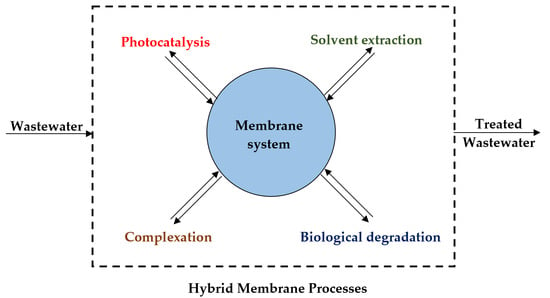
Application of Hybrid Membrane Processes Coupling Separation and Biological or Chemical Reaction in Advanced Wastewater Treatment
Abstract
1. Introduction
- (i)
- HMPs obtained by coupling membrane processes with photocatalysis (PC);
- (ii)
- HMPs obtained by coupling membrane processes with solvent extraction;
- (iii)
- HMPs obtained by coupling UF membranes with water soluble complexants;
- (iv)
- HMP obtained by coupling a photocatalytic membrane reactor and complexation–ultrafiltration (CP–UF) process;
- (v)
- other hybrid membrane processes.
2. HMPs Obtained by Coupling Membrane Processes with Photocatalysis
2.1. PMRs in the Treatment of Primary and Secondary Effluents
2.2. PMRs in the Photodegradation of Dyes in Aqueous Media
2.3. PMRs in the Photodegradation of Pharmaceuticals in Aqueous Media
3. HMPs Obtained by Coupling Membrane Processes with Solvent Extraction
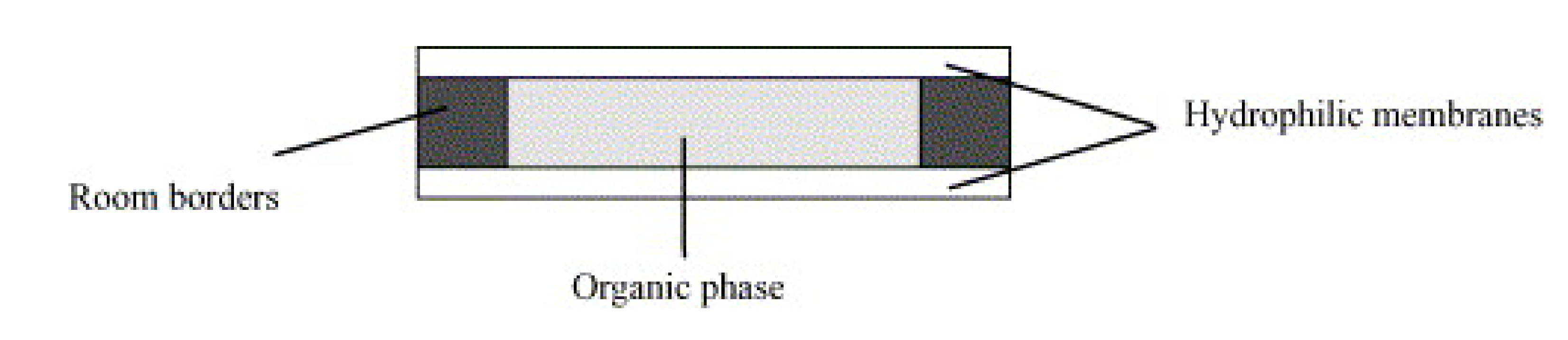
3.1. Liquid Membrane (LM)-Based Operations
3.2. Some Cases Studies on Application of Supported Liquid Membrane
3.3. Some Strategies Proposed in Literature to Improve LM System Stability
4. HMPs Obtained by Coupling Membrane Processes with Water Soluble Complexing Agents
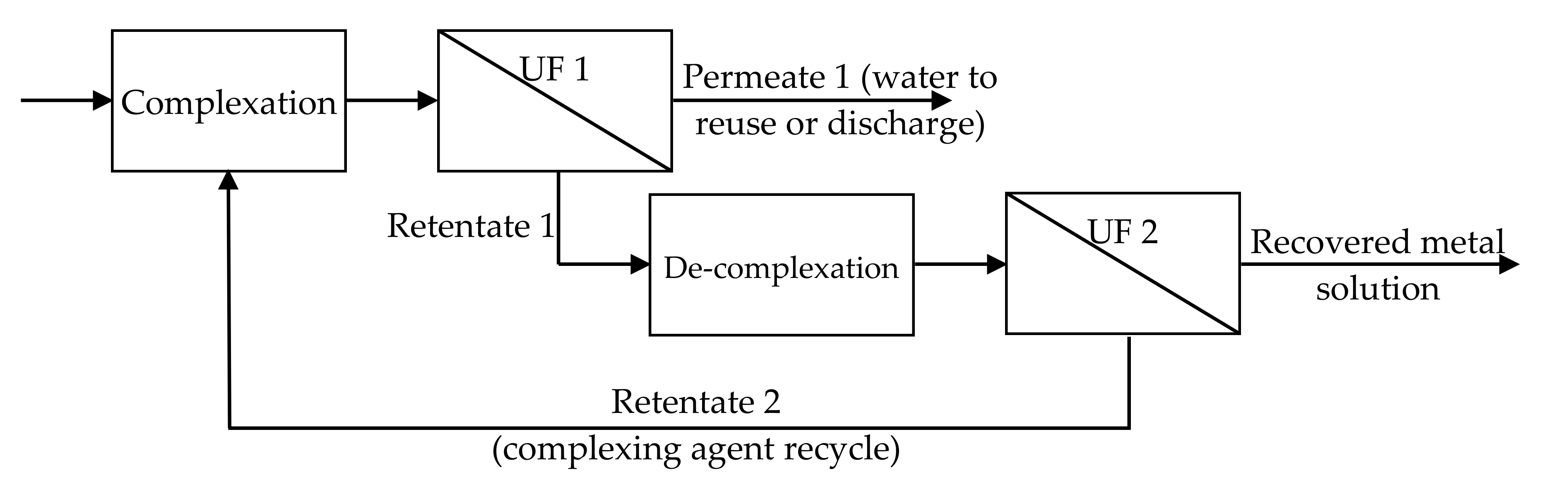
4.1. Foundamentals of Complexation–Ultrafiltration
4.2. Selective Separations by Complexation–Ultrafiltration
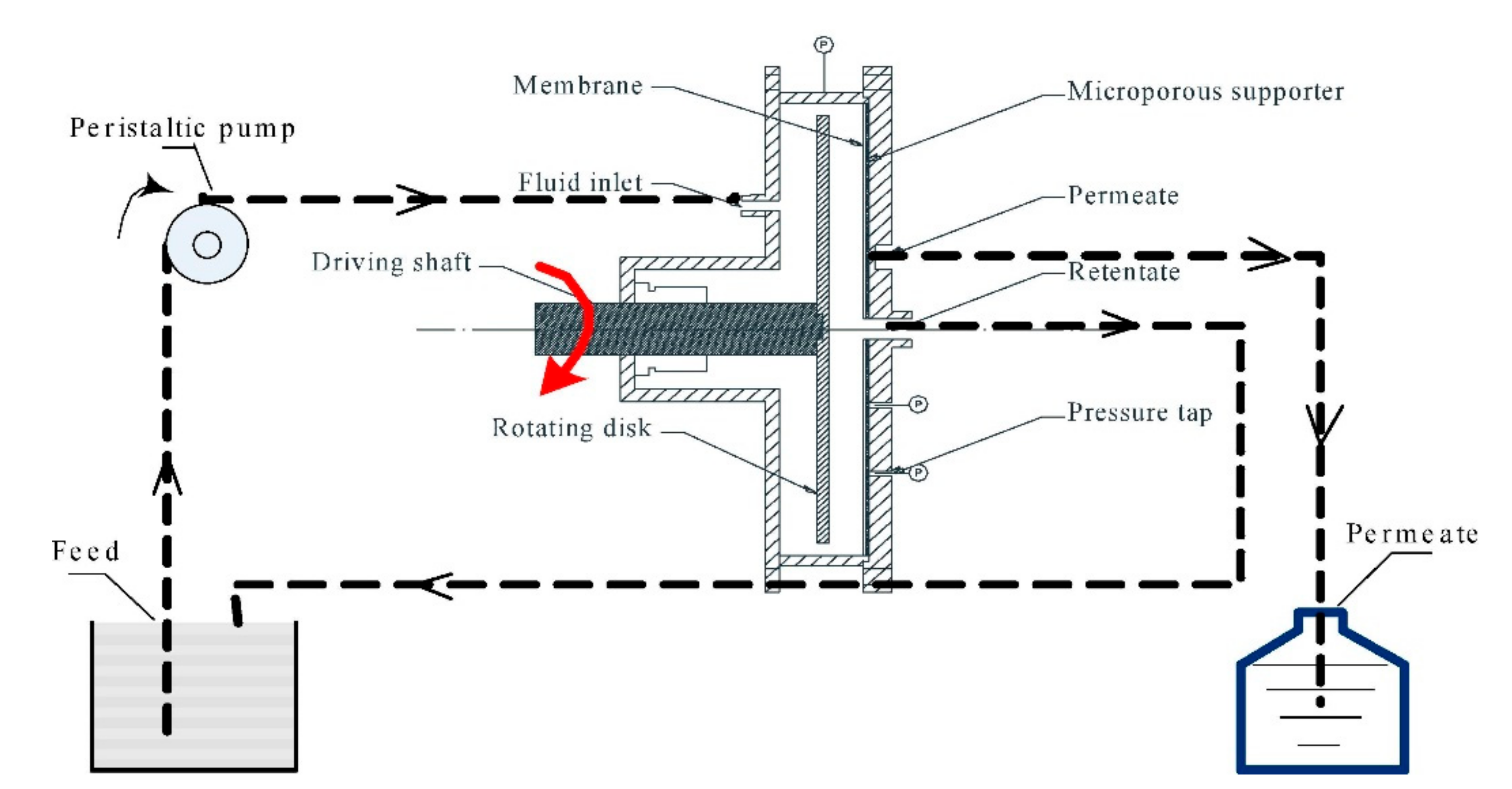
4.3. Stability of Polymer–Metal Complex in the Shear Field: A Possible Strategy for Achieving Both Polymer Regeneration and Separation Selectivity
