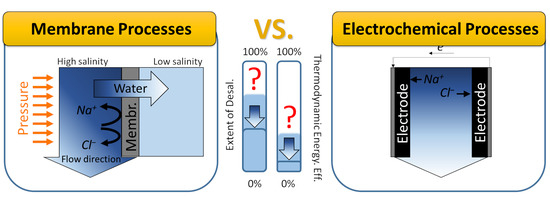
Membrane and Electrochemical Processes for Water Desalination: A Short Perspective and the Role of Nanotechnology
Abstract
1. Introduction
2. Membrane Process
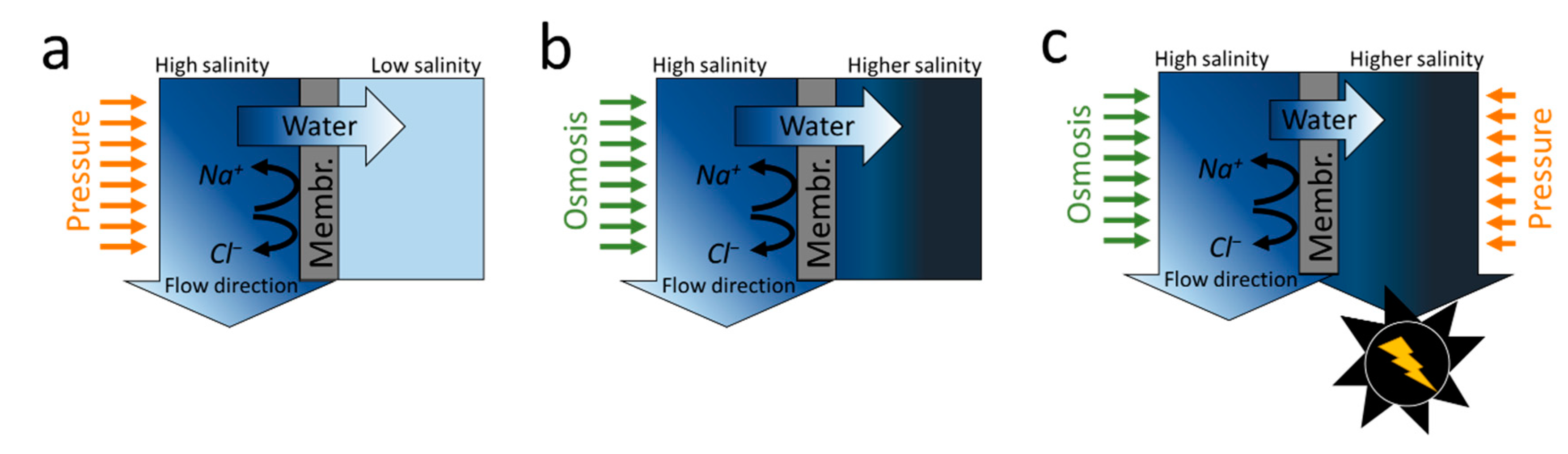
2.1. Reverse Osmosis
2.2. Forward Osmosis
2.3. Hybrid Membrane Processes
3. Electrochemical Cells
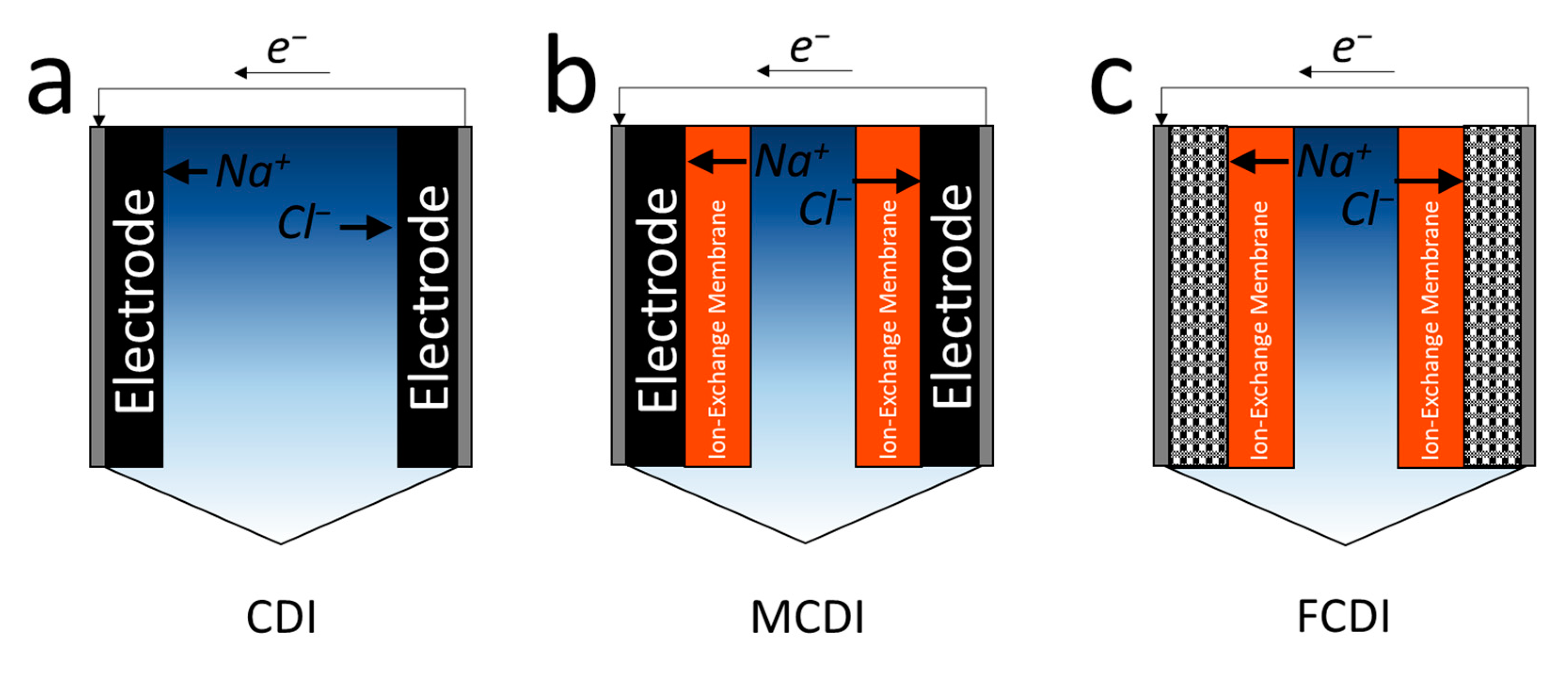
3.1. Capacitive Deionization
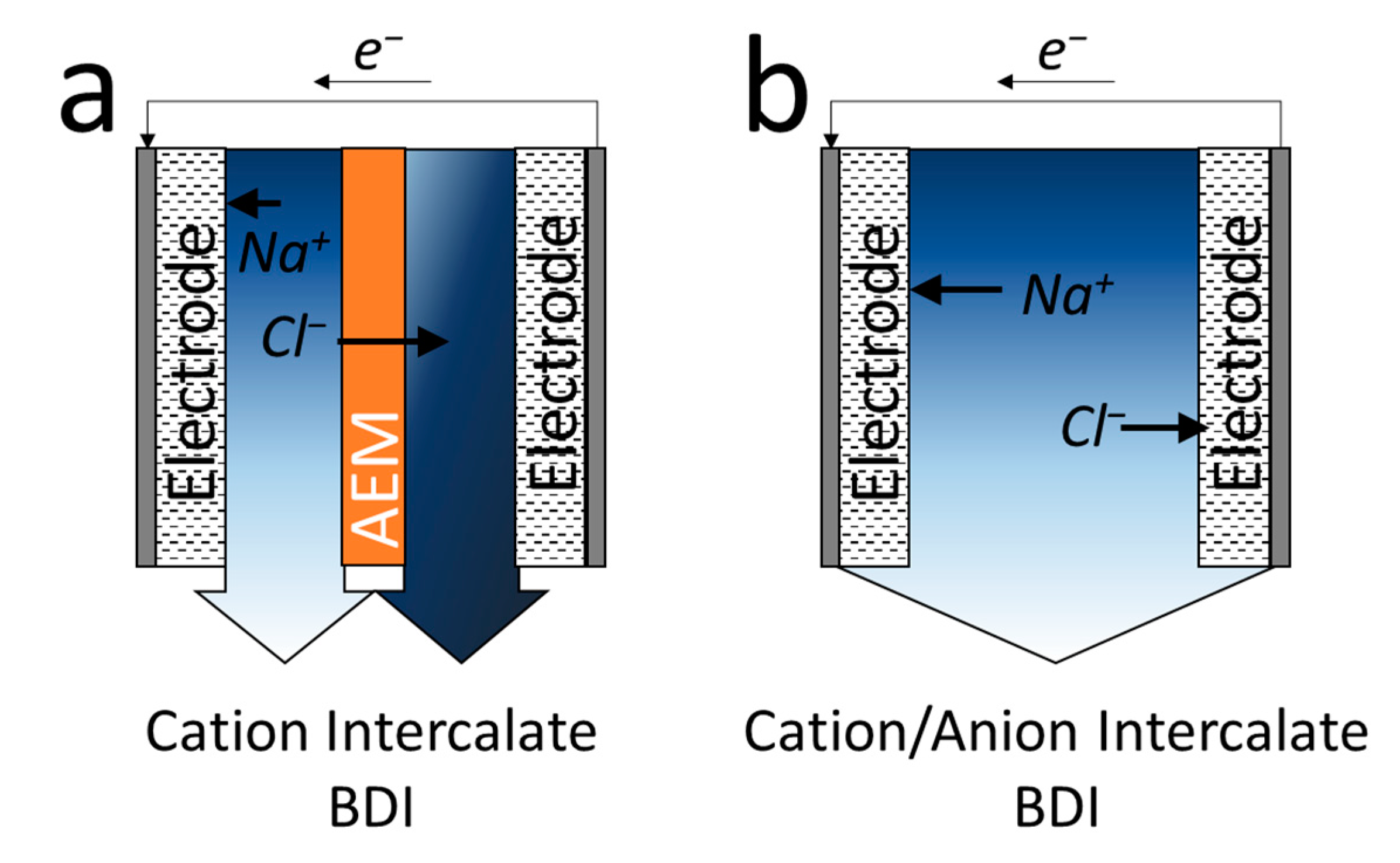
3.2. Battery Deionization
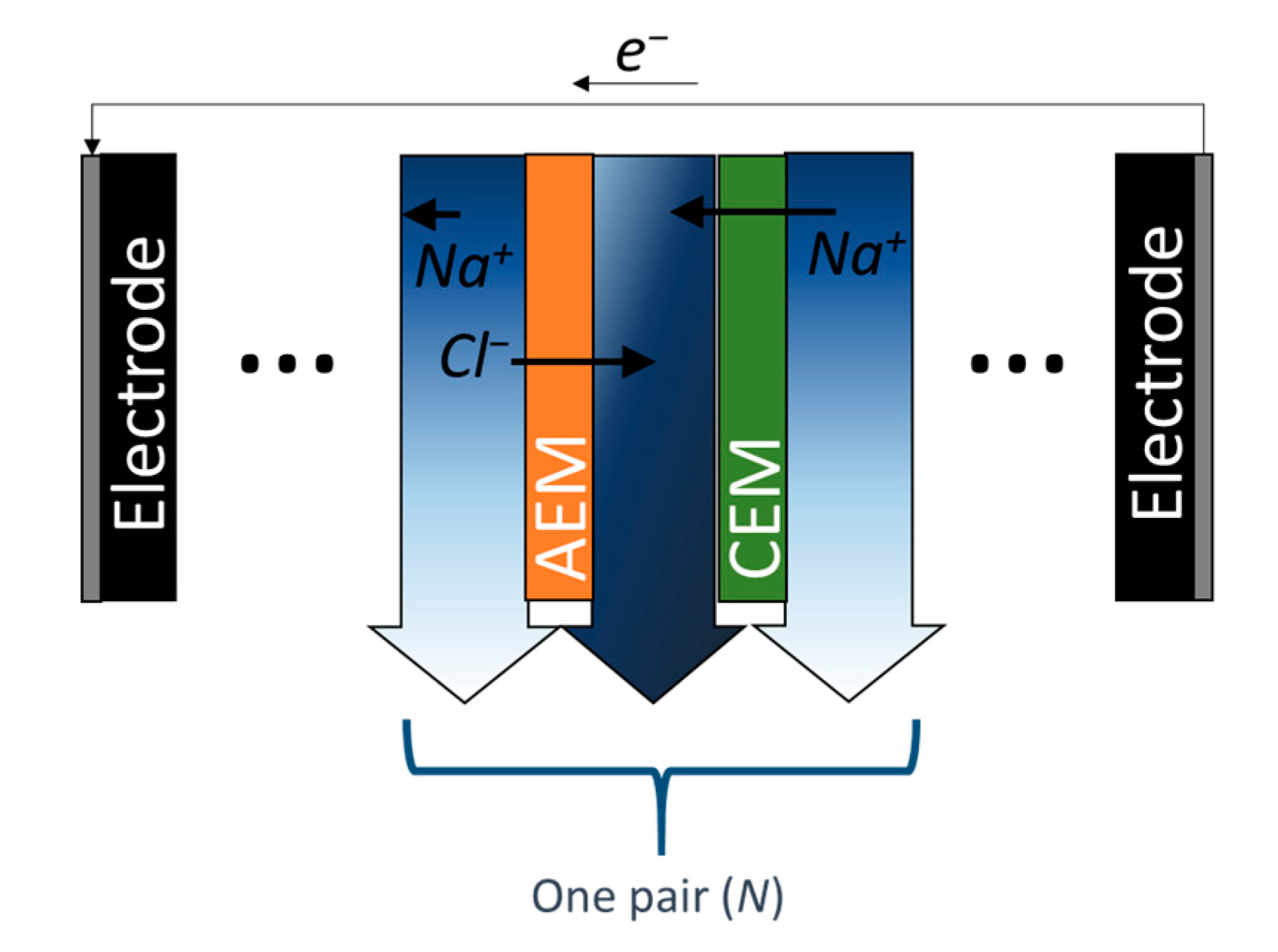
3.3. Electrodialysis
4. Energy and Resource Recovery
5. Perspectives for Future Desalination and the Role of Nanotechnology
Author Contributions
Funding
Conflicts of Interest
References
- Semiat, R. Energy Issues in Desalination Processes. Environ. Sci. Technol. 2008, 42, 8193–8201. [Google Scholar] [CrossRef]
- Al-Karaghouli, A.; Kazmerski, L.L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356. [Google Scholar] [CrossRef]
- Voutchkov, N. Energy use for membrane seawater desalination—Current status and trends. Desalination 2018, 431, 2–14. [Google Scholar] [CrossRef]
- Misdan, N.; Lau, W.J.; Ismail, A.F. Seawater Reverse Osmosis (SWRO) desalination by thin-film composite membrane—Current development, challenges and future prospects. Desalination 2012, 287, 228–237. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Avlonitis, S.; Kouroumbas, K.; Vlachakis, N. Energy consumption and membrane replacement cost for seawater RO desalination plants. Desalination 2003, 157, 151–158. [Google Scholar] [CrossRef]
- Sauvet-Goichon, B. Ashkelon desalination plant—A successful challenge. Desalination 2007, 203, 75–81. [Google Scholar] [CrossRef]
- Shrivastava, A.; Rosenberg, S.; Peery, M. Energy efficiency breakdown of reverse osmosis and its implications on future innovation roadmap for desalination. Desalination 2015, 368, 181–192. [Google Scholar] [CrossRef]
- Jeong, K.; Park, M.; Chong, T.H. Numerical model-based analysis of energy-efficient reverse osmosis (EERO) process: Performance simulation and optimization. Desalination 2019, 453, 10–21. [Google Scholar] [CrossRef]
- Kim, T.; Gorski, C.A.; Logan, B.E. Low energy desalination using battery electrode deionization. Environ. Sci. Technol. Lett. 2017, 4, 444–449. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive deionization (CDI) for desalination and water treatment—Past, present and future (a review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Tsiakis, P.; Papageorgiou, L.G. Optimal design of an electrodialysis brackish water desalination plant. Desalination 2005, 173, 173–186. [Google Scholar] [CrossRef]
- Qin, M.; Deshmukh, A.; Epsztein, R.; Patel, S.K.; Owoseni, O.M.; Walker, W.S.; Elimelech, M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 2019, 455, 100–114. [Google Scholar] [CrossRef]
- Ramachandran, A.; Oyarzun, D.I.; Hawks, S.A.; Campbell, P.G.; Stadermann, M.; Santiago, J.G. Comments on “Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis”. Desalination 2019, 461, 30–36. [Google Scholar] [CrossRef]
- Lin, S. Energy Efficiency of Desalination: Fundamental Insights from Intuitive Interpretation. Environ. Sci. Technol. 2020, 54, 76–84. [Google Scholar] [CrossRef]
- Wang, L.; Lin, S. Theoretical framework for designing a desalination plant based on membrane capacitive deionization. Water Res. 2019, 158, 359–369. [Google Scholar] [CrossRef]
- Wang, L.; Dykstra, J.E.; Lin, S. Energy Efficiency of Capacitive Deionization. Environ. Sci. Technol. 2019, 53, 3366–3378. [Google Scholar] [CrossRef]
