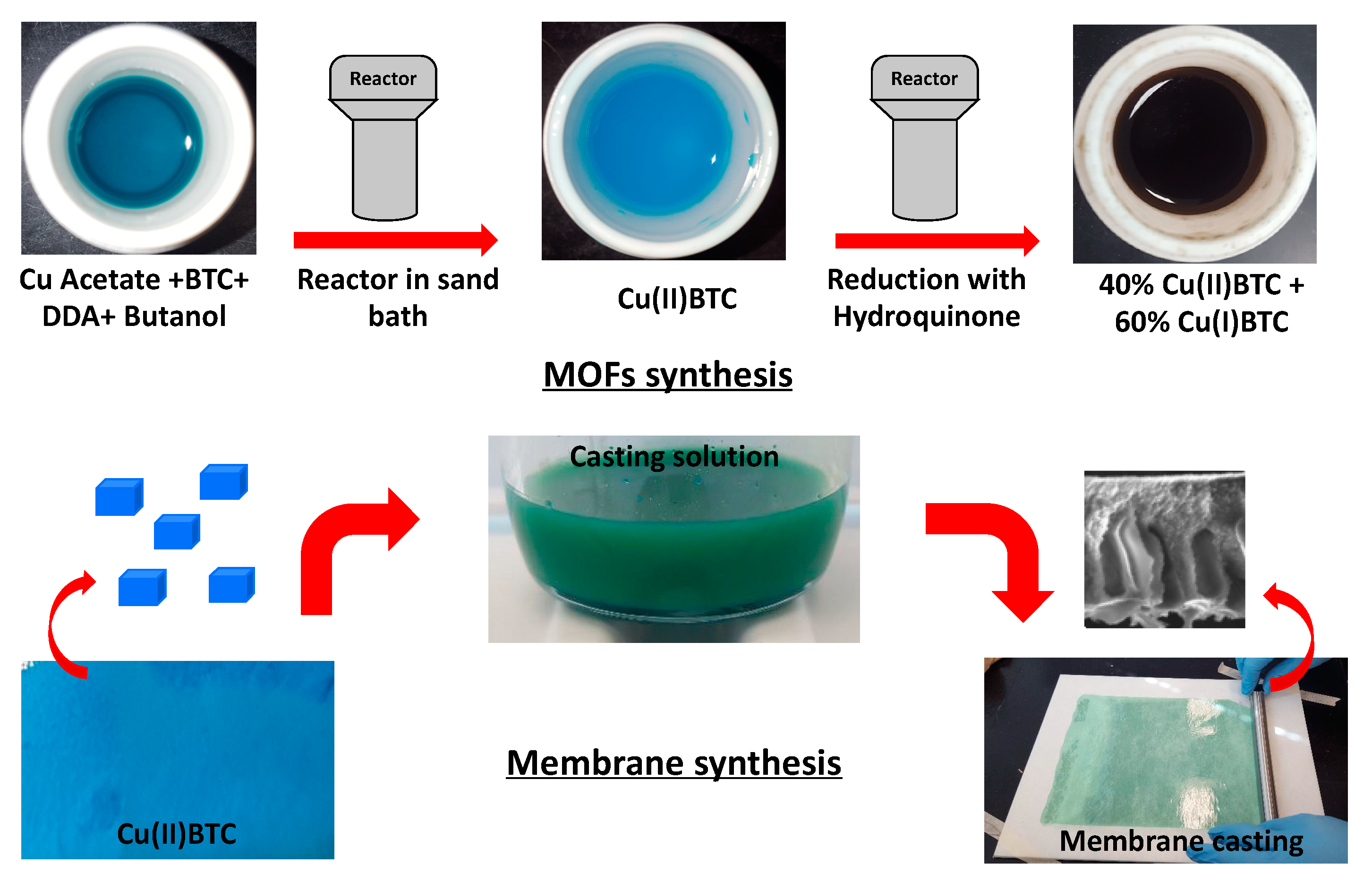
2.2. Synthesis of Cu(II)BTC
2.3. Reduction of Cu(II)BTC to Cu(I)BTC
2.4. Direct Synthesis of Cu(I)EDS
2.5. Synthesis of MMMs
2.6. Characterizations
2.7. Adsorption Studies
2.8. Membrane Performance
3. Results and Discussions
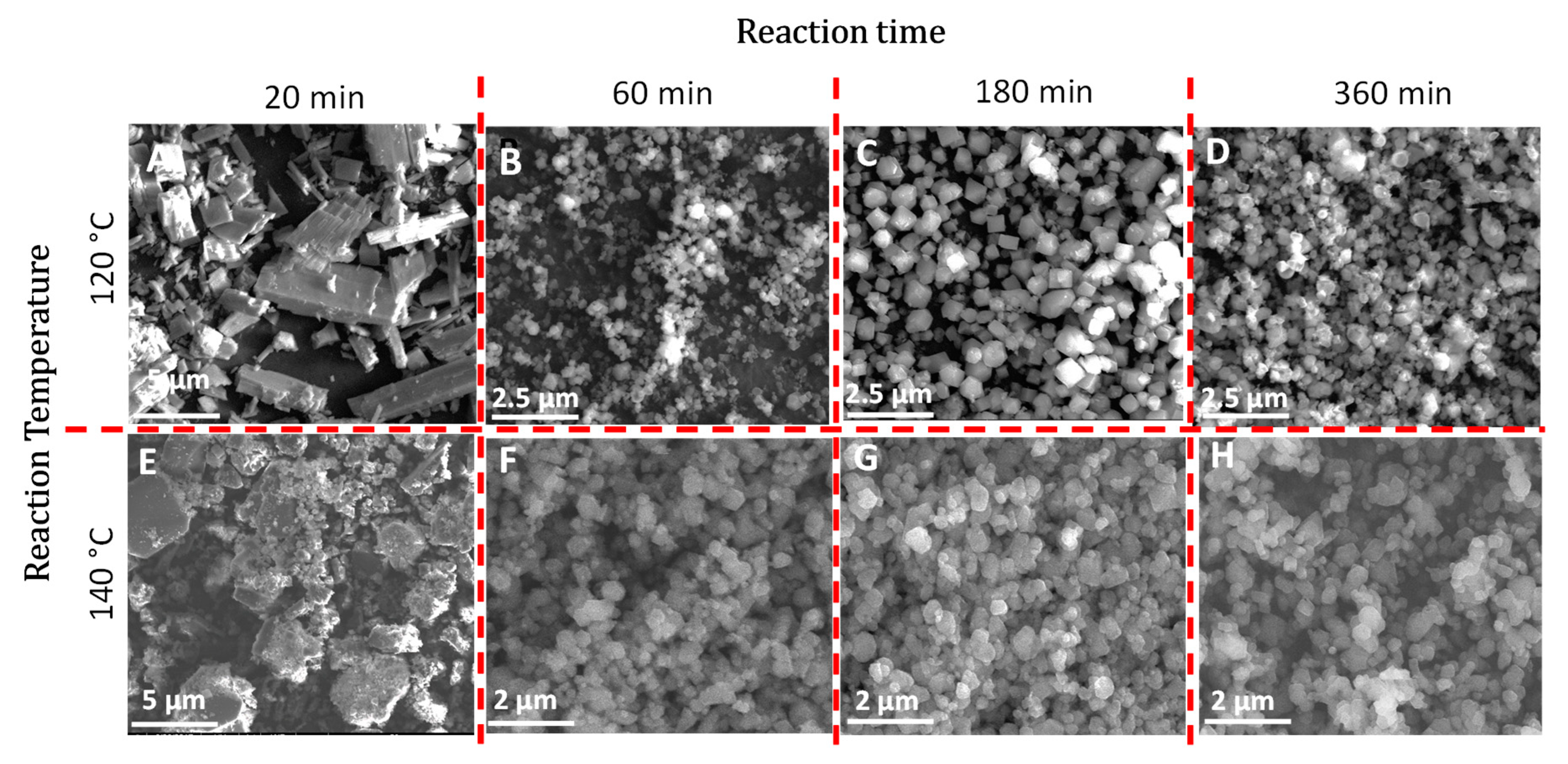
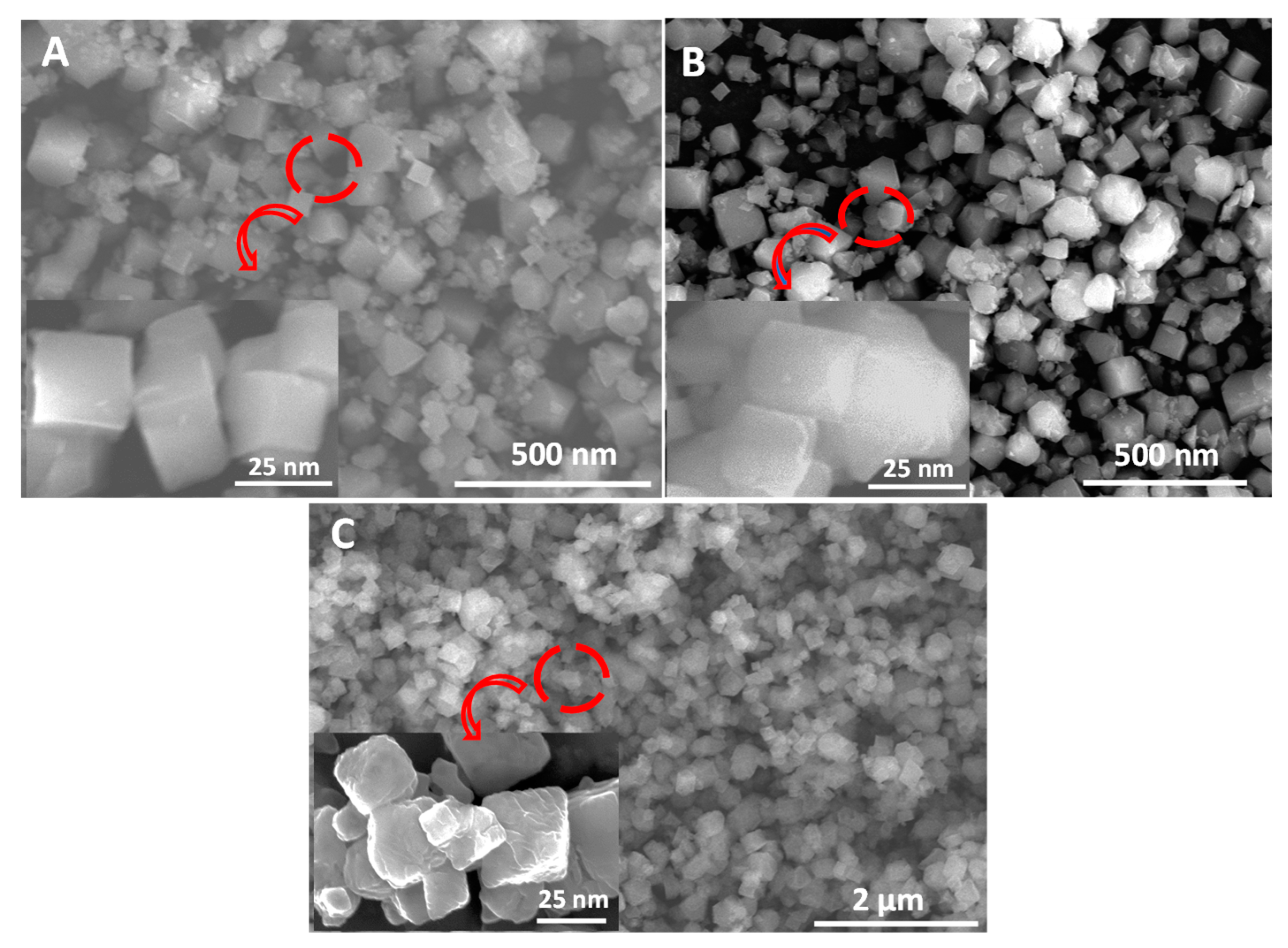
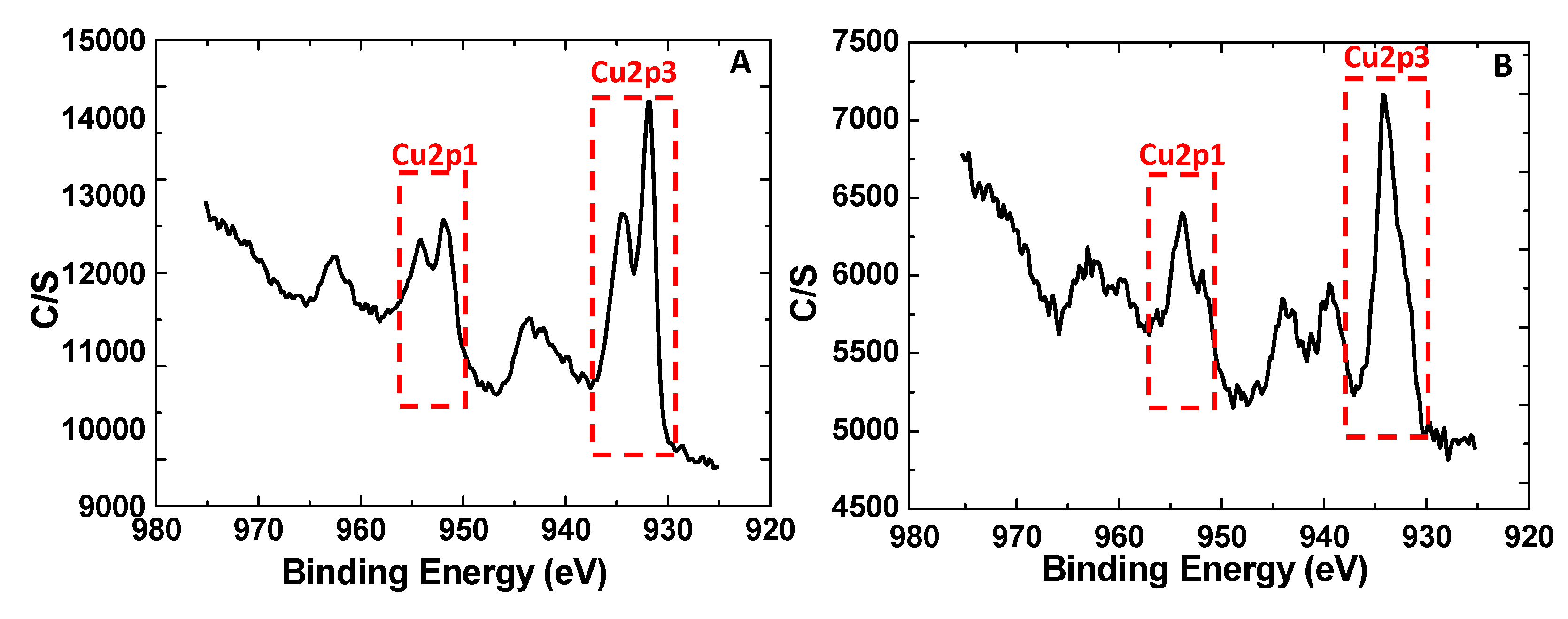
3.1. Synthesis and Characterization of MOFs
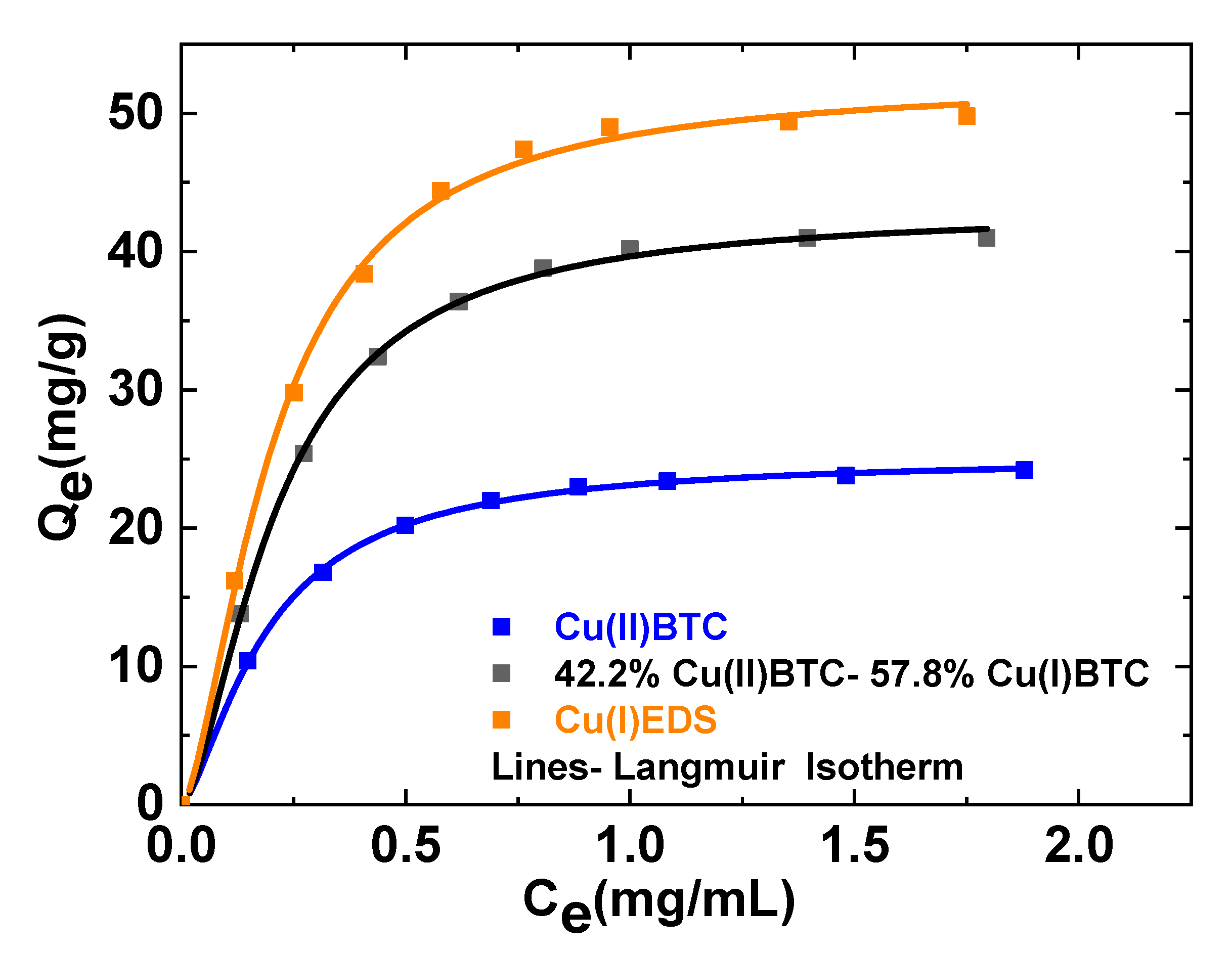
3.2. Adsorption Experiments
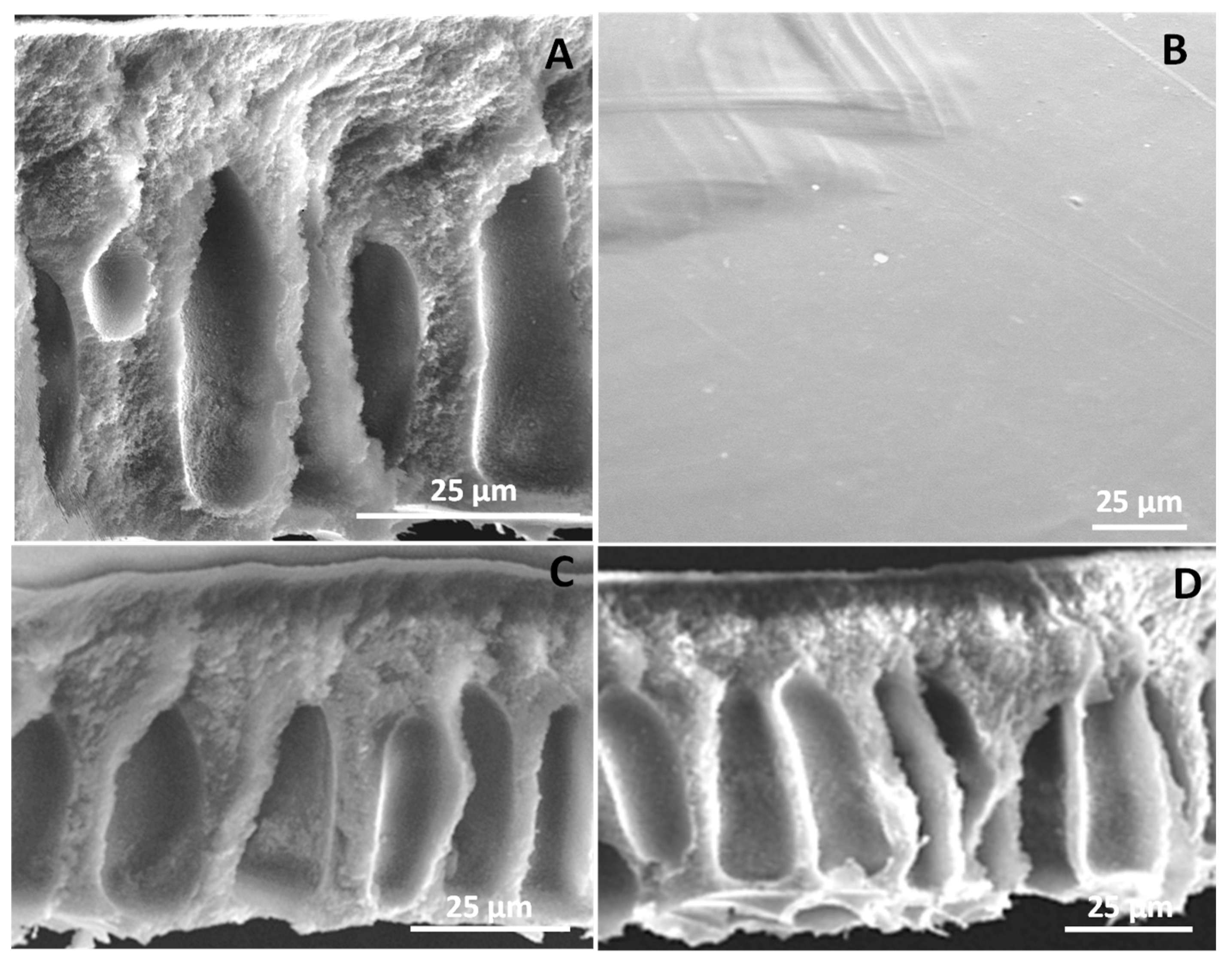
3.3. Membrane Characterization
3.4. Separation Experiments with n-Heptane/Toluene System
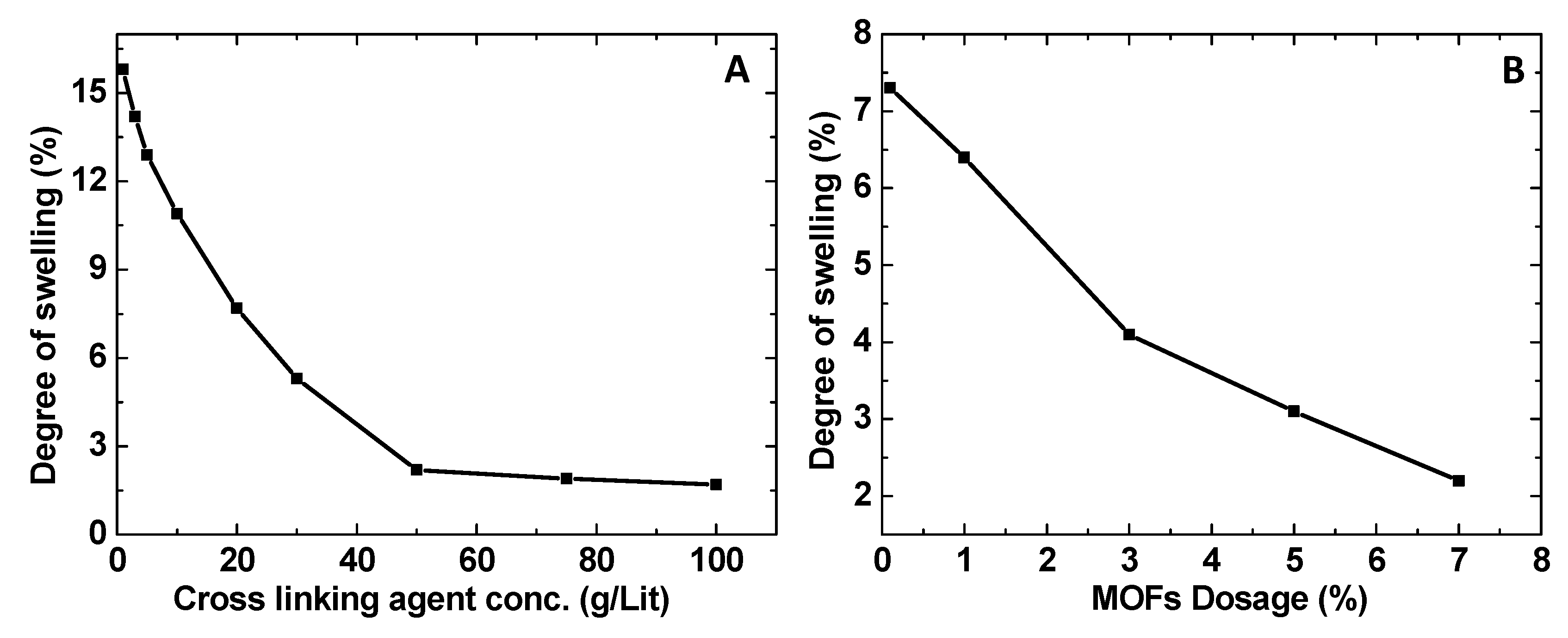
3.5. Swelling Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Villaluenga, J.G.; Tabe-Mohammadi, A. A review on the separation of benzene/cyclohexane mixtures by pervaporation processes. J. Membr. Sci. 2000, 169, 159–174. [Google Scholar] [CrossRef]
- Ribeiro, C.P.; Freeman, B.D.; Kalika, D.S.; Kalakkunnath, S. Aromatic polyimide and polybenzoxazole membranes for the fractionation of aromatic/aliphatic hydrocarbons by pervaporation. J. Membr. Sci. 2012, 390, 182–193. [Google Scholar] [CrossRef]
- Dong, Y.; Guo, H.; Su, Z.; Wei, W.; Wu, X. Pervaporation separation of benzene/cyclohexane through AAOM-ionic liquids/polyurethane membranes. Chem. Eng. Process. Process Intensif. 2015, 89, 62–69. [Google Scholar] [CrossRef]
- Kuila, S.B.; Ray, S.K. Separation of benzene–cyclohexane mixtures by filled blend membranes of carboxymethyl cellulose and sodium alginate. Sep. Purif. Technol. 2014, 123, 45–52. [Google Scholar] [CrossRef]
- Shen, J.-N.; Chu, Y.-X.; Ruan, H.-M.; Wu, L.-G.; Gao, C.-J.; Van der Bruggen, B. Pervaporation of benzene/cyclohexane mixtures through mixed matrix membranes of chitosan and Ag+/carbon nanotubes. J. Membr. Sci. 2014, 462, 160–169. [Google Scholar] [CrossRef]
