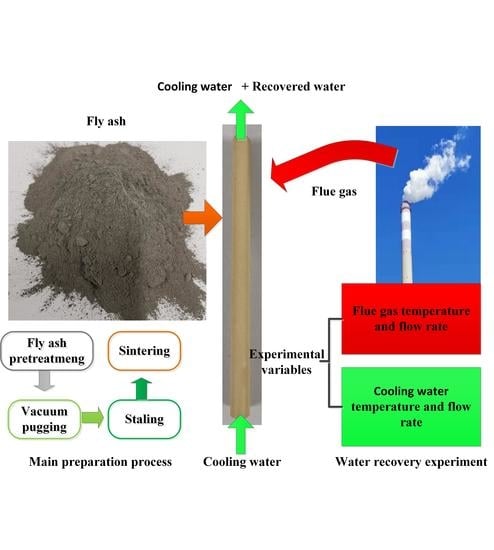
Study on the Preparation and Properties of Talcum-Fly Ash Based Ceramic Membrane Supports
Abstract
1. Introduction
2. Experimental
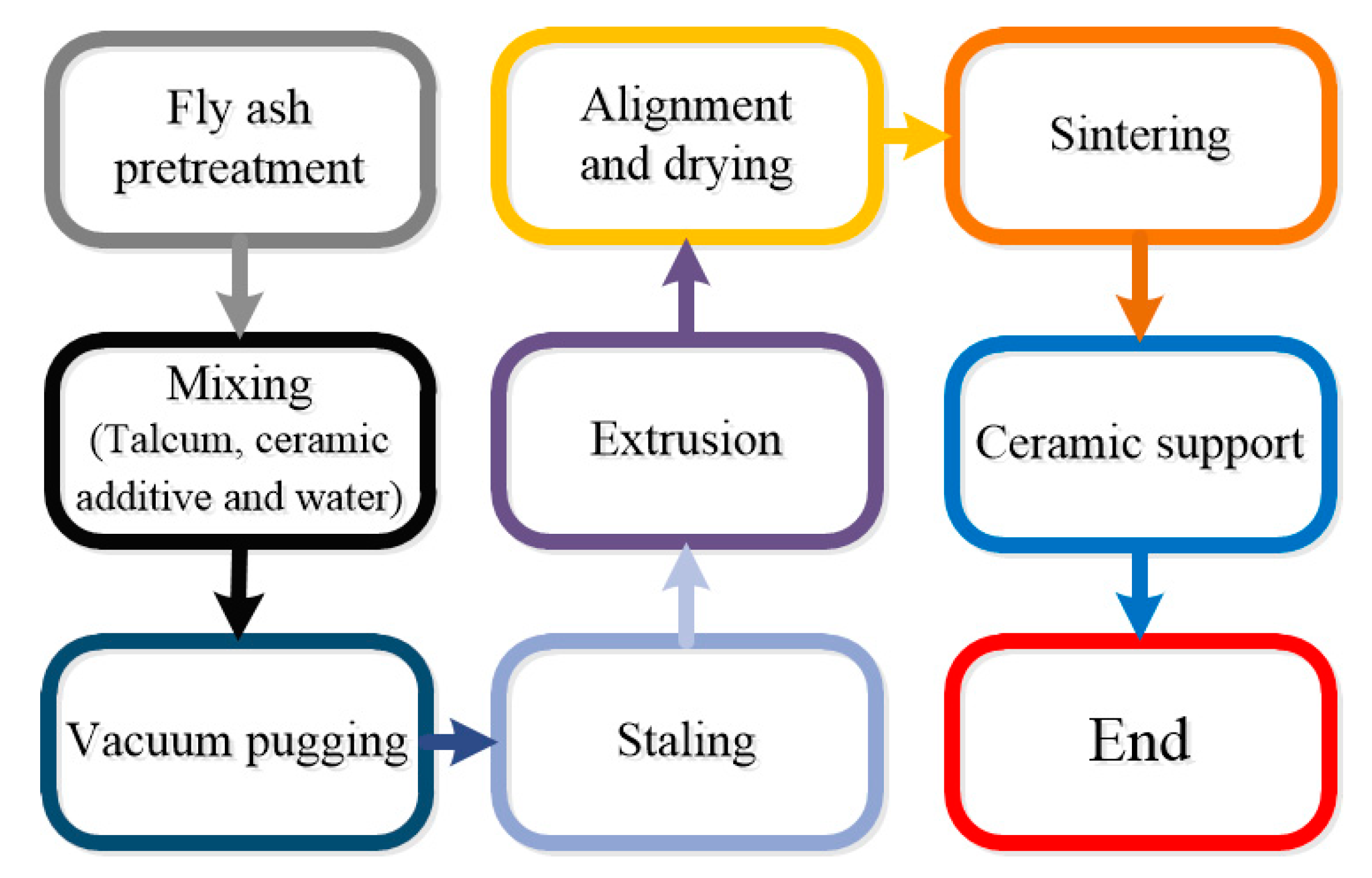
2.1. Membrane Preparation
2.2. Characterization Method
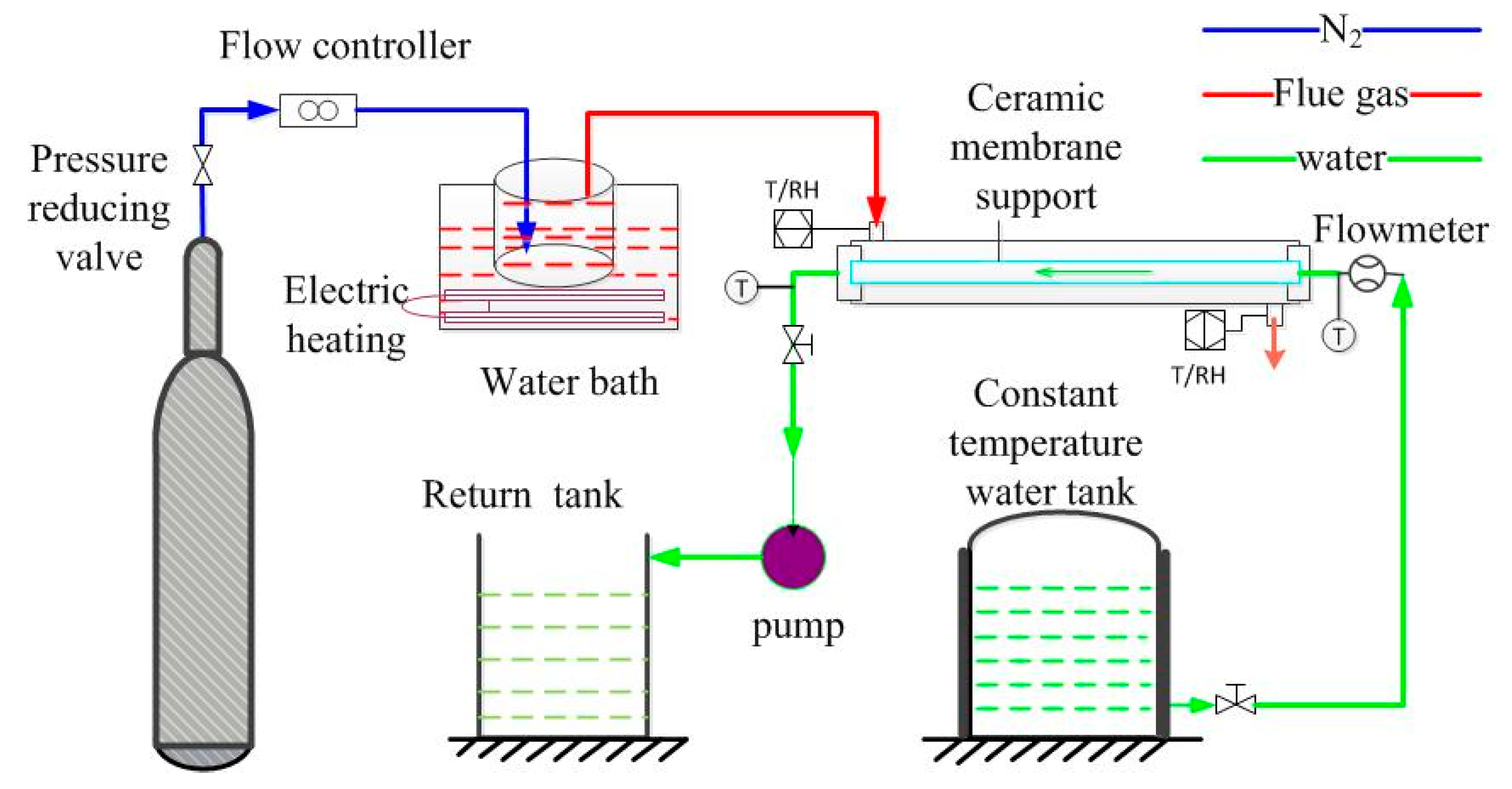
2.3. Water Recovery Experiment of Flue Gas
2.3.1. Experimental Devices
2.3.2. Experimental Performance Evaluation
2.4. Uncertainty Analysis
3. Results and Discussion
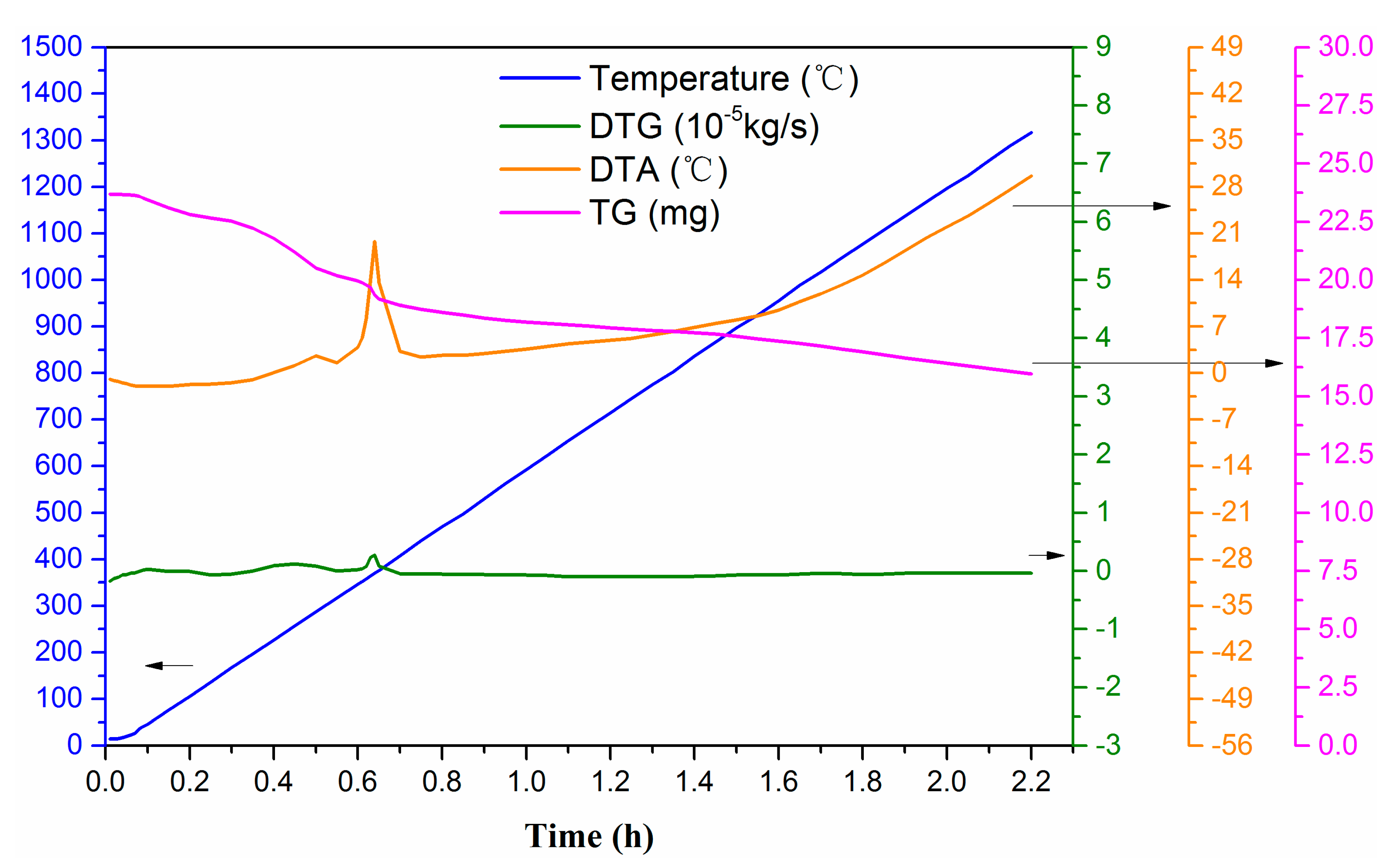
3.1. Characterization of Raw Materials
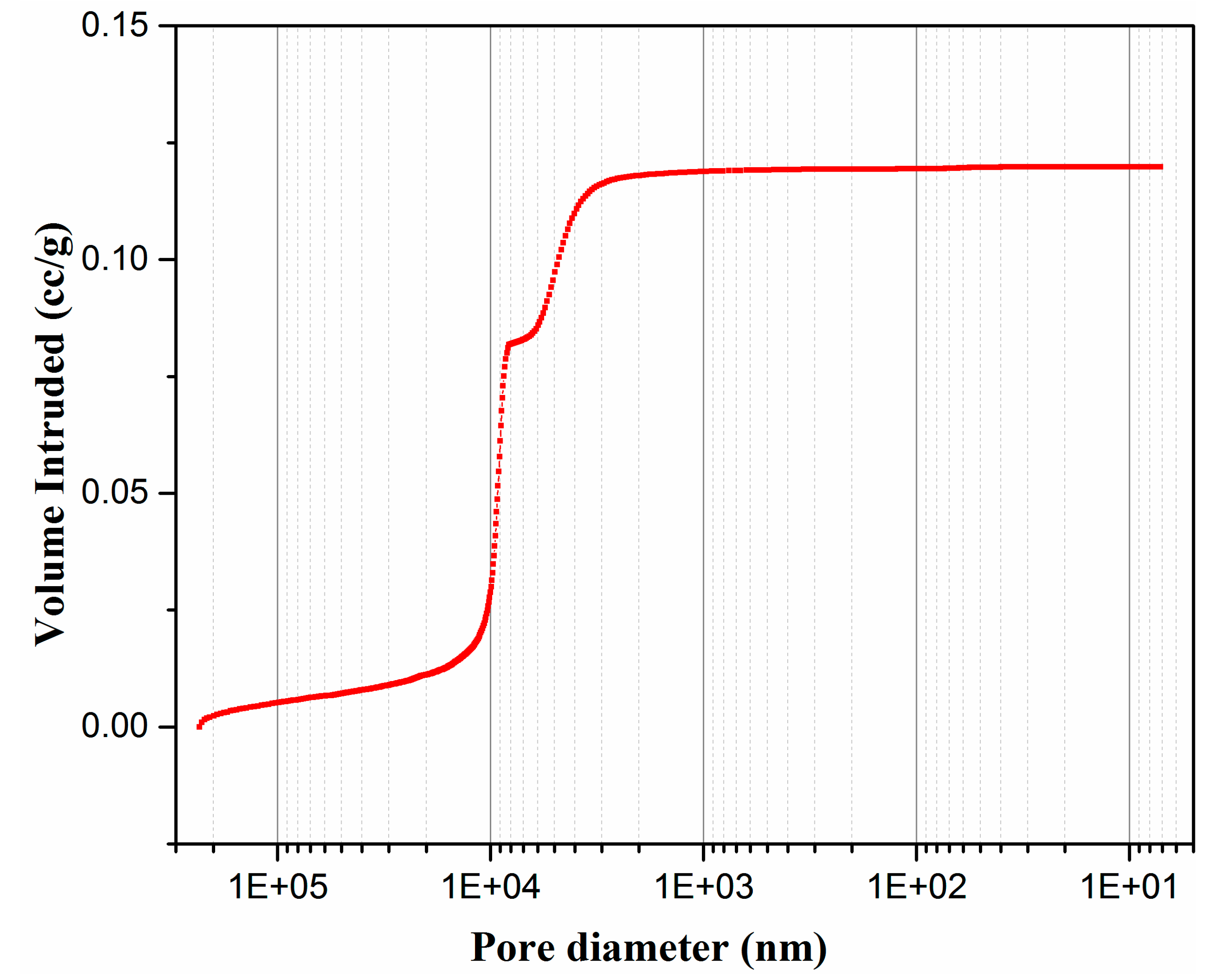
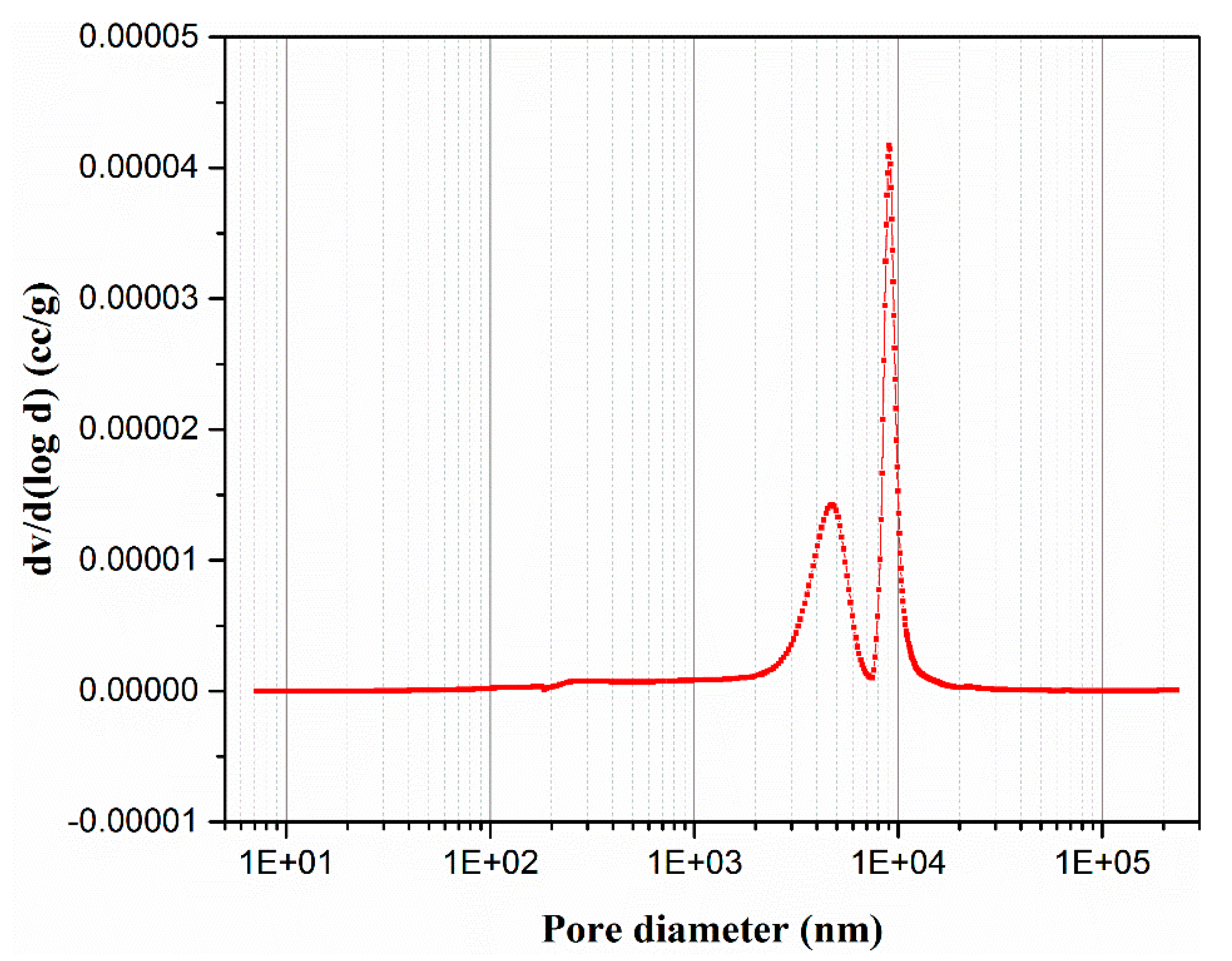
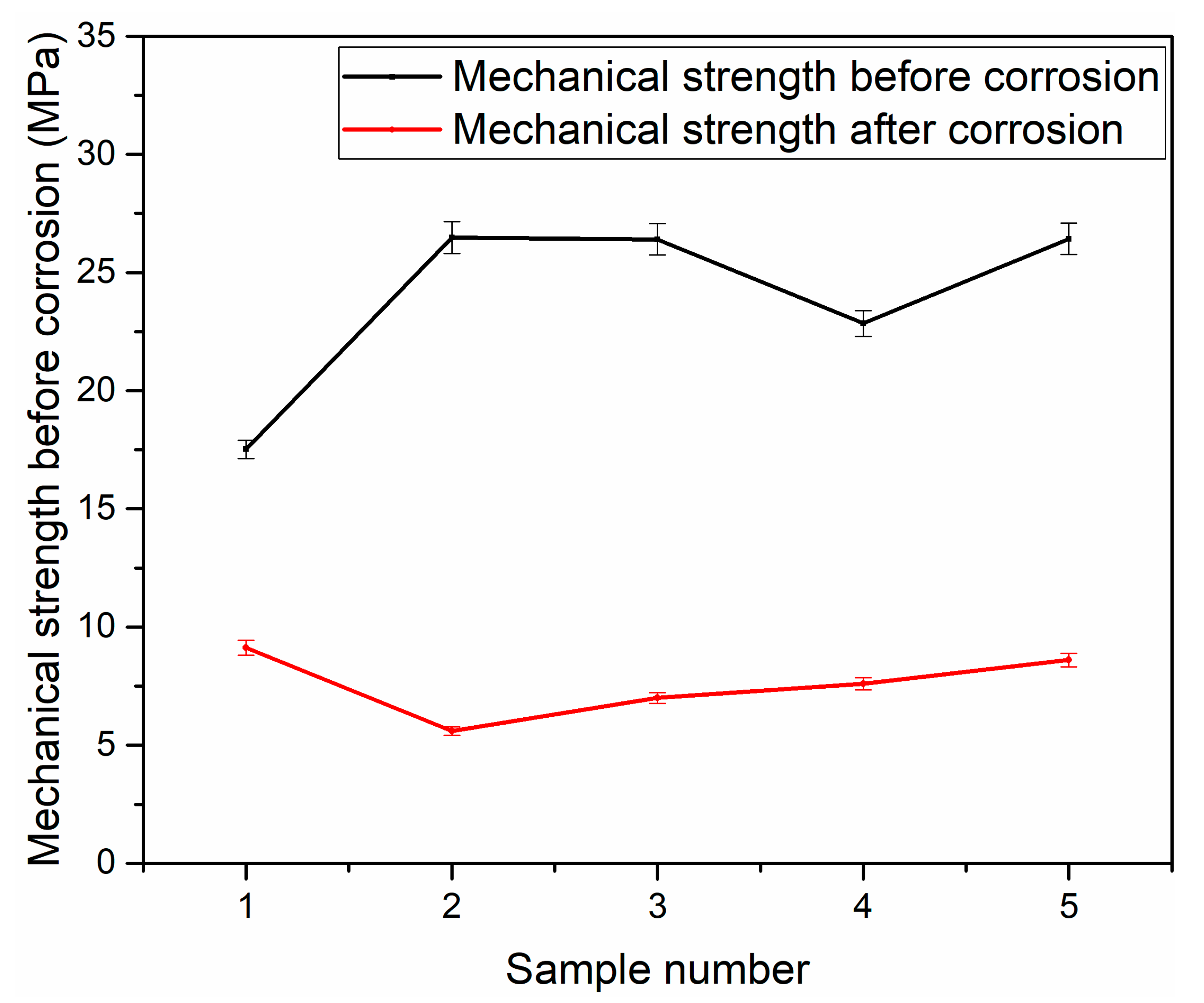
3.2. Characterization of Membrane Supports
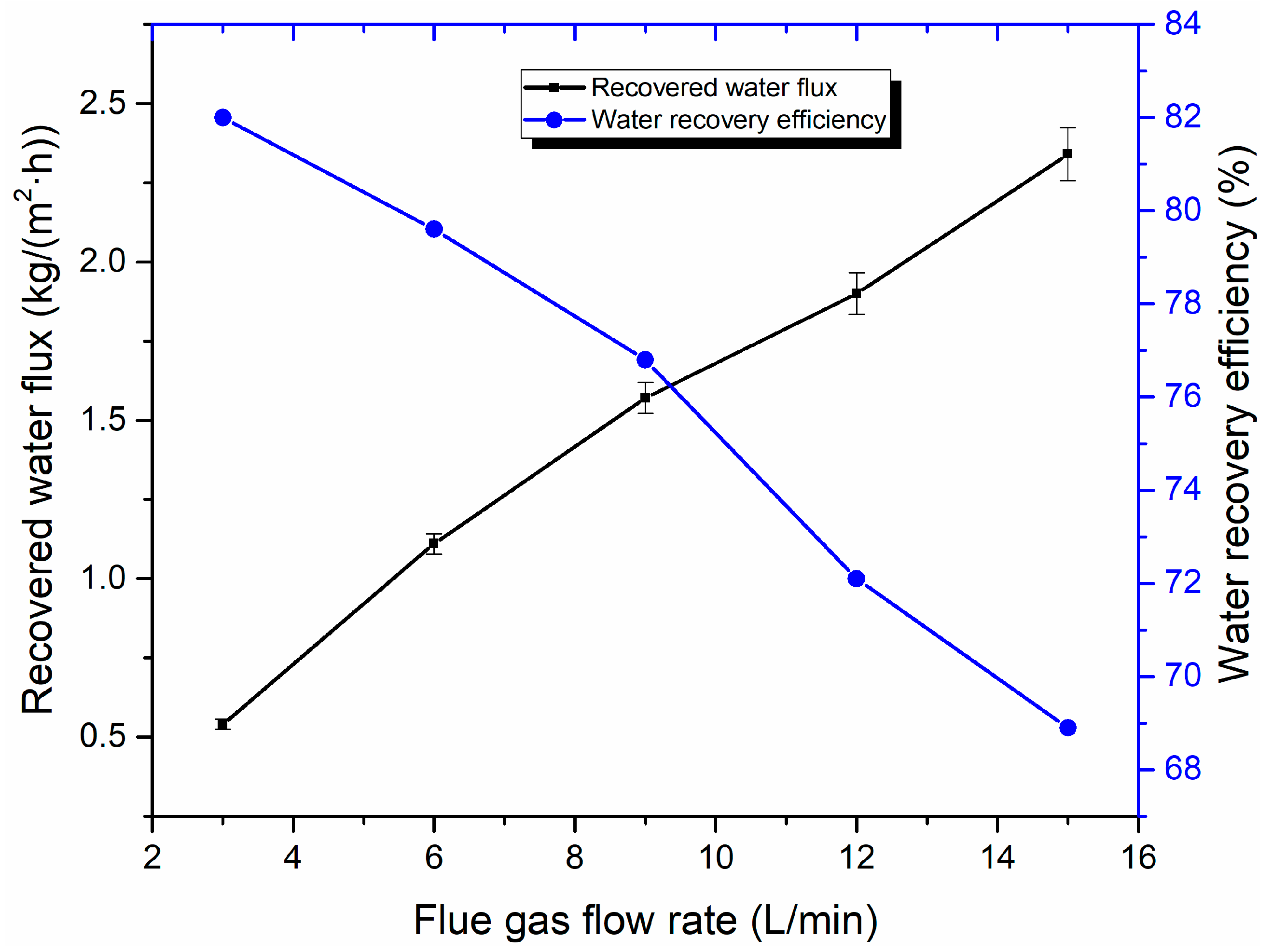
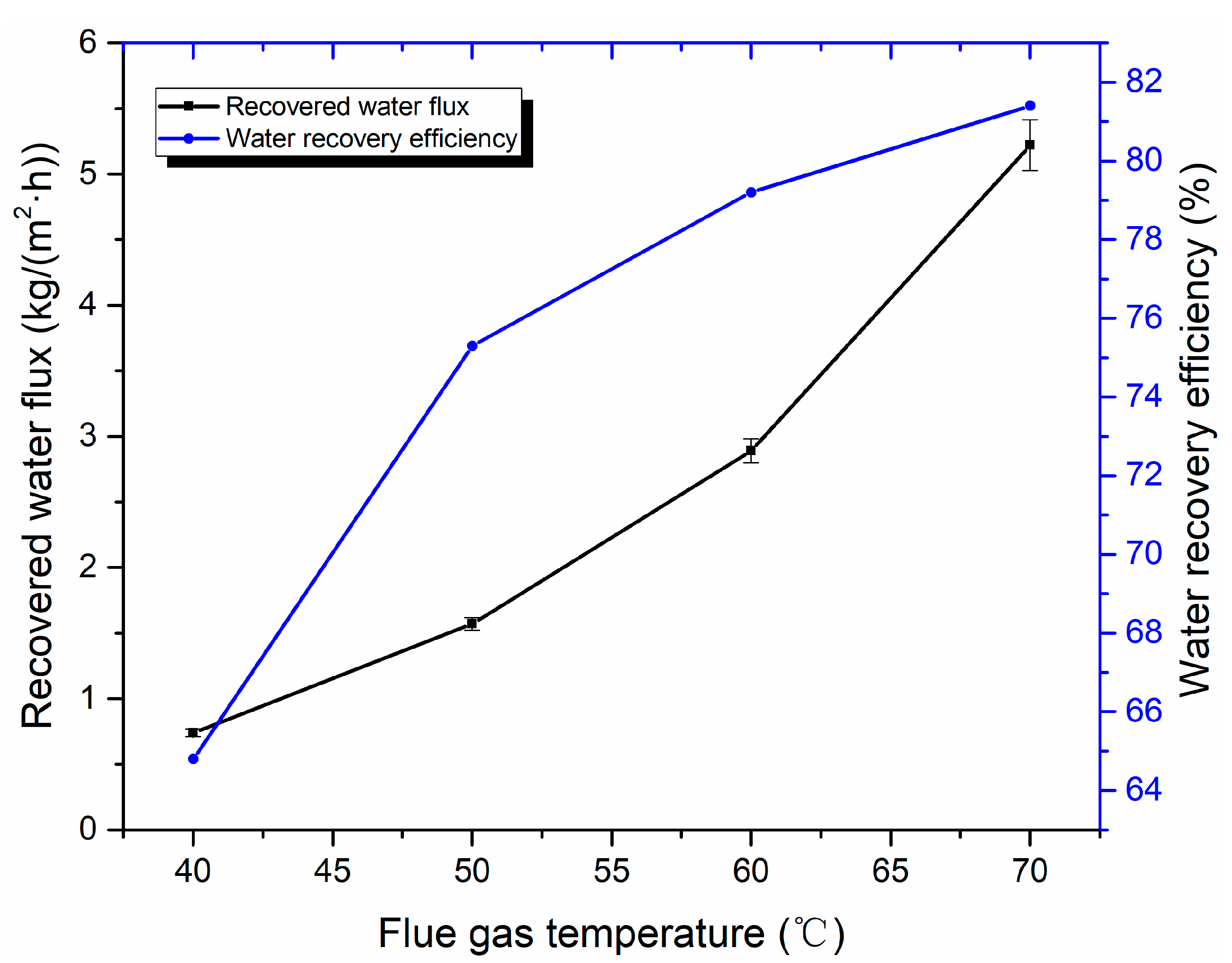
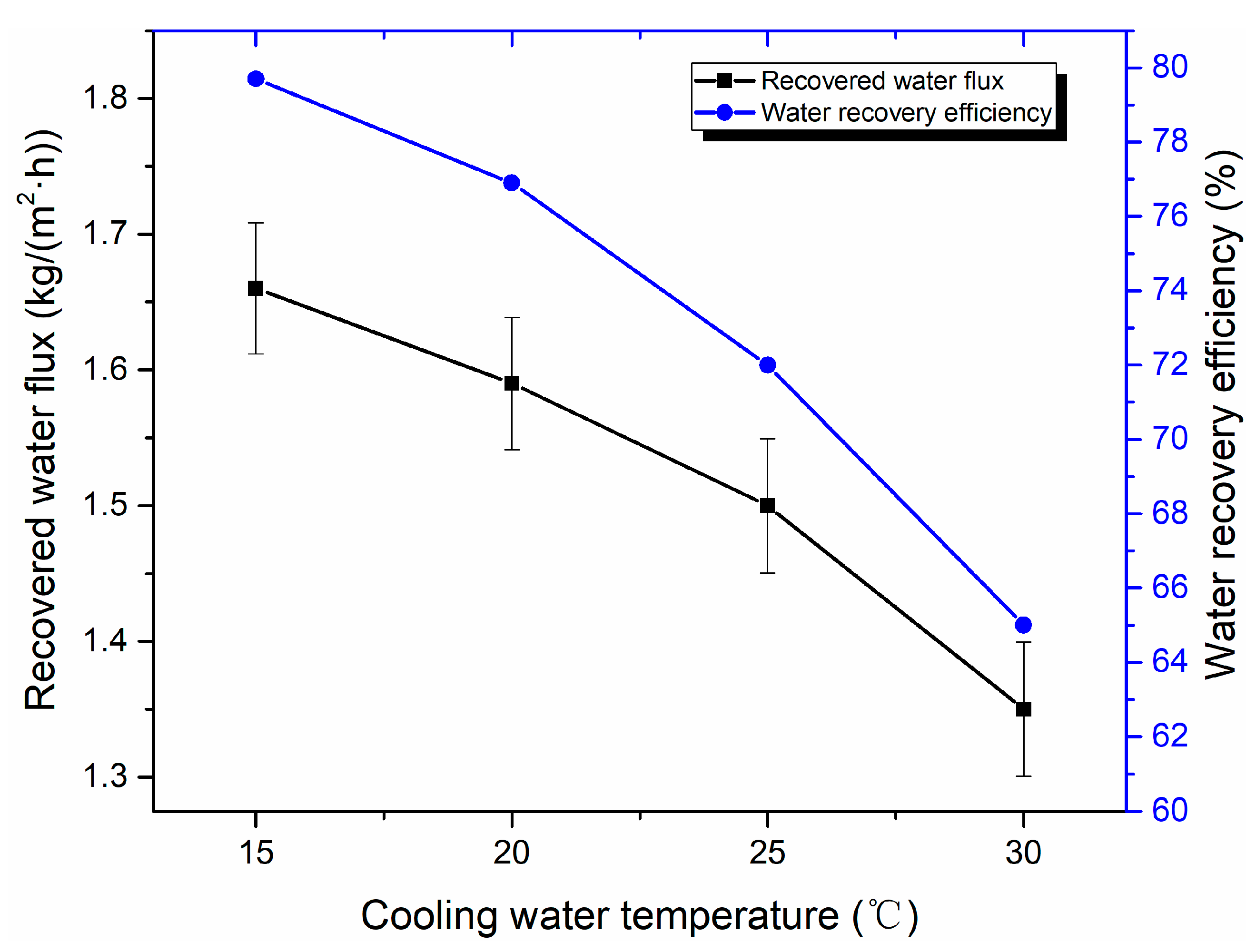
3.3. Water Recovery Performance Analysis
3.4. Performance Comparison and Cost Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, Z.; Dou, J. Current status of reclaimend water in China: An overview. J. Water Reuse Des. 2018, 8, 293–307. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Tang, Y.; Zhao, X.; Yang, H.; Gerbens-Leenes, P.W.; van Vliet, M.T.H.; Yan, J. China’s coal-fired power plants impose pressure on water resources. J. Clean. Prod. 2017, 161, 1171–1179. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Su, X.; Cao, S.; Liu, Y.; Gao, D.; An, L.; Chen, H.; Zhou, Y.; Su, X. Experimental study of water recovery from flue gas using hollow micro-nano porous ceramic composite membranes. J. Ind. Eng. Chem. 2018, 57, 349–355. [Google Scholar] [CrossRef]
- Shao, W.; Feng, J.; Liu, J.; Yang, G.; Yang, Z.; Wang, J. Research on the Status of Water Conservation in the Thermal Power Industry in China. Energy Procedia 2017, 105, 3068–3074. [Google Scholar] [CrossRef]
