1. Introduction
The production of hydrogen (H
2) can be carried out through different methods such as thermochemical method (heat and chemical reactions to produce H
2), reforming of hydrocarbons, biomass gasification, coal gasification, electrolytic method, and biological method. Usually, the thermochemical reforming methods are used to study the Thermochemical Energy Storage (TES) technology of H
2. The TES of H
2 can be produced from reforming reactions with high energy involved in chemical reaction. In particular, H
2 can be obtained from thermochemical conversion of CH
4 by driving endothermic reaction methods as the SRM and/or dry reforming of methane (DRM) [
1]. The choice of the SRM or DRM depends of the desired ending product. If H
2 is the desired ending product, then the SRM is preferable. H
2 can also provide environmental benefits as a promising renewable energy vector which can be used in several industrial processes [
2,
3]. Technological development efforts to produce renewable energy have been reported since last century. H
2 is one of the most important energy vectors in the universe accounting for almost 75% of the all universe mass. H
2 is considered a clean energy source for the future.
Membrane reformers (MRs) can be used to produce H
2 and purify it in packed-bed and/or fluidized-bed setups [
4,
5]. The production of H
2 inside MRs is still attracting the interest of researchers and engineers. This article is focused on the thermochemical conversion of CH
4 in a Packed-Bed Membrane Reformer (PBMR). In this setup, H
2 gas from shell side (reaction system) passes towards a permeation side through a Pd-based dense membrane [
6]. The permselectivity properties of a Pd-based dense membrane such as permeability and selectivity are able to enhance the production of H
2 from the thermochemical reforming reactions. The Pd-based dense membrane can act as a remover and it facilitates the selective removal of the gaseous molecule of H
2. The removal of H
2 through Pd-based dense membrane shifts the equilibrium of reforming reactions to the chosen direction according to the Le Chatelier principle [
7,
8,
9]. PBMRs offer many potential advantages such as enhanced conversion of hydrocarbons, reduced cost, improved yield, and high selectivity.
PBMR is an apparatus which integrates the reaction zone and permeation zone separated by a Pd-based dense membrane on the same physical equipment. The Pd-based dense membrane is a barrier that allows only certain component (H
2) to pass through it and it acts as separator [
10,
11]. Among the metals, Pd and its alloys have been applied for manufacturing H
2 separators as a function of their high membrane permeability towards H
2. The mathematical modelling from PBMRs is important to design and optimize this type reformer in order to understand its behavior for a given reaction system. The mathematical models of PBMRs (gas-solid system) can be developed using mass, energy and momentum balance equations for the gas phase as well as mass, energy and momentum balance equations to the solid phase [
3,
6,
12,
13,
14]. The dynamic performance of a state variable inside PBMRs can be investigated from an initial value up to a steady state of this variable. In this work, a Non-isothermal Pseudo-Homogeneous Dynamic (NIPHD) model is used to model the SRM method inside PBMR. The NIPHD model is described by a system of Nonlinear Partial Differential Equations (NPDEs) that couple to a complex kinetic model of the SRM.
NIPHD models can be used to simulate and analyze the SRM method inside PBMR. The application of NIPHD models can be an excellent alternative to predict faster solutions of systems of NPDEs due to the lower computational time [
15,
16]. In this context, the NIPHD model is able to predict the main characteristics of the SRM method’s dynamic performance in PBMR [
17]. In addition, the system of NPDEs represents a strong tool for facilitating the project, optimization, and PBMR reformer’s control [
3,
18,
19,
20]. The numerical solution of the system of NPDEs has been a great challenge due to the numerical stability. Given this, several numerical methodologies have been used to compute numerical solutions [
21,
22]. The choice of methodology is dependent on the desired accuracy of the stability and robustness of numerical results of the NPDE system to maintain computational efficiency. The NPDE system of this work has been transformed into a simpler Nonlinear Ordinary Differential Equations (NODEs) system using the Coupled Integral Equation Approach (CIEA) [
23,
24]. The NODE system was solved by the Runge-Kutta Gill method as well as the NODE from the permeation zone.
With the purpose of reducing the research cost and project time, mathematical modelling and computer simulation are extensively used to obtain a better understanding of design parameters in reformers. The approach and solution of physical-mathematical models are still a novelty of membrane reformers to obtain sustainable clean H2 and, thus, the topic is a very relevant in the literature. In comparison with traditional methodologies such as finite element, finite volume, etc., that have already been used before in the literature, our methodology can provide results faster than traditional methods and, therefore, the novelty of the present work lies in the determination of the solution method. A comparative analysis had been driven to investigate ERT, CR of CH4, and feed-based yield (FBY) of H2 inside FBR and PBMR. The effects of the H2O/CH4 ratio and Ssp on the ERT were numerically investigated in PBMR. After checking the effects of the H2O/CH4 ratio and Ssp on the ERT, the effects of these parameters were also studied on the CR of CH4 and CBY of H2. In addition, the ERT’s effect was verified on the ηtese, chemical energy, sensible heat, and heat loss. In addition, the selectivity of components (H2, CO, CO2, and CH4) was computed in PBMR.