- Okamoto, Y.; Lienhard, J.H. How RO membrane permeability and other performance factors affect process cost and energy use: A review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Jeong, K.; Park, M.; Ki, S.J.; Kim, J.H. A systematic optimization of Internally Staged Design (ISD) for a full-scale reverse osmosis process. J. Membr. Sci. 2017, 540, 285–296. [Google Scholar] [CrossRef]
- Manth, T.; Gabor, M.; Oklejas, E. Minimizing RO energy consumption under variable conditions of operation. Desalination 2003, 157, 9–21. [Google Scholar] [CrossRef]
- Kamada, T.; Ohara, T.; Shintani, T.; Tsuru, T. Controlled surface morphology of polyamide membranes via the addition of co-solvent for improved permeate flux. J. Membr. Sci. 2014, 467, 303–312. [Google Scholar] [CrossRef]
- Song, X.; Qi, S.; Tang, C.Y.; Gao, C. Ultra-thin, multi-layered polyamide membranes: Synthesis and characterization. J. Membr. Sci. 2017, 540, 10–18. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, P.C.Y.; Ho, W.S.W. High-flux reverse osmosis membranes incorporated with hydrophilic additives for brackish water desalination. Desalination 2013, 308, 225–232. [Google Scholar] [CrossRef]
- Son, M.; Choi, H.-G.; Liu, L.; Celik, E.; Park, H.; Choi, H. Efficacy of carbon nanotube positioning in the polyethersulfone support layer on the performance of thin-film composite membrane for desalination. Chem. Eng. J. 2015, 266, 376–384. [Google Scholar] [CrossRef]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. The Critical Need for Increased Selectivity, Not Increased Water Permeability, for Desalination Membranes. Environ. Sci. Technol. Lett. 2016, 3, 112–120. [Google Scholar] [CrossRef]
- Son, M.; Bae, J.; Park, H.; Choi, H. Continuous thermal-rolling of electrospun nanofiber for polyamide layer deposition and its detection by engineered osmosis. Polymer 2018, 145, 281–285. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Weyland, M.; Yuan, S.; Xia, Y.; Liu, H.; Jian, M.; Yang, J.; Easton, C.D.; Selomulya, C.; et al. Thermally Reduced Nanoporous Graphene Oxide Membrane for Desalination. Environ. Sci. Technol. 2019, 53, 8314–8323. [Google Scholar] [CrossRef]
- Zarrabi, H.; Yekavalangi, M.E.; Vatanpour, V.; Shockravi, A.; Safarpour, M. Improvement in desalination performance of thin film nanocomposite nanofiltration membrane using amine-functionalized multiwalled carbon nanotube. Desalination 2016, 394, 83–90. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Hoek, E.M.V.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Tang, C.Y.; Zhao, Y.; Wang, R.; Hélix-Nielsen, C.; Fane, A.G. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Tang, C.Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Cheng, Y.; Hao, Z.; Hao, C.; Deng, Y.; Li, X.; Li, K.; Zhao, Y. A review of modification of carbon electrode material in capacitive deionization. RSC Adv. 2019, 9, 24401–24419. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, J.; Han, J.; Yan, T.; Shi, L.; Zhang, D. N, P, S co-doped hollow carbon polyhedra derived from MOF-based core–shell nanocomposites for capacitive deionization. J. Mater. Chem. A 2018, 6, 15245–15252. [Google Scholar] [CrossRef]
- Peng, L.E.; Yao, Z.; Liu, X.; Deng, B.; Guo, H.; Tang, C.Y. Tailoring Polyamide Rejection Layer with Aqueous Carbonate Chemistry for Enhanced Membrane Separation: Mechanistic Insights, Chemistry-Structure-Property Relationship, and Environmental Implications. Environ. Sci. Technol. 2019, 53, 9764–9770. [Google Scholar] [CrossRef]
- Son, M.; Yang, W.; Bucs, S.S.; Nava-Ocampo, M.F.; Vrouwenvelder, J.S.; Logan, B.E. Polyelectrolyte-Based Sacrificial Protective Layer for Fouling Control in Reverse Osmosis Desalination. Environ. Sci. Technol. Lett. 2018, 5, 584–590. [Google Scholar] [CrossRef]
