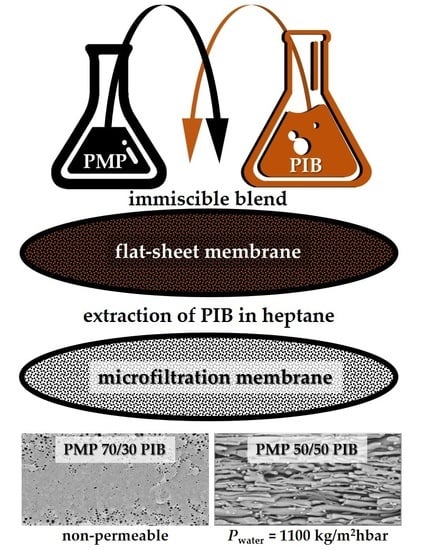
Formation of Microfiltration Membranes from PMP/PIB Blends: Effect of PIB Molecular Weight on Membrane Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Membrane Formation
2.2. Methods
3. Results and Discussion
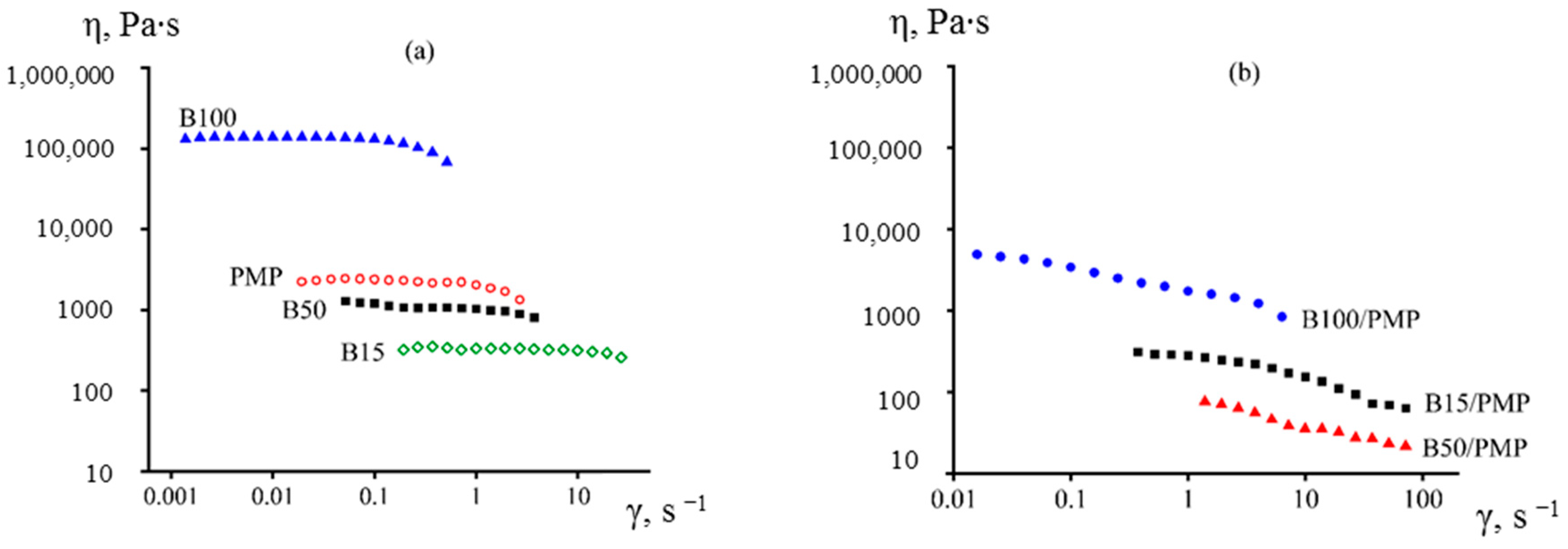
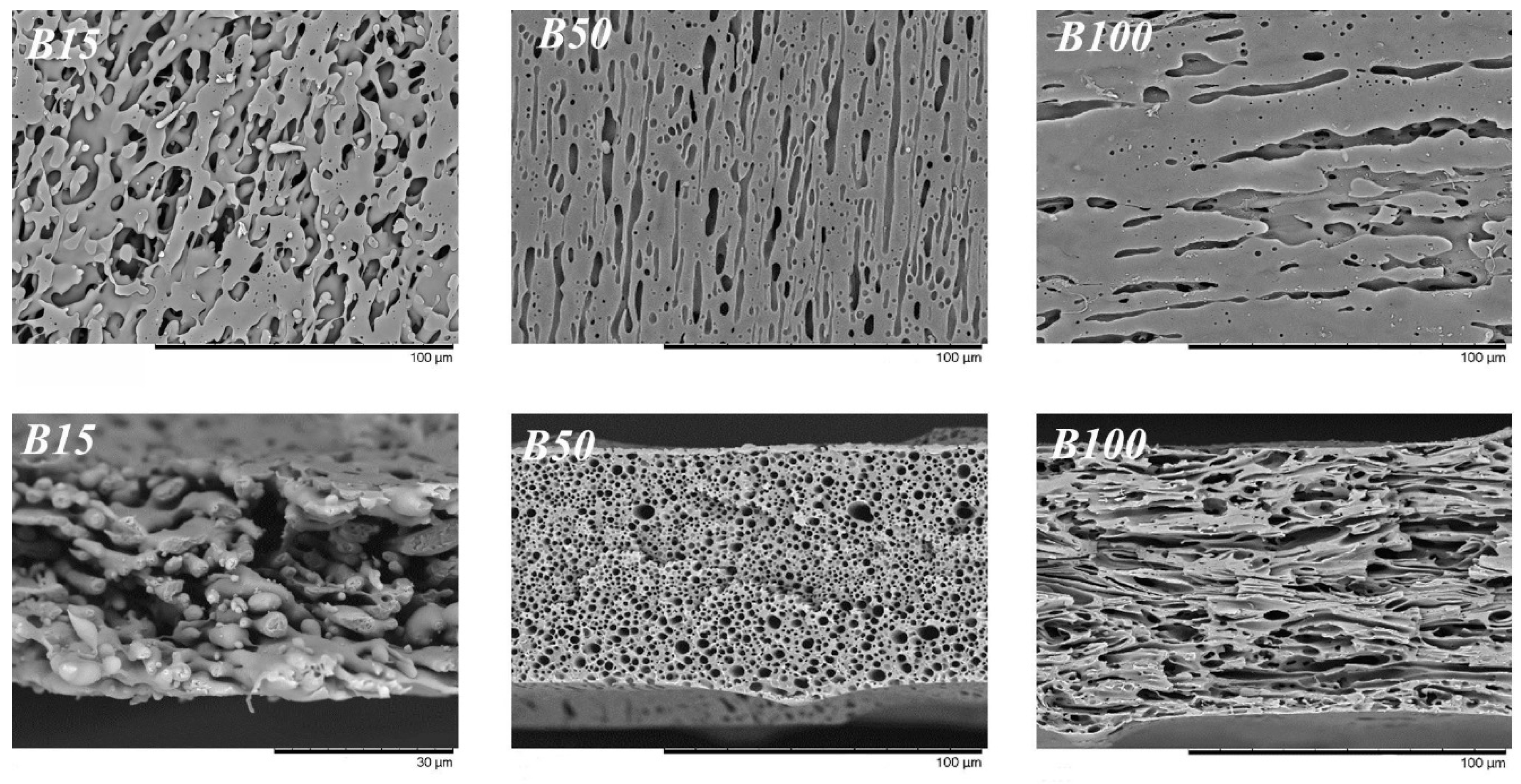
3.1. Effect of PIB Molecular Weight
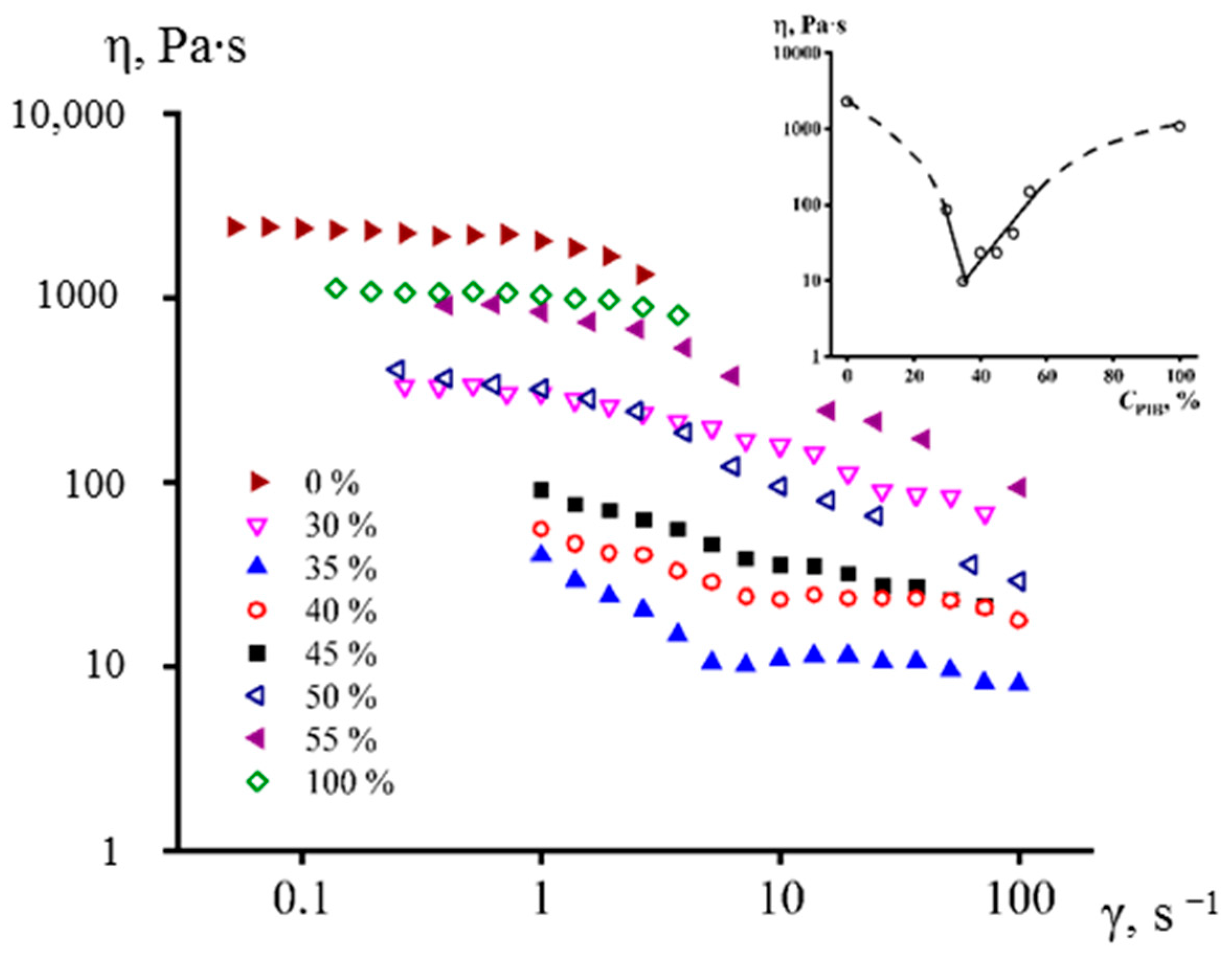
3.2. Effect of PMP/PIB Ratio
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Strathmann, H.; Kock, K. The formation mechanism of phase inversion membranes. Desalination 1977, 21, 241–255. [Google Scholar] [CrossRef]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef]
- Wienk, I.M.; Boom, R.M.; Beerlage, M.A.M.; Bulte, A.M.W.; Smolders, C.A.; Strathmann, H. Recent advances in the formation of phase inversion membranes made from amorphous or semi-crystalline polymers. J. Membr. Sci. 1996, 113, 361–371. [Google Scholar] [CrossRef]
- Kimmerle, K.; Strathmann, H. Analysis of the structure-determining process of phase inversion membranes. Desalination 1990, 79, 283–302. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Anokhina, T.S.; Ignatenko, V.Y.; Brantseva, T.V.; Volkov, A.V.; Antonov, S.V. Diffusion and phase separation at the morphology formation of cellulose membranes by regeneration from N-methylmorpholine N-oxide solutions. Cellulose 2018, 25, 2515–2530. [Google Scholar] [CrossRef]
- Wilczynski, A.P.; Liu, C.H.; Hsiao, C.C. Mechanics of polymer craze. J. Appl. Phys. 1977, 48, 1149–1154. [Google Scholar] [CrossRef]
- Sadeghi, F.; Ajji, A.; Carreau, P.J. Analysis of microporous membranes obtained from polypropylene films by stretchin. J. Memb. Sci. 2007, 292, 62–71. [Google Scholar] [CrossRef]
- Michaels, A.S.; Bixler, H.J.; Hopfenberg, H.B. Controllably crazed polystyrene: Morphology and permeability. J. Appl. Polym. Sci. 1968, 12, 991–1007. [Google Scholar] [CrossRef]