2. Physical-Mathematical Model
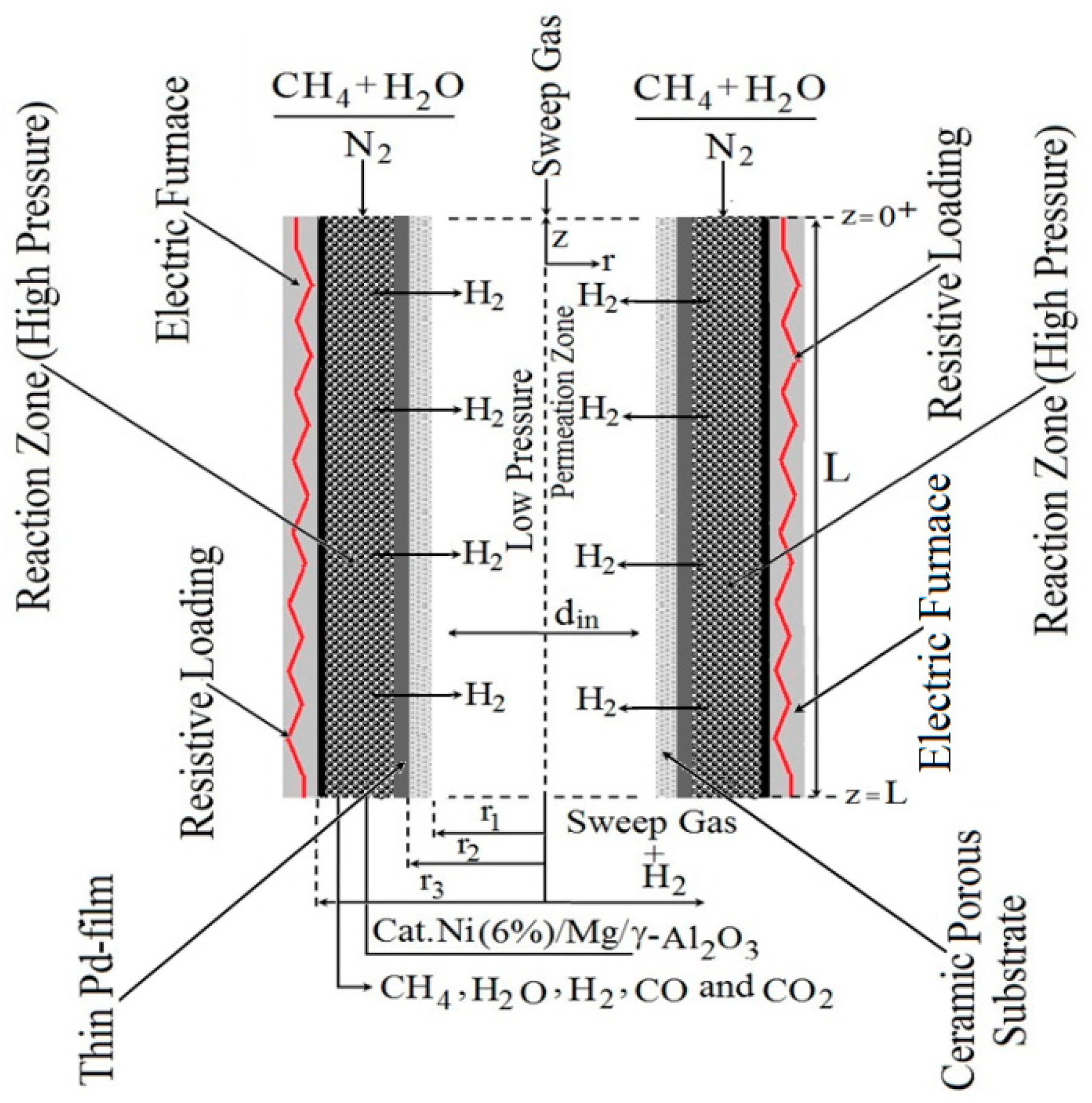
A schematic setup is used to study the SRM method’s thermochemical conversion in PBMR according to
Figure 1. The simplified setup from
Figure 1 involves a heating module (electric furnace), input reagents (CH
4, H
2O), Sweep gas (N
2), reaction zone, permeation zone, and outlet products (CH
4, H
2O, H
2, CO, and CO
2). The physical setup of the PBMR is built by two concentric tubes according to
Figure 1. The inner tube consists of a thin palladium (Pd) dense membrane which contains a permeation zone receiving H
2 from the reaction zone through the Pd-based dense membrane. The catalyst loading is placed between the tubes in the annular zone, named the fixed-bed.
2.1. Electric Power of the Electric Furnace
In
Figure 1, a resistive loading inside of an electric furnace has been used to heat the FBMR’s reaction zone and therefore the thermal energy storage is used to drive the reforming reactions. The electric power provided by the electric furnace is given for Equation (1) as follows.
The thermochemical energy storage is obtained by subtracting the product enthalpy (reaction heat) from the reagent enthalpy at room temperature. Thus, the PBMR’s chemical energy is obtained using Equation (2) as follows.
After cooling the products to the room temperature, the sensible heat of the products (ranging from outer temperature to the room temperature) can be used and therefore the sensible heat can be computed from Equation (3) as follows.
From Equations (1)–(3), it is possible to estimate the heat loss using Equation (4) as follows.