- Li, Y.; Yan, M.; Zhang, L.; Chen, G.; Cui, L.; Song, Z.; Chang, J.; Ma, C. Method of flash evaporation and condensation—Heat pump for deep cooling of coal-fired power plant flue gas: Latent heat and water recovery. Appl. Energy 2016, 172, 107–117. [Google Scholar] [CrossRef]
- Xu, G.; Xu, C.; Yang, Y.; Fang, Y.; Li, Y.; Song, X. A novel flue gas waste heat recovery system for coal-fired ultra-supercritical power plants. Appl. Therm. Eng. 2014, 67, 240–249. [Google Scholar] [CrossRef]
- Wang, C.; He, B.; Sun, S.; Wu, Y.; Yan, N.; Yan, L.; Pei, X. Application of a low pressure economizer for waste heat recovery from the exhaust flue gas in a 600MW power plant. Energy 2012, 48, 196–202. [Google Scholar] [CrossRef]
- Wang, C.; He, B.; Yan, L.; Pei, X.; Chen, S. Thermodynamic analysis of a low-pressure economizer based waste heat recovery system for a coal-fired power plant. Energy 2014, 65, 80–90. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, L.; Wang, X.; Sun, T.; Wang, J.; Zhang, S. Flue gas recovery system for natural gas combined heat and power plant with distributed peak-shaving heat pumps. Appl. Therm. Eng. 2017, 111, 599–607. [Google Scholar] [CrossRef]
- Westerlund, L.; Hermansson, R.; Fagerström, J. Flue gas purification and heat recovery: A biomass fired boiler supplied with an open absorption system. Appl. Energy 2012, 96, 444–450. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Sun, J.; Liu, Y.; Zhong, Y.; Du, W.; Lan, J. An experimental study of membranes for capturing water vapor from flue gas. J. Energy Inst. 2017, 91, 1–10. [Google Scholar] [CrossRef]
- Choi, O.; Ingole, P.G.; Lee, H.-K. Preparation and characterization of thin film composite membranefor the removal of water vapor from the flue gas at bench scale. Sep. Purif. Technol. 2019, 211, 401–407. [Google Scholar] [CrossRef]