- Jacques, C.H.M.; Hopfenberg, H.B.; Stannett, V. The effect of orientation on the morphology and kinetics of solvent crazing in polystyrene. J. Appl. Polym. Sci. 1974, 18, 223–233. [Google Scholar] [CrossRef]
- He, D.; Susanto, H.; Ulbricht, M. Photo-irradiation for preparation, modification and stimulation of polymeric membrane. Progr. Polym. Sci. 2009, 34, 62–98. [Google Scholar] [CrossRef]
- Apel, P. Track etching technique in membrane technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publisher: London, UK, 1997; p. 563. [Google Scholar]
- Matsuyama, H.; Okafuji, H.; Maki, T.; Teramoto, M.; Tsujioka, N. Membrane formation via thermally induced phase separation in polypropylene/polybutene/diluent system. J. Appl. Polym. Sci. 2002, 84, 1701–1708. [Google Scholar] [CrossRef]
- Esquirol, A.L.; Sarazin, P.; Virgilio, N. Tunable Porous Hydrogels from Cocontinuous Polymer Blends. Macromolecules 2014, 47, 3068–3075. [Google Scholar] [CrossRef]
- Zeng, M.; Fang, Z.; Xu, C. Novel method of preparing microporous membrane by selective dissolution of chitosan/polyethylene glycol blend membrane. J. Appl. Polym. Sci. 2004, 91, 2840–2847. [Google Scholar] [CrossRef]
- Zeng, M.; Fang, Z.; Xu, C. Effect of compatibility on the structure of the microporous membrane prepared by selective dissolution of chitosan/synthetic polymer blend membrane. J. Membr. Sci. 2004, 230, 175–181. [Google Scholar] [CrossRef]
- Trifkovic, M.; Hedegaard, A.; Huston, K.; Sheikhzadeh, M.; Macosko, C.W. Porous films via PE/PEO cocontinuous blends. Macromolecules 2012, 45, 6036–6044. [Google Scholar] [CrossRef]
- Anokhina, T.S.; Ilyin, S.O.; Ignatenko, V.Y.; Bakhtin, D.S.; Kostyuk, A.V.; Antonov, S.V.; Volkov, A.V. Formation of Porous Films with Hydrophobic Surface from a Blend of Polymers. Polym. Sci. Ser. A 2019, 61, 619–626. [Google Scholar] [CrossRef]