2.2. Thermochemical Kinetic Model
The reforming reactions of CH
4 are used to produce syngas (H
2 and CO) and they are highly endothermic [
3]. The SRM method has a limited equilibrium and it comprises three major reactions as follows.
The two reforming reactions, Equations (5) and (7), are highly endothermic reactions and they need high temperatures to obtain a high H2 productivity. On the other hand, Equation (6) is a slightly exothermic reaction and it works at low temperature when comparing to Equations (5) and (7).
The global rate equations of the three reactions, Equations (5)–(7), are based on the Langmuir-Hinshelwood kinetic model [
3]. The kinetic rates from Equations (5)–(7) are considered more general for nickel (Ni) catalyst and, therefore, the equations of the SRM method are presented as:
where β is given by Equation (11) as follows.
The partial pressures of chemical components i, i = CH
4, H
2O, CO, CO
2 and H
2, from Equations (8)–(11) are computed from Equations (12)–(16) below.
where,
The net rates (r
i) for each chemical component i (i = CH
4, H
2O, CO, CO
2 and H
2) are computed through Equation (18) as follows.
From Equation (18), the net rates of each chemical component i are obtained by Equations (19)–(23) as follows.
2.3. PBMR’s Mathematical Modelling
The mathematical modelling inside PBMR’s reaction zone is described through the NIPHD model. The development of the NIPHD model takes into account the following assumptions: (1) the NIPHD model is described under non-isothermal conditions inside the reaction zone, (2) the NIPHD model in the reaction zone is plug-flow with axial dispersion under transient condition, (3) the radial dispersion is negligible inside the reaction zone, (4) the gaseous mixture has constant density inside the reaction zone from PBMR, (5) the membrane is considered to be 100% H2-permselectivity, i.e., the selectivity of H2 is typically very high in dense metallic membranes, (6) the heat exchange between the reaction zone and permeation zone is negligible, (7) the molar flow rates in the reaction zone and permeation zone are constant, (8) the deposition effect of carbon at the surface of catalytic particles has been neglected, (9) the gas behavior in the reaction zone from PBMR was considered as an ideal gas mixture, (10) the bed porosity in the axial direction is considered constant, and (11) chemical reactions are assumed to take place at the surface of catalyst particles.
These premises are used to build the governing equations of the NIPHD model in PBMR’s reaction zone and permeation zone as follows.
2.3.1. Energy Balance of the Gas Phase in Reaction Zone
The developed equation provides clear information to drive the temperature distribution of the gas phase in porous medium from PBMR’s reaction zone. The energy transport in the gas phase inside the reaction zone is characterized by a balance equation in PBMR’s axial direction. Thus, a one-dimensional dynamic equation is modelled for the temperature of the gas phase as follows.
- -
Energy balance in the gas phase
The gas phase’s effective thermal conductivity is defined as a function of the gaseous mixture thermal conductivity as follows.
where
The suitable initial and boundary conditions from Equations are given as follows.
- -
Initial condition, i.e., t = 0
- -
At the inlet face surface (upper) of the reaction zone from PBMR, i.e., z = 0+
- -
At the outlet face surface (bottom) of the reaction zone from PBMR, i.e., z = L
2.3.2. Energy Balance of the Solid Phase in Reaction Zone
The spherical particle’s tortuous structure in the reaction zone could give rise to turbulences with an increase in heat transfer between the solid and gas phases. The thermal energy storage takes place on the solid particles to ensure sufficient energy for processing the endothermic reactions from the SRM method. A promising point is reported by thermal interactions at the surface of catalyst particles where SRM reactions are thermochemically converted. However, the energy balance for the temperature of reforming reactions at the surface of catalytic particles is given as follows.
- -
Energy balance at the surface of catalytic particles
The solid phase’s effective thermal conductivity is defined as a function of the thermal conductivity of the gaseous mixture according to Equation below.
The suitable initial and boundary conditions from Equations are given as follows.
- -
Initial condition at t = 0
- -
At the inlet face surface (upper) of the reaction zone from PBMR, i.e., z = 0+
- -
At the outlet face surface (bottom) of the reaction zone from PBMR, i.e., z = L
2.3.3. Transport Equations of Chemical Components in Reaction Zone
Based on assumptions mentioned in
Section 2.3, chemical components on the reaction zone from PBMR are modelled by Equation (35). Equation (35) reports only the transport equations for chemical components i (i = CH
4, H
2O, CO and CO
2) without H
2 as follows.
- -
Transport equations of chemical components i in reaction zone
The suitable initial and boundary conditions from Equations are presented as follows.
- -
Initial condition, i.e., t = 0
- -
At the inlet face surface (upper) of the reaction zone from PBMR, i.e., z = 0+
- -
At the outlet face surface (bottom) of the reaction zone from PBMR, i.e., z = L
As H2 permeates the Pd-based dense membrane, a transport equation is specifically developed for H2 inside the reaction zone. Thus, this equation is reported by Equation (39) as follows.