4.4. Influence of Membrane Characteristic on CP–UF Performance: A Case Study.
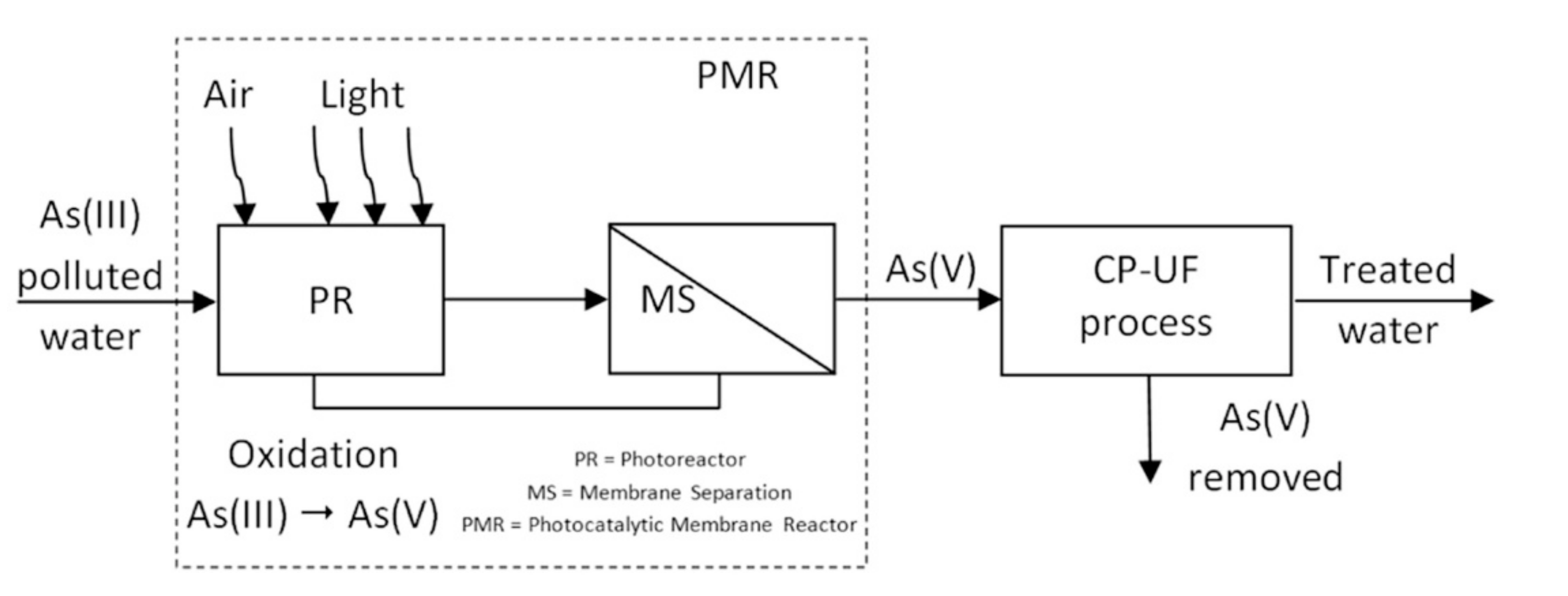
5. HMPs Obtained by Coupling PMRs and CP–UF Processes
6. Other Hybrid Membrane Processes
7. Perspectives/Future Directions
8. Conclusions
Funding
Conflicts of Interest
References
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, Q.; Wu, G.; Hu, H.-Y. Centralized water reuse system with multiple applications in urban areas: Lessons from China’s experience. Resour. Conserv. Recycl. 2017, 117, 125–136. [Google Scholar] [CrossRef]
- Bauer, S.; Linke, H.J.; Wagner, M. Combining industrial and urban water-reuse concepts for increasing the water resources in water-scarce regions. Water Environ. Res. 2020, 92, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Roshan, A.; Kumar, M. Water end-use estimation can support the urban water crisis management: A critical review. J. Environ. Manag. 2020, 268, 110663. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef] [PubMed]
- Pecson, B.M.; Trussell, R.S.; Triolo, S.C.; Trussell, R.R. Examining Reservoirs in Potable Reuse, Part 1: Groundwater Recharge and Surface Water Augmentation. J. Am. Water Work. Assoc. 2018, 110, 34–40. [Google Scholar] [CrossRef]
- Ahmed, W.; Kitajima, M.; Tandukar, S.; Haramoto, E. Recycled water safety: Current status of traditional and emerging viral indicators. Curr. Opin. Environ. Sci. Heal. 2020, 16, 62–72. [Google Scholar] [CrossRef]
- Rezaei, N.; Diaz-Elsayed, N.; Mohebbi, S.; Xie, X.; Zhang, Q. A multi-criteria sustainability assessment of water reuse applications: A case study in Lakeland, Florida. Environ. Sci. Water Res. Technol. 2019, 5, 102–118. [Google Scholar] [CrossRef]
- Helmecke, M.; Fries, E.; Schulte, C. Regulating water reuse for agricultural irrigation: Risks related to organic micro-contaminants. Environ. Sci. Eur. 2020, 32, 4. [Google Scholar] [CrossRef]
- Pedrero, F.; Grattan, S.; Ben-Gal, A.; Vivaldi, G.A. Opportunities for expanding the use of wastewaters for irrigation of olives. Agric. Water Manag. 2020, 241, 106333. [Google Scholar] [CrossRef]
- Anito, D.A.; Wang, T.-X.; Liu, Z.-W.; Ding, X.; Han, B.-H. Iminodiacetic acid-functionalized porous polymer for removal of toxic metal ions from water. J. Hazard. Mater. 2020, 400, 123188. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Argurio, P.; Poerio, T.; Gullone, G. Selective separation of copper(II) and nickel(II) from aqueous systems by polymer assisted ultrafiltration. Desalination 2006, 200, 728–730. [Google Scholar] [CrossRef]
- Peng, D.; Qiao, S.; Luo, Y.; Ma, H.; Zhang, L.; Hou, S.; Wu, B.; Xu, H. Performance of microbial induced carbonate precipitation for immobilizing Cd in water and soil. J. Hazard. Mater. 2020, 400, 123116. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Song, L.; Song, H.; Hui, K.; Lin, Z.; Wang, Q.; Xuan, L.; Wang, Z.; Gao, W. Remediation of copper contaminated sediments by granular activated carbon-supported titanium dioxide nanoparticles: Mechanism study and effect on enzyme activities. Sci. Total Environ. 2020, 741, 139962. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Argurio, P.; Poerio, T. Membrane Processes Based on Complexation Reactions of Pollutants as Sustainable Wastewater Treatments. Sustainability 2009, 1, 978–993. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, J.; Song, Y.; Cui, Y.; Zhang, H.; Zhao, X.; Li, Z.; Yao, J. Influence of operating conditions on the efficiency of domestic wastewater treatment in membrane bioreactors. Desalination 2009, 245, 73–81. [Google Scholar] [CrossRef]
- Villain-Gambier, M.; Bourven, I.; Guibaud, G.; Marrot, B. Influence of proteins and humic-like substances from soluble microbial products on membrane bioreactor fouling under normal and stress conditions. Process. Biochem. 2019, 78, 140–147. [Google Scholar] [CrossRef]
- Kitanou, S.; Taky, A.E.M.; Hafida, A.; Soukaina, B.; Mahi, M.; Taky, M.; Elmidaoui, A. Performance of an ultrafiltration membrane bioreactor (UF-MBR) in wastewater treatment. Desalination Water Treat. 2019, 157, 393–398. [Google Scholar] [CrossRef]
- Xing, C.-H.; Tardieu, E.; Qian, Y.; Wen, X. Ultrafiltration membrane bioreactor for urban wastewater reclamation. J. Membr. Sci. 2000, 177, 73–82. [Google Scholar] [CrossRef]