- Ignatenko, V.Y.; Anokhina, T.S.; Ilyin, S.O.; Kostyuk, A.V.; Bakhtin, D.S.; Antonov, S.V.; Volkov, A.V. Fabrication of microfiltration membranes from polyisobutylene/polymethylpentene blends. Polym. Int. 2019. [Google Scholar] [CrossRef]
- Chandavasu, C.; Xanthos, M.; Sirkar, K.K.; Gogos, C.G. Fabrication of microporous polymeric membranes by melt processing of immiscible blends. J. Membr. Sci. 2003, 211, 167–175. [Google Scholar] [CrossRef]
- Femmer, T.; Kuehne, A.J.C.; Torres-Rendon, J.; Walther, A.; Wessling, M. Print your membrane: Rapid prototyping of complex 3D-PDMS membranes via a sacrificial resist. J. Memb. Sci. 2015, 478, 12–18. [Google Scholar] [CrossRef]
- Fritzmann, C.; Hausmann, M.; Wiese, M.; Wessling, M.; Melin, T. Microstructured spacers for submerged membrane filtration systems. J. Memb. Sci. 2013, 446, 189–200. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tan, W.S.; An, J.; Chua, C.K.; Tang, C.Y.; Fane, A.G.; Tzyy, H.C. The potential to enhance membrane module design with 3D printing technology. J. Memb. Sci. 2016, 499, 480–490. [Google Scholar] [CrossRef]
- Femmer, T.; Kuehne, A.J.C.; Wessling, M. Print your own membrane: Direct rapid prototyping of polydimethylsiloxane. Lab Chip 2014, 14, 2610–2613. [Google Scholar] [CrossRef] [PubMed]
- Breiter, S. Membranes for oxygenators and plasma filters. In Biomaterials for Artificial Organs; Lysaght, M., Webster, T.J., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–33. [Google Scholar]
- Pflaum, M.; Peredo, A.S.; Dipresa, D.; De, A.; Korossis, S. Membrane bioreactors for (bio-)artificial lung. In Current Trends and Future Developments on (Bio-)Membranes; Basile, A., Annesini, M.C., Piemonte, V., Charcosset, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 45–75. [Google Scholar]