- -
Transport equation of H2 in reaction zone
- -
Initial condition, i.e., t = 0
- -
At the inlet face surface (upper) of the reaction zone from PBMR, i.e., z = 0+
- -
At the outlet face surface (bottom) of the reaction zone from PBMR, i.e., z = L
2.3.4. Transport of H2 within the Permeation Zone
The permeation rate of H
2 through the membrane from the high-pressure zone into the permeation zone is assumed to obey the half power pressure law. However, the permeation rate of H
2 from the reaction zone into the permeation zone is given as follows.
A differential model allows us to quantify the amount of H
2 in the permeation side, but the model has to be consistent with the permeation rate which passes through the Pd-based dense membrane. Thus, a transport equation is developed to estimate the production of H
2 in the permeation zone from PBMR as follows.
- -
At the inlet face surface (upper) of the permeation zone from PBMR, i.e., z = 0+
- -
At the outlet face surface (bottom) of the permeation zone from PBMR, i.e., z = L
2.4. Solution of the Mathematical Model
2.4.1. Application of the CIEA Method
The CIEA method can be considered as a powerful technique because of its low computer time relative to traditional methods (finite difference, finite volume, finite element, etc.). The CIEA methodology has been used to transform the NPDE system (Equations (24), (30), (35) and (39)) into an NODE system using the boundary conditions (Equations (28), (29), (33), (34), (37), (38), (41) and (42)) of each NPDE. The coefficients of Equations (47)–(50) can be found in
Appendix A of this work. Thus, NODEs (Equations (47)–(50)) are reported as follows.
- -
Transformed NODE for the gas phase
- -
Transformed NODE for the solid phase
- -
Transformed NODE for chemical components i in reaction zone
- -
Transformed NODE for H2 in reaction zone
2.4.2. Approximation of the Full Solution
Several numerical methods have been proposed to solve NPDE systems [
15]. The numeric methodology’s selection is limited to the desired accuracy on the consistency and robustness of numerical data of the NPDE system. Regarding NODEs, Equations (47)–(50) have been solved by the Runge-Kutta Gill method as well as the NODE in the permeation zone (Equation (44)). On the other hand, the full solution is obtained from Equations (51)–(54) as follows.