- Özkan, O.; Uyanık, I. Effect of Membrane Type for the Treatment of Organized Industrial Zone (OIZ) Wastewater with a Membrane Bioreactor (MBR): Batch Experiments. Water 2017, 9, 582. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Hilal, N.; Leo, C.P. A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 2015, 363, 2–18. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.-L.; Chang, J.-S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Dhangar, K.; Kumar, M. Tricks and tracks in removal of emerging contaminants from the wastewater through hybrid treatment systems: A review. Sci. Total Environ. 2020, 738, 140320. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Oatley-Radcliffe, D.L.; Williams, P.M. Foreward to Special Issue: Hybrid Systems. Desalination 2015, 363, 1. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Hybrid technologies: The future of energy efficient desalination—A review. Desalination 2020, 495, 114659. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nat. Cell Biol. 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P.; Szymański, K.; Darowna, D.; Mozia, S. Overview of Photocatalytic Membrane Reactors in Organic Synthesis, Energy Storage and Environmental Applications. Catalysts 2019, 9, 239. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- Jiang, L.; Choo, K.-H. Photocatalytic mineralization of secondary effluent organic matter with mitigating fouling propensity in a submerged membrane photoreactor. Chem. Eng. J. 2016, 288, 798–805. [Google Scholar] [CrossRef]
- Mozia, S.; Darowna, D.; Szymański, K.; Grondzewska, S.; Borchert, K.; Wróbel, R.; Morawski, A. Performance of two photocatalytic membrane reactors for treatment of primary and secondary effluents. Catal. Today 2014, 236, 135–145. [Google Scholar] [CrossRef]
- Tang, K.; Ooi, G.T.; Torresi, E.; Kaarsholm, K.M.; Hambly, A.; Sundmark, K.; Lindholst, S.; Sund, C.; Kragelund, C.; Christensson, M.; et al. Municipal wastewater treatment targeting pharmaceuticals by a pilot-scale hybrid attached biofilm and activated sludge system (Hybas™). Chemosphere 2020, 259, 127397. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Y.; Fent, K. Cardiovascular drugs and lipid regulating agents in surface waters at global scale: Occurrence, ecotoxicity and risk assessment. Sci. Total. Environ. 2020, 729, 138770. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, L.; Gala, I.V.; Comas, J.; Carbonell, S.M.; Rodríguez-Roda, I.; Gernjak, W. Integrated assessment of sulfate-based AOPs for pharmaceutical active compound removal from wastewater. J. Clean. Prod. 2020, 260, 121014. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Szymański, K.; Cristóvão, R.O.; Mendes, A.; Vilar, V.J.; Mozia, S. Performance of hybrid systems coupling advanced oxidation processes and ultrafiltration for oxytetracycline removal. Catal. Today 2019, 328, 274–280. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M. Graphene oxide based ultrafiltration membranes for photocatalytic degradation of organic pollutants in salty water. Water Res. 2015, 77, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Alyarnezhad, S.; Marino, T.; Basiri-Parsa, J.; Galiano, F.; Ursino, C.; García, H.; Puche, M.; Figoli, A. Polyvinylidene Fluoride-Graphene Oxide Membranes for Dye Removal under Visible Light Irradiation. Polymer 2020, 12, 1509. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.K.; Da Costa, J.C.D.; Da Costa, J.C.D. Recent progresses on fabrication of photocatalytic membranes for water treatment. Catal. Today 2014, 230, 47–54. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Simone, S.; Galiano, F.; Faccini, M.; Boerrigter, M.; Chaumette, C.; Drioli, E.; Figoli, A. Preparation and Characterization of Polymeric-Hybrid PES/TiO2 Hollow Fiber Membranes for Potential Applications in Water Treatment. Fibers 2017, 5, 14. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, T.; Shi, J.; Teng, K.; Wang, W.; Ma, M.; Li, J.; Qian, X.; Li, C.; Fan, J. Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Membr. Sci. 2016, 520, 281–293. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Quan, X.; Yu, H.; Zhao, H. Integration of microfiltration and visible-light-driven photocatalysis on g-C 3 N 4 nanosheet/reduced graphene oxide membrane for enhanced water treatment. Appl. Catal. B Environ. 2016, 194, 134–140. [Google Scholar] [CrossRef]
- Wangab, M.; Zhangb, Y.; Yuc, G.; Zhaoad, J.; Chend, X.; Yanad, F.; Liab, J.; Yinad, Z.; Heab, B. Monolayer porphyrin assembled SPSf/PES membrane reactor for degradation of dyes under visible light irradiation coupling with continuous filtration✰. J. Taiwan Inst. Chem. Eng. 2020, 109, 62–70. [Google Scholar] [CrossRef]
- Ashar, A.; Bhatti, I.A.; Ashraf, M.; Tahir, A.A.; Aziz, H.; Yousuf, M.; Ahmad, M.; Mohsin, M.; Bhutta, Z.A. Fe3+ @ ZnO/polyester based solar photocatalytic membrane reactor for abatement of RB5 dye. J. Clean. Prod. 2020, 246, 119010. [Google Scholar] [CrossRef]
- Zhang, Q.; Quan, X.; Wang, H.; Chen, S.; Su, Y.; Li, Z. Constructing a visible-light-driven photocatalytic membrane by g-C3N4 quantum dots and TiO2 nanotube array for enhanced water treatment. Sci. Rep. 2017, 7, 3128. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.-S.; Chen, C.-H.; Chen, Y.-R.; Huang, P.-H.; Tung, K.-L. Phosphorus-doped g-C3N4 integrated photocatalytic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 580, 1–11. [Google Scholar] [CrossRef]