- Nava-Ocampo, M.F.; Bucs, S.S.; Farinha, A.S.F.; Son, M.; Logan, B.E.; Vrouwenvelder, J.S. Sacrificial coating development for biofouling control in membrane systems. Desalination 2020, 496, 114650. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, T.; Lu, T.; Sun, Z.; Chua, D.H.C.; Pan, L. Nitrogen-doped porous carbon spheres for highly efficient capacitive deionization. Electrochim. Acta 2015, 158, 403–409. [Google Scholar] [CrossRef]
- Gao, T.; Du, Y.; Li, H. Preparation of nitrogen-doped graphitic porous carbon towards capacitive deionization with high adsorption capacity and rate capability. Sep. Purif. Technol. 2019, 211, 233–241. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, M.; Xu, X.; Lu, T.; Sun, C.Q.; Pan, L. Phosphorus-doped 3D carbon nanofiber aerogels derived from bacterial-cellulose for highly-efficient capacitive deionization. Carbon 2018, 130, 377–383. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Kim, S.-R.; Kang, J.; Kim, S.; Kim, C.; Yoon, J. Capacitive deionization with Ca-alginate coated-carbon electrode for hardness control. Desalination 2016, 392, 46–53. [Google Scholar] [CrossRef]
- Han, J.; Shi, L.; Yan, T.; Zhang, J.; Zhang, D. Removal of ions from saline water using N, P co-doped 3D hierarchical carbon architectures via capacitive deionization. Environ. Sci. Nano 2018, 5, 2337–2345. [Google Scholar] [CrossRef]
- Zhu, A.; Christofides, P.D.; Cohen, Y. Minimization of energy consumption for a two-pass membrane desalination: Effect of energy recovery, membrane rejection and retentate recycling. J. Membr. Sci. 2009, 339, 126–137. [Google Scholar] [CrossRef]
- Zhu, A.; Christofides, P.D.; Cohen, Y. On RO membrane and energy costs and associated incentives for future enhancements of membrane permeability. J. Membr. Sci. 2009, 344, 1–5. [Google Scholar] [CrossRef]
- Chae, S.H.; Kim, Y.M.; Park, H.; Seo, J.; Lim, S.J.; Kim, J.H. Modeling and Simulation Studies Analyzing the Pressure-Retarded Osmosis (PRO) and PRO-Hybridized Processes. Energies 2019, 12, 243. [Google Scholar] [CrossRef]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. Can batch or semi-batch processes save energy in reverse-osmosis desalination? Desalination 2017, 402, 109–122. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Song, L.; Hu, J.Y.; Ong, S.L.; Ng, W.J.; Elimelech, M.; Wilf, M. Performance limitation of the full-scale reverse osmosis process. J. Membr. Sci. 2003, 214, 239–244. [Google Scholar] [CrossRef]
- Qasim, M.; Darwish, N.A.; Sarp, S.; Hilal, N. Water desalination by forward (direct) osmosis phenomenon: A comprehensive review. Desalination 2015, 374, 47–69. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, X.M. A critical review on draw solutes development for forward osmosis. Desalination 2016, 391, 16–29. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Akther, N.; Sodiq, A.; Giwa, A.; Daer, S.; Arafat, H.A.; Hasan, S.W. Recent advancements in forward osmosis desalination: A review. Chem. Eng. J. 2015, 281, 502–522. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. A novel ammonia—Carbon dioxide forward (direct) osmosis desalination process. Desalination 2005, 174, 1–11. [Google Scholar] [CrossRef]
- Qin, M.; He, Z. Self-Supplied Ammonium Bicarbonate Draw Solute for Achieving Wastewater Treatment and Recovery in a Microbial Electrolysis Cell-Forward Osmosis-Coupled System. Environ. Sci. Technol. Lett. 2014, 1, 437–441. [Google Scholar] [CrossRef]