- Volkov, A.V.; Novitsky, E.G.; Borisov, I.L.; Vasilevsky, V.P.; Volkov, V.V. Porous condenser for thermally driven membrane processes: Gravity-independent operation. Sep. Purif. Technol. 2016, 171, 191–196. [Google Scholar] [CrossRef]
- Borisov, I.L.; Golubev, G.S.; Vasilevsky, V.P.; Volkov, A.V.; Volkov, V.V. Novel hybrid process for bio-butanol recovery: Thermopervaporation with porous condenser assisted by phase separation. J. Membr. Sci. 2017, 523, 291–300. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.A.; Zhou, J. Effect of starch addition on microstructure and properties of highly porous alumina ceramics. Ceram. Int. 2013, 39, 8833–8839. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, B.; Zhou, J.-e.; Zhang, X.; Ling, Y.; Liu, X.; Meng, G.; Hampshire, S. Corrosion resistance characterization of porous alumina membrane supports. Mater. Charact. 2011, 62, 409–418. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Effect of TiO2 addition on the fabrication of ceramic membrane supports: A study on the separation of oil droplets and bovine serum albumin (BSA) from its solution. Desalination 2011, 279, 104–114. [Google Scholar] [CrossRef]
- Wang, D.; Bao, A.; Kunc, W.; Liss, W. Coal power plant flue gas waste heat and water recovery. Appl. Energ. 2012, 91, 341–348. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Cao, S.; Li, X.; Su, X.; An, L.; Gao, D. Heat exchange and water recovery experiments of flue gas with using nanoporous ceramic membranes. Appl. Therm. Eng. 2017, 110, 686–694. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Zhang, H.; Chen, H. Moisture Recovery From Gas-fired Boiler Exhaust Using Membrane Module Array. J. Clean. Prod. 2019, 231, 1110–1121. [Google Scholar] [CrossRef]
- Wei, Z.; Jie, H.; Zhu, Z. High-aluminum fly ash recycling for fabrication of cost-effective ceramic membrane supports. J. Alloy Compd. 2016, 683, 474–480. [Google Scholar]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2018, 44, 4538–4560. [Google Scholar]