- Tang, J.; Sirkar, K.K.; Majumdar, S. Permeation and sorption of organic solvents and separation of their mixtures through an amorphous perfluoropolymer membrane in pervaporation. J. Membr. Sci. 2013, 447, 345–354. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and preparation of porous polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Zhang, R.; Li, J.; Zhao, C.; Wu, T.; Ji, S. Highly stable “pore-filling” tubular composite membrane by self-crosslinkable hyperbranched polymers for toluene/n-heptane separation. J. Membr. Sci. 2015, 474, 263–272. [Google Scholar] [CrossRef]
- Wu, T.; Wang, N.; Li, J.; Wang, L.; Zhang, W.; Zhang, G.; Ji, S. Tubular thermal crosslinked-PEBA/ceramic membrane for aromatic/aliphatic pervaporation. J. Membr. Sci. 2015, 486, 1–9. [Google Scholar] [CrossRef]
- Aouinti, L.; Roizard, D.; Hu, G.; Thomas, F.; Belbachir, M. Investigation of pervaporation hybrid polyvinylchloride membranes for the separation of toluene–n-heptane mixtures—Case of clays as filler. Desalination 2009, 241, 174–181. [Google Scholar] [CrossRef]
- Kononova, S.V.; Kremnev, R.V.; Suvorova, E.I.; Baklagina, Y.G.; Volchek, B.Z.; Uchytil, P.; Shabsels, B.M.; Romashkova, K.A.; Setnickova, K.; Reznickova, J. Pervaporation membranes with poly (γ-benzyl-l-glutamate) selective layers for separation of toluene–n-heptane mixtures. J. Membr. Sci. 2015, 477, 14–24. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, B.; Qu, L.; Ren, J.; Li, Y. A novel atmospheric dielectric barrier discharge (DBD) plasma graft-filling technique to fabricate the composite membranes for pervaporation of aromatic/aliphatic hydrocarbons. J. Membr. Sci. 2011, 371, 163–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Ji, S.; Zhang, R.; Zhao, C.; Li, J.-R. Metal–organic framework/poly (vinyl alcohol) nanohybrid membrane for the pervaporation of toluene/n-heptane mixtures. J. Membr. Sci. 2015, 489, 144–152. [Google Scholar] [CrossRef]