- Suna, T.; Liua, Y.; Shen, L.; Xua, Y.; Li, R.; Huangb, L.; Lin, H. Magnetic field assisted arrangement of photocatalytic TiO2 particles on membrane surface to enhance membrane antifouling performance for water treatment. J. Colloid Interface Sci. 2020, 570, 273–285. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, G.; Han, K.; Ye, H.; Wei, S.; Zhou, Y. One-step facile synthesis of graphene oxide/TiO2 composite as efficient photocatalytic membrane for water treatment: Crossflow filtration operation and membrane fouling analysis. Chem. Eng. Process. Process. Intensif. 2017, 120, 20–26. [Google Scholar] [CrossRef]
- Liu, G.; Han, K.; Zhou, Y.; Ye, H.; Zhang, X.; Hu, J.; Li, X. Facile Synthesis of Highly Dispersed Ag Doped Graphene Oxide/Titanate Nanotubes as a Visible Light Photocatalytic Membrane for Water Treatment. ACS Sustain. Chem. Eng. 2018, 6, 6256–6263. [Google Scholar] [CrossRef]
- Luo, J.; Chen, W.; Song, H.; Liu, J. Fabrication of hierarchical layer-by-layer membrane as the photocatalytic degradation of foulants and effective mitigation of membrane fouling for wastewater treatment. Sci. Total Environ. 2020, 699, 134398. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Peña-Bahamonde, J.; Bandara, P.C.; Rodrigues, D.F. Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. NPJ Clean Water 2020, 3, 1–15. [Google Scholar] [CrossRef]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Magnetic fluorinated mesoporous g-C3N4 for photocatalytic degradation of amoxicillin: Transformation mechanism and toxicity assessment. Appl. Catal. B Environ. 2019, 242, 337–348. [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Li, P.; Shao, J.; He, Y. Photo-Fenton degradation of amoxicillin via magnetic TiO2-graphene oxide-Fe3O4 composite with a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). J. Hazard. Mater. 2019, 373, 437–446. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 2017, 310, 389–398. [Google Scholar] [CrossRef]
- Kamaludin, R.; Rasdi, Z.; Jaafar, J.; Kadir, S.S.A.; Nor, N.S.M.; Khan, J.; Zain, W.N.I.W.M.; Ismail, A.; Rahman, M.A.; Jaafar, J.; et al. Visible-Light Active Photocatalytic Dual Layer Hollow Fiber (DLHF) Membrane and Its Potential in Mitigating the Detrimental Effects of Bisphenol A in Water. Membranes 2020, 10, 32. [Google Scholar] [CrossRef]
- Chakraborty, S.; Loutatidou, S.; Palmisano, G.; Kujawa, J.; Mavukkandy, M.O.; Al-Gharabli, S.; Curcio, E.; Arafat, H.A. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J. Environ. Chem. Eng. 2017, 5, 5014–5024. [Google Scholar] [CrossRef]
- Tugaoen, H.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Compact light-emitting diode optical fiber immobilized TiO2 reactor for photocatalytic water treatment. Sci. Total. Environ. 2018, 613, 1331–1338. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Tran, Q.B.; Ly, Q.V.; Hai, L.T.; Le, D.T.; Tran, M.B.; Ho, T.T.T.; Nguyen, X.C.; Shokouhimehr, M.; Vo, D.-V.N.; et al. Enhanced visible photocatalytic degradation of diclofen over N-doped TiO2 assisted with H2O2: A kinetic and pathway study. Arab. J. Chem. 2020. [Google Scholar] [CrossRef]
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Res. 2019, 163, 114841. [Google Scholar] [CrossRef]
- Lumbaque, E.C.; Sirtori, C.; Vilar, V.J. Heterogeneous photocatalytic degradation of pharmaceuticals in synthetic and real matrices using a tube-in-tube membrane reactor with radial addition of H2O2. Sci. Total. Environ. 2020, 743, 140629. [Google Scholar] [CrossRef] [PubMed]
- Quist-Jensen, C.A.; Ali, A.; Drioli, E.; Macedonio, F. Perspectives on mining from sea and other alternative strategies for minerals and water recovery—The development of novel membrane operations. J. Taiwan Inst. Chem. Eng. 2019, 94, 129–134. [Google Scholar] [CrossRef]
- Dai, Y.; Li, S.; Meng, D.; Yang, J.; Cui, P.; Wang, Y.; Zhu, Z.; Gao, J.; Ma, Y. Economic and Environmental Evaluation for Purification of Diisopropyl Ether and Isopropyl Alcohol via Combining Distillation and Pervaporation Membrane. ACS Sustain. Chem. Eng. 2019, 7, 20170–20179. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Poerio, T. Studies of various solid membrane supports to prepare stable sandwich liquid membranes and testing copper(II) removal from aqueous media. Sep. Purif. Technol. 2009, 70, 166–172. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Poerio, T. Flux enhancement of stagnant sandwich compared to supported liquid membrane systems in the removal of Gemfibrozil from waters. J. Membr. Sci. 2009, 340, 26–34. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Ansari, S.A.; Prabhu, D.R.; Kumar, D.; Mohapatra, P.K. Highly efficient separation of Am3+ and Eu3+ using an aqueous soluble sulfonated BTP derivative by hollow-fiber supported liquid membrane containing TODGA. Sep. Sci. Technol. 2019, 54, 1512–1520. [Google Scholar] [CrossRef]
- Chang, S.H. A Comparative Study of Batch and Continuous Bulk Liquid Membranes in the Removal and Recovery of Cu(II) Ions from Wastewater. Water Air Soil Pollut. 2018, 229, 22. [Google Scholar] [CrossRef]