- Mestre, S.; Gozalbo, A.; Lorente-Ayza, M.M.; Sánchez, E. Low-cost ceramic membranes: A research opportunity for industrial application. J. Eur. Ceram. Soc. 2019, 39, 3392–3407. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, J.-e.; Lin, B.; Wang, Y.; Wang, S.; Miao, L.; Lang, Y.; Liu, X.; Meng, G. Reaction-sintered porous mineral-based mullite ceramic membrane supports made from recycled materials. J. Hazard. Mater. 2009, 172, 180–186. [Google Scholar]
- Abdelaziz, E.; Najib, T. From a naturally occurring-clay mineral to the production of porous ceramic membranes. Micropor. Mesopor. Mat. 2018, 271, 52–58. [Google Scholar]
- Rashad, M.; Balasubramanian, M. Characteristics of porous mullite developed from clay and AlF3·3H2O. J. Eur. Ceram. Soc. 2018, 38, 3673–3680. [Google Scholar] [CrossRef]
- Hou, Z.; Cheng, L.; Liangliang, L.; Shaowei, Z. Microstructural evolution and densification behavior of porous kaolin-based mullite ceramic added with MoO3. Ceram. Int. 2018, 44, 17914–17918. [Google Scholar]
- Gonzalez, A.; Navia, R.; Moreno, N. Fly ashes from coal and petroleum coke combustion: Current and innovative potential applications. Waste Manag. 2009, 27, 976–987. [Google Scholar]
- Fang, J.; Qin, G.; Wei, W.; Zhao, X. Preparation and characterization of tubular supported ceramic microfiltration membranes from fly ash. Sep. Purif. Technol. 2011, 80, 585–591. [Google Scholar]
- Zhu, J.; Hong, Y. Microstructure and properties of mullite-based porous ceramics produced from coal fly ash with added Al2O3. Int. J. Min. Met. Mater. 2017, 24, 309–315. [Google Scholar]