- Iravaninia, M.; Mirfendereski, M.; Mohammadi, T. Pervaporation separation of toluene/n-heptane mixtures using a MSE-modified membrane: Effects of operating conditions. Chem. Eng. Res. Des. 2012, 90, 397–408. [Google Scholar] [CrossRef]
- Ye, H.; Li, J.; Lin, Y.; Chen, J.; Chen, C. Synthesis of polyimides containing fluorine and their pervaporation performances to aromatic/aliphatic hydrocarbon mixtures. J. Macromol. Sci. Part A Pure Appl. Chem. 2008, 45, 172–178. [Google Scholar] [CrossRef]
- Roizard, D.; Nilly, A.; Lochon, P. Preparation and study of crosslinked polyurethane films to fractionate toluene–n-heptane mixtures by pervaporation. Sep. Purif. Technol. 2001, 22, 45–52. [Google Scholar] [CrossRef]
- Frahn, J.; Malsch, G.; Matuschewski, H.; Schedler, U.; Schwarz, H.-H. Separation of aromatic/aliphatic hydrocarbons by photo-modified poly (acrylonitrile) membranes. J. Membr. Sci. 2004, 234, 55–65. [Google Scholar] [CrossRef]
- Billard, P.; Nguyen, Q.; Leger, C.; Clement, R. Diffusion of organic compounds through chemically asymmetric membranes made of semi-interpenetrating polymer networks. Sep. Purif. Technol. 1998, 14, 221–232. [Google Scholar] [CrossRef]
- Wang, N.; Ji, S.; Li, J.; Zhang, R.; Zhang, G. Poly (vinyl alcohol)–graphene oxide nanohybrid “pore-filling” membrane for pervaporation of toluene/n-heptane mixtures. J. Membr. Sci. 2014, 455, 113–120. [Google Scholar] [CrossRef]
- Sorribas, S.; Kudasheva, A.; Almendro, E.; Zornoza, B.; de la Iglesia, Ó.; Téllez, C.; Coronas, J. Pervaporation and membrane reactor performance of polyimide based mixed matrix membranes containing MOF HKUST-1. Chem. Eng. Sci. 2015, 124, 37–44. [Google Scholar] [CrossRef]