- Soniya, M.; Muthuraman, G. Comparative study between liquid–liquid extraction and bulk liquid membrane for the removal and recovery of methylene blue from wastewater. J. Ind. Eng. Chem. 2015, 30, 266–273. [Google Scholar] [CrossRef]
- Ahmad, A.; Zaulkiflee, N.D.; Kusumastuti, A.; Buddin, M.M.H.S. Removal of Acetaminophen from Aqueous Solution by Emulsion Liquid Membrane: Emulsion Stability Study. Ind. Eng. Chem. Res. 2018, 58, 713–719. [Google Scholar] [CrossRef]
- Rosly, M.B.; Jusoh, N.; Othman, N.; Rahman, H.A.; Sulaiman, R.N.R.; Noah, N.F.M. Stability of emulsion liquid membrane using bifunctional diluent and blended nonionic surfactant for phenol removal. Chem. Eng. Process. Process. Intensif. 2020, 148, 148. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Pirillo, F. Comparison between stagnant sandwich and supported liquid membranes in copper(II) removal from aqueous solutions: Flux, stability and model elaboration. J. Membr. Sci. 2005, 256, 158–168. [Google Scholar] [CrossRef]
- Argurio, P.; Tagarelli, A.; Molinari, R. A study on neodymium recovery from aqueous solutions for designing a new generation of sandwich liquid membrane. Journal of Membrane Science and Research 2019, 5, 147–156. [Google Scholar] [CrossRef]
- Duan, H.; Liu, H.; Hu, C.; Cheng, W.; Wang, X. Facilitated recovery of copper from ammoniacal solution by supported liquid membrane following multiple cooperative effects. Chem. Pap. 2020, 74, 3335–3345. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Highly selective transport of lithium across a supported liquid membrane. J. Fluor. Chem. 2020, 236, 109593. [Google Scholar] [CrossRef]
- Mahanty, B.; Mohapatra, P.K.; Leoncini, A.; Huskens, J.; Verboom, W. Americium pertraction across supported liquid membranes containing multiple diglycolamide ligands: Role of alkyl substitution and spacer length in carrier ligands. Chem. Eng. Res. Des. 2020, 159, 170–178. [Google Scholar] [CrossRef]
- Güell, R.; Fontàs, C.; Salvadó, V.; Anticó, E. Modelling of liquid–liquid extraction and liquid membrane separation of arsenic species in environmental matrices. Sep. Purif. Technol. 2010, 72, 319–325. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, L.; Song, C.; Zhang, L. Extraction of nicotine from local tobacco using double-supported liquid membranes technique. J. Membr. Sci. 2010, 356, 105–109. [Google Scholar] [CrossRef]
- Zidi, C.; Tayeb, R.; Ali, M.B.S.; Dhahbi, M. Liquid–liquid extraction and transport across supported liquid membrane of phenol using tributyl phosphate. J. Membr. Sci. 2010, 360, 334–340. [Google Scholar] [CrossRef]
- Djian, D.; Alloin, F.; Martinet, S.; Lignier, H.; Sanchez, J. Lithium-ion batteries with high charge rate capacity: Influence of the porous separator. J. Power Sources 2007, 172, 416–421. [Google Scholar] [CrossRef]
- Djian, D.; Alloin, F.; Martinet, S.; Lignier, H. Macroporous poly(vinylidene fluoride) membrane as a separator for lithium-ion batteries with high charge rate capacity. J. Power Sources 2009, 187, 575–580. [Google Scholar] [CrossRef]
- Chasib, K.F.; Mohsen, A.J.; Jisha, K.J.; Gardas, R.L. Extraction of Phenolic Pollutants from Industrial Wastewater Using a Bulk Ionic Liquid Membrane Technique. Environ. Technol. 2020, 1–12. [Google Scholar] [CrossRef]
- Kemperman, A.J.; Bargeman, D.; Boomgaard, T.V.D.; Strathmann, H. Stability of Supported Liquid Membranes: State of the Art. Sep. Sci. Technol. 1996, 31, 2733–2762. [Google Scholar] [CrossRef]
- Hill, C.; Dozol, J.-F.; Rouquette, H.; Eymard, S.; Tournois, B. Study of the stability of some supported liquid membranes. J. Membr. Sci. 1996, 114, 73–80. [Google Scholar] [CrossRef]
- Rehn, G.; Ayres, B.M.; Adlercreutz, P.; Grey, C. An improved process for biocatalytic asymmetric amine synthesis by in situ product removal using a supported liquid membrane. J. Mol. Catal. B Enzym. 2016, 123, 1–7. [Google Scholar] [CrossRef]
- Pei, L.; Wang, L.; Yu, G. Study on a novel flat renewal supported liquid membrane with D2EHPA and hydrogen nitrate for neodymium extraction. J. Rare Earths 2012, 30, 63–68. [Google Scholar] [CrossRef]
- Kohli, H.P.; Gupta, S.; Chakraborty, M. Applicability of Hollow Fiber Strip Dispersion for the Removal of Metal Ions from Aqueous Streams. J. Inst. Eng. (India) Ser. E 2020, 101, 91–97. [Google Scholar] [CrossRef]
- Pirom, T.; Arponwichanop, A.; Pancharoen, U.; Yonezawa, T.; Kheawhom, S. Yttrium (III) Recovery with D2EHPA in Pseudo-Emulsion Hollow Fiber Strip Dispersion System. Sci. Rep. 2018, 8, 7627. [Google Scholar] [CrossRef]
- Lu, S.; Pei, L. A study of zinc borne waste water treatment with dispersion supported liquid membrane. Int. J. Hydrogen Energy 2016, 41, 15717–15723. [Google Scholar] [CrossRef]
- Yang, X. Stabilization of supported liquid membranes by plasma polymerization surface coating. J. Membr. Sci. 2000, 168, 29–37. [Google Scholar] [CrossRef]