- Kim, J. Recent Progress on Improving the Sustainability of Membrane Fabrication. J. Membr. Sci. Res. 2019. [Google Scholar] [CrossRef]
- Grace, H.P. Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar] [CrossRef]
- Minale, M.; Moldenaers, P.; Mewis, J. Effect of shear history on the morphology of immiscible polymer blends. Macromolecules 1997, 30, 5470–5475. [Google Scholar] [CrossRef]
- Willemse, R.C.; Ramaker, E.J.J.; Van Dam, J.; De Boer, A.P. Morphology development in immiscible polymer blends: Initial blend morphology and phase dimensions. Polymer 1999, 40, 6651–6659. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.L.; Favis, B.D. The role of the blend interface type on morphology in cocontinuous polymer blends. Macromolecules 2005, 35, 2005–2016. [Google Scholar] [CrossRef]
- Minale, M. Models for the deformation of a single ellipsoidal drop: A review. Rheol. Acta 2010, 49, 789–806. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase behavior and rheology of miscible and immiscible blends of linear and hyperbranched siloxane macromolecules. Mater. Today Commun. 2020, 22, 100833. [Google Scholar] [CrossRef]
- Barnes, H.A. A review of the slip (wall depletion) of polymer solutions, emulsions and particle suspensions in viscometers: Its cause, character, and cure. J. Non-Newton. Fluid Mech. 1995, 56, 221–251. [Google Scholar] [CrossRef]
- Hatzikiriakos, S.G. Wall slip of molten polymers. Prog. Polym. Sci. 2012, 37, 624–643. [Google Scholar] [CrossRef]
- Kostyuk, A.; Ignatenko, V.; Smirnova, N.; Brantseva, T.; Ilyin, S.; Antonov, S. Rheology and adhesive properties of filled PIB-based pressure-sensitive adhesives. I. Rheology and shear resistance. J. Adhes. Sci. Technol. 2015, 29, 1831–1848. [Google Scholar] [CrossRef]
- Brantseva, T.; Antonov, S.; Kostyuk, A.; Ignatenko, V.; Smirnova, N.; Korolev, Y.; Tereshin, A.; Ilyin, S. Rheological and adhesive properties of PIB-based pressure-sensitive adhesives with montmorillonite-type nanofillers. Eur. Polym. J. 2016, 76, 228–244. [Google Scholar] [CrossRef]