- Inui, K.; Noguchi, T.; Miyata, T.; Uragami, T. Pervaporation characteristics of methyl methacrylate–methacrylic acid copolymer membranes ionically crosslinked with metal ions for a benzene/cyclohexane mixture. J. Appl. Polym. Sci. 1999, 71, 233–241. [Google Scholar] [CrossRef]
- Farshad, F.; Iravaninia, M.; Kasiri, N.; Mohammadi, T.; Ivakpour, J. Separation of toluene/n-heptane mixtures experimental, modeling and optimization. Chem. Eng. J. 2011, 173, 11–18. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K. The nature of. pi.-. pi. interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Ding, S.-Y. Bacterial protein complexes with potential applications in nanotechnology. In Microbial Bionanotechnology: Biological Self-Assembly Systems and Biopolymer-Based Nanostructures; Taylor & Francis: Abingdon, UK, 2006; pp. 269–305. [Google Scholar]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A review on contemporary metal–organic framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal–organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef]
- Ba, C.; Langer, J.; Economy, J. Chemical modification of P84 copolyimide membranes by polyethylenimine for nanofiltration. J. Membr. Sci. 2009, 327, 49–58. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Qian, X.; Wickramasinghe, S.R. Chemical modification of membrane surface—Overview. Curr. Opin. Chem. Eng. 2018, 20, 13–18. [Google Scholar] [CrossRef]
- Aouinti, L.; Roizard, D.; Belbachir, M. PVC–activated carbon based matrices: A promising combination for pervaporation membranes useful for aromatic–alkane separations. Sep. Purif. Technol. 2015, 147, 51–61. [Google Scholar] [CrossRef]
- Liu, X.; Jin, H.; Li, Y.; Bux, H.; Hu, Z.; Ban, Y.; Yang, W. Metal–organic framework ZIF-8 nanocomposite membrane for efficient recovery of furfural via pervaporation and vapor permeation. J. Membr. Sci. 2013, 428, 498–506. [Google Scholar] [CrossRef]