- He, T. Towards stabilization of supported liquid membranes: Preparation and characterization of polysulfone support and sulfonated poly (ether ether ketone) coated composite hollow fiber membranes. Desalination 2008, 225, 82–94. [Google Scholar] [CrossRef]
- Kemperman, A.J.B.; Damink, B.; Boomgaard, T.V.D.; Strathmann, H. Stabilization of supported liquid membranes by gelation with PVC. J. Appl. Polym. Sci. 1997, 65, 1205–1216. [Google Scholar] [CrossRef]
- Ren, X.; Jia, Y.; Lü, X.; Shi, T.; Ma, S. Preparation and characterization of PDMS-D2EHPA extraction gel membrane for metal ions extraction and stability enhancement. J. Membr. Sci. 2018, 559, 159–169. [Google Scholar] [CrossRef]
- Song, J.; Huang, T.; Qiu, H.; Niu, X.; Li, X.-M.; Xie, Y.; He, T. A critical review on membrane extraction with improved stability: Potential application for recycling metals from city mine. Desalination 2018, 440, 18–38. [Google Scholar] [CrossRef]
- Basu, R.; Sirkar, K.K. Hollow fiber contained liquid membrane separation of citric acid. AIChE J. 1991, 37, 383–393. [Google Scholar] [CrossRef]
- Gaikwad, A. Synergetic transport of europium through a contained supported liquid membrane using trioctylamine and tributyl phosphate as carriers. Talanta 2004, 63, 917–926. [Google Scholar] [CrossRef]
- Kislik, V.; Eyal, A. Hybrid liquid membrane (HLM) and supported liquid membrane (SLM) based transport of titanium (IV). J. Membr. Sci. 1996, 111, 273–281. [Google Scholar] [CrossRef]
- Kislik, V.; Eyal, A. Hybrid liquid membrane (HLM) system in separation technologies. J. Membr. Sci. 1996, 111, 259–272. [Google Scholar] [CrossRef]
- Galán, B.; Roman, F.S.; Irabien, A.; Ortiz, I. Viability of the separation of Cd from highly concentrated Ni−Cd mixtures by non-dispersive solvent extraction. Chem. Eng. J. 1998, 70, 237–243. [Google Scholar] [CrossRef]
- Raut, D.R.; Mohapatra, P.K. Non-Dispersive Solvent Extraction of Uranium from Nitric Acid Medium by Several Amides and their Mixture with TODGA using a Hollow Fiber Contactor. Sep. Sci. Technol. 2013, 48, 2436–2443. [Google Scholar] [CrossRef]
- Molinari, R.; Caruso, A.; Argurio, P.; Poerio, T. Diclofenac Transport through Stagnant Sandwich and Supported Liquid Membrane Systems. Ind. Eng. Chem. Res. 2006, 45, 9115–9121. [Google Scholar] [CrossRef]
- Abraham, M.R.; Susan, T.B. Water contamination with heavy metals and trace elements from Kilembe copper mine and tailing sites in Western Uganda; implications for domestic water quality. Chemosphere 2017, 169, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.T.; Lin, C.; Shern, C.-C.; Yeh, G.; Le, V.G.; Tran, H.T. Contamination, ecological risk and source apportionment of heavy metals in sediments and water of a contaminated river in Taiwan. Ecol. Indic. 2017, 82, 32–42. [Google Scholar] [CrossRef]
- Barkouch, Y.; Pineau, A.; Eddine, E.K.M. A New Approach to Understanding Well Water Contamination by Heavy Metals at a Mining Extract Region in Marrakech, Morocco. Pol. J. Environ. Stud. 2016, 25, 1347–1351. [Google Scholar] [CrossRef]
- Molinari, R.; Poerio, T.; Argurio, P. Selective separation of copper(II) and nickel(II) from aqueous media using the complexation–ultrafiltration process. Chemosphere 2008, 70, 341–348. [Google Scholar] [CrossRef]
- Gao, J.; Qiu, Y.; Hou, B.; Zhang, Q.; Zhang, X. Treatment of wastewater containing nickel by complexation- ultrafiltration using sodium polyacrylate and the stability of PAA-Ni complex in the shear field. Chem. Eng. J. 2018, 334, 1878–1885. [Google Scholar] [CrossRef]
- Molinari, R.; Poerio, T.; Argurio, P. Polymer assisted ultrafiltration for copper?citric acid chelate removal from wash solutions of contaminated soil. J. Appl. Electrochem. 2005, 35, 375–380. [Google Scholar] [CrossRef]
- Molinari, R.; Gallo, S.; Argurio, P. Metal ions removal from wastewater or washing water from contaminated soil by ultrafiltration–complexation. Water Res. 2004, 38, 593–600. [Google Scholar] [CrossRef]
- Shao, J.; Qin, S.; Davidson, J.; Li, W.; He, Y.; Zhou, H.S. Recovery of nickel from aqueous solutions by complexation-ultrafiltration process with sodium polyacrylate and polyethylenimine. J. Hazard. Mater. 2013, 244, 472–477. [Google Scholar] [CrossRef]
- Rivas, B.L.; Moreno-Villoslada, I. Prediction of the retention values associated to the ultrafiltration of mixtures of metal ions and high molecular weight water-soluble polymers as a function of the initial ionic strength. J. Membr. Sci. 2000, 178, 165–170. [Google Scholar] [CrossRef]
- Zeng, J.X.; Ye, H.Q.; Huang, N.D.; Liu, J.F.; Zheng, L.F. Selective separation of Hg(II) and Cd(II) from aqueous solutions by complexation–ultrafiltration process. Chemosphere 2009, 76, 706–710. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Qiu, Y.-R. Removal of copper(II) ions from aqueous solutions by complexation–ultrafiltration using rotating disk membrane and the shear stability of PAA–Cu complex. Chem. Eng. Res. Des. 2018, 136, 712–720. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, Y. Removal of Cd (II) from dilute aqueous solutions by complexation–ultrafiltration using rotating disk membrane and the shear stability of PAA–Cd complex. Chin. J. Chem. Eng. 2019, 27, 519–527. [Google Scholar] [CrossRef]