- Son, M.; Kim, T.; Yang, W.; Gorski, C.A.; Logan, B.E. Electro-forward osmosis. Environ. Sci. Technol. 2019, 53, 8352–8361. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-G.; Son, M.; Choi, H. Integrating seawater desalination and wastewater reclamation forward osmosis process using thin-film composite mixed matrix membrane with functionalized carbon nanotube blended polyethersulfone support layer. Chemosphere 2017, 185, 1181–1188. [Google Scholar] [CrossRef]
- Bamaga, O.A.; Yokochi, A.; Zabara, B.; Babaqi, A.S. Hybrid FO/RO desalination system: Preliminary assessment of osmotic energy recovery and designs of new FO membrane module configurations. Desalination 2011, 268, 163–169. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.D.; Tang, C.Y.; Le-Clech, P. Opportunities to reach economic sustainability in forward osmosis–reverse osmosis hybrids for seawater desalination. Desalination 2015, 363, 26–36. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.; Comas, J.; Rodriguez-Roda, I.; Le-Clech, P. Efficiently combining water reuse and desalination through forward osmosis—Reverse osmosis (FO-RO) hybrids: A critical review. Membranes 2016, 6, 37. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, H.; Shin, Y.; Jang, Y.; Lee, S. Economic evaluation of a hybrid desalination system combining forward and reverse osmosis. Membranes 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Altaee, A.; Sharif, A.; Zaragoza, G.; Ismail, A.F. Evaluation of FO-RO and PRO-RO designs for power generation and seawater desalination using impaired water feeds. Desalination 2015, 368, 27–35. [Google Scholar] [CrossRef]
- Kim, J.; Park, M.; Snyder, S.A.; Kim, J.H. Reverse osmosis (RO) and pressure retarded osmosis (PRO) hybrid processes: Model-based scenario study. Desalination 2013, 322, 121–130. [Google Scholar] [CrossRef]
- Kim, D.I.; Kim, J.; Shon, H.K.; Hong, S. Pressure retarded osmosis (PRO) for integrating seawater desalination and wastewater reclamation: Energy consumption and fouling. J. Membr. Sci. 2015, 483, 34–41. [Google Scholar] [CrossRef]
- Kim, Y.C.; Elimelech, M. Potential of osmotic power generation by pressure retarded osmosis using seawater as feed solution: Analysis and experiments. J. Membr. Sci. 2013, 429, 330–337. [Google Scholar] [CrossRef]
- Seo, J.; Kim, Y.M.; Chae, S.H.; Lim, S.J.; Park, H.; Kim, J.H. An optimization strategy for a forward osmosis-reverse osmosis hybrid process for wastewater reuse and seawater desalination: A modeling study. Desalination 2019, 463, 40–49. [Google Scholar] [CrossRef]
- Suss, M.; Porada, S.; Sun, X.; Biesheuvel, P.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Choi, J.; Dorji, P.; Shon, H.K.; Hong, S. Applications of capacitive deionization: Desalination, softening, selective removal, and energy efficiency. Desalination 2019, 449, 118–130. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, C.; Liu, X.; Xu, X.; Sun, Z.; Pan, L. Review on carbon-based composite materials for capacitive deionization. RSC Adv. 2015, 5, 15205–15225. [Google Scholar] [CrossRef]
- Patel, S.K.; Ritt, C.L.; Deshmukh, A.; Wang, Z.; Qin, M.; Epsztein, R.; Elimelech, M. The relative insignificance of advanced materials in enhancing the energy efficiency of desalination technologies. Energy Environ. Sci. 2020, 13, 1694–1710. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Tang, W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)—Problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Dorji, P.; Kim, D.I.; Hong, S.; Phuntsho, S.; Shon, H.K. Pilot-scale membrane capacitive deionisation for effective bromide removal and high water recovery in seawater desalination. Desalination 2020, 479, 114309. [Google Scholar] [CrossRef]