- Schwarz, H.-H.; Malsch, G. Polyelectrolyte membranes for aromatic–aliphatic hydrocarbon separation by pervaporation. J. Membr. Sci. 2005, 247, 143–152. [Google Scholar] [CrossRef]
- Wang, N.; Wu, T.; Wang, L.; Li, X.; Zhao, C.; Li, J.; Ji, S. Hyperbranched polymer composite membrane using water as solvent for separating aromatic/aliphatic hydrocarbon mixtures. Sep. Purif. Technol. 2017, 179, 225–235. [Google Scholar] [CrossRef]
- Dou, Z.; Yu, J.; Cui, Y.; Yang, Y.; Wang, Z.; Yang, D.; Qian, G. Luminescent metal–organic framework films as highly sensitive and fast-response oxygen sensors. J. Am. Chem. Soc. 2014, 136, 5527–5530. [Google Scholar] [CrossRef]
- Sabouni, R.; Kazemian, H.; Rohani, S. Carbon dioxide capturing technologies: A review focusing on metal organic framework materials (MOFs). Environ. Sci. Pollut. Res. 2014, 21, 5427–5449. [Google Scholar] [CrossRef]
- So, M.C.; Wiederrecht, G.P.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Metal–organic framework materials for light-harvesting and energy transfer. Chem. Commun. 2015, 51, 3501–3510. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M. Review on processing of metal–organic framework (MOF) materials towards system integration for hydrogen storage. Int. J. Energy Res. 2015, 39, 607–620. [Google Scholar] [CrossRef]
- Qian, X.; Yadian, B.; Wu, R.; Long, Y.; Zhou, K.; Zhu, B.; Huang, Y. Structure stability of metal-organic framework MIL-53 (Al) in aqueous solutions. Int. J. Hydrog. Energy 2013, 38, 16710–16715. [Google Scholar] [CrossRef]
- Wang, H.; Ugomori, T.; Wang, Y.; Tanaka, K.; Kita, H.; Okamoto, K.I.; Suma, Y. Sorption and pervaporation properties of crosslinked membranes of poly (ethylene oxide imide) segmented copolymer to aromatic/nonaromatic hydrocarbon mixtures. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1800–1811. [Google Scholar] [CrossRef]