- Qin, G.; Lü, X.; Wei, W.; Li, J.; Cui, R.; Hu, S. Microfiltration of kiwifruit juice and fouling mechanism using fly-ash-based ceramic membranes. Food Bioprod. Process. 2015, 96, 278–284. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G.; Uppaluri, R. Fly ash based ceramic microfiltration membranes for oil-water emulsion treatment: Parametric optimization using response surface methodology. J. Water Process Eng. 2016, 13, 27–43. [Google Scholar] [CrossRef]
- Zou, D.; Qiu, M.; Chen, X.; Drioli, E.; Fan, Y. One step co-sintering process for low-cost fly ash based ceramic microfiltration membrane in oil-in-water emulsion treatment. Sep. Purif. Technol. 2019, 210, 511–520. [Google Scholar] [CrossRef]
- Fu, M.; Liu, J.; Dong, X.; Zhu, L.; Dong, Y.; Hampshire, S. Waste recycling of coal fly ash for design of highly porous whisker-structured mullite ceramic membranes. J. Eur. Ceram. Soc. 2019, 39, 5320–5331. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, S.; Zhang, X.; Yang, J.; Liu, X.; Meng, G. Fabrication and characterization of low cost tubular mineral-based ceramic membranes for micro-filtration from natural zeolite. J. Membr. Sci. 2006, 281, 592–599. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, X.; Ma, Q.; Meng, G. Preparation of cordierite-based porous ceramic micro-filtration membranes using waste fly ash as the main raw materials. J. Membr. Sci. 2006, 285, 173–181. [Google Scholar] [CrossRef]
- Jedidi, I.; Khemakhem, S.; Saïdi, S.; Larbot, A.; Elloumi-Ammar, N.; Fourati, A.; Charfi, A.; Salah, A.B.; Amar, R.B. Preparation of a new ceramic microfiltration membrane from mineral coal fly ash: Application to the treatment of the textile dying effluents. Powder Technol. 2011, 208, 427–432. [Google Scholar] [CrossRef]
- Hedfi, I.; Hamdi, N.; Rodriguez, M.A.; Srasra, E. Development of a low cost micro-porous ceramic membrane from kaolin and Alumina, using the lignite as porogen agent. Ceram. Int. 2015, 42, 5089–5093. [Google Scholar] [CrossRef]