- -
Gas phase’s full solution
- -
Solid phase’s full solution
- -
Full solution for chemical components i
- -
Full solution for chemical components H2.
4. Conclusions and Future Work
The present work has been focused on a numerical analysis of physical-mathematical modelling and computer simulation to describe the performance of reformers for the production of H2 using a reference method of steam reforming CH4. The model equations that describe the gas temperature in the reaction zone, endothermic reaction’s temperature in the reaction zone, molar flow of the components i in the reaction zone, molar flow of H2 in the reaction zone, and molar flow of H2 in the permeation zone have been reported and discussed. As a solution to the model equations, the main focus has been the CIEA method as a powerful technique to reduce the NPDE system of this work into a NODE system using the boundary conditions of each NPDE. The work’s results highlighted the importance of the mathematical model developed to describe the performance from FBR and PBMR. In this context, the main conclusions are summarized as follows.
The computed results of MFCs are compared with experimental results from the literature under same operating conditions for FBR and PBMR.
Figure 2a compares the computed results of MFCs against experimental results of MFCs of components CH
4, CO, CO
2, H
2, and H
2O at the outlet from FBR with AREs around
,
,
and
respectively. On the other hand,
Figure 2b also confronts the simulated results of MFCs against the experimental results of the MFCs of components CH
4, CO, CO
2, H
2, and H
2O at the outlet from FMBR with AREs of about
,
,
,
and
, respectively.
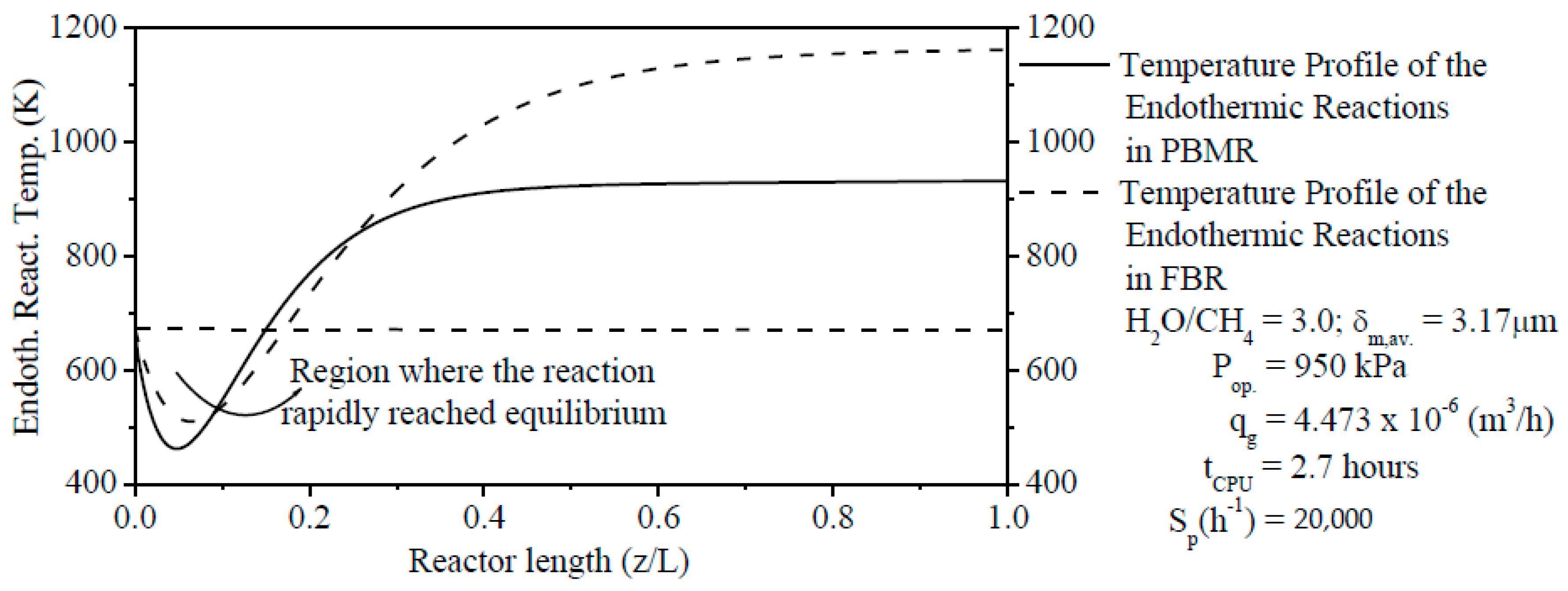
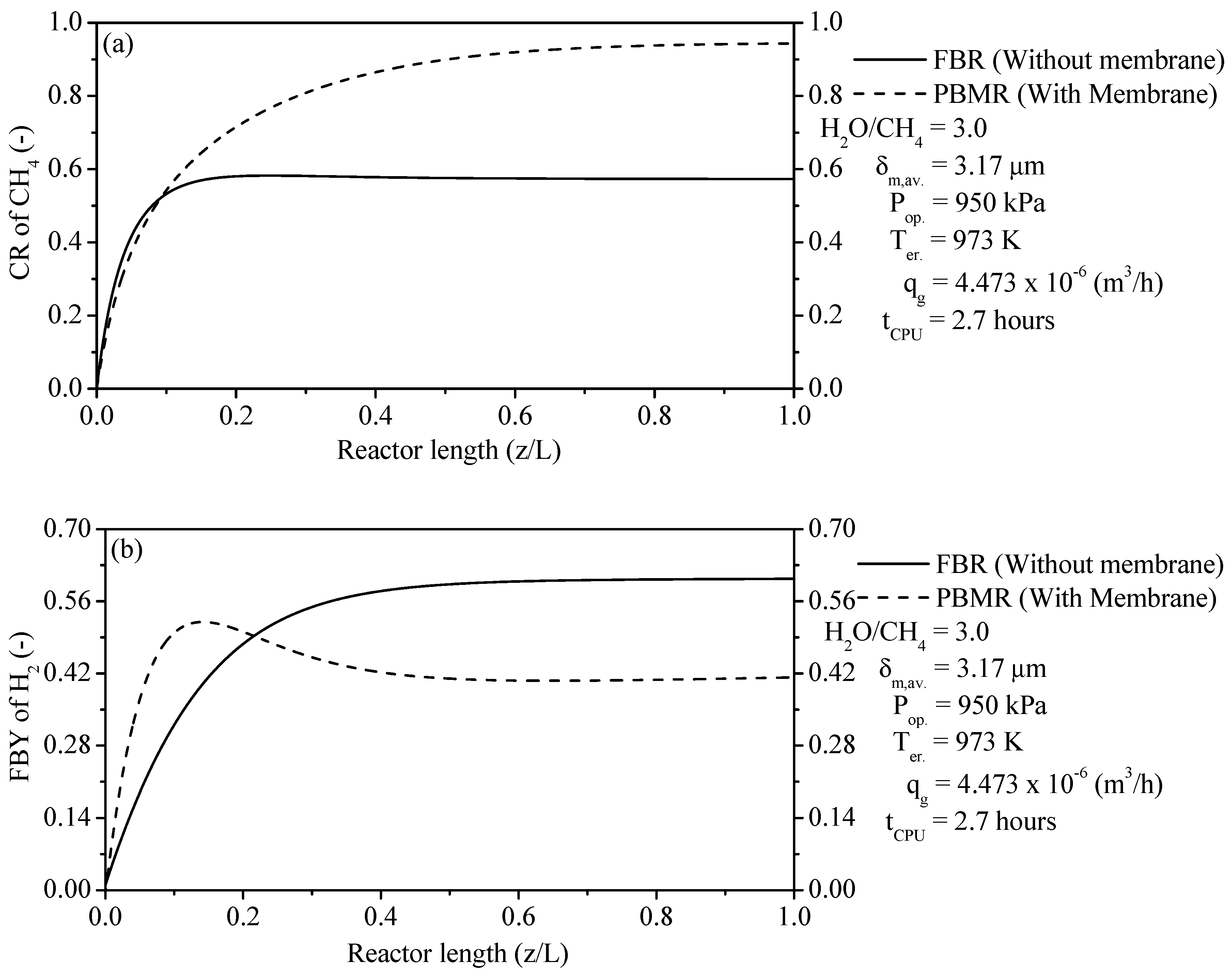
The thermochemical conversion of CH4 and production of H2 are considered the two main parameters of FBR and PBMR. The ERT has a positive effect on these two parameters. When comparing the two reformer types, the production of H2 and thermochemical conversion of CH4 on the PBMR can be obtained at lower ERT due to the removal of H2 through a membrane from PBMR. This point is a great advantage from PBMR in relation to FBR because PBMR can be operated at lower ERT than FBR and to obtain higher results of these two parameters.