- Utracki, L.A. On the viscosity-concentration dependence of immiscible polymer blends. J. Rheol. 1991, 35, 1615–1637. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G.; Shaulov, A.Y.; Stegno, E.V.; Berlin, A.A.; Patlazhan, S.A. Rheological properties of polyethylene/metaboric acid thermoplastic blends. Rheol. Acta 2014, 53, 467–475. [Google Scholar] [CrossRef]
- Benderly, D.; Siegmann, A.; Narkis, M. Polymer encapsulation of glass filler in ternary PP/PA-6/glass blends. Polym. Compos. 1996, 17, 86–95. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Petrukhina, N.N.; Kostyuk, A.V.; Dzhabarov, E.G.; Filatova, M.P.; Antonov, S.V.; Maksimov, A.L. Hydrogenation of Indene–Coumarone Resin on Palladium Catalysts for Use in Polymer Adhesives. Russ. J. Appl. Chem. 2019, 92, 1143–1152. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Kostyuk, A.V.; Ignatenko, V.Y.; Smirnova, N.M.; Alekseeva, O.A.; Petrukhina, N.N.; Antonov, S.V. The Effect of Tackifier on the Properties of Pressure-Sensitive Adhesives Based on Styrene–Butadiene–Styrene Rubber. Russ. J. Appl. Chem. 2018, 91, 1945–1956. [Google Scholar] [CrossRef]
- Fisher, I.; Siegmann, A.; Narkis, M. The effect of interface characteristics on the morphology, rheology and thermal behavior of three-component polymer alloys. Polym. Compos. 2002, 23, 34–48. [Google Scholar] [CrossRef]

| PIB | MPIB, kDa | Melting | Crystallization | σadh, kPa | σstr, MPa | E, GPa | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tm, °C | ΔHm, J/g | ΔHm/CPMP, J/g | Tcr, °C | ΔHcr, J/g | ΔHcr/CPMP, J/g | |||||
| - | - | 233.0 | 24.6 | 24.6 | 202.9 | 26.1 | 26.1 | 0 | 14.4 | 0.36 |
| B15 | 75 | 228.6 | 16.6 | 30.3 | 205.4 | 11.7 | 21.3 | 10 | 5.1 | 0.17 |