- Hashemifard, S.A.; Ghodrati, M.S.; Rezaei, M.; Izadpanah, A.A. Experimental study of gas dehydration via PDMS/CaCO3 NP-coated PVC hollow fiber membrane contactor. Chem. Eng. Res. Des. 2020, 162, 62–73. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Jordana, R.; Nijmeijer, K.; Wessling, M. Highly hydrophilic, rubbery membranes for CO2 capture and dehydration of flue gas. Int. J. Greenh. Gas Control 2011, 5, 26–36. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Chen, H. Application of transport membrane condenser for recovering water in a coal-fired power plant: A pilot study. J. Clean. Prod. 2020, 261, 121229. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Chen, H.; Huang, J.; Fu, H. Water vapor capture using microporous ceramic membrane. Desalination 2020, 482, 114405. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, S.; Wang, D.K.; Wei, Y.; Qi, H.; Wu, T.; Feron, P.H.M. Simultaneous heat and water recovery from flue gas by membrane condensation: Experimental investigation. Appl. Therm. Eng. 2017, 113, 843–850. [Google Scholar] [CrossRef]
- Macedonio, F.; Cersosimo, M.; Brunetti, A.; Barbieri, G.; Drioli, E. Water recovery from humidified waste gas streams: Quality control using membrane condenser technology. Chem. Eng. Process. Process Intensif. 2014, 86, 196–203. [Google Scholar] [CrossRef]
- Horikawa, T.; Do, D.D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface 2011, 169, 40–58. [Google Scholar] [CrossRef]
- Khan, H.A.; Castel, A.; Khan, M.S.H. Corrosion investigation of fly ash based geopolymer mortar in natural sewer environment and sulphuric acid solution. Corros. Sci. 2020, 168, 108586. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, H.; Chen, H. Experimental study on water recovery and SO2 permeability of ceramic membranes with different pore sizes. Int. J. Energy Res. 2020, 44, 6313–6324. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, B.; Chen, J.; Wang, J. Preparation and characterization of high performance alumina ceramic membrane support. Foshan Ceram. 2019, 29, 10–14. [Google Scholar]