- Patel, S.K.; Qin, M.; Walker, W.S.; Elimelech, M. Energy Efficiency of Electro-Driven Brackish Water Desalination: Electrodialysis Significantly Outperforms Membrane Capacitive Deionization. Environ. Sci. Technol. 2020, 54, 3663–3677. [Google Scholar] [CrossRef]
- Jeon, S.-i.; Park, H.-R.; Yeo, J.-G.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Jeong, K.; Yoon, N.; Park, S.; Son, M.; Lee, J.; Park, J.; Cho, K.H. Optimization of a nanofiltration and membrane capacitive deionization (NF-MCDI) hybrid system: Experimental and modeling studies. Desalination 2020, 493, 114658. [Google Scholar] [CrossRef]
- Chung, H.J.; Kim, J.; Kim, D.I.; Gwak, G.; Hong, S. Feasibility study of reverse osmosis–flow capacitive deionization (RO-FCDI) for energy-efficient desalination using seawater as the flow-electrode aqueous electrolyte. Desalination 2020, 479, 114326. [Google Scholar] [CrossRef]
- Kang, J.; Kim, T.; Shin, H.; Lee, J.; Ha, J.-I.; Yoon, J. Direct energy recovery system for membrane capacitive deionization. Desalination 2016, 398, 144–150. [Google Scholar] [CrossRef]
- Pernía, A.M.; Norniella, J.G.; Martín-Ramos, J.A.; Díaz, J.; Martínez, J.A. Up–Down Converter for Energy Recovery in a CDI Desalination System. IEEE Trans. Power Electron. 2012, 27, 3257–3265. [Google Scholar] [CrossRef]
- Zhao, R.; Porada, S.; Biesheuvel, P.M.; van der Wal, A. Energy consumption in membrane capacitive deionization for different water recoveries and flow rates, and comparison with reverse osmosis. Desalination 2013, 330, 35–41. [Google Scholar] [CrossRef]
- Tang, W.; Liang, J.; He, D.; Gong, J.; Tang, L.; Liu, Z.; Wang, D.; Zeng, G. Various cell architectures of capacitive deionization: Recent advances and future trends. Water Res. 2019, 150, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Reale, E.R.; Shrivastava, A.; Smith, K.C. Effect of conductive additives on the transport properties of porous flow-through electrodes with insulative particles and their optimization for Faradaic deionization. Water Res. 2019, 165, 114995. [Google Scholar] [CrossRef] [PubMed]
- Porada, S.; Shrivastava, A.; Bukowska, P.; Biesheuvel, P.M.; Smith, K.C. Nickel Hexacyanoferrate Electrodes for Continuous Cation Intercalation Desalination of Brackish Water. Electrochim. Acta 2017, 255, 369–378. [Google Scholar] [CrossRef]
- Smith, K.C. Theoretical evaluation of electrochemical cell architectures using cation intercalation electrodes for desalination. Electrochim. Acta 2017, 230, 333–341. [Google Scholar] [CrossRef]
- Son, M.; Kolvek, E.; Kim, T.; Yang, W.; Vrouwenvelder, J.S.; Gorski, C.A.; Logan, B.E. Stepwise ammonium enrichment using selective battery electrodes. Environ. Sci. Water Res. Technol. 2020, 6, 1649–1657. [Google Scholar] [CrossRef]
- Son, M.; Aronson, B.L.; Yang, W.; Gorski, C.A.; Logan, B.E. Recovery of ammonium and phosphate using battery deionization in a background electrolyte. Environ. Sci. Water Res. Technol. 2020, 6, 1688–1696. [Google Scholar] [CrossRef]
- Son, M.; Pothanamkandathil, V.; Yang, W.; Vrouwenvelder, J.S.; Gorski, C.A.; Logan, B.E. Improving the Thermodynamic Energy Efficiency of Battery Electrode Deionization Using Flow-Through Electrodes. Environ. Sci. Technol. 2020, 54, 3628–3635. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Wang, R.; Wu, Y.; Xu, S.; Wang, J. Performance comparison and energy consumption analysis of capacitive deionization and membrane capacitive deionization processes. Desalination 2013, 324, 127–133. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Choi, J.-H. Enhanced desalination efficiency in capacitive deionization with an ion-selective membrane. Sep. Purif. Technol. 2010, 71, 70–75. [Google Scholar] [CrossRef]