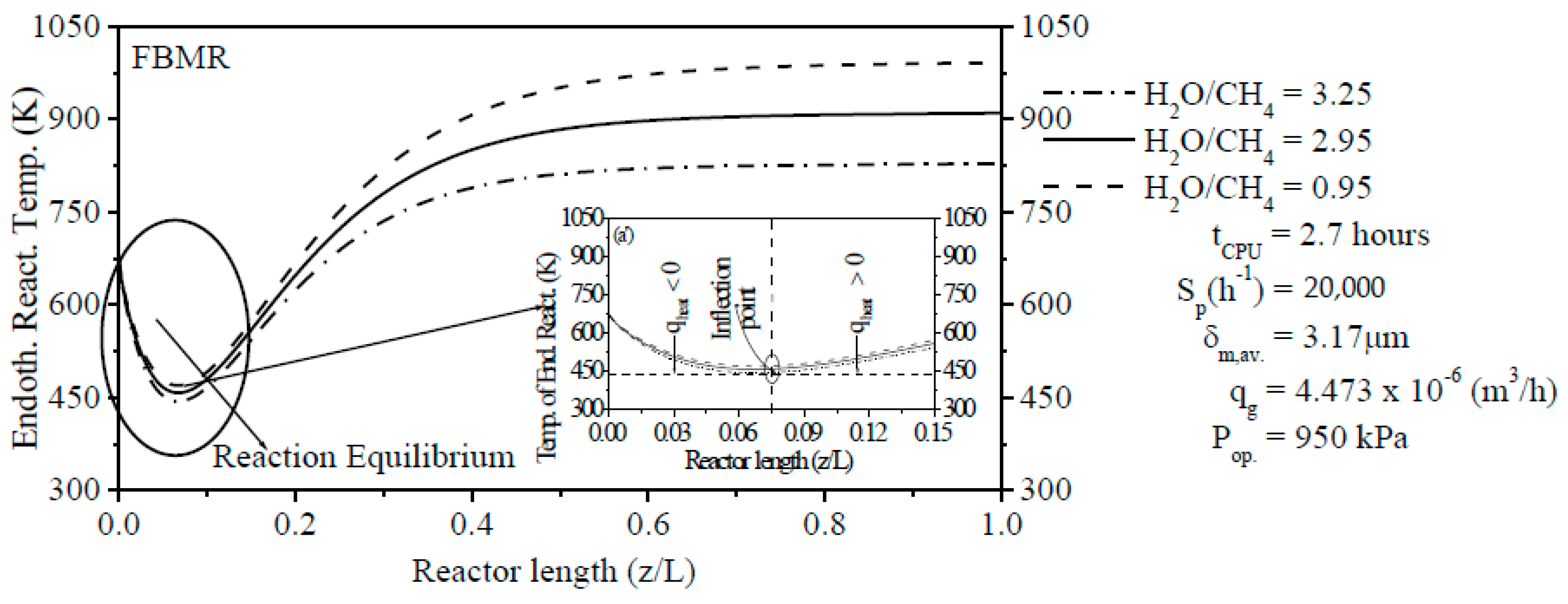
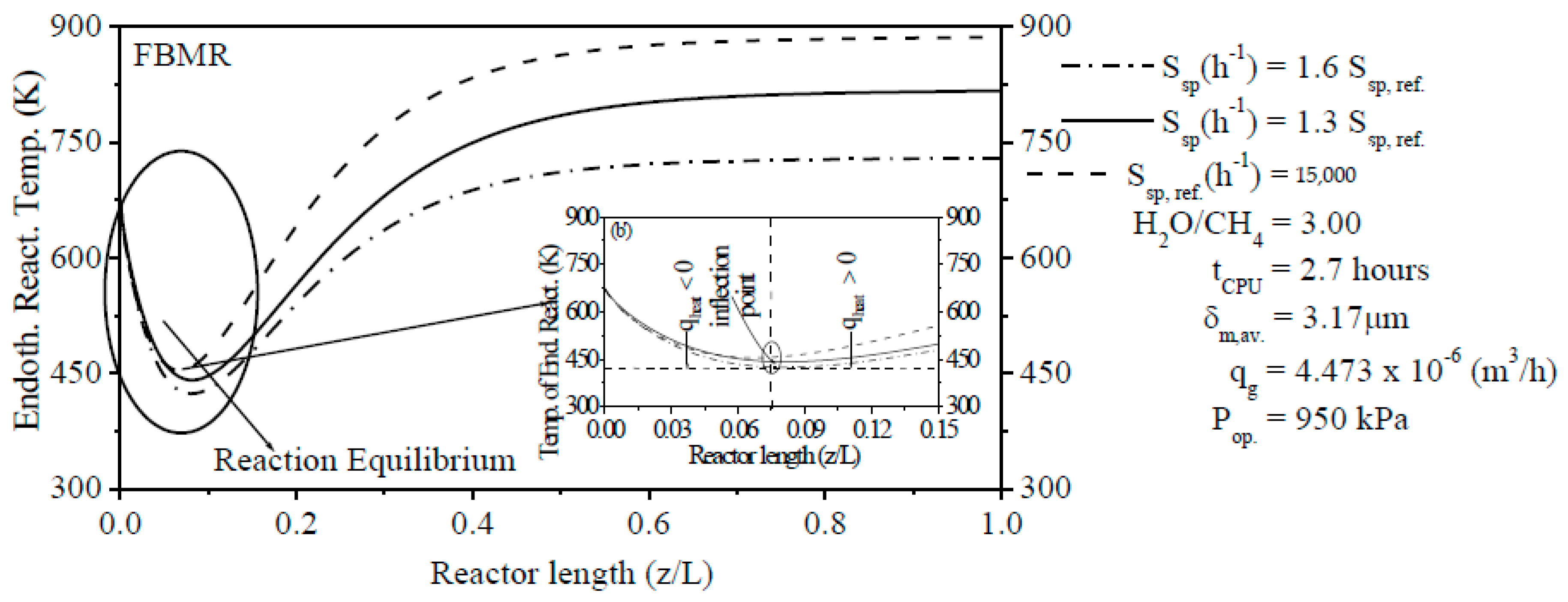
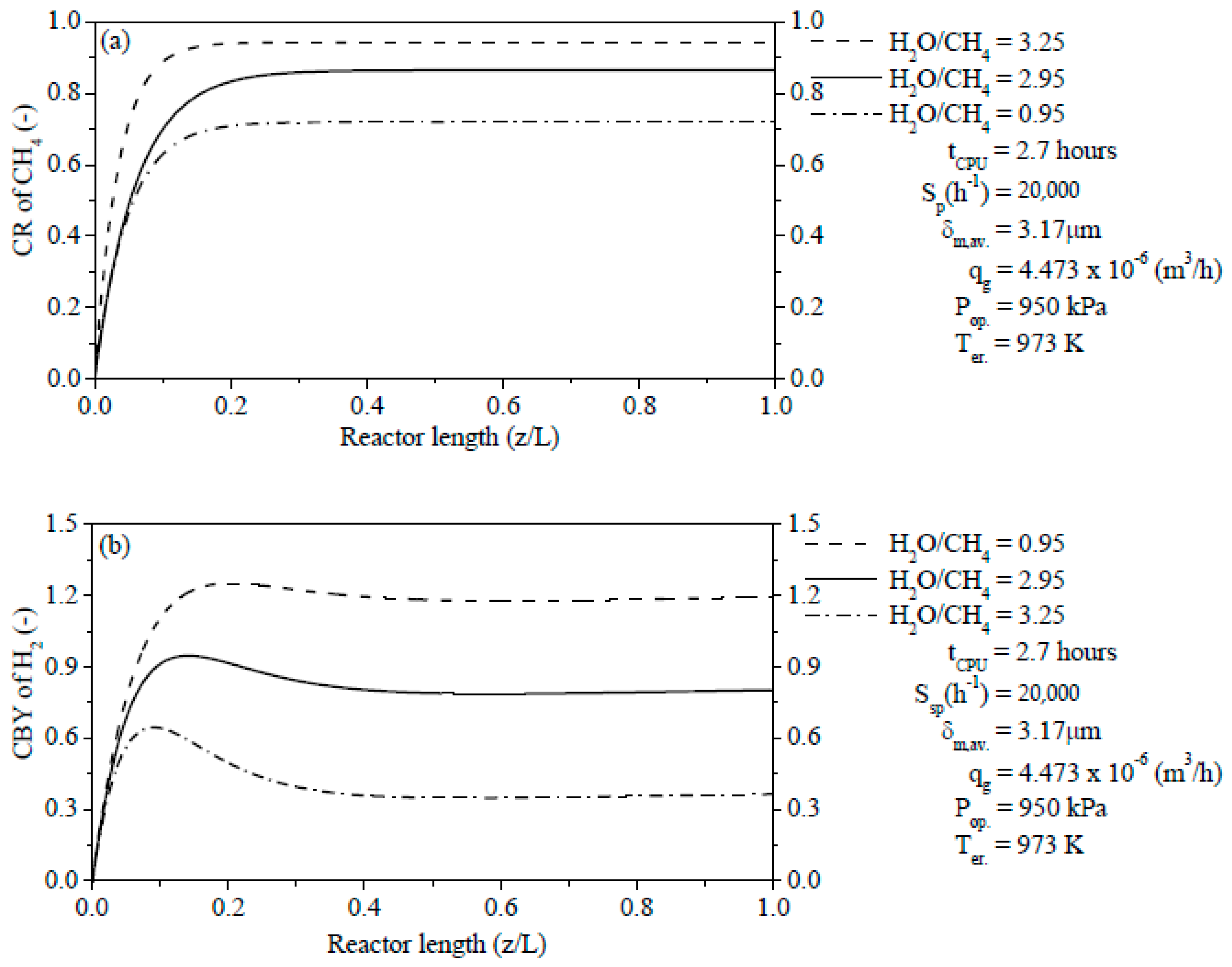
The steam content has an important effect on the ERT in PBMR, i.e., when the H2O/CH4 ratio is low, the ERT is notably increased. In contrast, when the H2O/CH4 ratio is high, the ERT is remarkably decreased. The Ssp has also a significant effect on the ERT and CR of CH4. Similar to steam content, when the Ssp is small, the ERT is remarkably increased. On the other hand, the ERT is reduced with the increase of the Ssp. For a lower Ssp value, we observed a higher CR of CH4 in PBMR. Conversely, a higher Ssp value points to a low CR of CH4 in PBMR.