- Li, L.; Chen, M.; Dong, Y.; Dong, X.; Cerneaux, S.; Hampshire, S.; Cao, J.; Zhu, L.; Zhu, Z.; Liu, J. A low-cost alumina-mullite composite hollow fiber ceramic membrane fabricated via phase-inversion and sintering method. J. Eur. Ceram. Soc. 2016, 36, 2057–2066. [Google Scholar] [CrossRef]






| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | Loss of Ignition |
|---|---|---|---|---|---|---|---|---|---|
| Fly ash | 50.6 | 27.1 | 7.1 | 2.8 | 1.2 | 0.5 | 1.3 | 0.3 | 8.2 |
| Talcum | 58 | 20 | / | 1.8 | 18 | / | / | / | / |
| Materials | Specifications | Sources |
|---|---|---|
| Fly ash | 100 Mesh | A power plant of China Datang corporation |
| Talcum | 3000 Mesh industrial grade | Shijiazhuang, Hebei |
| Carboxymethylcellulose | Industrial grade high viscosity | Cangzhou, Hebei |
| Dextrin | Industrial grade high viscosity | Ji’nan, Shandong |
| Glycerin | 99% Purity | A chemical plant in Beijing |
| Experimental Apparatus | Model | Parameters | Precision | Manufacturer |
|---|---|---|---|---|
| Gas flow controller | D07-9E | 30 SLM | ±2% | Beijing Sevenstar, China |
| Temperature and humidity transmitter | TH-21E | Temperature Range: −40 °C to 125 °C RH Range: 0–100% | ≤±0.2 °C ≤±2% | Guangzhou Anymetre, China |
| Eight-loop digital display device | HT-MK807-01-23-KL | / | 0.5% FS | Hantang Precision Instrument, Shanghai, China |
| Thermocouple | PT100 | −50 °C to 200 °C | A Class | Hangzhou Sinomeasure, China |
| Miniature electric diaphragm pump | PLD-1205 | Max flow rate: 3.2 L/min | / | Shijiazhaung Pulandi, China |
| Flow meter | LZT-M15 | Range: 0.2–2.0 LPM | ±4% | VAKADA, Beijing, China |