- Peng, F.; Lu, L.; Hu, C.; Wu, H.; Jiang, Z. Significant increase of permeation flux and selectivity of poly (vinyl alcohol) membranes by incorporation of crystalline flake graphite. J. Membr. Sci. 2005, 259, 65–73. [Google Scholar] [CrossRef]
- Diring, S.; Furukawa, S.; Takashima, Y.; Tsuruoka, T.; Kitagawa, S. Controlled multiscale synthesis of porous coordination polymer in nano/micro regimes. Chem. Mater. 2010, 22, 4531–4538. [Google Scholar] [CrossRef]
- Ahmed, A.; Robertson, C.M.; Steiner, A.; Whittles, T.; Ho, A.; Dhanak, V.; Zhang, H. Cu(i)Cu(ii)BTC, a microporous mixed-valence MOF via reduction of HKUST-1. Rsc Adv. 2016, 6, 8902–8905. [Google Scholar] [CrossRef]
- Fei, H.; Rogow, D.L.; Oliver, S.R. Reversible anion exchange and catalytic properties of two cationic metal−organic frameworks based on Cu (I) and Ag (I). J. Am. Chem. Soc. 2010, 132, 7202–7209. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, S.; Lv, L.; Su, B.; Hu, M.Z. High solvent-resistant and integrally crosslinked polyimide-based composite membranes for organic solvent nanofiltration. J. Membr. Sci. 2018, 564, 10–21. [Google Scholar] [CrossRef]
- Fagerlund, G. Determination of specific surface by the BET method. Matériaux Et Constr. 1973, 6, 239–245. [Google Scholar] [CrossRef]
- Ding, Y.; Sartaj, M. Statistical analysis and optimization of ammonia removal from aqueous solution by zeolite using factorial design and response surface methodology. J. Environ. Chem. Eng. 2015, 3, 807–814. [Google Scholar] [CrossRef]
- Shi, G.M.; Hua, D.; Chung, T.S. Chapter 6—Pervaporation and vapor separation. In Membrane Separation Principles and Applications; Ismail, A.F., Rahman, M.A., Othman, M.H.D., Matsuura, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–231. [Google Scholar]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; Van der Bruggen, B. Metal–organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef]