The effect of the H2O/CH4 ratio played a significant role on the CR of CH4 and CBY of H2. An increase of the H2O/CH4 ratio is associated with a significant improvement due to the coke reduction on the catalytic particles, i.e., there is a rise of the CR of CH4. Conversely, a decrease of the H2O/CH4 ratio has a poor effect owing to the increase of the coke formation and thus the CR of CH4 is reduced. Unlike the CR of CH4, a rise of the H2O/CH4 ratio led to a decrease of the CBY of H2. On the other hand, a reduction of the H2O/CH4 ratio led to an increase of the CBY of H2.
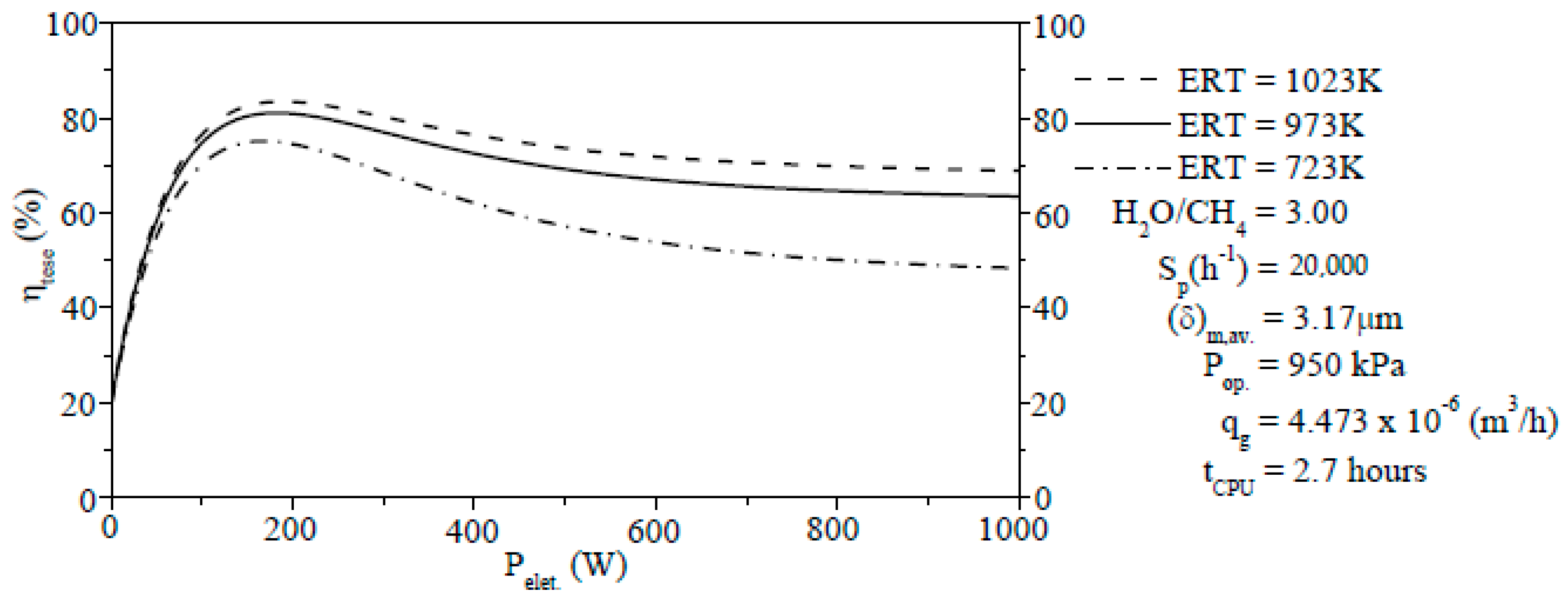
An increase of the ERT led to a rise of the ηtese. Meanwhile, a reduction of ERT resulted in an decrease of the ηtese. A rise of the ERT has a positive effect on the chemical storage, i.e., the chemical energy gradually rose with the increase of ERT (156.01 W (573 K)–384.96 W (973 K)). The selectivity of components (H2, CO, CO2, and CH4) was computed for different δm. Among these components, H2 showed higher amounts (60.98% to 73.18%) of the selectivity.
In future research, the exploration of novel materials like open-cell foams can be explored in the context of reforming reactions. Solid open-cell foams constitute a class of porous materials with low density and improved thermal properties. In addition, open-cell foams can be considered as potential candidates for catalyst support in the gas-solid reaction field due to their high external surface area, high porosity, and low drop pressure. Thus, solid open-cell foams are future trends for reforming methods such as steam reforming of CH4, dry reforming of CH4, etc.