| Reference | Pore Size | Component | Model | Q/A (m3/h/m2) | Water Flux kg/(m2·h) | Experimental Conditions |
|---|---|---|---|---|---|---|
| [43] | 7 nm | Air/water vapor | Single tube | 191 | 4.50 | Inlet gas temperature and flow rate were 100 °C and 6.7 L/min, respectively. Cooling water flow rate was 3.3 L/h. |
| [20] | 1 μm | Gas-fired boiler flue gas | Bundle | 2286 | 15.77 | Inlet gas temperature and flow rate were 46 °C and 1600 m3/h, respectively. Cooling water temperature and flow rate were 23 °C and 1150 L/h, respectively. |
| [47] | 50 nm | Air/water vapor | Single tube | 30 | 4.82 | Inlet gas temperature and flow rate were 60 °C and 15 L/min, respectively. Cooling water temperature and flow rate were 20 °C and 1 L/min, respectively. |
| This paper | 5–9 μm | N2/water vapor | Single tube | 18 | 5.22 | Inlet gas temperature and flow rate were 70 °C and 9 L/min, respectively. Cooling water temperature and flow rate were 20 °C and 1 L/min, respectively. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Fu, H.; Wu, J.; Zhang, H.; Chen, H. Study on the Preparation and Properties of Talcum-Fly Ash Based Ceramic Membrane Supports. Membranes 2020, 10, 207. https://doi.org/10.3390/membranes10090207
Cheng C, Fu H, Wu J, Zhang H, Chen H. Study on the Preparation and Properties of Talcum-Fly Ash Based Ceramic Membrane Supports. Membranes. 2020; 10(9):207. https://doi.org/10.3390/membranes10090207
Chicago/Turabian StyleCheng, Chao, Hongming Fu, Jun Wu, Heng Zhang, and Haiping Chen. 2020. "Study on the Preparation and Properties of Talcum-Fly Ash Based Ceramic Membrane Supports" Membranes 10, no. 9: 207. https://doi.org/10.3390/membranes10090207
APA StyleCheng, C., Fu, H., Wu, J., Zhang, H., & Chen, H. (2020). Study on the Preparation and Properties of Talcum-Fly Ash Based Ceramic Membrane Supports. Membranes, 10(9), 207. https://doi.org/10.3390/membranes10090207