- Basu, S.; Maes, M.; Cano-Odena, A.; Alaerts, L.; De Vos, D.E.; Vankelecom, I.F.J. Solvent resistant nanofiltration (SRNF) membranes based on metal-organic frameworks. J. Membr. Sci. 2009, 344, 190–198. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal–organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Vishnyakov, A.; Ravikovitch, P.I.; Neimark, A.V.; Bülow, M.; Wang, Q.M. Nanopore structure and sorption properties of Cu−BTC metal−organic framework. Nano Lett. 2003, 3, 713–718. [Google Scholar] [CrossRef]

| Properties | Cu(II)BTC | Cu(I/II)BTC | Cu(I)EDS |
|---|---|---|---|
| Particle size (nm) | 25.1 | 24.9 | 22.6 |
| BET surface area (m2g−1) | 1123.7 | 1069.2 | 983.4 |
| Membranes | Temperature (°C) | Toluene Concentration in the Feed | Flux (g·m−2·h−1) | Separation Factor |
|---|---|---|---|---|
| Polyurethanes cross-linked with PEGHT (polyethylene glycol-isocyanate-2,2′,2”-nitrilotriethanol) [20] | 50 | 50 wt% | 1700 | 9.0 |
| 80 | 20 wt% | 4600 | 8.0 | |
| Poly (oxyethylene 360 methacrylates) /poly(acrylonitrile) [21] | 80 | 20 wt% | 330 | 6.6 |
| Poly (oxyethylene 400 methacrylates) /poly(acrylonitrile) [21] | 80 | 20 wt% | 1270 | 6.7 |
| Cellulosic ester - polyethylene glycol-600-dimethacrylate [22] | 80 | 20 wt% | 580 | 8.9 |
| 2,2-bis(3,4-dicarboxyphenyl) hexafluoro propane dianhydride/2,2-bis [4-(4-amino phenoxy) phenyl] hexafluoro propane/Polyimide [19] | 80 | 20 wt% | 760 | 6.5 |
| PolyAn modified aromatic selective polyvinylchloride [18] | 75 | 40 wt% | 230 | 6.0 |
| Sulfoethyl cellulose/benzyl dodecyl dimethylammonium chloride [36] | 80 | 20 wt% | 740 | 3.4 |
| Methyl methacrylate-co-methacrylic acid (3-sulfopropyl ester) potassium salt/benzyl dodecyl dimethylammonium chloride [36] | 80 | 20 wt% | 2190 | 3.5 |
| Surface modified polyacrylonitrile (PolyAn) [18] | 45 | 40 wt% | 3920 | 3.26 |
| 85 | 20 wt% | 5900 | 3.63 | |
| Cu(II)BTC/poly (vinyl alcohol) tubular membrane [17] | 45 | 50 wt% | 150 | 17.9 |