- Chen, F.; Huang, Y.; Guo, L.; Ding, M.; Yang, H.Y. A dual-ion electrochemistry deionization system based on AgCl-Na0. 44MnO2 electrodes. Nanoscale 2017, 9, 10101–10108. [Google Scholar] [CrossRef]
- Chen, F.; Huang, Y.; Guo, L.; Sun, L.; Wang, Y.; Yang, H.Y. Dual-ions electrochemical deionization: A desalination generator. Energy Environ. Sci. 2017, 10, 2081–2089. [Google Scholar] [CrossRef]
- Missoni, L.L.; Marchini, F.; del Pozo, M.; Calvo, E.J. A LiMn2O4-polypyrrole system for the extraction of LiCl from natural brine. J. Electrochem. Soc. 2016, 163, A1898–A1902. [Google Scholar] [CrossRef]
- Nam, D.-H.; Choi, K.-S. Bismuth as a new chloride-storage electrode enabling the construction of a practical high capacity desalination battery. J. Am. Chem. Soc. 2017, 139, 11055–11063. [Google Scholar] [CrossRef] [PubMed]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Jang, J.; Kang, Y.; Han, J.-H.; Jang, K.; Kim, C.-M.; Kim, I.S. Developments and future prospects of reverse electrodialysis for salinity gradient power generation: Influence of ion exchange membranes and electrodes. Desalination 2020, 491, 114540. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Cui, Y.; La Mantia, F. A Desalination Battery. Nano Lett. 2012, 12, 839–843. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Yoon, J. Rocking Chair Desalination Battery Based on Prussian Blue Electrodes. ACS Omega 2017, 2, 1653–1659. [Google Scholar] [CrossRef]
- Plata, S.L.; Childress, A.E. Limiting power density in pressure-retarded osmosis: Observation and implications. Desalination 2019, 467, 51–56. [Google Scholar] [CrossRef]
- Benjamin, J.; Arias, M.E.; Zhang, Q. A techno-economic process model for pressure retarded osmosis based energy recovery in desalination plants. Desalination 2020, 476, 114218. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Kim, T.; Logan, B.E.; Gorski, C.A. High power densities created from salinity differences by combining electrode and Donnan potentials in a concentration flow cell. Energy Environ. Sci. 2017, 10, 1003–1012. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, E.; Kim, H.; Johnson, C.; Cho, J.; Kim, Y. Rechargeable seawater battery and its electrochemical mechanism. ChemElectroChem 2015, 2, 328–332. [Google Scholar] [CrossRef]
- Iddya, A.; Hou, D.; Khor, C.M.; Ren, Z.; Tester, J.; Posmanik, R.; Gross, A.; Jassby, D. Efficient ammonia recovery from wastewater using electrically conducting gas stripping membranes. Environ. Sci. Nano 2020, 7, 1759–1771. [Google Scholar] [CrossRef]
- Hou, D.; Jassby, D.; Nerenberg, R.; Ren, Z.J. Hydrophobic Gas Transfer Membranes for Wastewater Treatment and Resource Recovery. Environ. Sci. Technol. 2019, 53, 11618–11635. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Ye, Y.; Ma, H.; Zhang, Y.; Guo, W.; Du, B.; Wei, Q.; Wei, D.; Ngo, H.H. A critical review on membrane hybrid system for nutrient recovery from wastewater. Chem. Eng. J. 2018, 348, 143–156. [Google Scholar] [CrossRef]
- Kim, T.; Gorski, C.A.; Logan, B.E. Ammonium Removal from Domestic Wastewater Using Selective Battery Electrodes. Environ. Sci. Technol. Lett. 2018, 5, 578–583. [Google Scholar] [CrossRef]