| B50 | 340 | 229.5 | 13.9 | 25.4 | 204.1 | 12.2 | 22.2 | 0.8 | 9.1 | 0.20 |
| B100 | 1100 | 229.6 | 16.4 | 29.8 | 203.8 | 11.7 | 21.3 | 0.3 | 8.2 | 0.14 |
| PIB | Pwater, kg/m2hbar | R240 nm, % | R38 nm, % |
|---|---|---|---|
| B15 | 31,000 | 93 | 8 |
| B50 | 1.9 | 99 | 39 |
| B100 | 76 | 58 | 3 |
| CPIB(B50), % | σstr, MPa | E, GPa | Pwater, kg/m2hbar | R240 nm, % | R38 nm, % |
|---|---|---|---|---|---|
| 0 | 14.4 | 0.36 | non-permeable | ||
| 30 | 13.2 | 0.27 | |||
| 35 | 11.7 | 0.22 | |||
| 40 | 9.5 | 0.21 | 0.05 | - | - |
| 45 | 9.1 | 0.20 | 1.9 | 99 | 39 |
| 50 | 7.9 | 0.13 | 1100 | 91 | 36 |
| 55 | 3.3 | 0.09 | 3790 | 87 | 29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyin, S.; Ignatenko, V.; Anokhina, T.; Bakhtin, D.; Kostyuk, A.; Dmitrieva, E.; Antonov, S.; Volkov, A. Formation of Microfiltration Membranes from PMP/PIB Blends: Effect of PIB Molecular Weight on Membrane Properties. Membranes 2020, 10, 9. https://doi.org/10.3390/membranes10010009
Ilyin S, Ignatenko V, Anokhina T, Bakhtin D, Kostyuk A, Dmitrieva E, Antonov S, Volkov A. Formation of Microfiltration Membranes from PMP/PIB Blends: Effect of PIB Molecular Weight on Membrane Properties. Membranes. 2020; 10(1):9. https://doi.org/10.3390/membranes10010009
Chicago/Turabian StyleIlyin, Sergey, Viktoria Ignatenko, Tatyana Anokhina, Danila Bakhtin, Anna Kostyuk, Evgenia Dmitrieva, Sergey Antonov, and Alexey Volkov. 2020. "Formation of Microfiltration Membranes from PMP/PIB Blends: Effect of PIB Molecular Weight on Membrane Properties" Membranes 10, no. 1: 9. https://doi.org/10.3390/membranes10010009
APA StyleIlyin, S., Ignatenko, V., Anokhina, T., Bakhtin, D., Kostyuk, A., Dmitrieva, E., Antonov, S., & Volkov, A. (2020). Formation of Microfiltration Membranes from PMP/PIB Blends: Effect of PIB Molecular Weight on Membrane Properties. Membranes, 10(1), 9. https://doi.org/10.3390/membranes10010009






.png)