| Aromatic polyimide and polybenzoxazole [2] | 80 | 40 wt% | 15–46 | 4.7 |
| Poly(γ-benzyl-l-glutamate) (PBG) on a micro-porous poly(amide-imide) [15] | 50 | 40 wt% | 320 | 3.8 |
| Poly(ethylene glycol) methacrylate (PEO526OHMA)- poly(acrylonitrile) [16] | 80 | 20 wt% | 1860 | 7.8 |
| Poly(vinyl alcohol)-graphene oxide [12] | 40 | 50 wt% | 27 | 12.9 |
| Polyvinylchloride/Nanocor clay [14] | 74 | 50 wt% | 690 | 4.0 |
| Poly(ether-block-amide) (PEBA) [3] | 40 | 50 wt% | 65 | 4.3 |
| Poly(vinyl chloride) (PVC)–activated carbon (Maxsorb SPD30) [34] | 74 | 50 wt% | 90 | 6.3 |
| Polyimide + 7% Cu(II)BTC [This work) | 35 | 58 mol% | 293 | 1.47 |
| Polyimide + 7% [42.2% Cu(II)BTC-57.8% Cu(I)BTC [This work] | 35 | 58 mol% | 263 | 1.67 |
| Polyimide + 7% Cu(I)EDS [This work] | 35 | 58 mol% | 223 | 1.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Upadhyaya, L.; Chiao, Y.-H.; Wickramasinghe, S.R.; Qian, X. Cu(I/II) Metal–Organic Frameworks Incorporated Nanofiltration Membranes for Organic Solvent Separation. Membranes 2020, 10, 313. https://doi.org/10.3390/membranes10110313
Upadhyaya L, Chiao Y-H, Wickramasinghe SR, Qian X. Cu(I/II) Metal–Organic Frameworks Incorporated Nanofiltration Membranes for Organic Solvent Separation. Membranes. 2020; 10(11):313. https://doi.org/10.3390/membranes10110313
Chicago/Turabian StyleUpadhyaya, Lakshmeesha, Yu-Hsuan Chiao, S. Ranil Wickramasinghe, and Xianghong Qian. 2020. "Cu(I/II) Metal–Organic Frameworks Incorporated Nanofiltration Membranes for Organic Solvent Separation" Membranes 10, no. 11: 313. https://doi.org/10.3390/membranes10110313
APA StyleUpadhyaya, L., Chiao, Y.-H., Wickramasinghe, S. R., & Qian, X. (2020). Cu(I/II) Metal–Organic Frameworks Incorporated Nanofiltration Membranes for Organic Solvent Separation. Membranes, 10(11), 313. https://doi.org/10.3390/membranes10110313