- Broséus, R.; Cigana, J.; Barbeau, B.; Daines-Martinez, C.; Suty, H. Removal of total dissolved solids, nitrates and ammonium ions from drinking water using charge-barrier capacitive deionisation. Desalination 2009, 249, 217–223. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Song, J.; He, C.; Waite, T.D. Continuous ammonia recovery from wastewaters using an integrated capacitive flow electrode membrane stripping system. Environ. Sci. Technol. 2018, 52, 14275–14285. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; He, D.; Waite, T.D. Capacitive membrane stripping for ammonia recovery (CapAmm) from dilute wastewaters. Environ. Sci. Technol. Lett. 2018, 5, 43–49. [Google Scholar] [CrossRef]
- Shi, W.; Liu, X.; Ye, C.; Cao, X.; Gao, C.; Shen, J. Efficient lithium extraction by membrane capacitive deionization incorporated with monovalent selective cation exchange membrane. Sep. Purif. Technol. 2019, 210, 885–890. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and sustainability—An appraisal and current perspective. Water Res. 2016, 89, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Mossad, M.; Zou, L. Study of fouling and scaling in capacitive deionisation by using dissolved organic and inorganic salts. J. Hazard. Mater. 2013, 244, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Hassanvand, A.; Chen, G.Q.; Webley, P.A.; Kentish, S.E. An investigation of the impact of fouling agents in capacitive and membrane capacitive deionisation. Desalination 2019, 457, 96–102. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.; Bai, L.; Xie, B.; Gan, Z.; Xing, J.; Li, G.; Liang, H. Scaling behavior of iron in capacitive deionization (CDI) system. Water Res. 2020, 171, 115370. [Google Scholar] [CrossRef] [PubMed]
- Lindstrand, V.; Sundström, G.; Jönsson, A.-S. Fouling of electrodialysis membranes by organic substances. Desalination 2000, 128, 91–102. [Google Scholar] [CrossRef]
- Lee, H.-J.; Choi, J.-H.; Cho, J.; Moon, S.-H. Characterization of anion exchange membranes fouled with humate during electrodialysis. J. Membr. Sci. 2002, 203, 115–126. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of capacitive deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, S.; Wan, J.; Tang, H.; Chang, L.; He, L.; Zhao, H.; Gao, Y.; Tang, Z. Three-dimensional graphene/metal oxide nanoparticle hybrids for high-performance capacitive deionization of saline water. Adv. Mater. 2013, 25, 6270–6276. [Google Scholar] [CrossRef]
- Jung, H.-H.; Hwang, S.-W.; Hyun, S.-H.; Lee, K.-H.; Kim, G.-T. Capacitive deionization characteristics of nanostructured carbon aerogel electrodes synthesized via ambient drying. Desalination 2007, 216, 377–385. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Hilal, N. Nano-enabled membranes technology: Sustainable and revolutionary solutions for membrane desalination? Desalination 2016, 380, 100–104. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Ng, B.C. Carbon nanotubes for desalination: Performance evaluation and current hurdles. Desalination 2013, 308, 2–14. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Salty water desalination using carbon nanotube sheets. Desalination 2010, 258, 182–186. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Daer, S.; Kharraz, J.; Giwa, A.; Hasan, S.W. Recent applications of nanomaterials in water desalination: A critical review and future opportunities. Desalination 2015, 367, 37–48. [Google Scholar] [CrossRef]
- Lee, B.; Park, N.; Kang, K.S.; Ryu, H.J.; Hong, S.H. Enhanced Capacitive Deionization by Dispersion of CNTs in Activated Carbon Electrode. ACS Sustain. Chem. Eng. 2018, 6, 1572–1579. [Google Scholar] [CrossRef]
- Khalid, A.; Al-Juhani, A.A.; Al-Hamouz, O.C.; Laoui, T.; Khan, Z.; Atieh, M.A. Preparation and properties of nanocomposite polysulfone/multi-walled carbon nanotubes membranes for desalination. Desalination 2015, 367, 134–144. [Google Scholar] [CrossRef]