- Mateur, M.N.; Ortiz, D.G.; Ennigrou, D.J.J.; Horchani-Naifer, K.; Bechelany, M.; Miele, P.; Pochat-Bohatier, C. Porous Gelatin Membranes Obtained from Pickering Emulsions Stabilized with h-BNNS: Application for Polyelectrolyte-Enhanced Ultrafiltration. Membranes 2020, 10, 144. [Google Scholar] [CrossRef]
- Bhowmik, A.K.; Alamdar, A.; Katsoyiannis, I.; Shen, H.; Ali, N.; Ali, S.M.; Bokhari, H.; Schäfer, R.B.; Eqani, S.A.M.A.S. Mapping human health risks from exposure to trace metal contamination of drinking water sources in Pakistan. Sci. Total. Environ. 2015, 538, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rahman, M.M.; Ramanathan, A.L.; Naidu, R. Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: Health risk index. Sci. Total. Environ. 2016, 539, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Argurio, P. Arsenic removal from water by coupling photocatalysis and complexation-ultrafiltration processes: A preliminary study. Water Res. 2017, 109, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Trivunac, K.; Stevanovic, S. Removal of heavy metal ions from water by complexation-assisted ultrafiltration. Chemosphere 2006, 64, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Rivas, B.L.; Aguirre, M.D.C.; Pereira, E. Retention properties of arsenate anions of water-soluble polymers by a liquid-phase polymer-based retention technique. J. Appl. Polym. Sci. 2006, 102, 2677–2684. [Google Scholar] [CrossRef]
- Rivas, B.L.; Sánchez, J.; Pooley, S.A.; Basaez, L.; Pereira, E.; Bucher, C.; Royal, G.; Aman, E.S.; Moutet, J.-C. Water-Soluble Polyelectrolytes with Ability to Remove Arsenic. Macromol. Symp. 2010, 296, 416–428. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, T.; Liu, X.; Zhang, W. Simultaneous oxidation and adsorption of As(III) from water by cerium modified chitosan ultrafine nanobiosorbent. J. Hazard. Mater. 2016, 308, 1–10. [Google Scholar] [CrossRef]
- Molinari, R.; Caruso, A.; Argurio, P.; Poerio, T. Degradation of the drugs Gemfibrozil and Tamoxifen in pressurized and de-pressurized membrane photoreactors using suspended polycrystalline TiO2 as catalyst. J. Membr. Sci. 2008, 319, 54–63. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Rohit, G.; Holkar, C.R.; Pinjari, D.; Bhanvase, B.; Pandit, A.B. Investigation of TiO2 photocatalyst performance for decolorization in the presence of hydrodynamic cavitation as hybrid AOP. Ultrason. Sonochem. 2016, 28, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Lelario, F.; Brienza, M.; A Bufo, S.; Scrano, L. Effectiveness of different advanced oxidation processes (AOPs) on the abatement of the model compound mepanipyrim in water. J. Photochem. Photobiol. A Chem. 2016, 321, 187–201. [Google Scholar] [CrossRef]
- Molinari, R.; Poerio, T.; Argurio, P. Chemical and operational aspects in running the polymer assisted ultrafiltration for separation of copper(II)–citrate complexes from aqueous media. J. Membr. Sci. 2007, 295, 139–147. [Google Scholar] [CrossRef]
- Moser, P.B.; Silva, G.R.D.A.; Lima, L.S.F.; Moreira, V.R.; Lebron, Y.A.R.; De Paula, E.C.; Amaral, M.C. Effect of organic and inorganic draw solution on recalcitrant compounds build up in a hybrid ultrafiltration-osmotic membrane reactor treating refinery effluent. Chem. Eng. J. 2021, 403, 126374. [Google Scholar] [CrossRef]
- Naddeo, V.; Secondes, M.F.N.; Borea, L.; Hasan, S.W.; Ballesteros, F.; Belgiorno, V. Removal of contaminants of emerging concern from real wastewater by an innovative hybrid membrane process—UltraSound, Adsorption, and Membrane ultrafiltration (USAMe®). Ultrason. Sonochem. 2020, 68, 105237. [Google Scholar] [CrossRef]
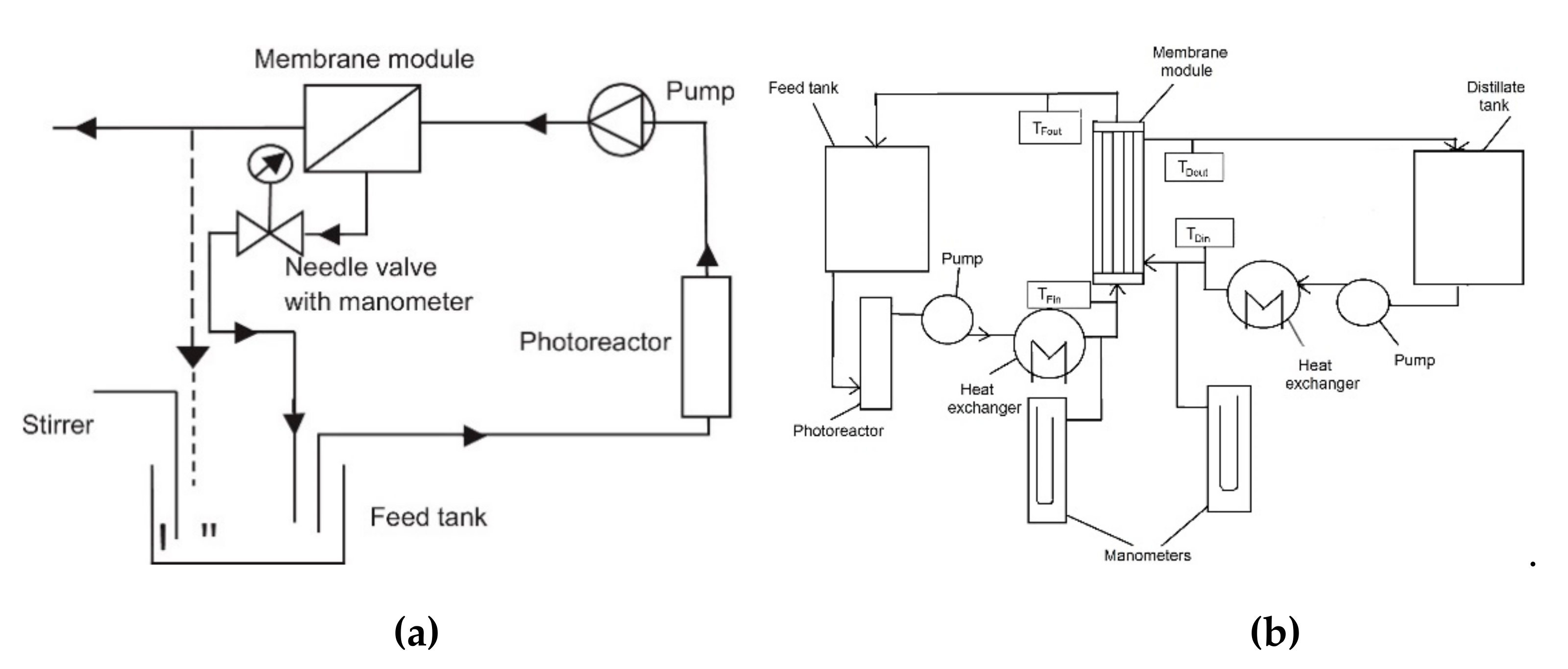
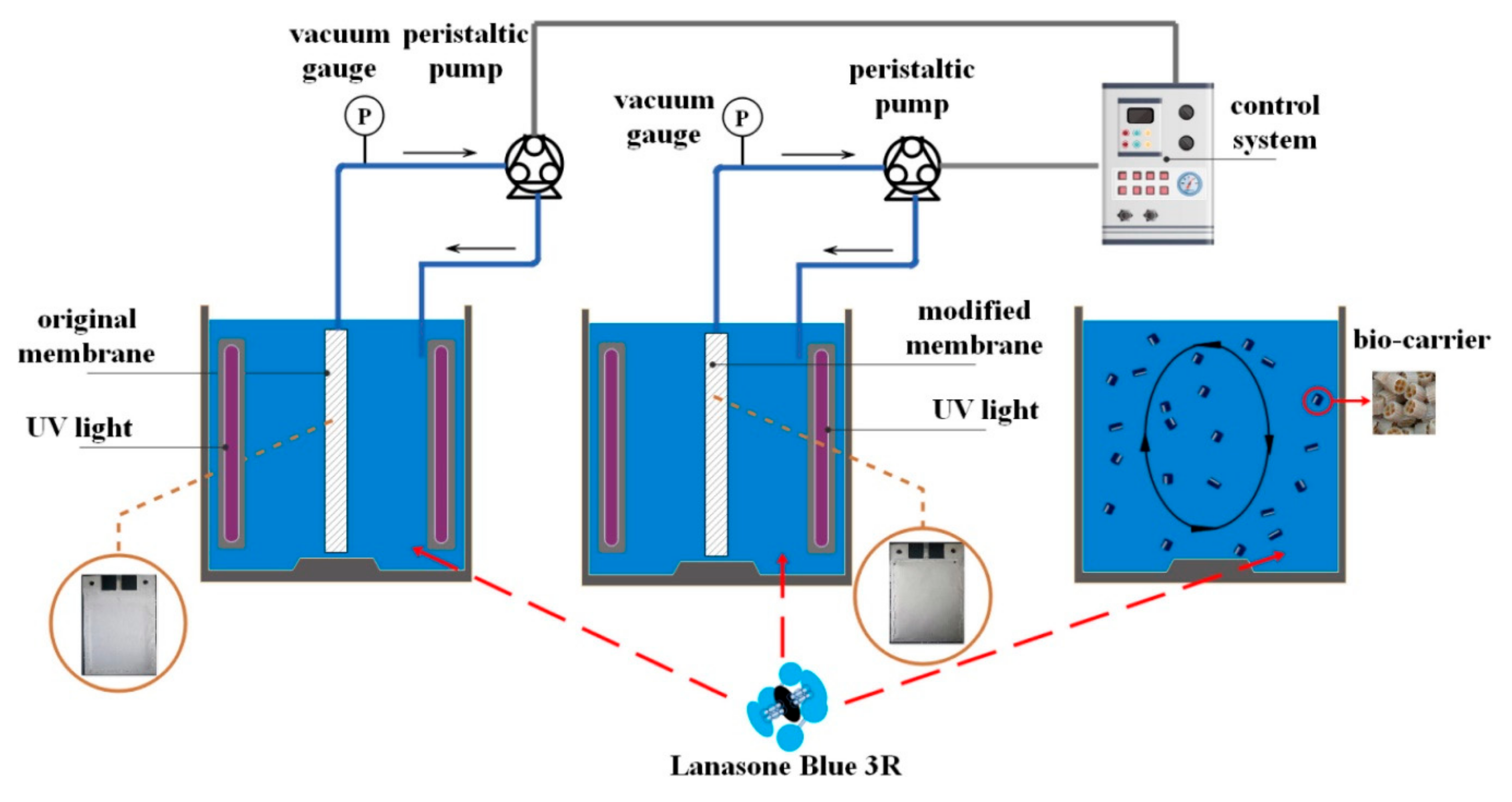
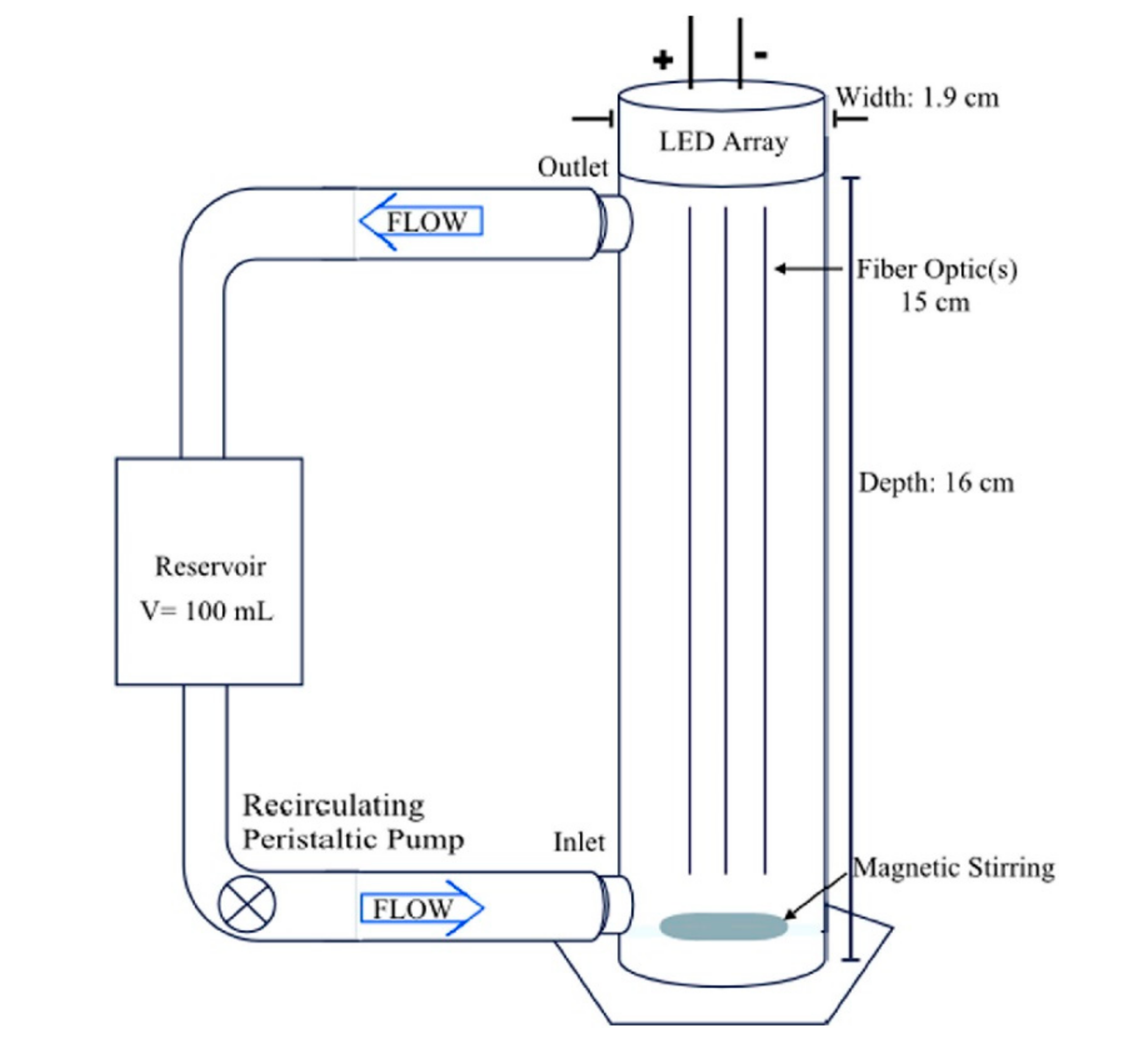
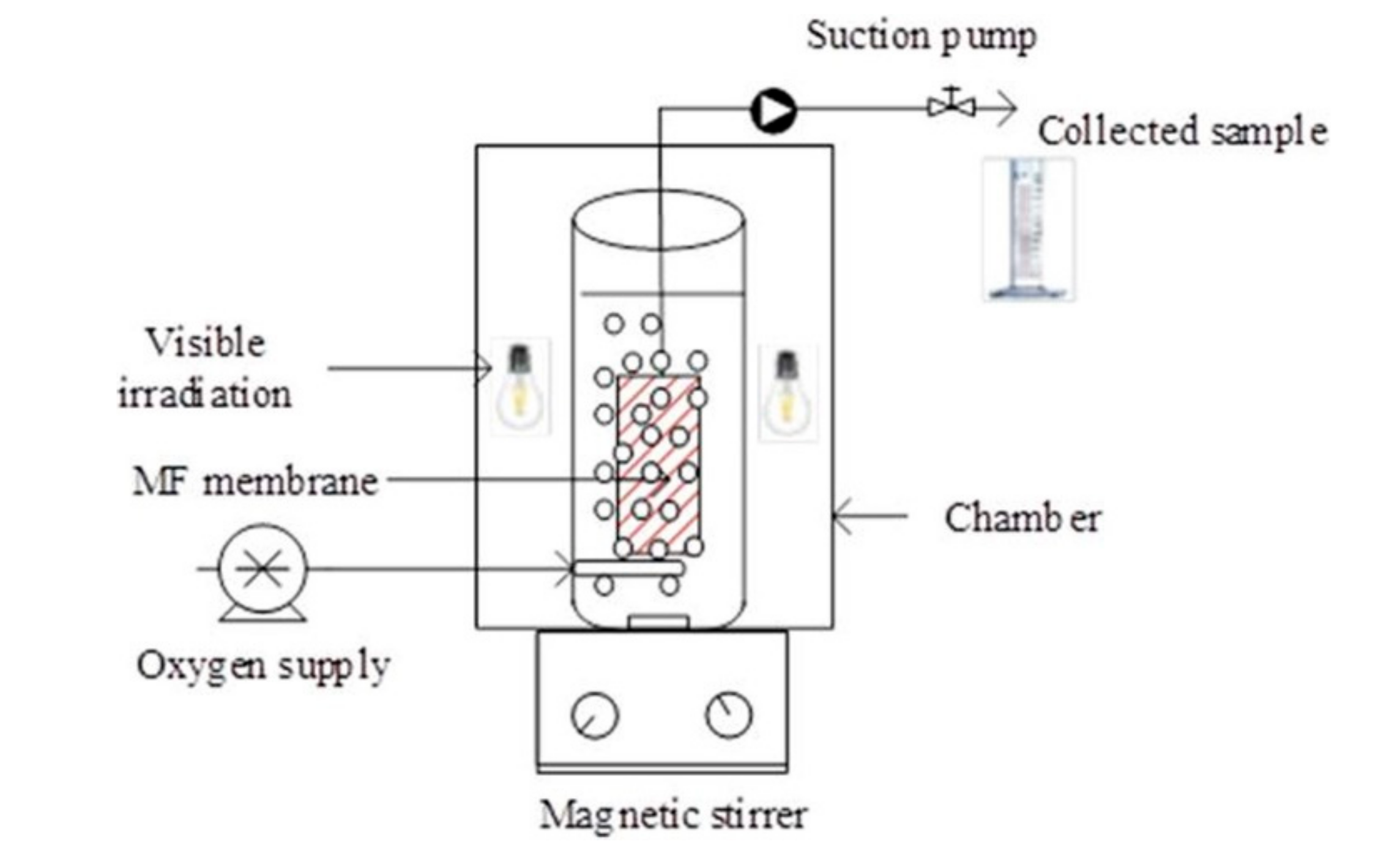
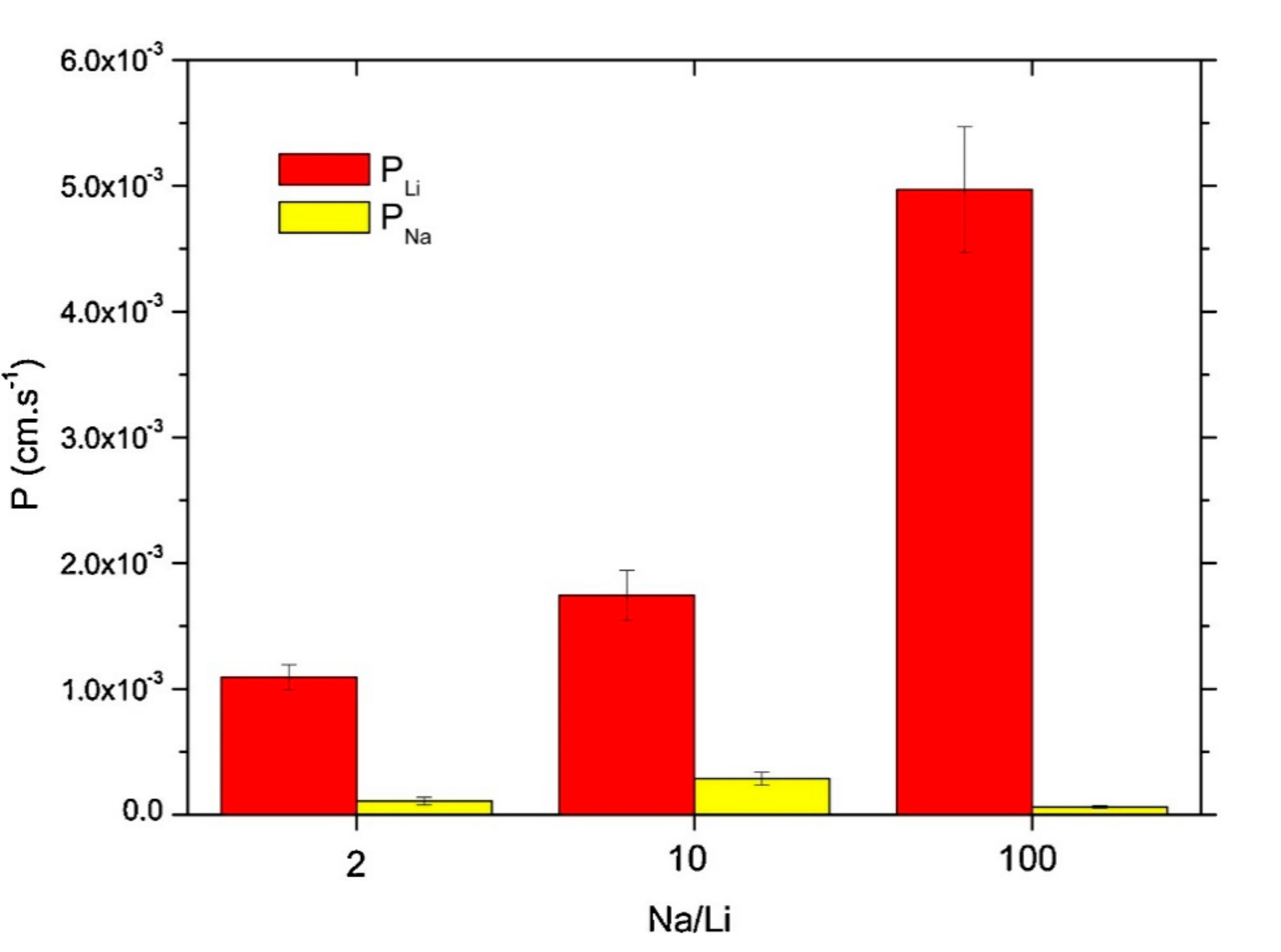
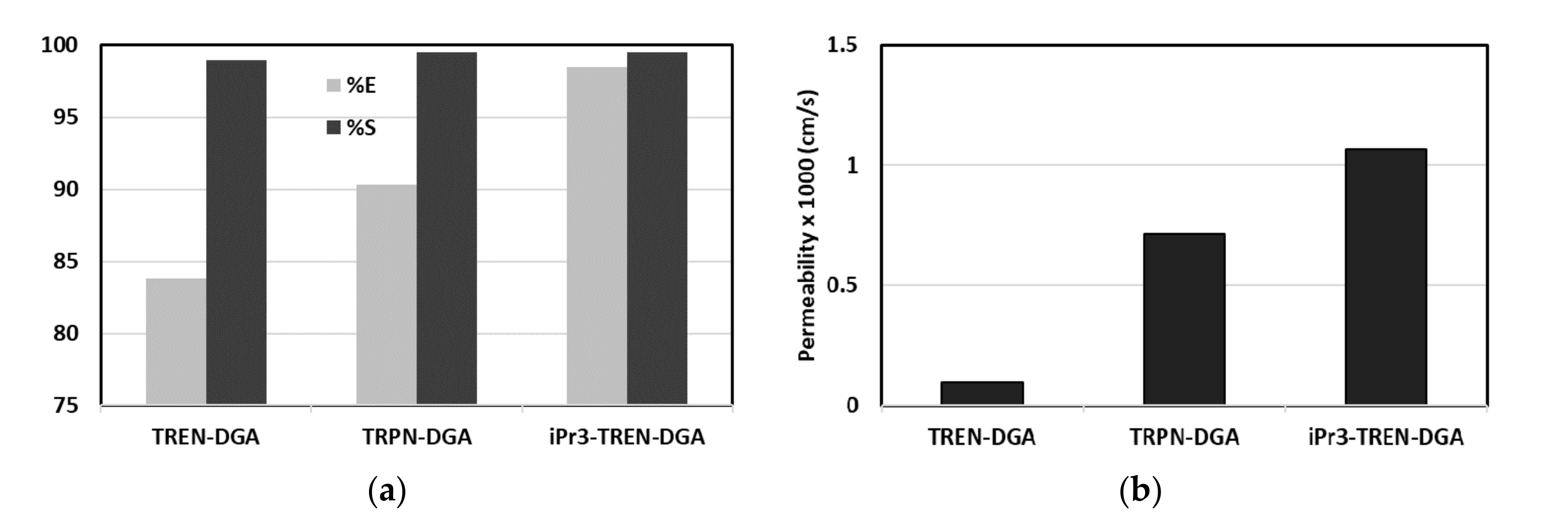
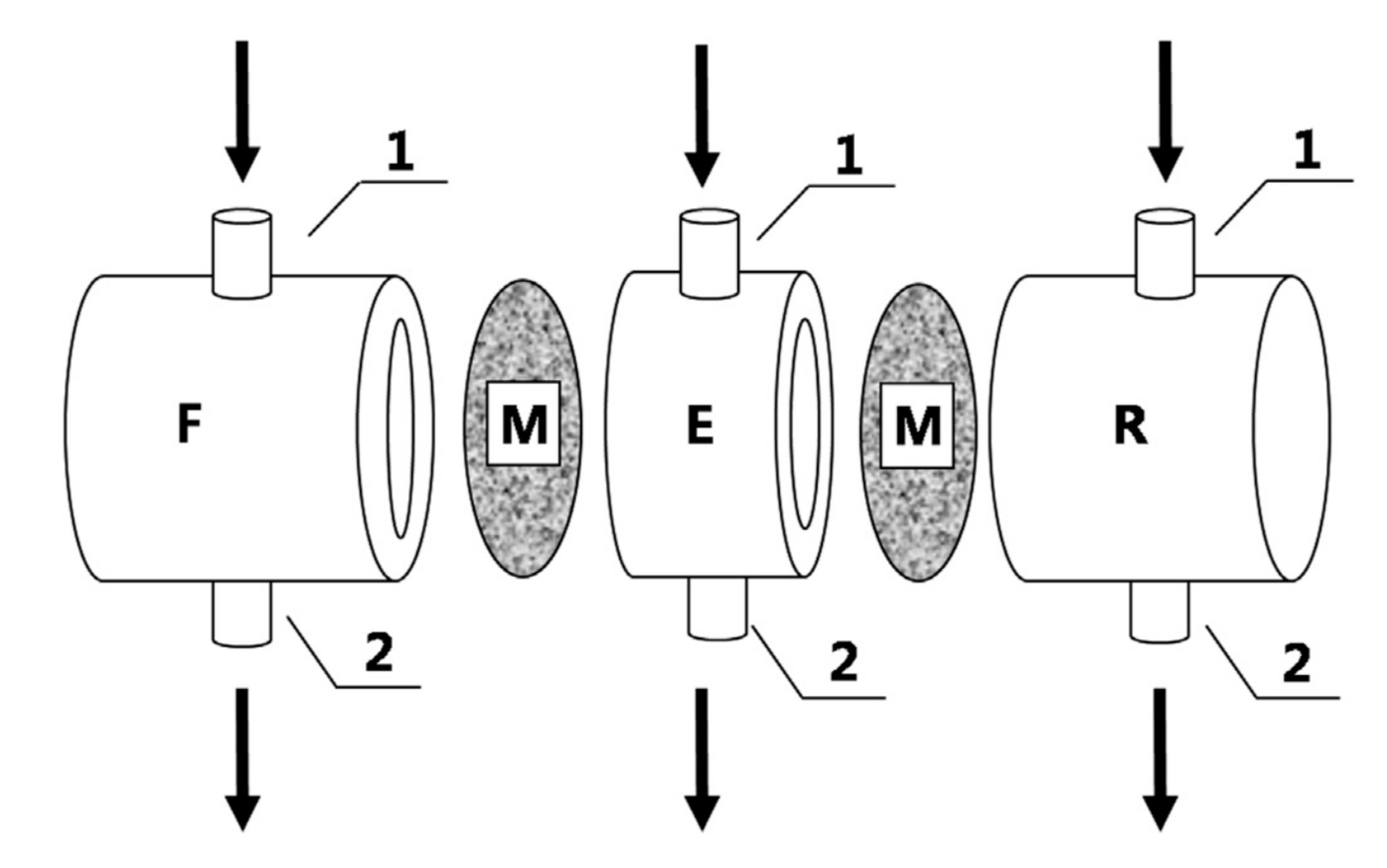
| Application | Types of Coupled Processes in the HMP | Main Advantages | Ref |
|---|---|---|---|
| Removal of secondary effluent organic matter (SEOM) from secondary effluents (SE) | Photocatalytic membrane reactor (PMR) | SEOM degradation > 60%reduction of membrane fouling propensity | [30] |
| Treatment of primary (PE) and secondary effluents (SE) coming from municipal wastewater treatment plant | PMR with UF and direct contact membrane distillation (DCMD) | The coupling photocatalysis-membrane processes resulted in increased the permeate flux only in the case of UF | [31] |
| Treatment of SE coming from a municipal wastewater treatment plant for removing pharmaceuticals | PMR coupling UF with: UVC/H2O2 UVC/TiO2 UVC | 100% OTC removal 49% mineralization in 5 h in the UVC/TiO2-UF system | [35] |