- Zhu, B.; Hong, Z.; Milne, N.; Doherty, C.M.; Zou, L.; Lin, Y.S.; Hill, A.J.; Gu, X.; Duke, M. Desalination of seawater ion complexes by MFI-type zeolite membranes: Temperature and long term stability. J. Membr. Sci. 2014, 453, 126–135. [Google Scholar] [CrossRef]
- Qi, S.; Wang, R.; Chaitra, G.K.M.; Torres, J.; Hu, X.; Fane, A.G. Aquaporin-based biomimetic reverse osmosis membranes: Stability and long term performance. J. Membr. Sci. 2016, 508, 94–103. [Google Scholar] [CrossRef]
- Pereira, M.F.R.; Figueiredo, J.L.; Órfão, J.J.M.; Serp, P.; Kalck, P.; Kihn, Y. Catalytic activity of carbon nanotubes in the oxidative dehydrogenation of ethylbenzene. Carbon 2004, 42, 2807–2813. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Jia, H.-P.; Bielawski, C.W. Graphene Oxide: A Convenient Carbocatalyst for Facilitating Oxidation and Hydration Reactions. Angew. Chem. Int. Ed. 2010, 49, 6813–6816. [Google Scholar] [CrossRef]

| Process | Type of Membranes (RO) or Electrodes (CDI) | Performances Enhanced | Performances to be Further Explored | Refs. |
|---|---|---|---|---|
| RO | Multi-layered polyamide | - ~60% flux increase (1.66 ± 0.20 LMH/bar) and higher salt rejection (98%) a - Better fouling resistance to bovine serum albumin (BSA) | - Fouling experiment with seawater | [23] |
| Hydrophilic additives incorporated polyamide | - Flux enhancement (up to 5.77 LMH/bar) b - Salt rejection of >98.8% | - Fouling resistance | [24] | |
| Co-solvent induced polyamide | - Twice water flux (2.78 LMH/bar) c - Salt rejection of 99% | - Long-term stability- Fouling resistance | [22] | |
| Polyamide synthesized under controlled solution pH | - Flux of > 1.55 LMH/bar b - Salt rejection of >97% | - Fouling resistance- Chlorine tolerance | [36] | |
| Polyelectrolyte coated polyamide | - Organic and biofouling control - ~10% flux reduction with a slight increase in salt rejection (>98%) d | - Seawater tests | [37,38] | |
| CDI | Nitrogen-doped porous carbon | - Salt adsorption capacity of 14.91 mg g−1 e - Specific capacitance of 290 F g−1 | - Long-term stability of >1000 min | [39] |
| Nitrogen-doped graphitic porous carbon | - Salt adsorption capacity of 17.73 mg g−1 f - Specific capacitance of 307 F g−1 | [40] | ||
| Phosphorus-doped 3D carbon nanofiber | - Salt adsorption capacity of 16.20 mg g−1 e - Specific capacitance of 295 F g−1 | [41] | ||
| Ca-alginate coated-carbon | - Salt adsorption capacity of 14.20 mg g−1 g | [42] | ||
| N, P co-doped 3D hierarchical carbon | - Salt adsorption capacity of 26.80 mg g−1 h - Specific capacitance of 221 F g−1 | - Fouling tests | [43] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, M.; Cho, K.H.; Jeong, K.; Park, J. Membrane and Electrochemical Processes for Water Desalination: A Short Perspective and the Role of Nanotechnology. Membranes 2020, 10, 280. https://doi.org/10.3390/membranes10100280
Son M, Cho KH, Jeong K, Park J. Membrane and Electrochemical Processes for Water Desalination: A Short Perspective and the Role of Nanotechnology. Membranes. 2020; 10(10):280. https://doi.org/10.3390/membranes10100280
Chicago/Turabian StyleSon, Moon, Kyung Hwa Cho, Kwanho Jeong, and Jongkwan Park. 2020. "Membrane and Electrochemical Processes for Water Desalination: A Short Perspective and the Role of Nanotechnology" Membranes 10, no. 10: 280. https://doi.org/10.3390/membranes10100280
APA StyleSon, M., Cho, K. H., Jeong, K., & Park, J. (2020). Membrane and Electrochemical Processes for Water Desalination: A Short Perspective and the Role of Nanotechnology. Membranes, 10(10), 280. https://doi.org/10.3390/membranes10100280