| Photocatalytic degradation of red blue 5 (RB5) | PMR | 88.89% RB5 degradation by using ZnO in 180 min 98.34% by using Fe3+@ZnO in 180 min | [44] |
| Photocatalytic degradation of rhodamine B (RhB) | PMR | RhB degradation > 60% increased antifouling ability using a visible-light-driven g-C3N4/TNA membrane | [45] |
| Photocatalytic degradation of methylene blue (MB) | PMR | MB degradation > 90% for four consecutive runs using P-doped g-C3N4 (PCN) as photocatalyst | [46] |
| Photocatalytic degradation of MB | PMR | 90% MB degradation after 120 min under visible light by using Ag/GO/TNTs | [48] |
| Photocatalytic degradation of MB | PMR | 83.5% MB degradation under simulated solar light irradiation | [37] |
| Photocatalytic degradation of lanasol blue 3R (LB)e | PMR | up to 91.42% LB degradation also after 5 degradation cycles excellent self-cleaning ability using the modified (TiO2/PSS) membrane showed | [50] |
| Photocatalytic degradation of bisphenol A (BPA) | PMR | 90% BPA removal under UV with dual-layer hollow fiber membrane (DLHF) 81.6% BPA removal under visible light using the N-doped TiO2 DLHF | [56] |
| Photocatalytic degradation of diclofenac (DCF) | Submerged PMR (SMPR) with suspended photocatalyst | Pseudo-first-order kinetic Beneficial effect of H2O2 of on system performance | [59] |
| Photocatalytic degradation of paracetamol (PCT), furosemide (FRS), nimesulide (NMD), diazepam (DZP) | Tube-in-tube PMR | 27.4% (PCT), 35.0% (FRS), 24.2% (NMD) and 30.0% (DZP) degradation in synthetic wastewater at steady-state for the UVC/H2O2/TiO2 system. Lower degradations by using an urban wastewater after secondary treatment | [61] |
| Recovery and concentration of neodymium from acidic media | Supported liquid membrane (SLM) | 0.381 cm min−1 membrane permeability 99.14% Nd recovery 114 feed/strip concentration ratio | [72] |
| Selective recovery of lithium from lithium-rich brines | SLM | High selectivity over lithium even for high sodium/lithium concentration ratio | [74] |
| Separation of actinides from lean acidic effluents | SLM | ca. 98% extraction ca. 99% stripping | [75] |
| Selective separation of Ni(II) from Cu(II) | CP–UF | 94% Cu(II) recovery in retentate 100% Ni(II) recovery in permeate Same selectivity but a higher membrane fouling by using a real aqueous effluent | [104] |
| Selective separation of Hg(II) from Cd(II) | CP–UF | 100% Hg rejection 10% Cd rejection. Diafiltration of the CP–UF retentate permitted increase separation selectivity | [110] |
| Recovery of nickel from wastewaters | CP–UF in a rotating disk system | 98.26% Ni2+ rejection at a rotating speed RS < 848 rpm polymer regeneration and nickel recovery at a RS > 848 rpm | [105] |
| Removal of copper(II) from aqueous solutions | CP–UF in a rotating disk system | 99.6% Cu(II) rejection. Shear stability and critical shear rate of the polymer–metal complex are important in view of industrial applications of CP–UF polymer regeneration and copper recovery at a RS > critical RS | [111] |
| Removal of arsenic ions from water | CP–UF coupled with photocatalysis | Quite complete As removal by coupling photocatalytic oxidation of As(III) to As(V) and CP–UF | [116] |
| Treatment of oil refinery effluent | Hybrid ultrafiltration-osmotic membrane bioreactor (UF-OMBR) | The use of CH3COONa instead of NaCl as salt in the draw solution (DS): favored the microbiological activity enhanced recalcitrant biodegradation favored the microbial growth over the membrane, thus leading to a biofouling layer formation with consequent decrease of flux (0.60 vs. 1.07 L m−2 h−1) | [125] |
| Removal of emerging contaminants (ECs) from SE | HMP called USAMe®, ultrasound irradiation (US), adsorption (A), membrane filtration (Me) | ECs removal of 99% by using the USAMe HMP. | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinari, R.; Lavorato, C.; Argurio, P. Application of Hybrid Membrane Processes Coupling Separation and Biological or Chemical Reaction in Advanced Wastewater Treatment. Membranes 2020, 10, 281. https://doi.org/10.3390/membranes10100281
Molinari R, Lavorato C, Argurio P. Application of Hybrid Membrane Processes Coupling Separation and Biological or Chemical Reaction in Advanced Wastewater Treatment. Membranes. 2020; 10(10):281. https://doi.org/10.3390/membranes10100281
Chicago/Turabian StyleMolinari, Raffaele, Cristina Lavorato, and Pietro Argurio. 2020. "Application of Hybrid Membrane Processes Coupling Separation and Biological or Chemical Reaction in Advanced Wastewater Treatment" Membranes 10, no. 10: 281. https://doi.org/10.3390/membranes10100281
APA StyleMolinari, R., Lavorato, C., & Argurio, P. (2020). Application of Hybrid Membrane Processes Coupling Separation and Biological or Chemical Reaction in Advanced Wastewater Treatment. Membranes, 10(10), 281. https://doi.org/10.3390/membranes10100281